Key Points
Question
Does mandatory reporting of CYP2C19 metabolizer status influence physicians’ decisions to switch P2Y12 inhibitors in the context of a clinical trial?
Findings
In this secondary analysis of a randomized clinical trial, prior to learning CYP2C19 metabolizer status, physicians were evenly split on how they intended to use the information. Prestipulated plans were not consistently implemented, with more than 68% of patients with reduced metabolizer status continuing to receive clopidogrel after results were provided to physicians.
Meaning
In the absence of definitive trial evidence of improvements in outcomes with a genetic testing strategy, clinicians are divided on how to implement genotyping data into clinical practice and are reluctant to alter pharmacotherapy based on provided results.
Abstract
Importance
Physician behavior in response to knowledge of a patient’s CYP2C19 clopidogrel metabolizer status is unknown.
Objective
To investigate the association of mandatory reporting of CYP2C19 pharmacogenomic testing, provided to investigators with no direct recommendations on how to use these results, with changes in P2Y12 inhibitor use, particularly clopidogrel, in the Randomized Trial to Compare the Safety of Rivaroxaban vs Aspirin in Addition to Either Clopidogrel or Ticagrelor in Acute Coronary Syndrome (GEMINI-ACS-1) clinical trial.
Design, Setting, and Participants
The GEMINI-ACS-1 trial compared rivaroxaban, 2.5 mg twice daily, with aspirin, 100 mg daily, plus open-label clopidogrel or ticagrelor (provided), in patients with recent acute coronary syndromes (ACS). The trial included 371 clinical centers in 21 countries and 3037 patients with ACS. Data were analyzed between May 2017 and February 2019.
Interventions
Investigators were required to prestipulate their planned response to CYP2C19 metabolizer status. In response to a regulatory mandate, results for all patients were reported to investigators approximately 1 week after randomization.
Main Outcomes and Measures
Reasons for switching P2Y12 inhibitors and occurrence of bleeding and ischemic events were collected.
Results
Of 3037 patients enrolled (mean [SD] age, 62.8 [9.0] years; 2275 men [74.9%], and 2824 white race/ethnicity [93.0%]), investigators initially treated 1704 (56.1%) with ticagrelor and 1333 (43.9%) with clopidogrel. Investigators prestipulated that they would use CYP2C19 metabolizer status to change P2Y12 inhibitor in 48.5% of genotyped clopidogrel-treated patients (n = 642 of 1324) and 5.5% of genotyped ticagrelor-treated patients (n = 93 of 1692). P2Y12 inhibitor switching for any reason occurred in 197 patients and was more common in patients treated with ticagrelor (146 of 1704 [8.6%]) compared with clopidogrel (51 of 1333 [3.8%]). Of patients initially treated with ticagrelor, only 1 (0.1% overall; 0.7% of all who switched) was switched based on CYP2C19 status. Of patients initially treated with clopidogrel, 23 (1.7% overall,;45.1% of all who switched) were switched owing to metabolizer status. Of 48 patients (3.6%) with reduced metabolizer status treated initially with clopidogrel, 15 (31.3%) were switched based on metabolizer status, including 48.1% (13 of 27) in which switching was prestipulated.
Conclusions and Relevance
Physicians were evenly split on how to respond to knowledge of CYP2C19 metabolizer status in clopidogrel-treated patients. Mandatory provision of this information rarely prompted P2Y12 inhibitor switching overall, including a minority of patients with reduced metabolizer status. These findings highlight the clinical equipoise among physicians regarding use of this information and the reluctance to use information from routine genotyping in the absence of definitive clinical trial data demonstrating the efficacy of this approach.
Clinical Trial Registration
ClinicalTrials.gov identifier: NCT02293395
This study investigates whether mandatory reporting of CYP2C19 metabolizer status influence physicians’ decisions to switch P2Y12 inhibitors in the context of a clinical trial.
Introduction
Platelet P2Y12 receptor inhibitors, together with aspirin, are recommended for patients with acute coronary syndromes (ACS). Clopidogrel is a prodrug that requires conversion into an active metabolite, with CYP2C19 being the most important enzyme involved in its activation.1 Patients who carry reduced-function CYP2C19 alleles generate lower levels of the clopidogrel active metabolite and have higher on-treatment platelet reactivity when treated with clopidogrel2 and an increased risk of ischemic events.3,4,5,6 The US Food and Drug Administration (FDA) in 2010 made modifications to the label indications for clopidogrel that suggested genotyping and subsequent adjustment of antiplatelet therapies for poor metabolizers; however, clinical guidelines do not reflect this approach.7
The FDA warning prompted regulatory requirements to perform routine genotyping for patients included in randomized clinical trials, evaluating experimental antithrombotic regimens vs active-control treatment arms that include clopidogrel. The association of knowledge of CYP2C19 metabolizer status with physician choice of P2Y12 inhibitor for the treatment of patients with ACS is unknown. We investigated the association of mandatory reporting of CYP2C19 genotype with changes in initial P2Y12 inhibitor use, particularly clopidogrel, in the Randomized Trial to Compare the Safety of Rivaroxaban vs Aspirin in Addition to Either Clopidogrel or Ticagrelor in Acute Coronary Syndrome (GEMINI-ACS-1) that evaluated rivaroxaban compared with aspirin together with clopidogrel or ticagrelor in patients with a recent ACS event.8,9
Methods
The design and features of the GEMINI-ACS-1 trial were previously published.8,9 The FDA required that the results of CYP2C19 metabolizer status be reported to investigators for all patients.9 Patients were designated as having ultrarapid metabolizer status, extensive metabolizer status, intermediate metabolizer status (IM), and reduced metabolizer status (RM) according to clinical pharmacogenetics implementation consortium guidelines10 based on CYP2C19 genotyping (eAppendix in the Supplement). No direct recommendations were given regarding P2Y12 inhibitor therapy choices.9 Investigators were required to prestipulate how they expected to use this information. The median duration of follow-up was 326 days.8 The protocol was approved by ethics committees at participating sites, and all patients provided written informed consent. Statistical methods are fully described in the eMethods in the Supplement.
Results
Rate and Timing of P2Y12 Switching
Of 3037 patients enrolled, 1704 (56.1%) were initially treated with ticagrelor while 1333 (43.9%) received clopidogrel. The initial choice of P2Y12 inhibitor was switched in 197 of 3037 enrolled patients (6.5%): 146 of 1704 patients (8.6%) initially treated with ticagrelor and 51 of 1333 patients (3.8%) initially treated with clopidogrel. Patients who were switched for any reason were slightly older, more likely to live in Western Europe or North America, and had less diabetes and hypertension, but were more likely to have undergone prior revascularization or have peripheral artery disease (eTable in the Supplement).
The association of the initial choice of clopidogrel or ticagrelor with P2Y12 inhibitor switching was nonproportional over time and occurred earlier for those switched from clopidogrel (eFigure 1 in the Supplement; Table 1). P2Y12 inhibitor switching was most frequent in both arms the first month after randomization but was uncommon subsequently among patients initially treated with clopidogrel. The most common reason for switching from ticagrelor as recorded by investigators was a nonbleeding adverse event, while CYP2C19 status was the most common reason for switching from clopidogrel (Table 1).
Table 1. Reasons for and Timing of P2Y12 Inhibitor Switching (All Patients).
| Indication for P2Y12 Inhibitor Switching by Stratum | No./Total No. (%) (N = 3037) | Time to Switch, Median (IQR), d |
|---|---|---|
| Total patients who switched P2Y12 | 197/3037 (6.5) | 40.0 (24.0-118.0) |
| Ticagrelor stratum | 146/1704 (8.6) | 62.0 (28.0-143.0) |
| Reasons | ||
| CYP2C19 metabolizer status | 1 (0.1) | 29.0 (29.0-29.0) |
| Nonbleeding adverse event | 81 (4.8) | 42.0 (28.0-94.0) |
| Other | 62 (3.6) | 100.0 (29.0-197.0) |
| Recurrent ischemic event | 2 (0.1) | 67.0 (1.0-133.0) |
| Clopidogrel stratum | 51/1333 (3.8) | 30.0 (11.0-59.0) |
| Reasons | ||
| CYP2C19 metabolizer status | 23 (1.7) | 29.0 (14.0-33.0) |
| Nonbleeding adverse event | 3 (0.2) | 91.0 (30.0-92.0) |
| Other | 16 (1.2) | 28.0 (2.5-37.0) |
| Recurrent ischemic event | 9 (0.7) | 70.0 (8.0-77.0) |
Abbreviations: IQR, interquartile range; NA, not applicable.
Results of P2Y12 Metabolizer Status
P2Y12 metabolizer status was reported to investigators in 3016 of 3037 patients (99%): 1039 (34.4%) were patients with ultrarapid metabolizer status, 1140 (37.8%) were patients with extensive metabolizer status, 739 (24.5%) were patients with IM status, and 98 (3.2%) were patients with RM status. Of the 1692 genotyped patients initially treated with ticagrelor, 7% to 11% of patients were switched across CYP2C19 metabolizer status categories (eFigure 2 in the Supplement). Of the 1324 genotyped patients initially treated with clopidogrel, 51 were switched: 33% of patients with RM status (n = 8 of 465) were switched as compared with 4.7% (n = 16 of 343), 2.4% (n = 11 of 468), and 1.7% (n = 8 of 465) of patients with intermediate, extensive, and ultrarapid metabolizer status, respectively (eFigure 2 in the Supplement).
CYP2C19 Genotyping and P2Y12 Inhibitor Switching: Patients Treated With Ticagrelor
Investigators prestipulated switching from ticagrelor based on CYP2C19 status in 93 patients; however, only 1 patient was switched based on this information (Table 2). In 68 patients with ultrarapid/extensive metabolizer status in which the investigator prestipulated switching, the actual rate of switching was only 1.5% (n = 1 of 68).
Table 2. Rates of Switching Based on CYP2C19 Metabolizer Status.
| Initial P2Y12 Inhibitor | No./Total No. (%) | |||
|---|---|---|---|---|
| Metabolizer | Total | |||
| Ultrarapid and Extensive | Intermediate | Reduced | ||
| Clopidogrel | ||||
| All | 1/933 (0.1) | 7/343 (2.0) | 15/48 (31.3) | 23/1324 (1.7) |
| Prestipulated | ||||
| Yes | 0/463 | 7/152 (4.6) | 13/27 (48.1) | 20/642 (3.4) |
| No | 1/470 (0.2) | 0/191 | 2/21 (9.5) | 3/682 (0.5) |
| Ticagrelor | ||||
| All | 1/1246 (0.1) | 0/396 | 0/50 | 1/1692 (0.1) |
| Prestipulated | ||||
| Yes | 1/68 (1.5) | 0/21 | 0/4 | 1/93 (1.1) |
| No | 0/1178 | 0/375 | 0/46 | 0/1599 |
CYP2C19 Genotyping and P2Y12 Inhibitor Switching: Patients Treated With Clopidogrel
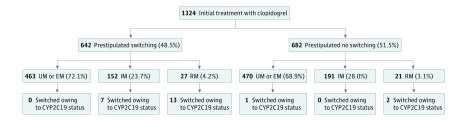
Switching was prestipulated in 642 of 1324 genotyped clopidogrel-treated patients with RM status (48.5%). Of the 152 patients with IM and 27 patients with RM status in which switching was prestipulated, 7 (4.6%) and 13 (48.1%), respectively, were switched based on CYP2C19 metabolizer status. Clopidogrel was switched based on CYP2C19 metabolizer status in 23 patients overall (1.7%), including 7 of 343 patients with IM status (2.0%) and 15 of 48 patients with RM status (31.3%) (Figure and Table 2).
Figure. Flowchart Depicting Switching by Metabolizer Status and Prestipulated Intent in Patients Initially Treated With Clopidogrel.
EM indicates extensive metabolizers; IM, intermediate metabolizers; RM, reduced metabolizers; UM, ultrarapid metabolizers.
Regional Variation
P2Y12 inhibitor switching was most likely among patients enrolled in North America and Western Europe (eFigure 3 in the Supplement). The number of patients with RM status receiving clopidogrel in these regions was limited (n = 2 [North America] and n = 1 [Western Europe]), and analysis of CYP2C19-influenced switching behavior by region is inconclusive (eFigure 4 in the Supplement).
Discussion
Our analysis represents, to our knowledge, the largest, most comprehensive evaluation of the association of knowledge of CYP2C19 metabolizer status with a physician’s choice of P2Y12 inhibitor for use in patients with ACS. Our findings highlight how investigators incorporate FDA-mandated provision of CYP2C19 metabolizer status into clinical practice when this information was provided without specific guidance on its use.
First, investigators using ticagrelor were less likely to prestipulate switching (6%) and ultimately did not use information on CYP2C19 status, even when they prestipulated intent to switch in patients with ultrarapid/extensive metabolizer status. Second, there was no consensus among investigators on how to use these data in patients receiving clopidogrel, with a nearly 50/50 split on whether these data would be used to inform P2Y12 inhibitor choice. This is especially notable in GEMINI-ACS-1, given that patients were randomized in a blinded fashion to no aspirin therapy, theoretically exposing reduced metabolizers to limited direct antiplatelet therapy. Second, investigators acted on this information in only 31% of patients with RM status (n = 15 of 48). Finally, mandatory reporting of this information in more than 3000 patients resulted in only 24 switches (0.8%), reflecting the low yield of mandatory requirements for CYP2C19 testing in clinical trials.
Previous evaluation of P2Y12 inhibitor switching based on limited reporting of CYP2C19 metabolizer status for patients treated with clopidogrel demonstrated similar findings. Data from a common pharmacy benefits management plan indicated that only 8% of 6000 US clopidogrel-treated patients with ACS accepted the offer to have free genotyping results reported to their physicians, and only one-fifth of patients with RM status were then switched from clopidogrel.11
Several analyses have evaluated the effect of routine reporting of CYP2C19 metabolizer status. A nonrandomized study implemented routine testing of CYP2C19 metabolizer status for 1815 US patients undergoing percutaneous coronary intervention with recommendations to use ticagrelor or prasugrel for the 518 patients with IM status (28.5%) and 54 patients with RM status (3%).12 In 58% of the patients with IM status and 87% of patients with RM status prescribed ticagrelor or prasugrel, a lower risk of adverse cardiovascular outcomes was observed; however, whether similar reductions in events might have been observed in patients with extensive or ultrarapid metabolizers was not assessed. Similar findings have been shown in analyses from national registries in the Netherlands and Spain.13,14 None of these studies prospectively evaluated how physicians responded to the CYP2C19 metabolizer status.
The Pharmacogenomic Approach to Selecting Antiplatelet Therapy in Patients With Acute Coronary Syndromes (PHARMCLO) trial is, to our knowledge, the first randomized, although unblinded, trial to assess a strategy of tailoring P2Y12 inhibitor choice based in part on genotyping information, suggesting that use of this information could lead to a decrease in combined ischemic and bleeding events.15 While provocative, recommendations and therapeutic choices were made on the basis of both genotypic and clinical information, and the number of patients whose therapies were altered and outcomes in these particular patients have not been presented. In addition, the use of P2Y12 inhibitors was similar in both arms, so the discrepancy in outcomes requires additional analyses.
Limitations
This study does not inform whether patients with RM status may have compromised clinical outcomes if treated with clopidogrel. The blinded treatment of patients with low-dose rivaroxaban compared with aspirin in our trial may have influenced patterns of P2Y12 inhibitor switching, although we would expect that this would encourage switching from clopidogrel. Unless the investigator indicated that metabolizer status was the primary reason for switching, we could not capture whether knowledge of metabolizer status influenced switching decisions in response to other adverse events. Although most patients were likely already discharged at the time genotyping data was provided (7 days after enrollment), all patients had mandatory follow-up at 4 weeks, at which time P2Y12 inhibitor choice could have been discussed in person. Investigators were asked about intention to switch if a reduced-function allele was identified; however, the FDA boxed warning is directed at patients with RM status (2 loss-of-function alleles).
Conclusions
Physicians exhibited equipoise on how to use information regarding routine genotyping of patients with ACS, which rarely prompted P2Y12 inhibitor switching overall, led to switching in less than one-third of patients with RM receiving clopidogrel, and was not acted on consistently with prespecified intent. Until trials demonstrate improved clinical outcomes with pharmacogenomics-guided strategies, routine genotyping of patients with ACS remains low yield. Physicians appear reluctant to incorporate this information into their clinical practice, and high-potency, more predictable P2Y12 inhibitors remain preferred agents in patients at high ischemic risk.
eMethods
eTable 1. Comparison of Patient Characteristics: Switching vs No Switching
eFigure 1. Frequency of P2Y12 Inhibitor Switching for Any Reason by Initial Choice of P2Y12 Inhibitor (Clopidogrel vs. Ticagrelor) Over Time
eFigure 2. Frequency of Overall P2Y12 Inhibitor Switching for Any Reason by Metabolizer Status and Initial P2Y12 Inhibitor Choice
eFigure 3. Time Course of P2Y12 Inhibitor Switching by Region
eFigure 4. Flow Chart Depicting Switching due to CYP2C19 Status by Metabolizer Status and Prestipulated Intent in Patients Initially Treated With Clopidogrel
eAppendix. CYP219 Genotype Interpretation
References
- 1.Hagihara K, Kazui M, Kurihara A, et al. A possible mechanism for the differences in efficiency and variability of active metabolite formation from thienopyridine antiplatelet agents, prasugrel and clopidogrel. Drug Metab Dispos. 2009;37(11):2145-2152. doi: 10.1124/dmd.109.028498 [DOI] [PubMed] [Google Scholar]
- 2.Gurbel PA, Shuldiner AR, Bliden KP, Ryan K, Pakyz RE, Tantry US. The relation between CYP2C19 genotype and phenotype in stented patients on maintenance dual antiplatelet therapy. Am Heart J. 2011;161(3):598-604. doi: 10.1016/j.ahj.2010.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doll JA, Neely ML, Roe MT, et al. ; TRILOGY ACS Investigators . Impact of CYP2C19 metabolizer status on patients with ACS treated with prasugrel versus clopidogrel. J Am Coll Cardiol. 2016;67(8):936-947. doi: 10.1016/j.jacc.2015.12.036 [DOI] [PubMed] [Google Scholar]
- 4.Holmes MV, Perel P, Shah T, Hingorani AD, Casas JP. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. JAMA. 2011;306(24):2704-2714. doi: 10.1001/jama.2011.1880 [DOI] [PubMed] [Google Scholar]
- 5.Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360(4):354-362. doi: 10.1056/NEJMoa0809171 [DOI] [PubMed] [Google Scholar]
- 6.Shuldiner AR, O’Connell JR, Bliden KP, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302(8):849-857. doi: 10.1001/jama.2009.1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes DR Jr, Dehmer GJ, Kaul S, Leifer D, O’Gara PT, Stein CM. ACCF/AHA clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents and the American Heart Association endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2010;56(4):321-341. doi: 10.1016/j.jacc.2010.05.013 [DOI] [PubMed] [Google Scholar]
- 8.Ohman EM, Roe MT, Steg PG, et al. Clinically significant bleeding with low-dose rivaroxaban versus aspirin, in addition to P2Y12 inhibition, in acute coronary syndromes (GEMINI-ACS-1): a double-blind, multicentre, randomised trial. Lancet. 2017;389(10081):1799-1808. doi: 10.1016/S0140-6736(17)30751-1 [DOI] [PubMed] [Google Scholar]
- 9.Povsic TJ, Roe MT, Ohman EM, et al. A randomized trial to compare the safety of rivaroxaban vs aspirin in addition to either clopidogrel or ticagrelor in acute coronary syndrome: the design of the GEMINI-ACS-1 phase II study. Am Heart J. 2016;174:120-128. doi: 10.1016/j.ahj.2016.01.004 [DOI] [PubMed] [Google Scholar]
- 10.Scott SA, Sangkuhl K, Stein CM, et al. ; Clinical Pharmacogenetics Implementation Consortium . Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94(3):317-323. doi: 10.1038/clpt.2013.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai NR, Canestaro WJ, Kyrychenko P, et al. Impact of CYP2C19 genetic testing on provider prescribing patterns for antiplatelet therapy after acute coronary syndromes and percutaneous coronary intervention. Circ Cardiovasc Qual Outcomes. 2013;6(6):694-699. doi: 10.1161/CIRCOUTCOMES.113.000321 [DOI] [PubMed] [Google Scholar]
- 12.Cavallari LH, Lee CR, Beitelshees AL, et al. ; IGNITE Network . Multisite investigation of outcomes with implementation of CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. JACC Cardiovasc Interv. 2018;11(2):181-191. doi: 10.1016/j.jcin.2017.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deiman BALM, Tonino PAL, Kouhestani K, et al. Reduced number of cardiovascular events and increased cost-effectiveness by genotype-guided antiplatelet therapy in patients undergoing percutaneous coronary interventions in the Netherlands. Neth Heart J. 2016;24(10):589-599. doi: 10.1007/s12471-016-0873-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sánchez-Ramos J, Dávila-Fajardo CL, Toledo Frías P, et al. Results of genotype-guided antiplatelet therapy in patients who undergone percutaneous coronary intervention with stent. Int J Cardiol. 2016;225:289-295. doi: 10.1016/j.ijcard.2016.09.088 [DOI] [PubMed] [Google Scholar]
- 15.Notarangelo FM, Maglietta G, Bevilacqua P, et al. Pharmacogenomic approach to selecting antiplatelet therapy in patients with acute coronary syndromes: the PHARMCLO trial. J Am Coll Cardiol. 2018;71(17):1869-1877. doi: 10.1016/j.jacc.2018.02.029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1. Comparison of Patient Characteristics: Switching vs No Switching
eFigure 1. Frequency of P2Y12 Inhibitor Switching for Any Reason by Initial Choice of P2Y12 Inhibitor (Clopidogrel vs. Ticagrelor) Over Time
eFigure 2. Frequency of Overall P2Y12 Inhibitor Switching for Any Reason by Metabolizer Status and Initial P2Y12 Inhibitor Choice
eFigure 3. Time Course of P2Y12 Inhibitor Switching by Region
eFigure 4. Flow Chart Depicting Switching due to CYP2C19 Status by Metabolizer Status and Prestipulated Intent in Patients Initially Treated With Clopidogrel
eAppendix. CYP219 Genotype Interpretation