This study examines the mechanism by which triptan drugs enter the central nervous system in individuals with migraine.
Key Points
Question
Does the antimigraine drug sumatriptan, a 5-HT1B receptor agonist, cross the blood-brain barrier in patients with migraine?
Findings
In this study of 8 patients with untreated episodic migraine without aura, administration of a clinically relevant dose of sumatriptan was associated with a statistically significant decrease in 5-HT1B receptor binding that corresponded to a mean 16.0% 5-HT1B receptor occupancy of sumatriptan.
Meaning
Reduction in 5-HT1B receptor binding after sumatriptan administration appeared to be associated with the binding of sumatriptan to central 5-HT1B receptors, but cerebral release of serotonin could also play a role.
Abstract
Importance
Triptans, the most efficient acute treatment for migraine attacks, are 5-HT1B/1D receptor agonists, but their precise mechanism of action is not completely understood. The extent to which triptans enter the central nervous system and bind to 5-HT1B receptors in the brain is unknown.
Objectives
To determine the occupancy of sumatriptan to central 5-HT1B receptors, and to investigate changes in brain serotonin levels during migraine attacks.
Design, Setting, and Participants
This study of 8 patients in Denmark used a within-participant design and was conducted from April 20, 2015, to December 5, 2016. Participants were otherwise healthy patients with untreated episodic migraine without aura, aged between 18 and 65 years, and recruited from the general community. Data analysis was performed from January 2017 to April 2018.
Interventions
All participants underwent positron emission tomographic scans after injection of [11C]AZ10419369, a specific 5-HT1B receptor radiotracer. All participants were scanned 3 times: (1) during an experimentally induced migraine attack, (2) after a subcutaneous injection of 6-mg subcutaneous sumatriptan, and (3) on a migraine attack–free day. Scans 1 and 2 were conducted on the same study day. Each scan lasted for 90 minutes.
Main Outcome and Measure
The primary outcome was the nondisplaceable binding potential of [11C]AZ10419369 across 7 brain regions involved in pain modulation. The binding potential reflects receptor density, and changes in binding potential reflects displacement of the radiotracer. The occupancy of sumatriptan was estimated from the 2 scans before and after sumatriptan administration.
Results
Eight patients with migraine were included in the study; of these participants, 7 (87%) were women. The mean (SD) age of participants on study day 1 was 29.5 (9.2) years and on study day 2 was 30.0 (8.9) years. Sumatriptan was associated with statistically significantly reduced 5-HT1B receptor binding across pain-modulating regions (mean [SD] binding potential, 1.20 [0.20] vs 1.02 [0.22]; P = .001), corresponding to a mean (SD) drug occupancy rate of 16.0% (5.3%). Furthermore, during migraine attacks, as compared with outside of attacks, 5-HT1B receptor binding was statistically significantly associated with reduced in pain-modulating regions (mean [SD] binding potential, 1.36 [0.22] vs 1.20 [0.20]; P = .02).
Conclusions and Relevance
Treatment with sumatriptan during migraine attacks appeared to be associated with a decrease in 5-HT1B receptor binding, a finding that is most likely associated with the binding of sumatriptan to central 5-HT1B receptors, but the contribution of ongoing cerebral serotonin release to the lower binding cannot be excluded; the migraine attack–associated decrease in binding could indicate that migraine attacks are associated with increases in endogenous serotonin.
Introduction
Triptans, which were introduced in the late 1980s,1 have revolutionized migraine management and remain the standard abortive therapy for migraine. Triptans are 5-HT1B/1D receptor agonists, but the specific mechanism of action relevant to their therapeutic effects is unknown. The antimigraine mechanism was originally thought to be exerted exclusively through triptans’ vasoconstrictor process,2,3 but preclinical studies suggest a more complex mode of action, such as impeding signals between the first- and second-order trigeminal neurons4,5 or preventing release of vasoactive and inflammatory substances from trigeminal nerve endings.6 In addition, the 5-HT1B receptor is expressed abundantly throughout the human brain,7 and common triptans-associated adverse events such as dizziness and somnolence8 suggest a possible central nervous system implication. However, to which extent triptans enter the brain and bind to 5-HT1B receptors in the brain parenchyma remains unclear.
Positron emission tomographic (PET) imaging with the specific 5-HT1B receptor radiotracer [11C]AZ10419369 is suitable for measuring endogenous brain 5-HT (serotonin) release9,10 and for assessing the occupancy of drugs binding to the 5-HT1B receptor (eg, triptans).11,12 Using [11C]AZ10419369, a study recently reported low 5-HT1B receptor binding across pain-modulating regions in patients with migraine on an attack-free day and that binding of 5-HT1B receptors in the raphe was associated with time since the most recent migraine attack.13 Because brain serotonin levels are believed to increase during migraine attacks,14 higher brain serotonin levels during attacks could result in a temporary downregulation of the 5-HT1B receptor. However, conclusive evidence is still lacking on increases in brain serotonin during a migraine attack and on binding of sumatriptan succinate to central 5-HT1B receptors during migraine attacks.
We investigated 5-HT1B receptor binding in patients with migraine during migraine attacks (ictal) and after treatment with sumatriptan (postictal). In addition, all participating patients underwent a PET scan on an attack-free day (interictal). We hypothesized that brain 5-HT1B receptor binding would decrease after administration of a clinically relevant dose of sumatriptan. Furthermore, we hypothesized that 5-HT1B receptor binding would be lower during the ictal phase compared with the interictal phase because of the competition between endogenous serotonin and [11C]AZ10419369 at the 5HT1B receptor site.
Methods
This study was approved by the Ethics Committee of the Capital Region of Denmark and the Danish Data Protection Agency, and it was registered at ClinicalTrials.gov (Identifier: NCT01896167). All participants provided written informed consent after being given detailed oral and written information about the study. The study was conducted in accordance with the Declaration of Helsinki of 196415 and was conducted from April 20, 2015, to December 5, 2016. Data analysis was performed from January 2017 to April 2018.
Participants
Patients were recruited through advertisement on a Danish website for recruiting participants for health research (http://www.forsogsperson.dk/) and through a local database. Data from 5 patients’ interictal scans have been published previously.13 The inclusion criteria were as follows: (1) aged 18 to 65 years; 2) verified diagnosis of migraine without aura according to the criteria of the International Classification of Headache Disorders, 3rd edition (beta version),16 (3) at least 1 migraine attack every other month but fewer than 5 migraine days per month; (4) previous experience of successful treatment of migraine attacks with sumatriptan; and (5) successful induction of migraine after cilostazol administration (assessed by preinclusion screening). None of the participants had a history of any other primary headache (except for tension-type headache for fewer than 5 days per month); any psychiatric, cerebrovascular, or cardiovascular disease; or any daily intake of medication. No participant was pregnant or nursing, and all participants were eligible for a magnetic resonance imaging scan.
Study Design
On the day of the interictal scan, all included participants had been migraine free for more than 48 hours. The date of their most recent migraine attack was registered. No medication intake was allowed for 24 hours before the scan. Patients were excluded if they reported a migraine attack less than 48 hours after the scan.
To ensure that participants would have a migraine attack on the day of their planned scan, we used a human migraine provocation model to investigate [11C]AZ10419369 binding during migraine attacks. We used cilostazol, a phosphodiesterase 3 inhibitor, that has proven to be both efficient and highly reproducible for experimental triggering of migraine.17,18 For practical reasons, we used cilostazol as it is easier to administer, compared with other migraine triggers such as nitroglycerin, because it can be taken orally; thus, this method was less invasive.
In the morning on the day of the scan, participants ingested 200-mg cilostazol. Intake of the drug was ensured by a video phone call to the participants from one of us (M.D.). After ingestion, the participants filled out a headache diary every hour until their arrival at the hospital. After arrival, the diary was filled out every 30 minutes until initiation of the first scan, after which the diary was filled out every 10 minutes continuing until the end of the second scan. The diary included questions on headache intensity score (0-10), associated symptoms, and premonitory symptoms. Because the patients could not move when lying in the scanner, they were asked to make a judgment as to whether their headache would worsen by coughing or by performing physical activity.
We defined migraine-like attacks according to the definition of migraine without aura in criteria C and D of the International Classification of Headache Disorders, 3rd edition (beta version).16 Criteria C headache has at least 2 of the following 4 characteristics: unilateral location, pulsating quality, moderate or severe pain intensity, and aggravation by or causing avoidance of routine physical activity (eg, walking or climbing stairs). Criteria D headache has at least 1 of the following 2 characteristics: nausea and/or vomiting, and photophobia and phonophobia. Immediately after the first scan, all patients were treated with 6-mg subcutaneous sumatriptan.
Data Acquisition and Analysis
On each scan day, 2 venous catheters were inserted in the cubital veins: 1 for injecting the radiotracer and 1 for drawing blood samples. All participants underwent 3 PET scans on 2 separate study days. The interictal scan was conducted on a separate day, and the ictal and the postictal scans were conducted on the same day (Figure 1). For practical reasons, we always conducted the interictal scans before the ictal and postictal scans. This decision was based on previous test-retest studies showing that the absolute mean differences in binding potential between the first and second PET scan were less than 3% in all serotonergic projection areas.19 Details of the imaging procedures have been described previously.13 In brief, the radiotracer [11C]AZ10419369 was synthesized using an automated radiosynthesis system. All participants were placed in a supine position on the scanner bed, with the head in a specialized holder to minimize movement. The radiotracer [11C]AZ10419369 was administered intravenously for 20 seconds, after which emission data were acquired for 90 minutes using the PET scanner (High Resolution Research Tomographic Imaging; CTI/Siemens). The PET images were reconstructed using 3-dimensional OP-OSEM (ordinary Poisson ordered-subsets expectation maximization), including point-spread function modeling and attenuation map improvements.20,21,22 On a separate day, all participants underwent a T1- and a T2-weighted structural magnetic resonance imaging scan (Prisma 3T Scanner; Siemens), and the magnetic resonance images were used to delineate regions of interest.
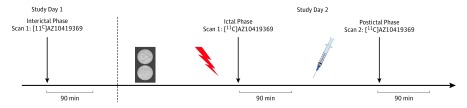
Figure 1. Study Design.
The median (range) time between study day 1 and 2 was 219.5 (2-326) days. On study day 2, the median (range) time from cilostazol ingestion to scan 1 was 5 (3.5-7.5) hours. The median (range) time between sumatriptan administration and scan 2 was 43 (33-48) minutes. The pills indicate cilostazol; the red flash, migraine attack; and the syringe, sumatriptan.
To compensate for head motion, each PET frame was aligned to a single PET frame (frame 27, first 5 minutes frame) using the scaled least squares cost function in Automated Image Registration 5.2.5 and with a 10-mm gaussian filter. The PET images were then aligned and coregistered to the corresponding T1-weighted magnetic resonance image using SPM8 (FIL Methods Group). Correct coregistration was ensured by visual inspection. Regions of interest were delineated on each participant’s magnetic resonance image and projected onto the PET images to extract regional time activity curves and gray matter volumes (GMVs). This process was done automatically using PVElab software (Neurobiology Research Unit, Rigshospitalet).23
Quantification of [11C]AZ10419369 Binding
Previous studies have shown that [11C]AZ10419369 binding can be quantified with the simplified reference tissue model using cerebellar gray matter (excluding vermis), which is devoid of 5-HT1B receptors, as a reference region.24 Therefore, we used this approach to calculate the nondisplaceable binding potential (BPND) for [11C]AZ10419369.
The main outcome measure was a GMV-weighted mean binding potential across 7 brain regions involved in pain modulation: dorsolateral and ventrolateral prefrontal cortex, orbitofrontal cortex, anterior cingulate cortex, sensorimotor cortex, and insula and amygdala brain regions. These regions were chosen to align with a previous study,13 in which lower 5-HT1B receptor binding was found in the interictal phase in patients with migraine compared with controls, and because these regions are relevant for comparing the interictal (pain free) with ictal (with pain) phase. In addition, because the occupancy of sumatriptan to the 5-HT1B receptor is expected to be equal across all regions, choosing specific regions will not lead to misinterpretation of the data.
After estimating the binding potential for each of the 7 regions, we calculated the mean binding potential across regions as follows: Mean BPND = Σ[BPND(regionx) × GMV(regionx)] / [Σ(GMV(regionx)]. Receptor occupancy was estimated from the 2 scans before and after sumatriptan administration as follows: Occupancy (%) = 100 × (BPND, attack – BPND, drug) / BPND, attack.
Statistical Analysis
Differences in demographics and PET variables between scans were assessed with 2-tailed, paired t test. Sample size was chosen according to the low test-retest variability described for binding potential of [11C]AZ10419369.19 We calculated that at 5% significance with 80% power, 5 participants would be sufficient to show a difference in binding potential between scans. Within-participant changes in binding potential between conditions (ictal vs postictal; interictal vs ictal) were assessed using a 1-tailed paired t test. Because we had a clear hypothesis of the direction (decrease) of the changes in binding potential and powered the study accordingly, we performed 1-tailed hypothesis testing. Difference in area under the curve (AUC) for headache intensity scores was analyzed using Wilcoxon matched-pairs signed rank test. One-sided or 2-sided P < .05 was considered statistically significant. No corrections for multiple comparisons were done.
Results
Demographics and PET Variables
Eight patients with migraine were included in the study; of these participants, 7 (87%) were women. The mean (SD) age of participants on study day 1 was 29.5 (9.2) years and on study day 2 was 30.0 (8.9) years. The mean (SD) injected dose was similar across scans: 593 (11) MBq for the ictal scan, 576 (14) MBq for the postictal scan, and 590 (14) MBq for the interictal scan. Postictal data from 1 participant were excluded because of intake of Buventol (salbutamol [albuterol]), a β2 agonist, which was not allowed according to the study protocol. Details on injected mass of AZ10419369 per kg and time-normalized AUC for the cerebellar time activity curve can be found in eTable 1 in the Supplement.
Changes in 5-HT1B Receptor Binding
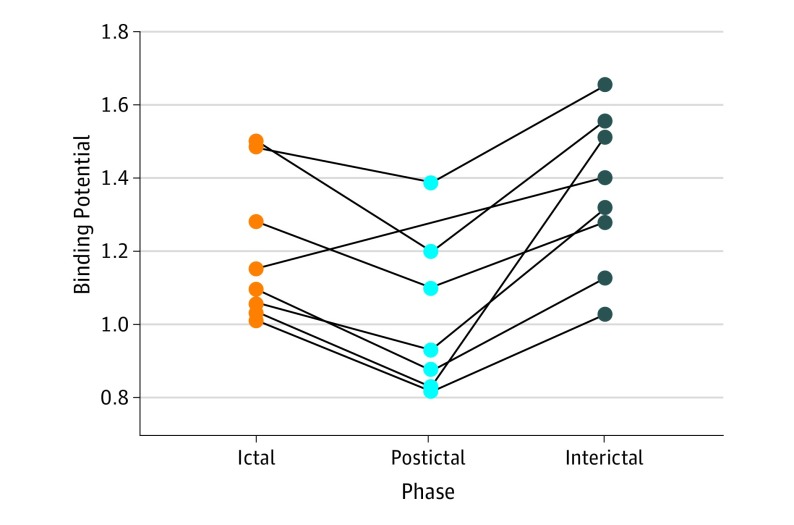
Compared with the ictal phase, 5-HT1B receptor binding decreased in the postictal phase, after sumatriptan administration in all participants (mean [SD] BPND, 1.20 [0.20] vs 1.02 [0.22]; P = .001) (Figure 2). This decrease in binding corresponded to a mean (SD) drug occupancy rate of 16.0% (5.3%). Furthermore, 5-HT1B receptor binding was reduced during migraine attacks (ictal phase), compared with the interictal phase (mean [SD] BPND, 1.36 [0.22] vs 1.20 [0.20]; P = .02).
Figure 2. Individual Changes in 5-HT1B Receptor Binding.
5-HT1B receptor binding decreased across 7 pain-modulating regions (dorsolateral and ventrolateral prefrontal cortex, orbitofrontal cortex, anterior cingulate cortex, sensorimotor cortex, and insula and amygdala) after sumatriptan administration (mean [SD] binding potential, 1.20 [0.20] vs 1.02 [0.22]; P = .001). During migraine attacks, binding was decreased compared with a migraine-free day (mean [SD] binding potential, 1.36 [0.22] vs 1.20 [0.20]; P = .02). Binding potential is the mean volume-weighted nondisplaceable binding potential.
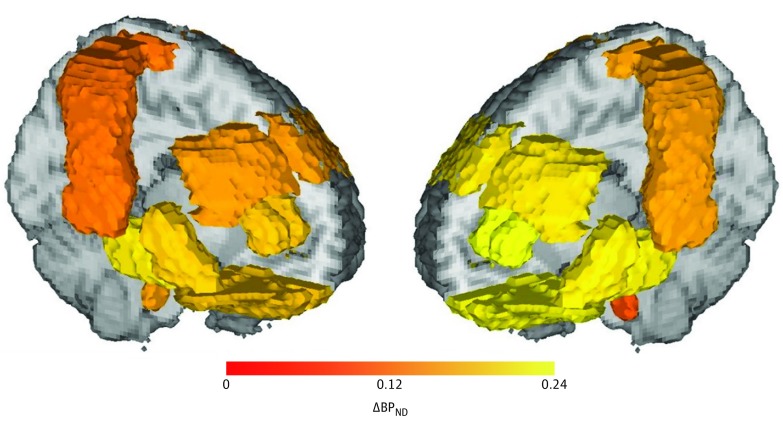
Because we used a volume-weighted mean across regions, we do not present the binding potentials for each of the 7 regions of interest. A visual representation of the regional distribution of changes in binding potential appears in Figure 3.
Figure 3. Group-Based Mean Difference in Nondisplaceable Binding Potential (BPND) for the Region of Interest Analyzed.
A, Change in BPND (interictal-ictal); B, Change in BPND (ictal-postictal after sumatriptan).
Headache Data
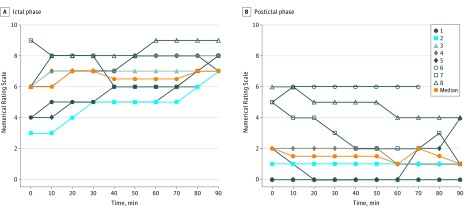
All patients fulfilled the criteria for migraine during the ictal scan, and the cilostazol-induced migraine attacks mimicked the patients' usual headache characteristics (eTable 2 in the Supplement). The AUC for the headache intensity was statistically significantly lower during the sumatriptan scan compared with the baseline scan (AUC0-90 min, P = .008; median [range], 62.5 [43-74.5] vs 10.25 [0-43]) (Figure 4). For 2 participants (participants 6 and 8), the headache still fulfilled the criteria for migraine during the postictal scan.
Figure 4. Headache Scores Measured From Radiotracer Injection and Throughout the Positron Emission Tomographic Scan.
Each line indicates individual headache scores for each participant, whereas the orange lines indicate median scores during the ictal phase (A) and postictal phase (B).
Discussion
A novel key finding of the present study is that administration of clinically relevant doses of sumatriptan during a migraine attack was associated with a statistically significant reduction in 5-HT1B receptor binding corresponding to an occupancy rate of 16.0% at central 5-HT1B receptors. These data suggest that sumatriptan crosses the blood-brain barrier and binds to central 5-HT1B receptors. Furthermore, the findings demonstrate that during pharmacologically induced migraine attacks, compared with outside of migraine attacks, patients with migraine had lower 5-HT1B receptor binding in pain-modulating regions. These data suggest ictal increases in endogenous brain serotonin levels.
Brain 5-HT1B Receptor Occupancy of Sumatriptan
When administered during the migraine attack, subcutaneous sumatriptan in clinically relevant doses was associated with a 16.0% reduction in 5-HT1B receptor binding. This decrease suggests that sumatriptan crosses the blood-brain barrier and binds to central 5-HT1B receptors during migraine attacks. This finding is in contrast to dihydroergotamine mesylate, another abortive migraine medication, which was shown not to cross the blood-brain barrier.25 Previously, drug occupancies of triptans were investigated only for zolmitriptan in healthy volunteers.11 Even though the occupancy was modest, this does not necessarily exclude a substantial clinical effect, particularly not when dealing with agonist compounds. For example, opioids exerted clinical effects at occupancies less than 10%,26 whereas 5-HT1A receptor agonists induced central, serotonergic adverse effects without substantial occupancies.27 Because a high intrinsic activity has been demonstrated for sumatriptan,28 the occupancies found in this study may be sufficient for antimigraine effects. A previous PET study found that administration of 6-mg subcutaneous sumatriptan decreased serotonin synthesis rate in the brain, an outcome that was not associated with reduction of pain intensity.29 In the present study, both pain intensity and 5-HT1B receptor binding were reduced in all participants after sumatriptan administration, but the migraine attack was only terminated in 6 of 8 patients. We can only speculate whether the therapeutic effect of sumatriptan was associated with activation of the central 5-HT1B receptors during attacks. Thus, even though this study found evidence that sumatriptan accesses the brain parenchyma and binds to central 5-HT1B receptors, the antimigraine efficacy of sumatriptan may be mediated through other sites of action than central 5-HT1B receptors.
We cannot exclude that the demonstrated drug occupancy could be partly associated with residual, elevated levels of endogenous serotonin; downregulation of the 5-HT1B receptor; or resolution of the migraine attack. A continuous increase in endogenous serotonin even after sumatriptan administration could explain the relatively high occupancies in the 2 patients who still experienced migraine during the postictal scan. On the other hand, 2 participants did not exhibit reductions in 5-HT1B receptor binding during attacks but had a large decrease after sumatriptan treatment (Figure 2). Furthermore, the ictal reductions in 5-HT1B receptor binding were larger for the pain-modulating regions compared with the neocortex, whereas the occupancy rates after sumatriptan administration were similar in these 2 regions. In addition, activation of the 5-HT1B autoreceptor could, theoretically, decrease serotonin levels in the brain, which could counteract prolonged increases in brain serotonin. Collectively, this theory speaks against residual binding of serotonin contributing substantially to the occupancy. Likewise, if the decrease in 5-HT1B receptor binding was associated with downregulation of the receptor, we would have expected to see a decrease in all participants during the migraine attack, which was not the case. We saw a reduction in binding in all participants regardless of the therapeutic effects of sumatriptan, indicating that resolution of the migraine attack itself is of less importance. Therefore, we consider the reduction in binding to be associated with sumatriptan partially blocking the 5-HT1B receptor.
Brain Serotonin Levels During Migraine Attacks
We interpret the decrease in 5-HT1B receptor binding during the migraine attack as being associated with a migraine-initiated acute increase in brain serotonin levels, which leads to the displacement of [11C]AZ10419369 binding. This interpretation is consistent with a previous PET study demonstrating an increase in brain serotonin synthesis during attacks.29 Another study found attack-associated normalization of visual and auditory evoked potentials compared with the interictal phase, which was interpreted as increases in central serotonergic activity.30 The question remains whether an increase in brain serotonin levels is a cause or a consequence of migraine pain. Pharmacologic interventions promoting the release of serotonin in the brain,31 as well as administration of m-chlorophenylpiperazine,32 a 5-HT2 receptor agonist, are known to provoke migraine attacks, supporting the idea that an increase in brain serotonin can elicit an attack. Because serotonin has higher affinity for the 5-HT1B/1D receptor than for the 5-HT2A receptor, it has been hypothesized that when present in low concentrations, serotonin binds to the antinociceptive 5-HT1B/1D receptor, but as serotonin concentrations increase, serotonin exerts its actions on the pronociceptive 5-HT2A receptor.33 We suggest that migraine attacks are partially initiated by increases in endogenous serotonin, which leads to a shift in pain modulation with decreased pain inhibition and increased pain facilitation.
Limitations
When using a reference tissue model for quantifying the binding potential in occupancy studies, it is important to assess whether drug administration affects the reference region (cerebellum) used for quantification of the binding of the radiotracer.34 We detected borderline changes in cerebellar radiotracer uptake in patients during migraine attacks, as compared with outside of migraine attacks (eTable 1 in the Supplement). However, uptake in cerebellum did not change after sumatriptan administration (eTable 1 in the Supplement). Based on this finding, we have no reason to believe that the change in uptake seen in patients with migraine is associated with specific binding in cerebellum. Instead, it could be associated with a global efficacy of cilostazol. In animal models, cilostazol enhances blood-brain barrier integrity.35,36 An increase in blood-brain barrier integrity could potentially reduce uptake of the radiotracer and thus the nondisplaceable distribution volume. However, if the nondisplaceable distribution volume decreases between the 2 conditions (interictal and ictal), we would overestimate the binding potential for the ictal scan and thus underestimate the change in binding potential between the 2 conditions. We acknowledge that there may be differences between normal attacks and those emulated in this study. However, all migraine attacks mimicked the patients’ normal migraine attacks (eTable 2 in the Supplement), and we believe it plausible to extrapolate our findings to spontaneous migraine attacks.
Conclusions
Sumatriptan, when given in clinically relevant doses, appeared to cross the blood-brain barrier and bind to central 5-HT1B receptors. Because of the relatively low lipophilicity of sumatriptan compared with the other triptans,37 we find it plausible that this finding can be extrapolated to all triptans. Whether the activation of central 5-HT1B receptors is necessary for the antimigraine efficacy of triptans remains to be determined. Furthermore, we demonstrate that brain serotonin levels are increased during migraine attacks, indicating that migraine attacks may be partly triggered by increases in endogenous brain serotonin.
eTable 1. PET Variables
eTable 2. Clinical Characteristics of Spontaneous and Cilostazol Induced Migraine Attacks
References
- 1.Doenicke A, Brand J, Perrin VL. Possible benefit of GR43175, a novel 5-HT1-like receptor agonist, for the acute treatment of severe migraine. Lancet. 1988;1(8598):1309-1311. doi: 10.1016/S0140-6736(88)92122-8 [DOI] [PubMed] [Google Scholar]
- 2.Humphrey PP, Feniuk W, Perren MJ, Beresford IJ, Skingle M, Whalley ET. Serotonin and migraine. Ann N Y Acad Sci. 1990;600:587-598. doi: 10.1111/j.1749-6632.1990.tb16912.x [DOI] [PubMed] [Google Scholar]
- 3.Feniuk W, Humphrey PPA, Perren MJ. The selective carotid arterial vasoconstrictor action of GR43175 in anaesthetized dogs. Br J Pharmacol. 1989;96(1):83-90. doi: 10.1111/j.1476-5381.1989.tb11787.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaube H, Hoskin KL, Goadsby PJ. Inhibition by sumatriptan of central trigeminal neurones only after blood-brain barrier disruption. Br J Pharmacol. 1993;109(3):788-792. doi: 10.1111/j.1476-5381.1993.tb13643.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy D, Jakubowski M, Burstein R. Disruption of communication between peripheral and central trigeminovascular neurons mediates the antimigraine action of 5HT 1B/1D receptor agonists. Proc Natl Acad Sci U S A. 2004;101(12):4274-4279. doi: 10.1073/pnas.0306147101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33(1):48-56. doi: 10.1002/ana.410330109 [DOI] [PubMed] [Google Scholar]
- 7.Varnäs K, Hall H, Bonaventure P, Sedvall G. Autoradiographic mapping of 5-HT(1B) and 5-HT(1D) receptors in the post mortem human brain using [(3)H]GR 125743. Brain Res. 2001;915(1):47-57. doi: 10.1016/S0006-8993(01)02823-2 [DOI] [PubMed] [Google Scholar]
- 8.Ferrari MD, Roon KI, Lipton RB, Goadsby PJ. Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet. 2001;358(9294):1668-1675. doi: 10.1016/S0140-6736(01)06711-3 [DOI] [PubMed] [Google Scholar]
- 9.Jørgensen LM, Weikop P, Svarer C, Feng L, Keller SH, Knudsen GM. Cerebral serotonin release correlates with [11C]AZ10419369 PET measures of 5-HT1B receptor binding in the pig brain. J Cereb Blood Flow Metab. 2018;38(7):1243-1252. doi: 10.1177/0271678X17719390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nord M, Finnema SJ, Halldin C, Farde L. Effect of a single dose of escitalopram on serotonin concentration in the non-human and human primate brain. Int J Neuropsychopharmacol. 2013;16(7):1577-1586. doi: 10.1017/S1461145712001617 [DOI] [PubMed] [Google Scholar]
- 11.Varnäs K, Jučaite A, McCarthy DJ, et al. A PET study with [11C]AZ10419369 to determine brain 5-HT1B receptor occupancy of zolmitriptan in healthy male volunteers. Cephalalgia. 2013;33(10):853-860. doi: 10.1177/0333102413476372 [DOI] [PubMed] [Google Scholar]
- 12.Varnäs K, Nyberg S, Karlsson P, et al. Dose-dependent binding of AZD3783 to brain 5-HT1B receptors in non-human primates and human subjects: a positron emission tomography study with [11C]AZ10419369. Psychopharmacology (Berl). 2011;213(2-3):533-545. doi: 10.1007/s00213-011-2165-z [DOI] [PubMed] [Google Scholar]
- 13.Deen M, Hansen HD, Hougaard A, et al. Low 5-HT1B receptor binding in the migraine brain: A PET study. Cephalalgia. 2018;38(3):519-527. doi: 10.1177/0333102417698708 [DOI] [PubMed] [Google Scholar]
- 14.Deen M, Christensen CE, Hougaard A, Hansen HD, Knudsen GM, Ashina M. Serotonergic mechanisms in the migraine brain - a systematic review. Cephalalgia. 2017;37(3):251-264. doi: 10.1177/0333102416640501 [DOI] [PubMed] [Google Scholar]
- 15.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 16.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808. doi: 10.1177/0333102413485658 [DOI] [PubMed] [Google Scholar]
- 17.Guo S, Olesen J, Ashina M. Phosphodiesterase 3 inhibitor cilostazol induces migraine-like attacks via cyclic AMP increase. Brain. 2014;137(Pt 11):2951-2959. doi: 10.1093/brain/awu244 [DOI] [PubMed] [Google Scholar]
- 18.Khan S, Deen M, Hougaard A, Amin FM, Ashina M. Reproducibility of migraine-like attacks induced by phosphodiesterase-3-inhibitor cilostazol. Cephalalgia. 2018;38(5):892-903. doi: 10.1177/0333102417719753 [DOI] [PubMed] [Google Scholar]
- 19.Nord M, Finnema SJ, Schain M, Halldin C, Farde L. Test-retest reliability of [11C]AZ10419369 binding to 5-HT(1B) receptors in human brain. Eur J Nucl Med Mol Imaging. 2014;41(2):301-307. doi: 10.1007/s00259-013-2529-1 [DOI] [PubMed] [Google Scholar]
- 20.Keller SH, Svarer C, Sibomana M. Attenuation correction for the HRRT PET-scanner using transmission scatter correction and total variation regularization. IEEE Trans Med Imaging. 2013;32(9):1611-1621. doi: 10.1109/TMI.2013.2261313 [DOI] [PubMed] [Google Scholar]
- 21.Hong IK, Chung ST, Kim HK, Kim YB, Son YD, Cho ZH. Ultra fast symmetry and SIMD-based projection-backprojection (SSP) algorithm for 3-D PET image reconstruction. IEEE Trans Med Imaging. 2007;26(6):789-803. doi: 10.1109/TMI.2007.892644 [DOI] [PubMed] [Google Scholar]
- 22.Sureau FC, Reader AJ, Comtat C, et al. Impact of image-space resolution modeling for studies with the high-resolution research tomograph. J Nucl Med. 2008;49(6):1000-1008. doi: 10.2967/jnumed.107.045351 [DOI] [PubMed] [Google Scholar]
- 23.Svarer C, Madsen K, Hasselbalch SG, et al. MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. Neuroimage. 2005;24(4):969-979. doi: 10.1016/j.neuroimage.2004.10.017 [DOI] [PubMed] [Google Scholar]
- 24.Varnäs K, Nyberg S, Halldin C, et al. Quantitative analysis of [11C]AZ10419369 binding to 5-HT1B receptors in human brain. J Cereb Blood Flow Metab. 2011;31(1):113-123. doi: 10.1038/jcbfm.2010.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schankin CJ, Maniyar FH, Seo Y, et al. Ictal lack of binding to brain parenchyma suggests integrity of the blood-brain barrier for 11C-dihydroergotamine during glyceryl trinitrate-induced migraine. Brain. 2016;139(Pt 7):1994-2001. doi: 10.1093/brain/aww096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melichar JK, Hume SP, Williams TM, et al. Using [11C]diprenorphine to image opioid receptor occupancy by methadone in opioid addiction: clinical and preclinical studies. J Pharmacol Exp Ther. 2005;312(1):309-315. doi: 10.1124/jpet.104.072686 [DOI] [PubMed] [Google Scholar]
- 27.Bantick RA, Rabiner EA, Hirani E, de Vries MH, Hume SP, Grasby PM. Occupancy of agonist drugs at the 5-HT1A receptor. Neuropsychopharmacology. 2004;29(5):847-859. doi: 10.1038/sj.npp.1300390 [DOI] [PubMed] [Google Scholar]
- 28.Martin GR, Robertson AD, MacLennan SJ, et al. Receptor specificity and trigemino-vascular inhibitory actions of a novel 5-HT1B/1D receptor partial agonist, 311C90 (zolmitriptan). Br J Pharmacol. 1997;121(2):157-164. doi: 10.1038/sj.bjp.0701041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakai Y, Dobson C, Diksic M, Aubé M, Hamel E. Sumatriptan normalizes the migraine attack-related increase in brain serotonin synthesis. Neurology. 2008;70(6):431-439. doi: 10.1212/01.wnl.0000299095.65331.6f [DOI] [PubMed] [Google Scholar]
- 30.Judit A, Sándor PS, Schoenen J. Habituation of visual and intensity dependence of auditory evoked cortical potentials tends to normalize just before and during the migraine attack. Cephalalgia. 2000;20(8):714-719. doi: 10.1111/j.1468-2982.2000.00122.x [DOI] [PubMed] [Google Scholar]
- 31.Panconesi A, Sicuteri R. Headache induced by serotonergic agonists—a key to the interpretation of migraine pathogenesis? Cephalalgia. 1997;17(1):3-14. doi: 10.1046/j.1468-2982.1997.1701003.x [DOI] [PubMed] [Google Scholar]
- 32.Leone M, Attanasio A, Croci D, et al. The serotonergic agent m-chlorophenylpiperazine induces migraine attacks: a controlled study. Neurology. 2000;55(1):136-139. doi: 10.1212/WNL.55.1.136 [DOI] [PubMed] [Google Scholar]
- 33.Sommer C. Is serotonin hyperalgesic or analgesic? Curr Pain Headache Rep. 2006;10(2):101-106. doi: 10.1007/s11916-006-0020-4 [DOI] [PubMed] [Google Scholar]
- 34.Takano A, Varrone A, Gulyás B, et al. Guidelines to PET measurements of the target occupancy in the brain for drug development. Eur J Nucl Med Mol Imaging. 2016;43(12):2255-2262. doi: 10.1007/s00259-016-3476-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horai S, Nakagawa S, Tanaka K, et al. Cilostazol strengthens barrier integrity in brain endothelial cells. Cell Mol Neurobiol. 2013;33(2):291-307. doi: 10.1007/s10571-012-9896-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yanai S, Toyohara J, Ishiwata K, Ito H, Endo S. Long-term cilostazol administration ameliorates memory decline in senescence-accelerated mouse prone 8 (SAMP8) through a dual effect on cAMP and blood-brain barrier. Neuropharmacology. 2017;116:247-259. doi: 10.1016/j.neuropharm.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 37.Goadsby PJ. Serotonin 5-HT1B/1D receptor agonists in migraine comparative pharmacology and its therapeutic implications. Mol Diagn Ther. 1998;10(4):271-286. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. PET Variables
eTable 2. Clinical Characteristics of Spontaneous and Cilostazol Induced Migraine Attacks