Key Points
Question
Is genetically elevated lipoprotein(a) associated with calcific aortic valve stenosis independently of coronary artery disease, and are first-degree relatives of patients with calcific aortic valve stenosis and high lipoprotein(a) levels more likely to have aortic valve microcalcification?
Findings
Genetically elevated lipoprotein(a) was strongly associated with calcific aortic valve stenosis, regardless of the presence of coronary artery disease. The first-degree relatives of patients with calcific aortic valve stenosis and high lipoprotein(a) levels were more likely to have calcific aortic valve stenosis or aortic valve microcalcification.
Meaning
Lipoprotein(a) is an independent risk factor for calcific aortic valve stenosis, and measuring it could be useful to identify individuals at risk of developing this condition.
This study combines data from multiple cohorts to assess the association of LPA variants with calcific aortic valve stenosis in patients undergoing heart surgery, patients with coronary artery disease, first-degree relatives of patients with coronary artery disease, and unrelated control participants.
Abstract
Importance
Genetic variants at the LPA locus are associated with both calcific aortic valve stenosis (CAVS) and coronary artery disease (CAD). Whether these variants are associated with CAVS in patients with CAD vs those without CAD is unknown.
Objective
To study the associations of LPA variants with CAVS in a cohort of patients undergoing heart surgery and LPA with CAVS in patients with CAD vs those without CAD and to determine whether first-degree relatives of patients with CAVS and high lipoprotein(a) (Lp[a]) levels showed evidence of aortic valve microcalcification.
Design, Setting, and Participants
This genetic association study included patients undergoing cardiac surgery from the Genome-Wide Association Study on Calcific Aortic Valve Stenosis in Quebec (QUEBEC-CAVS) study and patients with CAD, patients without CAD, and control participants from 6 genetic association studies: the UK Biobank, the European Prospective Investigation of Cancer (EPIC)–Norfolk, and Genetic Epidemiology Research on Aging (GERA) studies and 3 French cohorts. In addition, a family study included first-degree relatives of patients with CAVS. Data were collected from January 1993 to September 2018, and analysis was completed from September 2017 to September 2018.
Exposures
Case-control studies.
Main Outcomes and Measures
Presence of CAVS according to a weighted genetic risk score based on 3 common Lp(a)-raising variants and aortic valve microcalcification, defined as the mean tissue to background ratio of 1.25 or more, measured by fluorine 18–labeled sodium fluoride positron emission tomography/computed tomography.
Results
This study included 1009 individuals undergoing cardiac surgery and 1017 control participants in the QUEBEC-CAVS cohort; 3258 individuals with CAVS and CAD, 41 100 controls with CAD, 2069 individuals with CAVS without CAD, and 380 075 control participants without CAD in the UK Biobank, EPIC-Norfolk, and GERA studies and 3 French cohorts combined; and 33 first-degree relatives of 17 patients with CAVS and high Lp(a) levels (≥60 mg/dL) and 23 control participants with normal Lp(a) levels (<60 mg/dL). In the QUEBEC-CAVS study, each SD increase of the genetic risk score was associated with a higher risk of CAVS (odds ratio [OR], 1.35 [95% CI, 1.10-1.66]; P = .003). Each SD increase of the genetic risk score was associated with a higher risk of CAVS in patients with CAD (OR, 1.30 [95% CI, 1.20-1.42]; P < .001) and without CAD (OR, 1.33 [95% CI, 1.14-1.55]; P < .001). The percentage of individuals with a tissue to background ratio of 1.25 or more or CAVS was higher in first-degree relatives of patients with CAVS and high Lp(a) (16 of 33 [49%]) than control participants (3 of 23 [13%]; P = .006).
Conclusions and Relevance
In this study, a genetically elevated Lp(a) level was associated with CAVS independently of the presence of CAD. These findings support further research on the potential usefulness of Lp(a) cascade screening in CAVS.
Introduction
Lipoprotein(a) (Lp[a]) is an atherogenic lipoprotein particle that consists of a cholesterol-rich particle analogous to low-density lipoprotein in which apolipoprotein B-100 is linked to apolipoprotein(a) by a disulfide bond.1 In contrast with low-density lipoprotein, the Lp(a) particle carries a substantial amount of proinflammatory and procalcifying oxidized phospholipids, which bind to both the apolipoprotein(a) and the low-density lipoprotein moieties of Lp(a).2 The circulating level of Lp(a) is largely determined by genetic variation at the LPA locus, which is highly polymorphic. One of the strongest determinants of Lp(a) levels is a copy-number variation at this locus encoding Kringle IV–type 2 repeats, which influence the length of apolipoprotein(a) (with a smaller isoform size being linked to higher plasma Lp[a] levels).3 It has been estimated that up to 70% to 90% of the variance in circulating Lp(a) may be explained by genetic variations at the LPA locus.4
Approximately 10 years ago, a series of genetic association studies revealed an association between variants associated with high Lp(a) levels and the risk of coronary artery disease (CAD).5,6,7 Subsequent characterization of the LPA locus and its association with CAD risk makes it one of the strongest, most consistent, and best-characterized loci associated with CAD risk.8,9 Investigating the genetics of aortic valve calcium (AVC), Thanassoulis et al10 identified LPA as the genetic factor most strongly associated with the presence of AVC in an analysis of the Cohorts for Heart and Aging Research in Genetic Epidemiology consortium. Population-based studies and hospital cohorts later revealed a strong link between Lp(a) and calcific aortic valve stenosis (CAVS) risk and progression.11,12,13,14
Calcific aortic valve stenosis is the most common form of valvular heart disease, and its prevalence is steadily increasing in Western societies, where it now affects approximately 2% of the population older than 65 years.15 Similar to atherosclerotic cardiovascular diseases, the molecular mechanisms that initiate CAVS include infiltration of oxidized lipids and lipoproteins (such as oxidized low-density lipoprotein and Lp[a]) and inflammatory cells (such as macrophages and T cells), as well as extracellular matrix remodeling and calcification. Coronary artery disease and CAVS also share similar clinical risk factors, such as male sex, age, smoking, type 2 diabetes, hypercholesterolemia, and elevated Lp(a). Results of a recent analysis16 also suggested that risk factors associated with ideal cardiovascular health (an unhealthy diet, physical inactivity, smoking, obesity, type 2 diabetes, hypertension, and hypercholesterolemia), often referred to as life’s simple 7, were associated with CAVS incidence in a large population-based study. While there are considerable overlaps between pathobiological mechanisms and clinical risk factors between CAD and CAVS, whether these overlaps also exist for genetic factors is currently unknown. Also unknown is whether the association between genetically elevated Lp(a) levels and CAVS is independent of the presence of CAD.
Although the association between Lp(a) and CAVS is strong and consistent, there is currently little evidence supporting the routine assessment of Lp(a) levels in patients with CAVS. Routine measurement of Lp(a) levels in patients with CAVS could assist in the identification of family members of patients with CAVS and high Lp(a) levels who could be at increased risk of developing CAVS in the future. Whether first-degree relatives of patients with CAVS and high Lp(a) levels may show early signs of aortic valve disease or are at increased CAVS risk is unknown.
The objectives of this study were 3-fold. First, we sought to determine the association between genetically elevated Lp(a) levels and CAVS in a cohort of patients undergoing a heart surgery (including controls with CAD). The second aim was to determine whether the association between genetically elevated Lp(a) levels and CAVS was observed in patients with CAD vs those without CAD. Finally, this study assessed whether first-degree relatives of patients with CAVS and high Lp(a) levels were characterized by aortic valve macrocalcification or microcalcification per a newly developed aortic valve imaging technique that assesses active aortic valve microcalcification: fluorine 18–labeled sodium fluoride (18F–NaF) positron emission tomography (PET)/computed tomography (CT).
Methods
Study Populations
This analysis includes affected individuals and control participants from the Genome-Wide Association Study on Calcific Aortic Valve Stenosis in Quebec (QUEBEC-CAVS) study, the UK Biobank, the European Prospective Investigation of Cancer–Norfolk (EPIC-Norfolk) study, and the Genetic Epidemiology Research on Aging (GERA) study, as well as additional affected individuals and control participants from 3 French cohorts (individuals with CAVS from the l’Institut du Thorax, Nantes, France; individuals with CAVS from the combined GENERAC and COFRASA studies; and control populations from the combined Population de Référence du Grand Ouest and Data from the Epidemiological Study on the Insulin Resistance Syndrome studies). The characteristics of these cohorts are described in detail in the eMethods in the Supplement. This project was approved by the institutional review board of the l’Institut Universitaire de Cardiologie et de Pneumologie de Québec and the local institutional review boards of each cohort included in the genetic association study. All study participants provided informed consent.
In the QUEBEC-CAVS cohort, patients with a history of myocardial infarction, coronary artery stenosis on coronary angiography, or documented myocardial ischemia were included in the CAD group. In the UK Biobank, patients with self-reported myocardial infarction (n = 950) or CAD from International Statistical Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) or Office of Population Censuses and Surveys’ Classification of Surgical Operations and Procedures (4th Revision) codes (version 4.6; n = 25 162) were included in the CAD group. In the EPIC-Norfolk study, patients with documented myocardial ischemia or myocardial infarction or other ischemic heart disease on hospital admission were included in the CAD group. In the GERA study, CAD cases were defined using a diagnosis of myocardial infarction or CAD (ICD-9 codes 410-414); procedure codes for percutaneous coronary intervention or coronary artery bypass surgery.
The design and characteristics of the family study are also described in the eMethods in the Supplement. Briefly, a series of consecutive patients with mild to severe CAVS who did not undergo aortic valve replacement were recruited at the echocardiography lab of the l’Institut Universitaire de Cardiologie et de Pneumologie de Québec. Levels of Lp(a) were measured in patients with CAVS and the first-degree relatives (brothers, sisters, or children older than 40 years) of patients who had both CAVS and Lp(a) levels of 60 mg/dL or more (2.14 μmol/L or more), as well as a control group of participants with Lp(a) levels less than 60 mg/dL who were unrelated to patients with CAVS. These individuals underwent aortic valve microcalcification assessment, as described in the eMethods in the Supplement.
Genotyping, Single-Nucleotide Polymorphisms Selection, and Lp(a) Measurements
The associations between the selected single-nucleotide polymorphism (SNPs) and Lp(a) levels were obtained from the study of Emdin et al8 in which the effects of SNPs on Lp(a) levels were estimated in individuals of European ancestry in the Atherosclerosis Risk in Communities Study cohort (n = 2758). The SNPs that were selected for this study are presented in eTable 1 in the Supplement. β Coefficients are expressed in 1-SD (28 mg/dL) increase of Lp(a) level. We selected SNPs with a minor allele frequency superior to 1%. Only 2 SNPs (rs10455872 and rs3798220) were available from the EPIC-Norfolk study. A weighted genetic risk score (wGRS) was calculated in each cohort by adding the number of Lp(a)-raising alleles that the person had inherited at each variant that was included in the score, weighted by the effect of each variant on Lp(a) levels reported by Emdin et al.8 In the family study, plasma Lp(a) levels were measured by a turbidimetric assay using the Tina-quant lipoprotein(a) Gen.2 system (Cobas Integra 400/800; Roche Diagnostics).
Statistical Analyses
Participant characteristics were summarized by disease status for each study, and differences were assessed using 2-sample t tests or Pearson χ2 test for continuous and discrete characteristics, respectively. The Welch t test was used for samples who have unequal variances tested with F test. All continuous variables were tested for normal distribution with the Shapiro-Wilk test. In cases in which the null hypothesis (that the data are normally distributed) was rejected, the data were log transformed for statistical analyses and presented as medians and interquartile ranges. The association between the wGRS and CAVS was tested using logistic regression analyses that were adjusted for age, sex, and principal components, when available. In the GERA cohort, genetic variants were modeled using PLINK 2.017 in logistic regression models adjusted for age, age squared, sex, and the 10 principal components. We performed a random-effect meta-analysis using the inverse-variance weighted method as implemented in rmeta package version 3.0 in R version 3.5.1 (R Foundation for Statistical Computing). Sensitivity analysis were performed in the QUEBEC-CAVS cohort using only participants with CAVS and CAD, compared with control participants with CAD, and in the other cohorts using participants with CAVS with CAD compared with participants with CAVS and without CAD, control participants with CAD, and control participants without CAD (except for the French cohort data, which did not include the CAD status of control participants). The frequency of participants with CAVS and tissue to background ratios (TBR) of 1.25 or more in first-degree relatives compared with control participants with low Lp(a) levels was assessed using Pearson χ2 test, and the differences in mean TBRs between first-degree relatives and participants in the control group were tested using 2-sample t tests. The threshold P value considered significant was .05, 2-sided.
Results
This analysis includes 1009 affected individuals and 1017 control participants from the QUEBEC-CAVS study (mean [SD] age: affected individuals, 72.5 [8.4] years; controls, 70.9 [8.1] years; P < .001; males: affected individuals, 642 [63.6%]; controls, 660 [64.9%]; P = .55), 1350 affected individuals and 349 043 control participants from the UK Biobank (mean [SD] age: affected individuals, 62.5 [5.8] years; controls, 56.9 [7.9] years; P < .001; males: affected individuals, 899 [66.6%]; controls, 162 125 [46.4%]; P < .001), 508 affected individuals and 20 421 control participants from the EPIC-Norfolk study (mean [SD] age: affected individuals, 64.5 [7.6] years; controls, 59.0 [9.3] years; P < .001; males: affected individuals, 286 [56.3%]; controls, 9438 [46.2%]; P < .001), 3469 affected individuals and 51 711 control participants from the GERA study (mean [SD] age: affected individuals, 74.6 [8.5] years; controls, 67.4 [8.5] years; P < .001; males: affected individuals, 1943 [56.0%]; controls, 25 183 [48.7%]; P < .001), and 3123 affected individuals and 6530 control participants from the 3 French cohorts (mean [SD] age: affected individuals, 75 [8.4] years; male affected individuals, 1929 [62.7%]). Further details are available in eTables 2, 3, 4, 5, and 6 in the Supplement.
Genetic Association Study
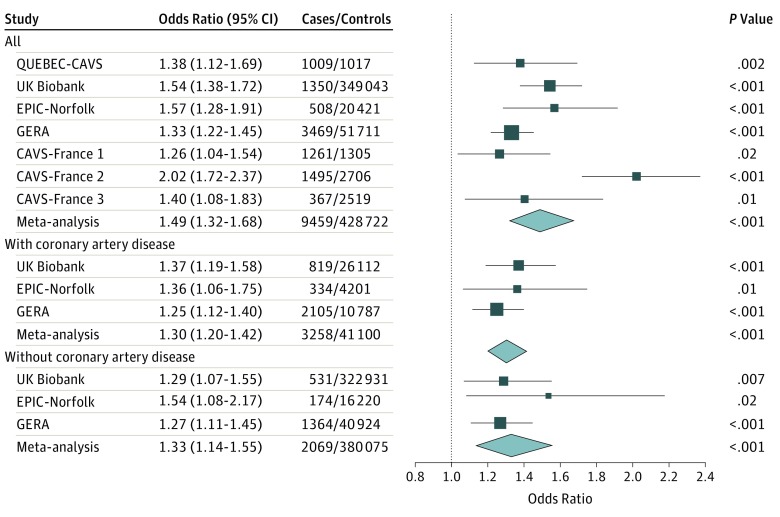
The association between the LPA wGRS and CAVS is presented in Figure 1. In QUEBEC-CAVS, each SD increase of the GRS was associated with a higher risk of CAVS (odds ratio [OR], 1.38 [95% CI, 1.12-1.69]; P = .002).
Figure 1. Association of Genetically Elevated Lipoprotein(a) Levels With Calcific Aortic Valve Stenosis in Individuals With Coronary Artery Disease and Those Without Coronary Artery Disease.
CAVS indicates calcific aortic valve stenosis; EPIC-Norfolk, European Prospective Investigation of Cancer–Norfolk; GERA, Genetic Epidemiology Research on Aging; QUEBEC-CAVS, the Genome-Wide Association Study on Calcific Aortic Valve Stenosis in Quebec; UK, United Kingdom.
In cohorts that included individuals with CAD and others without CAD, the wGRS was also positively associated with CAVS in sensitivity analyses performed in participants with CAD (3258 patients and 41 100 control participants) and participants without CAD (2069 patients and 380 075 control participants). Each SD increase of the wGRS was associated with a higher risk of CAVS in patients with CAD (OR, 1.30 [95% CI, 1.20-1.42]; P < .001) and patients without CAD (OR, 1.33 [95% CI, 1.14-1.55]; P < .001).
In the QUEBEC-CAVS cohort, each SD increase of the wGRS was associated with a higher risk of CAVS in patients with CAD (OR, 1.68 [95% CI, 1.33-2.12]; P < .001). That analysis included 586 patients and 989 control participants, all with CAD.
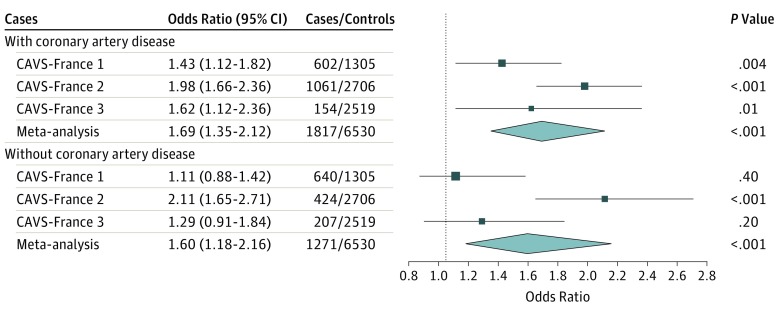
Because CAD status was not available for control participants included in the French cohort, results of the French populations are not presented in this sensitivity analysis (Figure 1). The association between the LPA wGRS and CAVS in cases of the French population stratified on the presence of CAD is presented in Figure 2. This analysis shows that the wGRS is associated with CAVS in patients with both CAVS and CAD (OR, 1.69 [95% CI, 1.35-2.12]; P < .001] and in patients with CAVS only (OR, 1.60 [95% CI, 1.18-2.16]; P < .001) compared with population controls.
Figure 2. Association of Genetically Elevated Lipoprotein(a) Levels With Calcific Aortic Valve Stenosis in Study Subgroups.
Patients with coronary artery disease and without coronary artery disease in the French cohorts, compared with control participants. CAVS indicates calcific aortic valve stenosis.
Family Study
The clinical characteristics of the 17 patients with CAVS, 33 of their first-degree relatives (including 6 with CAVS and 27 without CAVS), and a control group of 23 individuals with low Lp(a) levels is presented in the Table. Clinical characteristics of first-degree relatives and control participants with low Lp(a) levels were comparable, except for the percentage with CAD (patients with CAVS, 11 of 17 [64.7%]; first-degree relatives, 8 of 33 [24.2%]; control participants, 1 of 23 [4.4%]; P = .046), lipoprotein(a) levels (median [interquartile range], patients with CAVS, 93.4 [77.3-109.8] mg/dL; first-degree relatives, 36.7 [2.7-79.2] mg/dL; control participants, 3.12 [2.0-4.8] mg/dL; P < .001), transvalvular pressure gradients (mean [SD] peak gradient: patients with CAVS, 38.9 [17.3] mm Hg; first-degree relatives, 17.3 [16.9] mm Hg; P = .003; control participants, 7.7 [2.4] mm Hg; mean [SD] mean gradient: patients with CAVS, 21.8 [10.5] mm Hg; first-degree relatives, 9.4 [9.6] mm Hg; control participants, 4.2 [1.3] mm Hg; P = .004), and aortic valve area (mean [SD]: patients with CAVS, 1.07 [0.39] mm Hg; first-degree relatives, 1.96 [0.74] mm Hg; control participants, 2.41 [0.49] mm Hg; P = .02).
Table. Clinical Characteristics of Patients With Calcific Aortic Valve Stenosis, First-Degree Relatives, and a Control Group With Low Lipoprotein(a) (Lp[a]) Levels.
| Characteristic | Participants, No. (%) | |||
|---|---|---|---|---|
| Patients With CAVS | First-Degree Relatives | Control Participants With Low Lp(a) Levels | ||
| All | Without CAVS | |||
| No. | 17 | 33 | 27 | 23 |
| Age, mean (SD), y | 71.6 (7.7) | 63.9 (8.8) | 63.1 (8.7) | 60.6 (5.8) |
| Male, No. (%) | 11 (64.7) | 22 (66.7) | 17 (63.0) | 14 (60.9) |
| BMI, mean (SD) | 30.8 (5.4) | 29.3 (4.8) | 29.4 (5.1) | 28.1 (4.5) |
| Hypertension | 16 (94.1) | 18 (54.6) | 14 (51.9) | 8 (34.8) |
| Diabetes mellitus | 4 (23.5) | 6 (18.2) | 4 (14.8) | 2 (8.7) |
| Coronary artery disease | 11 (64.7) | 8 (24.2)a | 6 (22.2) | 1 (4.4) |
| Stroke | 0 | 0 | 0 | 1 (4.4) |
| Active smokers | 0 | 3 (9.1) | 3 (11.1) | 3 (13.0) |
| Lipid-lowering therapy | 12 (70.6) | 18 (54.6) | 14 (51.9) | 8 (34.8) |
| Aortic valve, mean (SD) | ||||
| Peak gradient, mm Hg | 38.9 (17.3) | 17.3 (16.9)b | 10.3 (4.8)c | 7.7 (2.4) |
| Mean gradient, mm Hg | 21.8 (10.5) | 9.4 (9.6)d | 5.4 (2.6)e | 4.2 (1.3) |
| Area, cm2 | 1.07 (0.39) | 1.96 (0.74)f | 2.20 (0.60) | 2.41 (0.49) |
| Lipoprotein(a), median (interquartile range), mg/dL | 93.4 (77.3-109.8) | 36.7 (2.7-79.2)g | 37.0 (3.6-86.7)h | 3.1 (2.0-4.8) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CAVS, calcific aortic valve stenosis.
P = .046 for first-degree relatives vs controls with low Lp(a) levels.
P = .003 for first-degree relatives vs controls with low Lp(a) levels.
P = .02 for first-degree relatives without CAVS vs controls with low Lp(a) levels.
P = .004 for first-degree relatives vs controls with low Lp(a) levels.
P = .03 for first-degree relatives without CAVS vs controls with low Lp(a) levels.
P = .02 for first-degree relatives vs controls with low Lp(a) levels.
P < .001 for first-degree relatives vs controls with low Lp(a) levels.
P < .001 for first-degree relatives without CAVS vs controls with low Lp(a) levels.
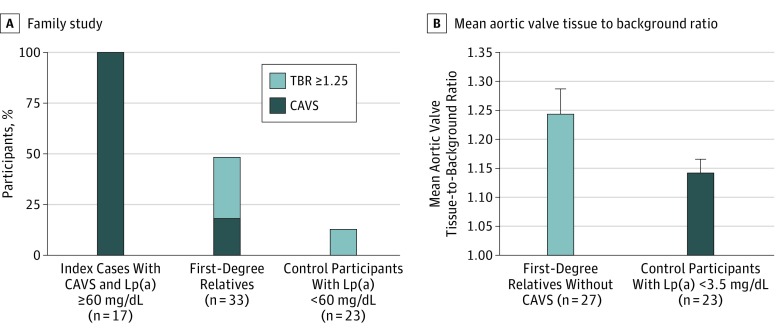
The percentage of participants with either CAVS or a TBR of 1.25 or more among first-degree relatives of patients with CAVS and high Lp(a) levels and the control groups is presented in Figure 3A. The percentage of individuals with a TBR of 1.25 or more or CAVS was higher in first-degree relatives of patients with CAVS (16 of 33 individuals [48.5%]) and high Lp(a) than controls (3 of 23 individuals [13%]; P = .006). No differences between the 2 groups were observed when we evaluated AVC by CT in first-degree relatives (data not shown). After excluding first-degree relatives with CAVS, the mean (SD) aortic valve TBR of first-degree relatives (without CAVS; 1.24 [0.23]) was higher than that of controls with low Lp(a) levels (1.14 [0.11]; P = .004; Figure 3B).
Figure 3. Distribution of Participant and Comparison Groups.
A, Distribution of patients with calcific aortic valve stenosis (CAVS) or a tissue to background (TBR) ratio of 1.25 or greater in the family study. B, Mean aortic valve TBR ratio in first-degree relatives without CAVS and control participants with low lipoprotein(a) (Lp[a]) levels.
Discussion
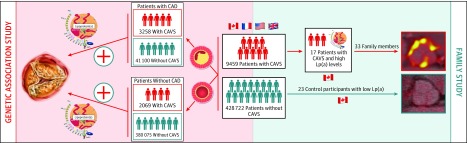
In this study, we found that genetic variation at the LPA locus associated with higher Lp(a) levels was strongly associated with CAVS risk in patients undergoing cardiac surgery. Investigating the association between a wGRS associated with high Lp(a) levels in 6 CAVS study cohorts stratified for the presence of CAD, we found that the association between genetically elevated Lp(a) levels and CAVS was independent of CAD status. Results of this study support the notion that the association between Lp(a) and CAVS is not mediated by the concomitant presence of CAD and individuals with high Lp(a) levels might have high CAVS risk even if they do not have CAD. In this proof-of-concept study, we found that first-degree relatives of patients with CAVS and elevated Lp(a) levels may be at higher risk of aortic valve microcalcification measured by 18F-NaF PET/CT compared with control participants with lower Lp(a) levels. These results consolidate the strong and independent association of Lp(a) with CAVS and support further research about the potential usefulness of routine measurement of Lp(a) levels in patients with CAVS and cascade screening for Lp(a) in the setting of CAVS (Figure 4).
Figure 4. Study Schematic.
Results of this study that included cohorts from 4 countries suggest that the association between genetically elevated lipoprotein(a) (Lp[a]) levels and calcific aortic valve stenosis (CAVS) is comparable in patients with coronary artery disease (CAD) vs those without and that first-degree relatives of patients with high Lp(a) levels and CAVS may have higher aortic valve calcification and could be at higher future risk of aortic valve calcification or CAVS.
It is well recognized that the presence of CAD might influence the symptom profile of CAVS and therefore the diagnosis of the disease, which is an important aspect to consider in genetic association studies of CAVS. Indeed, patients with CAD may become symptomatic at the stage of moderate aortic stenosis, whereas patients with severe aortic stenosis may remain asymptomatic in the absence of CAD. Another potential confounding factor is concomitant aortic valve replacement of a severe or moderate CAVS in patients undergoing coronary artery bypass surgery. In the setting of moderate aortic stenosis, aortic valve replacement is thus generally driven by the diagnosis of CAD and indication of coronary artery bypass. It must be recognized however that the presence of CAD relied on data from electronic medical records and was not validated by an independent committee in most of the cohorts. Additionally, study participants with subclinical CAD or symptomatic CAD without hospitalization might not have had their diagnosis captured in several cohorts and could have been assigned to the non-CAD group. Combined with previous work supporting a potentially causal role for Lp(a) in the causative mechanism of CAD,5,6 the finding that genetically elevated Lp(a) levels is associated with CAVS irrespective of the presence of CAD supports the notion that Lp(a) might be a strong risk factor and an important trigger for both coronary artery and aortic valve diseases. Altogether, these observations provide additional support to the hypothesis that Lp(a) may represent a therapeutic target of interest for CAVS.
Calcific aortic valve stenosis is a complex disease that was once thought to be the result of naturally occurring degenerative processes associated with aging. However, results of molecular and genetic association studies suggest that calcification of the aortic valve is a very tightly regulated process and that both genetic and environmental factors are pivotal for these processes.18 Imaging studies have also shown that years before the appearance of aortic calcification and anatomical disturbances of the aortic valve, the aortic valve may undergo a series of proinflammatory and osteogenic processes associated with early CAVS.19 The most compelling evidence that highlighted a genetic component in CAVS came from 2 studies performed in France that investigated the regional distribution of patients undergoing aortic valve replacement and the familial aggregation of the disease and concluded that CAVS has a strong genetic basis.20,21 More recently, Martinsson et al22 reported the results of a very large investigation of more than 6 million Swedish siblings and showed that the odds of developing CAVS were 3-fold higher if one had a sibling with CAVS and more than 30-fold higher if one had 2 siblings with CAVS. The association between Lp(a) levels and family risk of CAVS was not reported in these studies, however. Another recent investigation by Verweij et al23 included patients with atherosclerotic cardiovascular diseases and their first-degree relatives and found that first-degree relatives of patients with atherosclerotic cardiovascular diseases and Lp(a) levels of 50 mg/dL or more were twice as likely to be characterized by coronary artery calcium accumulation or to have had a cardiovascular event compared with those with lower Lp(a) levels. The link between Lp(a) levels and AVC accumulation was not reported in this family study.
The Working Group Recommendations to Reduce Lipoprotein(a)-Mediated Risk of Cardiovascular Disease and Aortic Stenosis recently included in its top priorities for Lp(a) research that clinical CAVS studies be performed to evaluate the role of Lp(a) in calcification with established techniques or with emerging techniques such as 18F-NaF PET/CT.24 Although previous studies have linked Lp(a) with aortic valve macrocalcification measured by CT,10,25 in this study, we used 18F-NaF PET/CT to assess microcalcification in first-degree relatives of patients with CAVS and high Lp(a) levels. The degree of CT-measured valve calcification reflects the amount of calcium regardless of when and at which rate the mineralization occurred in the course of CAVS, whereas the 18F-NaF PET/CT estimates the active mineral deposition in the aortic valve at a given point. Therefore, in contrast with CT, which detects quiescent macrocalcification, 18F-NaF PET/CT binds to active calcified nodules through chemical reactions with hydroxyapatite, a crystalline structure of calcium and phosphates.26 This technique is accurate and reproducible to detect and quantify inflammation (18F-fluorodeoxyglucose uptake) and mineralization activity (18F-NaF uptake into aortic valve hydroxyapatite).27,28,29,30 Both mineralization and inflammation are increased in patients with CAVS vs those without CAVS, and the activity of both rises steadily with increasing disease severity, as recently described by Dweck et al.27 However, mineralization is the culprit pathological process in CAVS, and the measurement of valvular fluorine 18–sodium fluoride (18F-NaF) uptake by PET/CT might provide a novel biomarker of early disease activity and progression.
In an effort to identify patients with high Lp(a) levels who may be at risk, the European Atherosclerosis Society Consensus Panel recommended measuring Lp(a) levels in individuals with premature CAD or with a family history of premature CAD, familial hypercholesterolemia, recurrent CAD despite statin treatment, or intermediate cardiovascular risk.31 Although it is tempting to suggest that Lp(a) levels should be assessed routinely in patients with CAVS, in the absence of guidelines about specific actions to be taken once a patient with CAVS and high Lp(a) is identified, this recommendation would be premature. Calcific aortic valve stenosis is the most common form of heart valve diseases. Surgical replacement of the aortic valve is the only effective treatment for CAVS. Given the strong association between Lp(a) and CAVS risk (independent of the presence of CAD), determining whether lowering Lp(a) levels in patients with mild-moderate CAVS would reduce the progression of CAVS or risk of future aortic valve replacement for severe CAVS would be the ultimate proof of a causal association between Lp(a) and CAVS, while simultaneously opening new therapeutic options to treat patients with CAVS and high Lp(a) levels. Results of this study reinforce the notion that cascade screening for Lp(a) (the measurement Lp[a] levels in patients with CAVS as well as in their first-degree relatives) could be clinically useful. In light of failed statin trials in CAVS, further studies should test whether targeting Lp(a) levels in these individuals would reduce future AVC accumulation or long-term CAVS risk. To our knowledge, only 1 agent (an antisense oligonucleotide targeting LPA) specifically designed to lower Lp(a) levels has been tested in a randomized clinical trial. Viney et al32 reported that treatment with AKCEA-APO(A)LRX was associated with a dose-response reduction in plasma Lp(a) levels of up to 80% to 90% in healthy individuals with Lp(a) levels equal to or greater than 60 mg/dL.
Limitations
Although active microcalcification assessed by 18F-NaF PET/CT is a factor associated with future AVC, it must be emphasized that, although these findings suggest that first-degree relatives of patients with CAVS and high Lp(a) levels have higher odds of being characterized by active microcalcification compared with controls with low Lp(a) levels, it is not possible to determine how many of them will show accumulation of AVC or develop clinically important CAVS in the future. Long-term follow-up of these individuals will help determine whether active microcalcification in the setting of high Lp(a) levels will translate into macroscopic AVC accumulation measured by CT. Additionally, because of the limited sample size, we could not determine whether these individuals have higher levels of aortic valve microcalcification because they have high mean Lp(a) levels or because they have family history of CAVS.
Conclusions
In conclusion, this investigation provides new genetic findings supporting an important role of Lp(a) in the causative mechanism of CAVS that is independent of the presence of CAD. Although the outcome of Lp(a)-lowering therapy in patients with mild-moderate CAVS or patients at high risk of developing CAVS still has to be established, these results provide evidence that the routine assessment of Lp(a) levels in patients with CAVS, as well as in their first-degree relatives, could be clinically useful.
eMethods. Methods
eReferences.
eTable 1. Characteristics of the selected single nucleotide polymorphism associated with Lp(a) concentrations characteristics.
eTable 2. Clinical characteristics of the Québec-CAVS cohort.
eTable 3. Clinical characteristics of the UK Biobank cohort.
eTable 4. Clinical characteristics of the EPIC-Norfolk cohort.
eTable 5. Clinical characteristics of the GERA cohort.
eTable 6. Clinical characteristics of the French cohorts.
References
- 1.Tsimikas S. A test in context: Lipoprotein(a), diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69(6):692-711. doi: 10.1016/j.jacc.2016.11.042 [DOI] [PubMed] [Google Scholar]
- 2.Leibundgut G, Scipione C, Yin H, et al. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a). J Lipid Res. 2013;54(10):2815-2830. doi: 10.1194/jlr.M040733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kronenberg F, Utermann G. Lipoprotein(a): resurrected by genetics. J Intern Med. 2013;273(1):6-30. doi: 10.1111/j.1365-2796.2012.02592.x [DOI] [PubMed] [Google Scholar]
- 4.Rao F, Schork AJ, Maihofer AX, et al. Heritability of biomarkers of oxidized lipoproteins: twin pair study. Arterioscler Thromb Vasc Biol. 2015;35(7):1704-1711. doi: 10.1161/ATVBAHA.115.305306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke R, Peden JF, Hopewell JC, et al. ; PROCARDIS Consortium . Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361(26):2518-2528. doi: 10.1056/NEJMoa0902604 [DOI] [PubMed] [Google Scholar]
- 6.Trégouët DA, König IR, Erdmann J, et al. ; Wellcome Trust Case Control Consortium; Cardiogenics Consortium . Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat Genet. 2009;41(3):283-285. doi: 10.1038/ng.314 [DOI] [PubMed] [Google Scholar]
- 7.Schunkert H, König IR, Kathiresan S, et al. ; Cardiogenics; CARDIoGRAM Consortium . Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43(4):333-338. doi: 10.1038/ng.784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emdin CA, Khera AV, Natarajan P, et al. ; CHARGE–Heart Failure Consortium; CARDIoGRAM Exome Consortium . Phenotypic characterization of genetically lowered human Lipoprotein(a) levels. J Am Coll Cardiol. 2016;68(25):2761-2772. doi: 10.1016/j.jacc.2016.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zekavat SM, Ruotsalainen S, Handsaker RE, et al. ; NHLBI TOPMed Lipids Working Group . Deep coverage whole genome sequences and plasma lipoprotein(a) in individuals of European and African ancestries. Nat Commun. 2018;9(1):2606. doi: 10.1038/s41467-018-04668-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thanassoulis G, Campbell CY, Owens DS, et al. ; CHARGE Extracoronary Calcium Working Group . Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368(6):503-512. doi: 10.1056/NEJMoa1109034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63(5):470-477. doi: 10.1016/j.jacc.2013.09.038 [DOI] [PubMed] [Google Scholar]
- 12.Arsenault BJ, Boekholdt SM, Dubé MP, et al. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis: a prospective mendelian randomization study and replication in a case-control cohort. Circ Cardiovasc Genet. 2014;7(3):304-310. doi: 10.1161/CIRCGENETICS.113.000400 [DOI] [PubMed] [Google Scholar]
- 13.Chen HY, Dufresne L, Burr H, et al. Association of LPA variants with aortic stenosis: a large-scale study using diagnostic and procedural codes from electronic health records. JAMA Cardiol. 2018;3(1):18-23. doi: 10.1001/jamacardio.2017.4266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capoulade R, Chan KL, Yeang C, et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol. 2015;66(11):1236-1246. doi: 10.1016/j.jacc.2015.07.020 [DOI] [PubMed] [Google Scholar]
- 15.Otto CM. Calcific aortic stenosis—time to look more closely at the valve. N Engl J Med. 2008;359(13):1395-1398. doi: 10.1056/NEJMe0807001 [DOI] [PubMed] [Google Scholar]
- 16.Perrot N, Boekholdt SM, Mathieu P, Wareham NJ, Khaw K-T, Arsenault BJ. Life’s simple 7 and calcific aortic valve stenosis incidence in apparently healthy men and women. Int J Cardiol. 2018;269:226-228. doi: 10.1016/j.ijcard.2018.07.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang C. Plink2.0. https://www.cog-genomics.org/plink/2.0/. Published April 29, 2019. Accessed May 1, 2019.
- 18.Bossé Y, Mathieu P, Pibarot P. Genomics: the next step to elucidate the etiology of calcific aortic valve stenosis. J Am Coll Cardiol. 2008;51(14):1327-1336. doi: 10.1016/j.jacc.2007.12.031 [DOI] [PubMed] [Google Scholar]
- 19.Aikawa E, Otto CM. Look more closely at the valve: imaging calcific aortic valve disease. Circulation. 2012;125(1):9-11. doi: 10.1161/CIRCULATIONAHA.111.073452 [DOI] [PubMed] [Google Scholar]
- 20.Probst V, Le Scouarnec S, Legendre A, et al. Familial aggregation of calcific aortic valve stenosis in the western part of France. Circulation. 2006;113(6):856-860. doi: 10.1161/CIRCULATIONAHA.105.569467 [DOI] [PubMed] [Google Scholar]
- 21.Le Gal G, Bertault V, Bezon E, Cornily JC, Barra JA, Blanc JJ. Heterogeneous geographic distribution of patients with aortic valve stenosis: arguments for new aetiological hypothesis. Heart. 2005;91(2):247-249. doi: 10.1136/hrt.2004.037093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinsson A, Li X, Zöller B, et al. Familial aggregation of aortic valvular stenosis: a nationwide study of sibling risk. Circ Cardiovasc Genet. 2017;10(6):10. doi: 10.1161/CIRCGENETICS.117.001742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verweij SL, de Ronde MWJ, Verbeek R, et al. Elevated lipoprotein(a) levels are associated with coronary artery calcium scores in asymptomatic individuals with a family history of premature atherosclerotic cardiovascular disease. J Clin Lipidol. 2018;12(3):597-603.e1. doi: 10.1016/j.jacl.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 24.Tsimikas S, Fazio S, Ferdinand KC, et al. NHLBI working group recommendations to reduce lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol. 2018;71(2):177-192. doi: 10.1016/j.jacc.2017.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vongpromek R, Bos S, Ten Kate GJ, et al. Lipoprotein(a) levels are associated with aortic valve calcification in asymptomatic patients with familial hypercholesterolaemia. J Intern Med. 2015;278(2):166-173. doi: 10.1111/joim.12335 [DOI] [PubMed] [Google Scholar]
- 26.Agnese I, Alex TV, David YL, et al. Identifying active vascular microcalcification by 18F-sodium fluoride positron emission tomography. Nat Commun. 2015;7495:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dweck MR, Jones C, Joshi NV, et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation. 2012;125(1):76-86. doi: 10.1161/CIRCULATIONAHA.111.051052 [DOI] [PubMed] [Google Scholar]
- 28.Dweck MR, Khaw HJ, Sng GK, et al. Aortic stenosis, atherosclerosis, and skeletal bone: is there a common link with calcification and inflammation? Eur Heart J. 2013;34(21):1567-1574. doi: 10.1093/eurheartj/eht034 [DOI] [PubMed] [Google Scholar]
- 29.Marincheva-Savcheva G, Subramanian S, Qadir S, et al. Imaging of the aortic valve using fluorodeoxyglucose positron emission tomography increased valvular fluorodeoxyglucose uptake in aortic stenosis. J Am Coll Cardiol. 2011;57(25):2507-2515. doi: 10.1016/j.jacc.2010.12.046 [DOI] [PubMed] [Google Scholar]
- 30.Hyafil F, Messika-Zeitoun D, Burg S, et al. Detection of 18fluoride sodium accumulation by positron emission tomography in calcified stenotic aortic valves. Am J Cardiol. 2012;109(8):1194-1196. doi: 10.1016/j.amjcard.2011.11.060 [DOI] [PubMed] [Google Scholar]
- 31.Nordestgaard BG, Chapman MJ, Ray K, et al. ; European Atherosclerosis Society Consensus Panel . Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31(23):2844-2853. doi: 10.1093/eurheartj/ehq386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viney NJ, van Capelleveen JC, Geary RS, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388(10057):2239-2253. doi: 10.1016/S0140-6736(16)31009-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Methods
eReferences.
eTable 1. Characteristics of the selected single nucleotide polymorphism associated with Lp(a) concentrations characteristics.
eTable 2. Clinical characteristics of the Québec-CAVS cohort.
eTable 3. Clinical characteristics of the UK Biobank cohort.
eTable 4. Clinical characteristics of the EPIC-Norfolk cohort.
eTable 5. Clinical characteristics of the GERA cohort.
eTable 6. Clinical characteristics of the French cohorts.