Key Points
Question
Does mortality risk in patients with alopecia areata differ from that in the general population?
Findings
In this nationwide population-based cohort study, patients with alopecia areata had a higher mortality risk associated with intentional self-harm/psychiatric diseases than controls. Mortality risk associated with smoking-associated malignant diseases including lung cancer was also increased in patients with alopecia totalis/universalis; however, mortality risk associated with diabetes mellitus was decreased in patients with alopecia areata.
Meaning
The psychiatric burden of alopecia areata may have contributed to increased mortality associated with self-harm, psychiatric diseases, and smoking-associated malignant diseases.
Using the National Health Insurance Service database and National Death Registry of Korea, this cohort study investigates all-cause and cause-specific mortality to estimate risk in patients with alopecia areata compared with a control group.
Abstract
Importance
Alopecia areata is associated with diverse systemic and psychiatric diseases. However, whether all-cause and cause-specific mortality in patients with alopecia areata differs from that of the general population remains unclear.
Objective
To investigate all-cause and cause-specific mortality risk in patients with alopecia areata.
Design, Setting, and Participants
Using the National Health Insurance Service database and National Death Registry of Korea, a retrospective cohort study of participants identified in 2006, with investigation of mortality until 2016, was carried out. Patients with alopecia areata with at least 3 documented visits to a dermatologist with an International Statistical Classification of Diseases (tenth revision) code of L63 during 2002 to 2006 were included. For comparison, 1:10 age- and sex-matched controls without documented visits with a code of L63 until 2016 were included.
Exposures
Patients with alopecia areata and controls without alopecia areata.
Main Outcomes and Measures
The study population was followed from January 1, 2007, for a period of 10 years to estimate all- and cause-specific mortality.
Results
The study comprised 73 107 patients with alopecia areata and 731 070 age- and sex-matched controls. Of these, 6023 were patients with alopecia totalis/universalis. No differences in all-cause mortality risk between the cohorts were found (HR, 0.97; 95% CI, 0.87-1.09). However, mortality associated with intentional self-harm/psychiatric diseases was greater in patients than in participants in the control group (HR, 1.21; 95% CI, 1.04-1.41). Adult patients aged 35 years or younger (HR, 1.68; 95% CI, 1.32-2.12) and those with alopecia totalis/universalis (HR, 1.85; 95% CI, 1.25-2.75) were particularly affected. Mortality associated with lung cancer was greater in patients with alopecia totalis/universalis (HR, 2.16; 95% CI, 1.41-3.33). However, mortality associated with diabetes mellitus was significantly lower in patients with alopecia areata (HR, 0.53; 95% CI, 0.36-0.79).
Conclusions and Relevance
Patients with alopecia areata have a higher risk of mortality associated with self-harm, psychiatric diseases, and smoking-associated malignant diseases including lung cancer. For better outcomes, clinicians should appropriately treat patients to ensure emotional and psychological well-being.
Introduction
Alopecia areata (AA) is a chronic disorder resulting in nonscarring hair loss. Alopecia areata is a major contributor to the global burden of skin diseases,1 with a prevalence of 0.1% to 0.2%.2 Alopecia areata was thought to have limited systemic significance, but appeared to be associated with psychiatric comorbidities given the psychosocial deprivation caused by cosmetic disfigurement.3 However, recent studies have suggested a significant association with systemic conditions in addition to mental health.4 Recent meta-analyses5,6,7 indicated that diverse conditions including atopic diseases, thyroid diseases, and vitamin D deficiency were more prevalent in patients with AA, in addition to higher risk of autoimmune diseases.8 In contrast, diabetes mellitus (DM)9,10,11 and some malignant diseases12,13,14 are reported to be less prevalent. Although evidence suggests an altered prevalence or risk for specific diseases, few studies have investigated mortality risk.
We postulated that all-cause and cause-specific mortality risk in patients with AA could be different from those in the general population. A nationwide insurance database and death registry might be a useful source of information owing to the relative rarity of AA and the difficulty of collecting mortality data for a non–life-threatening disease. This study investigated all-cause and cause-specific mortality to compare estimated risk in patients with AA and controls.
Methods
Data Sources
This nationwide population-based cohort study was carried out using the customized database of the National Health Insurance Service (NHIS) of Korea.15 Korea has a single universal health coverage system providing insurance to over 99% of the entire population. Accordingly, the NHIS has a comprehensive database of socioeconomic profiles, health care behavior, diagnoses, and treatment. Since 2014, the NHIS has made the Bigdata Sharing Service available to researchers; the database includes information since 2002.16 However, because it does not contain information on deaths, a linkage to the National Death Registry was needed to investigate the outcome for this study.17 This database includes the primary cause and date of deaths for all deceased individuals. Linkage was undertaken using anonymized identification numbers. Health examination results and lifestyle information obtained from questionnaires were collected from the general health examination database. This examination is offered (bi)annually to all employees, householders, or any citizen aged 40 years or older. All data were anonymized to protect privacy. This study was approved by the Korean National Institute for Bioethics Policy (NHIS-2018-1-307) and a waiver of informed consent was granted owing to the deidentified data used.
Study Population
The population was identified from the NHIS database in 2006, and participants who were 18 years or older and alive at the end of 2006 were selected. The AA cohort comprised participants with a documented visit to a dermatologist from 2002 to 2006 with an International Statistical Classification of Diseases, tenth revision (ICD-10) code of L63. To reduce misclassification, only participants who had at least 3 documented visits were considered patients with AA. Patients with alopecia totalis/universalis (AT/AU) (ICD-10 code of L63.0/L63.1) were isolated for subgroup analyses. Finally, only patients who had a general health examination at least once during 2002 to 2006 were retained to identify characteristics that may be associated with mortality. Patients were matched 1:10 for age and sex with controls without AA. The control cohort also comprised participants who had a general health examination during 2002 to 2006 but had never visited any medical facility with an ICD code of L63 until 2016. We set the 5-year recruiting period for both cohorts before the index date for follow-up and included only participants who had general health examination (1) to recruit as many patients with AA as possible, (2) to ensure full access to baseline characteristics, and (3) to exclude critically ill or immobilized participants who may have severely impaired health care behavior for non–life-threatening diseases, such as AA, from both cohorts.
Validation of Criteria for Identification of Patients With AA
Although most AA can be simply diagnosed by dermatologists and misdiagnoses are not common, we evaluated the reliability of our diagnostic criteria. We used the same criteria to retrieve electronic medical records of patients with AA who visited our institution during 2002 to 2006. Two AA experts reviewed each case to confirm the diagnosis, and the positive predictive value (PPV) using our criteria was calculated.
Mortality Outcomes
By the study design, the entire population was alive at the end of 2006. The population was followed for 10 years from January 1, 2007, to December 31, 2016, to estimate all-cause and cause-specific mortality risk in both cohorts. The primary cause and date of death were extracted from the database, and mortalities were classified using the ICD-10 code (eTable 1 in the Supplement). Deaths caused by congenital abnormalities, injury other than self-harm, and any other undetermined cause were not analyzed for cause-specific mortality. The mortality risk was compared between cohorts with or without adjustment for covariates including socioeconomic status, history of illness, and smoking and drinking profiles. In addition, disease-specific mortality risk was assessed for 10 leading internal causes of death in patients with AA.
Statistical Analysis
The baseline characteristics at study entry were summarized using the mean with standard deviation (SD) and frequencies with percentages as appropriate. All-cause and cause-specific death rates per 10 000 person-years were calculated. Although the controls were age- and sex-matched at an individual level, the survival analysis was a more appropriate statistical model for this study because the observation was limited at a 10-year period (right-censored). After testing the proportional hazard assumption, Cox proportional hazard models were fit to estimate the all-cause and cause-specific hazard ratio (HR) and 95% confidence interval (CI) in patients with AA compared with those in the control group (eTable 2 in the Supplement). The estimates from the crude model (model 1) and fully-adjusted model (model 4) were reported using forest plots. These comparisons were repeated for subgroup analyses between patients with AT/AU and controls with adjustments for age and sex. To assess the robustness of our results, sensitivity analysis excluding participants who died during the first 2 years (2007-2008) was performed to further eliminate critical illness. All statistical analyses were performed using SAS statistical software (version 9.4; SAS Institute, Inc) and R statistical software (version 3.4.1, R Foundation) at a significance level of 5%.
Results
Baseline Characteristics
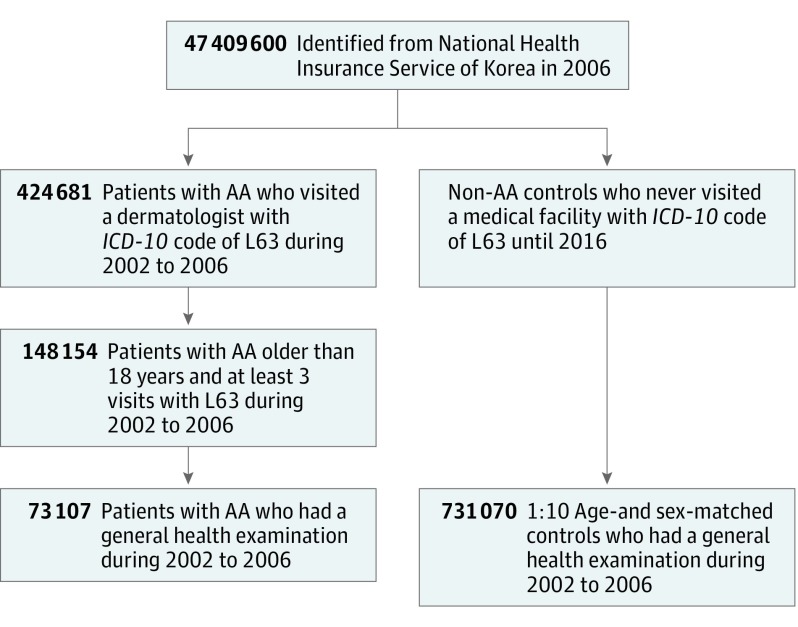
A total of 73 107 patients with AA and 731 070 age- and sex-matched controls were included (Figure 1), with demographics summarized in the Table. The proportion of men (433 240 [59.26%]) was higher than that of women and the mean (SD) age was 38.03 (11.42) years. The controls had a slightly higher positive rate of self-reported medical history of hypertension, DM, and cancer. The patients included the proportion with the highest income. Reported subjective stress level was more severe than in controls. The proportion of current smokers was higher among patients, whereas participants who consumed alcohol more than 3 days per week were more frequent among those in the control group.
Figure 1. Flowchart of Study Population Selection.
AA Indicates alopecia areata; ICD-10, International Statistical Classification of Diseases, tenth revision. A total of 73 107 patients with alopecia areata and 731 070 controls (1:10 age- and sex-matched) were identified from the National Health Insurance Service database.
Table. Baseline Characteristics of the Study Population.
| Characteristics | No. (%) | |||
|---|---|---|---|---|
| Control Cohort | AA Cohort | |||
| Total | PA | AT/AU | ||
| No. (%) | 731 070 (100.0) | 73 107 (100.0) | 67 083 (91.76) | 6024 (8.24) |
| Age, mean (SD) | 38.03 (11.42) | 38.03 (11.42) | 38.00 (11.39) | 38.37 (11.71) |
| Sex | ||||
| Men | 433 240 (59.26) | 43 324 (59.26) | 39 761 (59.27) | 3563 (59.15) |
| Women | 297 830 (40.74) | 29 783 (40.74) | 27 322 (40.73) | 2461 (40.85) |
| Self-reported personal history of illnessa | ||||
| Hypertension | 25 410 (3.48) | 2336 (3.20) | 2144 (3.20) | 192 (3.19) |
| Diabetes mellitus | 10 045 (1.37) | 848 (1.16) | 770 (1.15) | 78 (1.29) |
| Cancer | 1740 (0.24) | 142 (0.19) | 132 (0.20) | 10 (0.17) |
| Stroke | 1496 (0.20) | 111 (0.15) | 102 (0.15) | 9 (0.15) |
| Heart diseases | 2957 (0.40) | 300 (0.41) | 275 (0.41) | 25 (0.42) |
| Liver diseases | 5497 (0.75) | 549 (0.75) | 508 (0.76) | 41 (0.68) |
| Income quartilea | ||||
| Lowest | 131 674 (18.94) | 11 910 (17.11) | 10 912 (17.08) | 998 (17.35) |
| Lower | 148 730 (21.39) | 13 714 (19.70) | 12 603 (19.73) | 1111 (19.32) |
| Higher | 206 615 (29.72) | 20 332 (29.20) | 18 632 (29.17) | 1700 (29.56) |
| Highest | 208 221 (29.95) | 23 665 (33.99) | 21 723 (34.01) | 1942 (33.77) |
| Location of residencea | ||||
| Metropolitan | 502 887 (68.79) | 51 121 (69.94) | 46 979 (70.04) | 4142 (68.72) |
| Countryside | 228 183 (31.21) | 21 976 (30.06) | 20 094 (29.96) | 1882 (31.24) |
| No. of household membersa | ||||
| ≥2 | 565 947 (77.41) | 56 190 (76.87) | 51 602 (76.93) | 4588 (71.16) |
| 1 | 165 123 (22.59) | 16 907 (23.13) | 15 471 (23.07) | 1436 (23.84) |
| Subjective stressa | ||||
| None to mild | 271 230 (39.63) | 24 983 (36.40) | 22 940 (36.43) | 2043 (36.07) |
| Moderate | 321 183 (46.93) | 33 441 (48.72) | 30 686 (48.73) | 2755 (48.64) |
| Severe | 91 977 (13.44) | 10 210 (14.88) | 9344 (14.84) | 866 (15.29) |
| Smoking statusa | ||||
| Never smoker | 396 261 (54.87) | 37 836 (51.75) | 34 704 (52.29) | 3132 (51.99) |
| Ex-smoker | 65 552 (9.08) | 6591 (9.02) | 6061 (9.13) | 530 (8.80) |
| Current smoker | 260 342 (36.05) | 27 967 (38.25) | 25 606 (38.58) | 2361 (39.19) |
| Drinking frequency, d/wka | ||||
| No drinking | 282 614 (38.99) | 27 637 (38.09) | 25 342 (38.06) | 2104 (35.30) |
| ≤2 | 348 459 (48.07) | 36 504 (50.31) | 33 486 (50.30) | 3018 (50.46) |
| ≥3 | 93 847 (12.95) | 8416 (11.60) | 7748 (11.64) | 668 (11.17) |
| Alcohol intake per drink, ga,b | ||||
| ≤75.6 | 591 875 (80.96) | 58 691 (80.89) | 53 835 (80.86) | 4856 (81.19) |
| >75.6 | 139 195 (19.04) | 13 866 (19.11) | 12 741 (19.14) | 1125 (18.81) |
Abbreviations: AA, alopecia areata; AT/AU, alopecia totalis/universalis; PA, patchy alopecia.
The most recent record during 2002 to 2006 was used when multiple data were available.
The amount of alcohol was estimated based on 1 bottle of soju, a popular alcoholic beverage in Korea.
Validation of Criteria for Identification of Patients With AA
Among 386 patients who visited our institution with an ICD code of L63 during 2002 to 2006, 220 who visited at least 3 times were considered as study patients according to our criteria. Collectively, 209 were confirmed to have AA based on our review. The PPV was 95.0% (95% CI, 91.8%-97.0%), indicating the reliability of our criteria for identifying patients with AA from the NHIS database.
All-Cause and Cause-Specific Mortality Risk
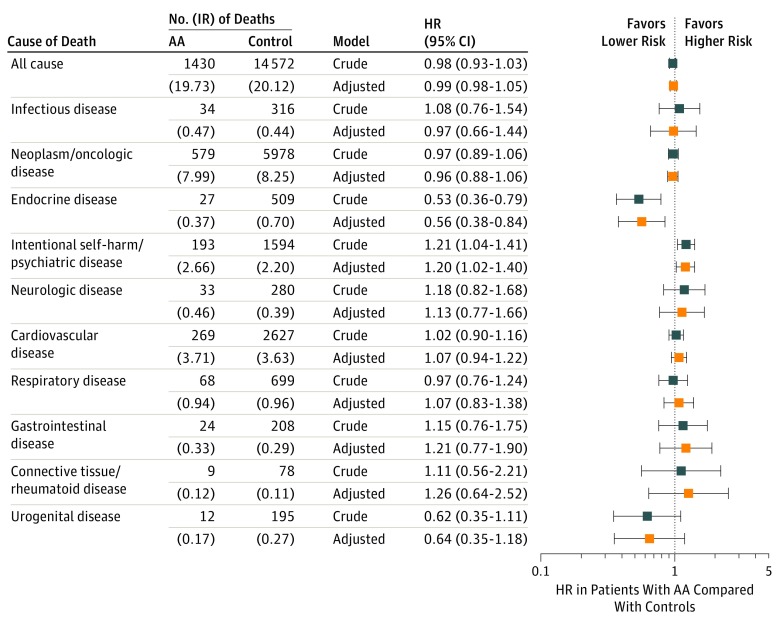
Figure 2 shows all-cause and cause-specific mortality in patients with AA compared with controls (see eFigure 1 in the Supplement for cumulative incidence functions and eTable 3 in the Supplement for statistical details). There was no significant difference in all-cause mortality risk between cohorts. However, the mortality risk associated with intentional self-harm/psychiatric diseases was greater in patients (HR, 1.21; 95% CI, 1.04–1.41). In age group-stratified analysis (eFigure 2 in the Supplement), adult patients younger than 35 years (HR, 1.84; 95% CI, 1.38-2.46) accounted for the increased risk. In particular, patients younger than 25 years (HR, 2.35; 95% CI, 1.53-3.60) were markedly affected. However, patients aged 35 years or older showed no difference from controls. The mortality risk associated with endocrine diseases was lower in patients with AA (HR, 0.53; 95% CI, 0.36-0.79). Although it did not reach statistical significance, their risk associated with urogenital diseases tended to be lower (HR, 0.62; 95% CI, 0.35-1.11). In patients with AT/AU (eTable 4 in the Supplement), their risk associated with intentional self-harm/psychiatric diseases was significantly increased (HR, 1.85; 95% CI, 1.25-2.75) (eFigure 3A in the Supplement).
Figure 2. All-Cause and Cause-Specific Death Rates and Hazard Ratio in Patients With Alopecia Areata.
AA Indicates alopecia areata; HR, hazard ratio; IR, incidence rate. All-cause and cause-specific death rates (IR per 10 000 person-years) and HRs in patients with AA were compared with those in controls. Estimates for model 1 (crude HR) and model 4 (adjusted HR) were reported using forest plot.
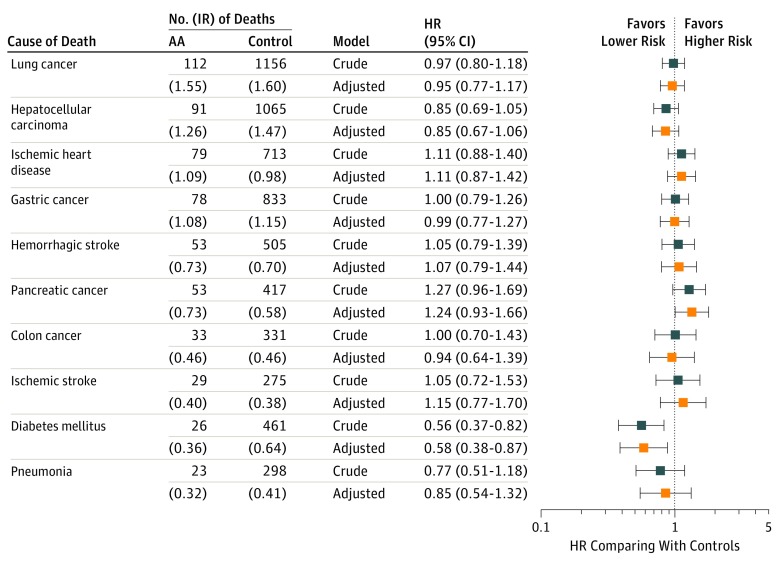
Figure 3 shows the disease-specific mortality of 10 leading internal causes in patients with AA. Although statistically nonsignificant, the mortality risk associated with pancreatic cancer tended to be higher in patients (HR, 1.27; 95% CI, 0.96-1.69). However, the risk associated with DM was significantly lower (HR, 0.56; 95% CI, 0.37-0.82) (eFigure 4 in the Supplement). Although chronic kidney disease (CKD) was not one of the 10 leading causes, mortality associated with CKD was calculated to clarify the contribution of lower mortality risk associated with urogenital diseases. Although statistically nonsignificant, the risk tended to be lower (HR, 0.56; 95% CI, 0.26-1.19). In patients with AT/AU, the risk associated with lung cancer was markedly increased (HR, 2.16; 95% CI, 1.41-3.33) (eFigure 3B in the Supplement).
Figure 3. Disease-Specific Rates and Relative Hazard Ratios for 10 Leading Internal Causes of Death in Patients With Alopecia Areata.
AA Indicates alopecia areata; HR, hazard ratio; IR, incidence rate. Disease-specific rates for 10 leading internal causes in patients with AA were investigated. Estimates for model (crude HR) and model 4 (adjusted HR) were reported using forest plot.
Sensitivity Analysis
A sensitivity analysis was performed, excluding participants who died during the first 2 years (eFigure 5 in the Supplement). There was no difference between the interpretation of the results of original analysis and that of sensitivity analysis. In addition, our study population mainly consisted of younger patients, possibly leading to false-negative associations for some diseases of elderly persons. In subgroup analysis of participants aged 65 years or older (eTable 5 in the Supplement), most results showed no difference in interpretation from those of the original analysis, except for the comparable mortality risk associated with intentional self-harm/psychiatric diseases. However, the statistical power was limited owing to a small number of mortality events for some outcomes.
Discussion
Main Findings
There was no difference in all-cause mortality between the cohorts. However, the mortality risk associated with intentional self-harm/psychiatric diseases was significantly increased in patients. Although statistically nonsignificant, the mortality risk associated with pancreatic cancer tended to be increased. In addition, mortality associated with lung cancer was markedly increased in patients with AT/AU. In contrast, the mortality risk associated with endocrine diseases including DM was lower in patients with AA.
Mortality Risk Associated With Self-harm and Psychiatric Diseases
Several previous studies showed a significant burden of psychiatric morbidity in AA.4,5,18 In this study, young adult patients and those with AT/AU had approximately a 2-fold higher risk. A comparable risk observed in older patients may have resulted from reduced interest in social activities or cosmetic appearance. Although the importance of mental and emotional support has been consistently emphasized, this suggested a current deficit in appropriate psychological treatment. Given several reports on hair regrowth following psychological intervention19 using antidepressants,20 aromatherapy,21 and hypnotherapy,22 clinicians are encouraged to provide appropriate interventions for unmet needs to achieve better outcomes in patients.
Mortality Risk Associated With DM
This study revealed a lower mortality risk associated with endocrine diseases in patients with AA. Notably, DM caused about 47% fewer deaths. Several studies have suggested lower prevalence of DM in patients, with a meta-analyzed odds ratio of 0.64.5,9,10,11 Therefore, the lower prevalence may have contributed to decreased mortality risk. However, because most mortality associated with DM was documented as type II or unspecified type in the study population, additional studies are needed to clarify the risk associated with type I DM. In addition, the mortality risk associated with CKD tended to be lower in the patients, possibly in association with the lower prevalence of DM because it accounts for 48% of CKD in the Korean population.23 However, the mechanism of lower DM prevalence has not been clarified.
Mortality Risk Associated With Smoking-Associated Cancers
A few nationwide population-based studies have reported the risk of incident cancer in patients. However, studies by Lee et al24 and Chen et al25 reported an increased or decreased risk of overall cancer, respectively, resulting in conflicting results. The mortality risk associated with neoplasm/oncological diseases did not differ in this study.
For organ-specific cancers, heterogeneous risks have been reported among previous studies.12,13,14,24,25,26 However, findings of this study suggest that patients tended to have increased mortality associated with pancreatic cancer. Moreover, patients with AT/AU showed a markedly increased mortality risk associated with lung cancer. Although statistically significant, these findings should be cautiously interpreted owing to its small number of mortality events. Based on previous studies, AA itself was not likely to have a definite biological role in independently increasing the risk of neoplasms. Alternatively, we considered that these results were owing to differences in the baseline characteristics of the study population. The AA cohort had a larger proportion of current smokers, possibly associated with the finding that patients were more likely to report feeling more severe stress than those in the control group. This difference may result in a higher mortality risk associated with smoking-associated cancers in patients. However, even after adjusting for smoking status, mortality risk was still higher. Because smoking status was based on self-response to questionnaires and was not followed up after the index date, this might have resulted in inadequate adjustment. Although additional data on smoking status from the cohort is required, the AA cohort may have consisted of a much higher proportion of smokers during follow-up owing to the chronic nature and psychological burdens of AA.
Interpretation of Other Findings
A few reports have investigated the risk of ischemic heart disease, ischemic stroke, and hemorrhagic stroke in patients with AA with contradicting results.27,28 In this study, the mortality risk associated with cardiovascular diseases was comparable between the 2 cohorts. To date, although it is still unclear whether the risk is increased or decreased in patients with AA, our results suggest that the risk of mortality associated with cardiovascular diseases in patients with AA does not differ from that in the general population. However, because those conditions are rare in the younger population and may require a longer observational period, additional studies would be required to fully determine the risk.
Although some studies have reported an increased risk of incident autoimmune or rheumatoid diseases in patients with AA,4,8 this study showed a minimal difference in the mortality risk associated with connective tissue/rheumatoid diseases. However, additional studies are warranted to better elucidate the risk because the number of deaths resulting from those was very small in this study.
Other causes, including infectious, neurological, respiratory, and gastrointestinal diseases, have been considered to have minimal association with AA, and this study also did not show any differences in terms of cause-specific mortality risk.
Generalization of Study Findings
Although estimates of this nationwide study may represent the actual risk of patients with AA in Korea as of 2006, these findings should be interpreted in the context of the characteristics of the study population. The mean age of participants analyzed in the study was somewhat lower than in other similar studies.29,30,31 This may have resulted from the distinctive population dynamics of Korea, a rapidly aging country. The pattern of the population pyramid in Korea as of 2006 showed a lower proportion of the elderly compared with the US or Western Europe.32,33 Although age- and sex-matched controls were compared, the demographic characteristics of the study population may have provided insufficient statistical power to reveal the associations with some diseases significantly associated with age, resulting in false-negative findings. Accordingly, we separately determined the risk in patients aged 65 years or older to resolve this limitation in part. Nonetheless, further studies involving additional elderly populations, mortality events, and a longer follow-up period will be required.
Strengths and Limitations
To date, the mortality of patients with AA has not been adequately investigated owing to small cohort sizes and the relatively low prevalence rate. We recruited the study participants using a nationwide insurance database covering almost the entire Korean population; thus, this study achieved adequate statistical power to determine mortality risks. In addition, the study ascertained outcome using a very reliable source through data linkage with the National Death Registry, in which the causes of deaths are managed accurately and systematically.
However, this study has several limitations. First, misclassification bias may have arisen while identifying patients with AA from the database. However, our working definition for identifying patients had a PPV of 95% when applied to our institutional cohort. Second, allocation bias could have occurred, whereby “healthier” participants may have been assigned to the AA cohort because health care behavior associated with non–life-threatening diseases would be considerably limited in critically ill patients. Therefore, we included only those participants who could undergo a general health examination within 5 years before the index date to exclude such participants from the study. Moreover, we performed sensitivity analysis to further compensate for any potential bias in the allocation and to ensure the robustness of our results. Third, because conditions such as hypertension and DM were rare in our younger study population, statistical adjustments may have been less efficient. Future studies involving investigation of more prevalent diseases such as atopic and thyroid diseases in patients with AA would be helpful.
Conclusions
This study found that the risk of cause-specific mortality of patients with AA differed from that in the general population in Korea, although all-cause mortality risk did not differ. Although the importance of treatment for psychiatric illnesses in patients with AA has been consistently emphasized, these may eventually lead to a substantial increase in mortality associated with intentional self-harm/psychological diseases compared with the general population. In addition, higher mortality from smoking-associated cancers may also be associated with a higher degree of severe stress and the higher proportion of current smokers. To improve outcomes, clinicians are encouraged to provide more appropriate treatment for unmet needs, including psychological interventions, in addition to providing therapeutic regimens for hair regrowth in patients with AA.
eTable 1. International Statistical Classification of Diseases 10th revision codes for cause of death
eTable 2. Cox proportional-hazards models for estimating all-cause and cause-specific mortality risks in patients with alopecia areata and controls
eTable 3. All-cause and cause-specific mortality risks in patients with alopecia areata
eTable 4. All-cause and cause-specific mortality risks in patients with alopecia totalis/universalis
eTable 5. All-cause and cause-specific mortality risks in patients with alopecia areata aged ≥65 years
eFigure 1. Cumulative incidence functions for all-cause and cause-specific mortality in patients with alopecia areata
eFigure 2. Age group-stratified cumulative incidence functions for cause-specific deaths from intentional self-harm/psychiatric diseases
eFigure 3. Cumulative incidence functions for cause-specific deaths from lung cancer and intentional self-harm/psychiatric diseases in patients with alopecia totalis/universalis
eFigure 4. Cumulative incidence functions for diabetes mellitus according to subtype
eFigure 5. Comparison of the original analysis and sensitivity analysis
References
- 1.Karimkhani C, Dellavalle RP, Coffeng LE, et al. . Global skin disease morbidity and mortality: an update from the global burden of disease study 2013. JAMA Dermatol. 2017;153(5):406-412. doi: 10.1001/jamadermatol.2016.5538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ III. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70(7):628-633. doi: 10.4065/70.7.628 [DOI] [PubMed] [Google Scholar]
- 3.Hunt N, McHale S. The psychological impact of alopecia. BMJ. 2005;331(7522):951-953. doi: 10.1136/bmj.331.7522.951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang KP, Mullangi S, Guo Y, Qureshi AA. Autoimmune, atopic, and mental health comorbid conditions associated with alopecia areata in the United States. JAMA Dermatol. 2013;149(7):789-794. doi: 10.1001/jamadermatol.2013.3049 [DOI] [PubMed] [Google Scholar]
- 5.Lee S, Lee H, Lee CH, Lee WS. Comorbidities in alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80(2):466-477.e16. doi: 10.1016/j.jaad.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 6.Lee S, Lee YB, Kim BJ, Lee WS. Screening of thyroid function and autoantibodies in patients with alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;80(5):1410-1413e4. doi: 10.1016/j.jaad.2018.10.066 [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Kim BJ, Lee CH, Lee WS. Increased prevalence of vitamin D deficiency in patients with alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2018;32(7):1214-1221. doi: 10.1111/jdv.14987 [DOI] [PubMed] [Google Scholar]
- 8.Chen CH, Wang KH, Lin HC, Chung SD. Follow-up study on the relationship between alopecia areata and risk of autoimmune diseases. J Dermatol. 2016;43(2):228-229. doi: 10.1111/1346-8138.13165 [DOI] [PubMed] [Google Scholar]
- 9.Conic RZ, Miller R, Piliang M, Bergfeld W, Atanaskova Mesinkovska N. Comorbidities in patients with alopecia areata. J Am Acad Dermatol. 2017;76(4):755-757. doi: 10.1016/j.jaad.2016.12.007 [DOI] [PubMed] [Google Scholar]
- 10.Chu SY, Chen YJ, Tseng WC, et al. . Comorbidity profiles among patients with alopecia areata: the importance of onset age, a nationwide population-based study. J Am Acad Dermatol. 2011;65(5):949-956. doi: 10.1016/j.jaad.2010.08.032 [DOI] [PubMed] [Google Scholar]
- 11.Thomas EA, Kadyan RS. Alopecia areata and autoimmunity: a clinical study. Indian J Dermatol. 2008;53(2):70-74. doi: 10.4103/0019-5154.41650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mostaghimi A, Qureshi S, Joyce C, Guo Y, Huang KP. Reduced incidence of skin cancer in patients with alopecia areata: a retrospective cohort study. Cancer Epidemiol. 2016;41:129-131. doi: 10.1016/j.canep.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 13.Conic RZ, Rambhia P, Atanaskova-Mesinkovska N, Piliang M, Bergfeld W. Lack of an association between alopecia areata and visceral or hematopoietic cancers. J Am Acad Dermatol. 2017;77(5):981-982. doi: 10.1016/j.jaad.2017.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conic RRZ, Miller R, Piliang M, Bergfeld W, Atanaskova Mesinkovska N. Risk of sun-induced skin cancers in patients with alopecia areata, alopecia totalis and alopecia universalis. J Eur Acad Dermatol Venereol. 2018;32(11):e409-e411. doi: 10.1111/jdv.15002 [DOI] [PubMed] [Google Scholar]
- 15.Cheol Seong S, Kim YY, Khang YH, et al. . Data Resource Profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46(3):799-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korea NHIS. National Health Insurance Bigdata Sharing Service. https://nhiss.nhis.or.kr/bd/ab/bdaba011eng.do. Accessed Jan 26, 2019.
- 17.Kim IS. Epidemiological usefulness of registered death information in Korea. Epidemiol Health. 1989;11(2):143-149. [Google Scholar]
- 18.Rencz F, Gulácsi L, Péntek M, Wikonkál N, Baji P, Brodszky V. Alopecia areata and health-related quality of life: a systematic review and meta-analysis. Br J Dermatol. 2016;175(3):561-571. doi: 10.1111/bjd.14497 [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Lee WS. Management of alopecia areata: updates and algorithmic approach. J Dermatol. 2017;44(11):1199-1211. doi: 10.1111/1346-8138.13933 [DOI] [PubMed] [Google Scholar]
- 20.Gupta MA, Guptat AK. The use of antidepressant drugs in dermatology. J Eur Acad Dermatol Venereol. 2001;15(6):512-518. doi: 10.1046/j.1468-3083.2001.00278.x [DOI] [PubMed] [Google Scholar]
- 21.Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy: successful treatment for alopecia areata. Arch Dermatol. 1998;134(11):1349-1352. doi: 10.1001/archderm.134.11.1349 [DOI] [PubMed] [Google Scholar]
- 22.Willemsen R, Vanderlinden J, Deconinck A, Roseeuw D. Hypnotherapeutic management of alopecia areata. J Am Acad Dermatol. 2006;55(2):233-237. doi: 10.1016/j.jaad.2005.09.025 [DOI] [PubMed] [Google Scholar]
- 23.Ji E, Kim YS. Prevalence of chronic kidney disease defined by using CKD-EPI equation and albumin-to-creatinine ratio in the Korean adult population. Korean J Intern Med. 2016;31(6):1120-1130. doi: 10.3904/kjim.2015.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JH, Song Y, Do Han K, et al. . Cancer risk by the subtype of alopecia. Sci Rep. 2018;8(1):9748. doi: 10.1038/s41598-018-28142-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen CC, Chang YT, Liu HN, Chen YJ. Cancer risk in patients with alopecia areata: a nationwide population-based matched cohort study. Cancer Med. 2018;7(5):2153-2159. doi: 10.1002/cam4.1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo HM, Han SS, Kim JS. Cancer risks among patients with alopecia areata: a population-based case-control study in Korea. J Am Acad Dermatol. 2018;78(1):209-211. doi: 10.1016/j.jaad.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 27.Kang JH, Lin HC, Kao S, Tsai MC, Chung SD. Alopecia areata increases the risk of stroke: a 3-year follow-up study. Sci Rep. 2015;5:11718. doi: 10.1038/srep11718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang KP, Joyce CJ, Topaz M, Guo Y, Mostaghimi A. Cardiovascular risk in patients with alopecia areata (AA): a propensity-matched retrospective analysis. J Am Acad Dermatol. 2016;75(1):151-154. doi: 10.1016/j.jaad.2016.02.1234 [DOI] [PubMed] [Google Scholar]
- 29.Gelfand JM, Troxel AB, Lewis JD, et al. . The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143(12):1493-1499. doi: 10.1001/archderm.143.12.1493 [DOI] [PubMed] [Google Scholar]
- 30.Maradit-Kremers H, Dierkhising RA, Crowson CS, Icen M, Ernste FC, McEvoy MT. Risk and predictors of cardiovascular disease in psoriasis: a population-based study. Int J Dermatol. 2013;52(1):32-40. doi: 10.1111/j.1365-4632.2011.05430.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thyssen JP, Skov L, Egeberg A. Cause-specific mortality in adults with atopic dermatitis. J Am Acad Dermatol. 2018;78(3):506-510. doi: 10.1016/j.jaad.2017.10.032 [DOI] [PubMed] [Google Scholar]
- 32.Korenjak-Černe S, Kejžar N, Batagelj V. A weighted clustering of population pyramids for the world’s countries, 1996, 2001, 2006. Popul Stud (Camb). 2015;69(1):105-120. doi: 10.1080/00324728.2014.954597 [DOI] [PubMed] [Google Scholar]
- 33.PopulationPyramid.net Population Pyramid of Korea in 2006. https://www.populationpyramid.net/republic-of-korea/2006/. Accessed Jan 26, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. International Statistical Classification of Diseases 10th revision codes for cause of death
eTable 2. Cox proportional-hazards models for estimating all-cause and cause-specific mortality risks in patients with alopecia areata and controls
eTable 3. All-cause and cause-specific mortality risks in patients with alopecia areata
eTable 4. All-cause and cause-specific mortality risks in patients with alopecia totalis/universalis
eTable 5. All-cause and cause-specific mortality risks in patients with alopecia areata aged ≥65 years
eFigure 1. Cumulative incidence functions for all-cause and cause-specific mortality in patients with alopecia areata
eFigure 2. Age group-stratified cumulative incidence functions for cause-specific deaths from intentional self-harm/psychiatric diseases
eFigure 3. Cumulative incidence functions for cause-specific deaths from lung cancer and intentional self-harm/psychiatric diseases in patients with alopecia totalis/universalis
eFigure 4. Cumulative incidence functions for diabetes mellitus according to subtype
eFigure 5. Comparison of the original analysis and sensitivity analysis