Key Points
Question
Does the addition of hydroxychloroquine sulfate to gemcitabine hydrochloride and nab-paclitaxel improve overall survival among patients with metastatic pancreatic adenocarcinoma?
Findings
In this phase 2 randomized clinical trial of 112 patients, the addition of hydroxychloroquine to chemotherapy did not improve overall survival at 12 months. A statistically significant increase in the overall response rate from 21% to 38% was shown with the addition of hydroxychloroquine.
Meaning
Hydroxychloroquine added to chemotherapy did not improve overall survival among patients with metastatic pancreatic cancer.
Abstract
Importance
Autophagy is a mechanism of treatment resistance to chemotherapy that has a role in the maintenance of pancreatic cancer. Hydroxychloroquine sulfate (HCQ) is an inhibitor of autophagy that inhibits the fusion of the autophagosome to the lysosome.
Objective
To determine whether HCQ improves overall survival at 1 year in combination with gemcitabine hydrochloride and nab-paclitaxel (GA) among patients with metastatic pancreatic cancer.
Design, Setting, and Participants
Open-label, phase 2 randomized clinical trial conducted between March 18, 2013, and November 16, 2017, at the University of Pennsylvania, HonorHealth, and The Johns Hopkins University among 112 patients with previously untreated metastatic or advanced pancreatic ductal adenocarcinoma, Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate marrow and organ function. All efficacy analyses were performed for the intention-to-treat population.
Interventions
Patients were randomized in a 1:1 ratio to receive GA with or without HCQ. All patients received standard doses of GA, and those randomized to receive HCQ were treated continuously with 600 mg orally twice daily.
Main Outcome and Measure
Overall survival at 1 year.
Results
A total of 112 patients (45 women and 67 men; median age, 65 years; range, 43-86 years) were enrolled; 55 were randomized to receive GA plus HCQ, and 57 to receive GA. Overall survival at 12 months was 41% (95% CI, 27%-53%) in the HCQ group and 49% (95% CI, 35%-61%) in the non-HCQ group. Median progression-free survival was 5.7 months (95% CI, 4.0-9.3 months) in the HCQ group and 6.4 months (95% CI, 4.5-7.6 months) in the non-HCQ group. Median overall survival was 11.1 months (95% CI, 9.0-14.2 months) in the HCQ group and 12.1 months (95% CI, 9.3-15.5 months) in the non-HCQ group. Overall response rate was 38.2% (n = 21) in the HCQ group and 21.1% (n = 12) in the non-HCQ group (P = .047). Treatment-related grade 3 or 4 adverse events that differed between the HCQ and non-HCQ groups were neutropenia (23 of 54 [42.6%] vs 12 of 53 [22.6%]), anemia (2 of 54 [3.7%] vs 9 of 53 [17.0%]), fatigue (4 of 54 [7.4%] vs 0), nausea (5 of 54 [9.3%] vs 0), peripheral neuropathy (7 of 54 [13.0%] vs 3 of 53 [5.7%]), visual changes (3 of 54 [5.6%] vs 0), and neuropsychiatric symptoms (3 of 54 [5.6%] vs 0).
Conclusions and Relevance
The addition of HCQ to block autophagy did not improve the primary end point of overall survival at 12 months. These data do not support the routine use of GA plus HCQ for metastatic pancreatic cancer in the absence of a biomarker. However, improvement seen in the overall response rate with HCQ may indicate a role for HCQ in the locally advanced setting, where tumor response may permit resection.
Trial Registration
ClinicalTrials.gov identifier: NCT01506973
This phase 2 randomized clinical trial examines whether hydroxychloroquine sulfate improves overall survival at 1 year in combination with gemcitabine hydrochloride and nab-paclitaxel among previously untreated adults with metastatic pancreatic cancer.
Introduction
Chemotherapy remains the only effective treatment of metastatic pancreatic ductal adenocarcinoma (PDAC).1 Both FOLFIRINOX (fluorouracil, irinotecan, and oxaliplatin) and gemcitabine hydrochloride with nab-paclitaxel (GA) improve overall survival (OS) compared with gemcitabine alone and are associated with occasional long-term survival, suggesting a substantial effect on a subset of patients.2,3 Nonetheless, essentially all patients experience acquired resistance to therapy, as manifested by regrowth of the tumor.
Autophagy is a cellular defense mechanism thought to have evolved to protect cells from adverse environmental conditions, including nutritional deprivation, hypoxia, or therapeutic stress. On activation, autophagy mediates regulated catabolism of cellular organelles, which are encapsulated first in autophagosomes and metabolized when these fuse to lysosomes. Inhibition of autophagy can be accomplished pharmacologically with hydroxychloroquine sulfate (HCQ), which inhibits the fusion of the autophagosome to the lysosome.4 Amaravadi et al5 first demonstrated that targeting autophagy with chloroquine derivatives enhanced the efficacy of chemotherapy. Pancreatic cancer, in particular, may be especially reliant on autophagy for growth and survival, and multiple preclinical studies have demonstrated the activity of HCQ in pancreatic cancer models.6,7,8
A phase 1 trial of GA with HCQ found that all agents were tolerable at full doses.9 Based on these findings, we report herein the subsequent randomized phase 2 trial of GA vs GA plus HCQ for previously untreated patients with metastatic pancreatic cancer.
Methods
Patient Selection
Eligible patients were adults with previously untreated metastatic or advanced PDAC with measurable disease.10 Adjuvant chemotherapy or radiotherapy was allowed if it had been administered at least 4 months prior. All patients had an Eastern Cooperative Oncology Group performance status of 0 or 1 and adequate marrow and organ function. Patients with a known allergy to HCQ, glucose-6-phosphate-dehydrogenase deficiency, severe psoriasis, porphyria, macular degeneration, or severe diabetic retinopathy were ineligible because of potential HCQ toxic effects in individuals with these conditions. The study was approved by the institutional review board at the University of Pennsylvania, HonorHealth, and The Johns Hopkins University and was conducted in accordance with US and international standards for Good Clinical Practice (US Food and Drug Administration Title 21 part 312 and International Conference on Harmonization guidelines). Written informed consent was obtained from each patient prior to study entry.
Study Design and Treatment
This study was a 3-institution, open-label, phase 2 randomized clinical trial conducted from March 18, 2013, to November 16, 2017 (trial protocol in Supplement 1). Patients were randomized in a 1:1 ratio using simple block randomization to the treatment group with GA plus HCQ or to GA alone. No placebo was given to the control group.
All patients received GA at standard doses. Dose modifications of chemotherapy were consistent with the recommendations on the US Food and Drug Administration labels, and alternative dosing strategies (eg, chemotherapy on days 1 and 8 of a 21-day cycle) were permitted. If one agent was stopped because of toxic effects, the other could be continued.
Patients randomized to the HCQ group were treated with 600 mg of HCQ orally twice daily throughout the 28-day cycle. In the case of any grade 3 or greater adverse event at least possibly related to HCQ, HCQ was withheld until the adverse event resolved to grade 1 or less and was reinitiated at either the same dose or at a reduced dose at the discretion of the investigator.
Assessments
The investigators evaluated tumor response every 8 weeks by means of computed tomography or magnetic resonance imaging. Serial measurements of the carbohydrate antigen (CA) 19-9 level were performed at baseline and every 4 weeks thereafter. Patients were followed up for survival until death or withdrawal of consent for follow-up.
Study End Points
The primary end point of the study was 1-year OS; secondary end points included toxic effects, progression-free survival (PFS), overall response rate, and median OS. Additional end points analyzed were disease control rate (defined as stable disease for at least 16 weeks or partial or complete response) and maximum reduction in CA 19-9 level from baseline. Treatment-related adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.11
Statistical Analysis
All efficacy analyses were performed for the intention-to-treat population. Final analysis was performed using a data cutoff date of November 16, 2017. Overall survival was analyzed with the use of the Kaplan-Meier method and a log-rank test. Progression-free survival and overall response rate were determined by investigator assessments. The sample size calculations were based on an estimated median OS of 12.2 months in the control group.12 A sample size of 90 patients yields 81% power to detect an improvement in 1-year OS of 23% (from 50% to 73%), with a 1-sided significance α level of .10 assuming a binomial distribution.
Genomic Analysis
Next-generation sequencing was performed for the subset of patients for whom there was sufficient archival tissue. A next-generation sequencing panel from the Clinical Laboratory Improvement Amendments–certified University of Pennsylvania Center for Personal Diagnostics (eAppendix in Supplement 2) or a commercially available next-generation sequencing panel from Foundation Medicine were used. When DNA quantity was limited, next-generation sequencing was performed using a limited platform of 20 genes, including p53 and KRAS (OMIM 190070).
Results
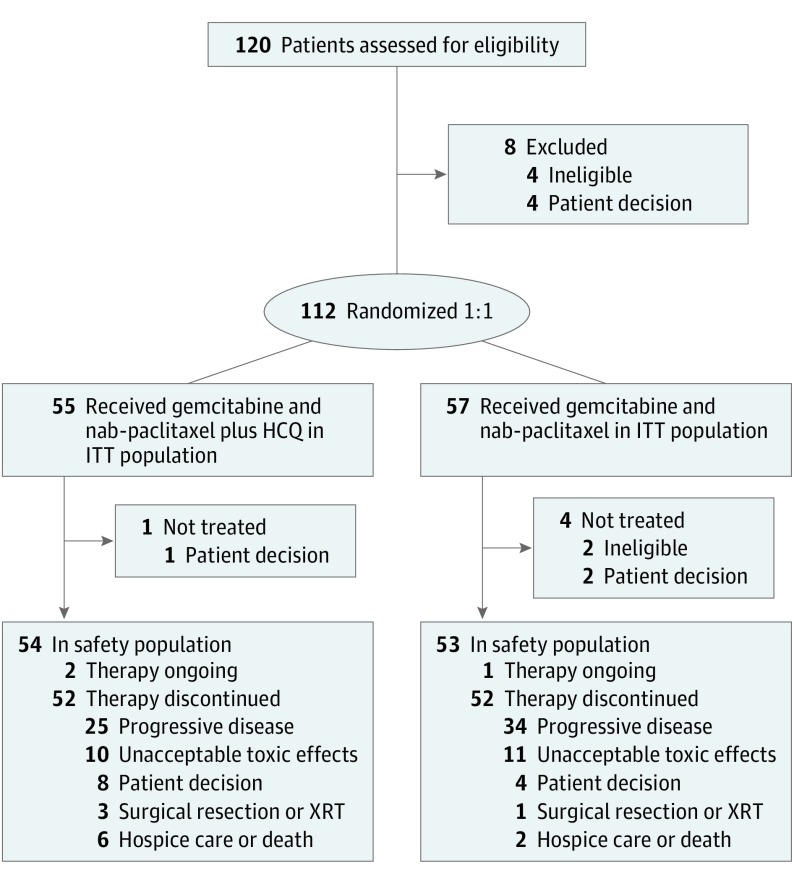
Between March 18, 2013, and November 16, 2017, 112 patients (45 women and 67 men; median age, 65 years; range, 43-86 years) were enrolled; 55 were randomized to receive GA plus HCQ, and 57 were randomized to receive GA (Figure 1). The 2 groups were well matched in baseline characteristics (eTable 1 in Supplement 2). Ninety-seven patients were enrolled at the University of Pennsylvania, 8 patients at HonorHealth, and 7 patients at The Johns Hopkins University. No differences in baseline characteristics between groups were statistically significant.
Figure 1. CONSORT Diagram.
HCQ indicates hydroxychloroquine; ITT, intention-to-treat; and XRT, external beam radiotherapy.
Efficacy
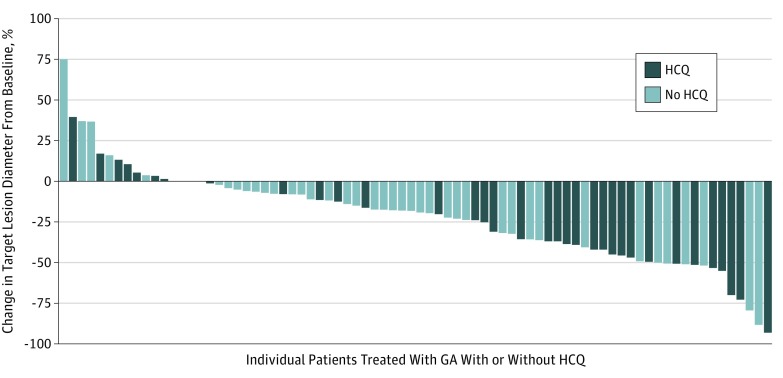
A total of 88 patients were evaluable for response, and 24 patients (who were included in the intention-to-treat analysis; 12 in each group) were not evaluable for response (Table 1). The overall response rate in the intention-to-treat population was 38.2% (n = 21) in the HCQ group and 21.1% (n = 12) in the non-HCQ group (P = .047); all responses were partial responses (Figure 2). The disease control rate was 49.1% in both groups (27 patients in the HCQ group and 28 patients in the non-HCQ group). Median decreases in CA 19-9 level were similar between the 2 groups (83.6% of patients in the HCQ group and 82.2% of patients in the non-HCQ group), and the proportion of patients achieving a decrease in CA 19-9 level of more than 90% was also similar (38.9% of the patients [14 of 36] in the HCQ group and 36.6% of the patients [15 of 41] in the non-HCQ group).
Table 1. Response Rate by Treatment Group.
| Best Response | Patients, No. (%) | |
|---|---|---|
| GA Plus HCQ (n = 55) | GA (n = 57) | |
| Partial response | 21 (38.2) | 12 (21.1) |
| Stable disease | 19 (34.5) | 27 (47.4) |
| Progressive disease | 3 (5.5) | 6 (10.5) |
| Not evaluable | 12 (21.8) | 12 (21.1) |
| Disease control rate (stable disease ≥16 wk + partial response) | 27 (49.1) | 28 (49.1) |
Abbreviations: GA, gemcitabine and nab-paclitaxel; HCQ, hydroxychloroquine.
Figure 2. Best Objective Response.
Waterfall plot of the best objective response according to treatment group, measured as the maximum change from baseline in the sum of the longest diameter of each target lesion. HCQ indicates hydroxychloroquine; GA, gemcitabine and nab-paclitaxel.
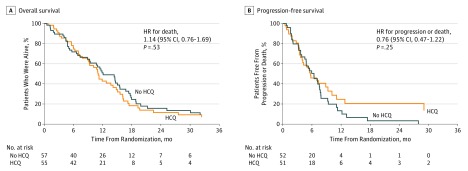
Overall survival at 12 months was 41% (95% CI, 27%-53%) in the HCQ group and 49% (95% CI, 35%-61%) in the non-HCQ group (P = .44). Median PFS (Figure 3A) was 5.7 months (95% CI, 4.0-9.3 months) in the HCQ group and 6.4 months (95% CI, 4.5-7.6 months) in the non-HCQ group (hazard ratio, 0.76; 95% CI, 0.47-1.22; P = .25). Four patients in the HCQ group had PFS greater than 20 months (21, 29, and 30 months as well as an ongoing response at 45 months), while only 1 patient in the non-HCQ group had PFS greater than 20 months (28 months). The median OS (Figure 3B) was 11.1 months (95% CI, 9.0-14.2 months) in the HCQ group and 12.1 months (95% CI, 9.3-15.5 months) in the non-HCQ group (hazard ratio, 1.14; 95% CI, 0.76-1.69; P = .53).
Figure 3. Kaplan-Meier Curves for Progression-Free Survival and Overall Survival in the Intention-to-Treat Population.
HCQ indicates hydroxychloroquine; HR, hazard ratio.
Toxic Effects
The most common treatment-related grade 3 or 4 adverse events (Table 2) that differed between the HCQ and non-HCQ groups were neutropenia (23 of 54 [42.6%] vs 12 of 53 [22.6%]; P = .03), anemia (2 of 54 [3.7%] vs 9 of 53 [17.0%]; P = .03), fatigue (4 of 54 [7.4%] vs 0%; P = .12), nausea (5 of 54 [9.3%] vs 0%; P = .06), peripheral neuropathy (7 of 54 [13.0%] vs 3 of 53 [5.7%]; P = .32), visual changes (3 of 54 [5.6%] vs 0%; P = .24), and neuropsychiatric symptoms (3 of 54 [5.6%] vs 0%; P = .24). Two thromboembolic events occurred in the non-HCQ group and none in the HCQ group.
Table 2. Treatment-Related Adverse Events.
| Adverse Event | Patients, No. (%) | |||||
|---|---|---|---|---|---|---|
| GA Plus HCQ (n = 54) | GA (n = 53) | |||||
| Grade 2 | Grade 3 | Grade 4 | Grade 2 | Grade 3 | Grade 4 | |
| Neutropenia | 12 (22.2) | 19 (35.2) | 4 (7.4) | 9 (17.0) | 9 (17.0) | 3 (5.7) |
| Thrombocytopenia | 8 (14.8) | 4 (7.4) | 0 | 0 | 5 (9.4) | 0 |
| Anemia | 8 (14.8) | 2 (3.7) | 0 | 10 (18.9) | 9 (17.0) | 0 |
| Fatigue | 20 (37.0) | 4 (7.4) | 0 | 22 (41.5) | 0 | 0 |
| Nausea | 19 (35.2) | 5 (9.3) | 0 | 22 (41.5) | 0 | 0 |
| Diarrhea | 20 (37.0) | 0 | 0 | 21 (39.6) | 0 | 0 |
| Peripheral neuropathy | 12 (22.2) | 7 (13.0) | 0 | 7 (13.2) | 3 (5.7) | 0 |
| Neuropsychiatric symptoms | 3 (5.6) | 3 (5.6) | 0 | 0 | 0 | 0 |
| Visual changes | 3 (5.6) | 3 (5.6) | 0 | 0 | 0 | 0 |
Abbreviations: GA, gemcitabine and nab-paclitaxel; HCQ, hydroxychloroquine.
The mean dose intensities of GA were similar between the 2 treatment groups (eTable 2A in Supplement 2). Ten of 54 patients (18.5%) in the HCQ group and 11 of 53 patients (20.8%) in the non-HCQ group discontinued therapy because of toxic effects. Dose reductions or dose delays of HCQ occurred for 10 of 54 patients (18.5%), 3 for neuropsychiatric symptoms, 2 for rash, 3 for visual changes, 1 for nausea or vomiting, and 1 for thrombocytopenia (eTable 2B in Supplement 2). Five patients (9.2%) were either not rechallenged or did not tolerate HCQ at a reduced dose, but no patients discontinued study therapy solely because of HCQ toxic effects.
Genomic Analysis
A total of 45 patients (40.2% of the total group) underwent limited genomic analysis (eTable 3 in Supplement 2). All 45 samples had sequencing of KRAS and p53, 32 samples had sequencing of SMAD4 (OMIM 600993), and 13 samples had sequencing of p16. No other mutations were identified in more than a single individual. There was an imbalance in KRAS mutation status between the 2 groups, with 16 of 24 patients (66.7%) having a KRAS mutation in the non-HCQ group compared with all 21 patients (100%) with a KRAS mutation in the HCQ group. An unplanned analysis determined that both PFS and OS were prolonged among patients without the KRAS mutation (eFigure 2A-D in Supplement 2). Removing these 8 patients without the KRAS mutation from the non-HCQ group decreased the median PFS and the median OS in this group (eFigure 2E and F in Supplement 2).
We also performed an analysis of the effect of p53 status on outcome. The proportion of p53 loss-of-function mutations was similar between the groups (17 of 21 patients [81.0%] in the HCQ group and 16 of 24 patients [66.7%] in the non-HCQ group). There was no apparent deleterious effect of p53 mutation on OS among patients in the combined treatment groups or among patients treated with HCQ (eFigure 3A and B in Supplement 2).
Discussion
The addition of HCQ to standard chemotherapy to reverse autophagy did not achieve the primary end point of improved OS at 12 months. Similarly, no improvements were demonstrated in median OS, with a trend toward worsening of OS. A phase 3 study of GA plus HCQ in an unselected population with metastatic PDAC is not warranted based on these results.
Despite the lack of survival benefit, the overall response rate was significantly higher in the HCQ group, with a trend toward improved PFS and with several patients with PFS of more than 20 months suggesting that a subpopulation of patients may benefit from inhibition of autophagy. Additional work to define a biomarker associated with susceptibility is needed. Increased response rate without improved OS was also demonstrated with the addition of HCQ to FOLFOX (folinic acid, fluorouracil, and oxaliplatin) and bevacizumab for the treatment of metastatic colorectal cancer.13 The activity of GA plus HCQ in PDAC has also been shown in the neoadjuvant setting, with improvements in pathologic responses as determined by the Evans grade.14 The improvement in response with HCQ may be most useful in the locally advanced or neoadjuvant setting, where tumor shrinkage may enable surgical resection.
The toxic effects of HCQ were typically modest, and fewer than 10% of patients required discontinuation of HCQ (5 of 54 [9.3%]). Increases in neutropenia, fatigue, nausea, peripheral neuropathy, visual changes, and neuropsychiatric symptoms were seen with HCQ, but these effects did not lead to decreased chemotherapy intensity or treatment discontinuation. This finding suggests that the additional toxic effects of HCQ were not sufficient to account for the lack of survival benefit in the experimental group. There was also a lack of thromboembolic events in the HCQ group, which supports recent findings that hydroxychloroquine therapy may decrease the risk of thrombosis.15
Although multiple murine models have demonstrated the antitumor effects of autophagy inhibition in PDAC, some studies have suggested that HCQ accelerated PDAC growth in the setting of concurrent KRAS mutation and p53 loss.16,17,18 The retrospective genomic analysis performed for 45 patients in our study does not support this observation and demonstrated no deleterious effects of p53 mutation on OS among patients receiving HCQ. This limited genomic analysis also demonstrated an imbalance in KRAS mutations between the 2 groups and prolonged PFS and OS among the patients with KRAS wild-type, all of whom were in the non-HCQ group. The improved prognosis in KRAS wild-type PDAC is consistent with previous observations, and this baseline imbalance may have inflated survival in the control group.19,20 However, the lack of genomic data from most patients limits conclusions that can be made in the overall study population based on KRAS mutation status because the imbalance may be artifactual owing to sampling.
Limitations
This study has some limitations. The availability of GA off-study and the lack of a placebo control for HCQ led to a higher-than-expected dropout rate and may have diminished differences between the treatment groups. Overall response rate was a secondary end point, and the improvement with HCQ must be considered hypothesis generating.
Conclusions
The addition of HCQ to GA was tolerable and significantly improved the response rate but did not improve OS among patients with metastatic PDAC. Further mechanistic studies are needed to understand the discrepancy between response and survival and to identify predictive biomarkers for HCQ. Exploration of HCQ in the setting of locally advanced PDAC should be considered.
Trial Protocol
eAppendix. Methods
eTable 1. Baseline Characteristics
eTable 2. Chemotherapy Dose Reductions by Arm and HCQ Dose Delays and Reductions
eTable 3. Genomic Analysis
eFigure 1. Survival by KRAS Status
eFigure 2. Overall Survival By p53 Status
Data Sharing Statement
References
- 1.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039-1049. doi: 10.1056/NEJMra1404198 [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, et al. ; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup . FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825. doi: 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691-1703. doi: 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17(9):528-542. doi: 10.1038/nrc.2017.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amaravadi RK, Yu D, Lum JJ, et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117(2):326-336. doi: 10.1172/JCI28833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perera RM, Stoykova S, Nicolay BN, et al. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature. 2015;524(7565):361-365. doi: 10.1038/nature14587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang A, Rajeshkumar NV, Wang X, et al. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov. 2014;4(8):905-913. doi: 10.1158/2159-8290.CD-14-0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang S, Wang X, Contino G, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25(7):717-729. doi: 10.1101/gad.2016111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Hara MH Gallagher M Teitelbaum UR, et al. Phase 1 trial of gemcitabine/nab-paclitaxel in combination with the autophagy inhibitor hydroxychloroquine in previously untreated patients with metastatic pancreatic adenocarcinoma. J Clin Oncol. 2015;33(15_suppl):e15213. doi: 10.1200/jco.2015.33.15_suppl.e15213 [DOI] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 11.US Dept of Health and Human Services Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Published May 28, 2009. Accessed April 22, 2019.
- 12.Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29(34):4548-4554. doi: 10.1200/JCO.2011.36.5742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Hara MH, Karasic TB, Vasilevskaya I, et al. Phase II trial of the autophagy inhibitor hydroxychloroquine with FOLFOX and bevacizumab in front line treatment of metastatic colorectal cancer. J Clin Oncol. 2017;35(15_suppl):3545. [Google Scholar]
- 14.Miller-Ocuin JL, Bahary NS, Singhi AD, et al. Inhibition of autophagy improves pathologic and biomarker response to preoperative gemcitabine/nab-paclitaxel in potentially resectable pancreatic cancer: a phase II randomized controlled trial. Ann Surg Oncol. 2017;24:S6-S7. [Google Scholar]
- 15.Boone BA, Murthy P, Miller-Ocuin J, et al. Chloroquine reduces hypercoagulability in pancreatic cancer through inhibition of neutrophil extracellular traps. BMC Cancer. 2018;18(1):678. doi: 10.1186/s12885-018-4584-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenfeldt MT, O’Prey J, Morton JP, et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature. 2013;504(7479):296-300. doi: 10.1038/nature12865 [DOI] [PubMed] [Google Scholar]
- 17.Kinsey C, Balakrishnan V, O’Dell MR, et al. Plac8 links oncogenic mutations to regulation of autophagy and is critical to pancreatic cancer progression. Cell Rep. 2014;7(4):1143-1155. doi: 10.1016/j.celrep.2014.03.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang A, Herter-Sprie G, Zhang H, et al. Autophagy sustains pancreatic cancer growth through both cell-autonomous and nonautonomous mechanisms. Cancer Discov. 2018;8(3):276-287. doi: 10.1158/2159-8290.CD-17-0952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Windon AL, Loaiza-Bonilla A, Jensen CE, Randall M, Morrissette JJD, Shroff SG. A KRAS wild type mutational status confers a survival advantage in pancreatic ductal adenocarcinoma. J Gastrointest Oncol. 2018;9(1):1-10. doi: 10.21037/jgo.2017.10.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim ST, Lim DH, Jang KT, et al. Impact of KRAS mutations on clinical outcomes in pancreatic cancer patients treated with first-line gemcitabine-based chemotherapy. Mol Cancer Ther. 2011;10(10):1993-1999. doi: 10.1158/1535-7163.MCT-11-0269 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix. Methods
eTable 1. Baseline Characteristics
eTable 2. Chemotherapy Dose Reductions by Arm and HCQ Dose Delays and Reductions
eTable 3. Genomic Analysis
eFigure 1. Survival by KRAS Status
eFigure 2. Overall Survival By p53 Status
Data Sharing Statement