Key Points
Question
Among patients with subjective debilitating tinnitus and audiometric normal to mild hearing loss, does treatment with tinnitus retraining therapy with conventional sound generators result in better outcomes than tinnitus retraining therapy with placebo sound generators or standard of care?
Findings
In this randomized clinical trial of 151 participants with 18 months of follow-up, average tinnitus distress decreased in all 3 groups. There was no clinically meaningful difference in extent of reduction in tinnitus distress or other important end points among patients in the 3 intervention groups.
Meaning
Tinnitus retraining therapy with conventional sound generators is no better than tinnitus retraining therapy with placebo generators or standard of care.
Abstract
Importance
Tinnitus retraining therapy (TRT) is an internationally recognized, but controversial, protocol of uncertain efficacy that uses tinnitus-specific educational counseling (TC) and sound therapy (ST) to reduce the patient’s tinnitus-evoked negative reaction to, and awareness of, tinnitus.
Objective
To compare the efficacy of TRT and its components, ST and TC, with the standard of care (SoC) in reducing the negative effect of tinnitus on quality of life.
Design, Setting, and Participants
A randomized, placebo-controlled, multicenter phase 3 trial was conducted from August 4, 2011, to June 20, 2017, at 6 US military hospitals, the study chairs’ office, and a data coordinating center, among 151 active-duty and retired military personnel and dependents with functionally adequate hearing sensitivity and moderate to severe subjective tinnitus. All analyses were based on intention to treat.
Interventions
Central randomized allocation to TRT (TC and ST with conventional sound generators), partial TRT (TC with placebo sound generators), or SoC.
Main Outcomes and Measures
The primary outcome was mean change on the Tinnitus Questionnaire (TQ), assessed longitudinally between baseline and 18 months after start of therapy. The secondary outcomes were changes in TQ subscales, Tinnitus Functional Index (TFI), and Tinnitus Handicap Inventory (THI) total and subscales, as well as a 10-point visual analog scale (VAS).
Results
Among the 151 participants in the study (44 women and 107 men; mean [SD] age, 50.6 [11.3] years), 51 were randomized to receive TRT, 51 to receive partial TRT, and 49 to receive standard of care. Longitudinal analyses showed no difference between partial TRT or TRT compared with SoC, or partial TRT compared with TRT, on TQ, TFI, or THI total scores. Comparison of changes in mean score from baseline to the 18-month visit also showed no difference between treatment groups. Significant improvement was observed at 18 months in all treatment groups on TQ scores for TRT (effect size, −1.32; 95% CI, −1.78 to −0.85), partial TRT (effect size, −1.16; 95% CI, −1.56 to −0.76), and SoC (effect size, −1.01; 95% CI, −1.41 to −0.61). Compared with baseline scores, at 18 months there were reductions in scores by 7 points or more on the TQ score for 86 of 111 participants (77.55%; 95% CI, 69.7%-85.2%), 13 points or more on the TFI for 52 of 111 participants (46.8%; 95% CI, 37.6%-56.1%), 7 points or more on the THI for 63 of 111 participants (56.8%; 95% CI, 47.5%-66.0%), and 2 points or more on the VAS for 45 of 93 participants (48.4%; 95% CI, 38.2%-58.5%).
Conclusions and Relevance
There were few differences between treatment groups. About half of participants showed clinically meaningful reductions in the effect of tinnitus.
Trial Registration
ClinicalTrials.gov identifier: NCT01177137
This randomized clinical trial compares the efficacy of tinnitus retraining therapy and its components, sound therapy and tinnitus-specific educational counseling, with the standard of care on tinnitus-related quality of life.
Introduction
Tinnitus is the perception of sound in the absence of a corresponding external sound. The prevalence of continuous subjective tinnitus among adults ranges from 5.1% to 42.7%.1 Although tinnitus is often associated with hearing impairment, survey estimates suggest that about 13 million persons in the United States deny hearing loss, but experience tinnitus.2 Based on Centers for Disease Control and Prevention estimates, tinnitus is severely debilitating in about 2 million persons in the United States, impairing their well-being and ability to lead a normal life.3
There are no reliable means of curing tinnitus at its source.4,5 Current efforts thus aim to reduce the effect of tinnitus on health-related quality of life, with some evidence for the efficacy of cognitive behavioral therapy.6 Tinnitus retraining therapy (TRT), an internationally recognized habituation-based treatment grounded in the neurophysiological model of tinnitus,7 also aims to reduce the effect of tinnitus on health-related quality of life. Tinnitus retraining therapy combines tinnitus-specific educational counseling (TC), synonymous with directive counseling,8,9 and low-level broadband sound therapy (ST) to habituate the patient to perceived tinnitus so as to lessen the patient’s associated negative emotional reactions (eg, annoyance or anxiety) and improve the patient’s quality of life.8 The model hypothesizes that the distress associated with tinnitus arises from abnormal activation of subconscious nonauditory mechanisms, mediated primarily by the limbic and autonomic nervous systems.
Various uncontrolled studies9,10,11,12,13,14 and a few randomized clinical trials15,16,17,18 have compared TRT with other interventions. Henry et al15 compared TRT with a tinnitus masking intervention. Compared with masking, TRT resulted in greater improvement in individuals with more severe tinnitus at baseline, but this study had a low quality of evidence. In a second quasirandomized trial, Bauer and Brozoski16 found that both TRT and general counseling reduced the annoyance and effect of tinnitus in persons with normal hearing, with TRT having a greater effect size (1.13) compared with general counseling (0.78). A randomized trial with adequate allocation concealment comparing a cognitive behavioral therapy–based treatment, acceptance and commitment therapy, with TRT found a larger reduction in the effect of tinnitus for acceptance and commitment therapy than for TRT (Cohen d, 1.04).17 Tyler et al18 reported a positive effect of either TRT ST or masking compared with counseling equivalent to TRT counseling, but with a high proportion of dropouts in all treatment arms. Bauer and colleagues19 recently compared TRT implemented with aided sound generators (SGs) (TRT group) vs aural rehabilitation and open-canal hearing aids (control group). Both groups showed meaningful improvement, with the TRT group achieving greater improvement than the control group when analyzed using an intention-to-treat analysis, but not with a per-protocol analysis.
In the Tinnitus Retraining Therapy Trial (TRTT), a randomized, placebo-controlled, multicenter trial reported here, we compared TRT with best practice and evaluated the separate effects of TC and ST in functionally normal-hearing individuals.
Methods
Design
The TRTT, conducted from August 4, 2011, to June 20, 2017, assessed the efficacy of TRT and its component parts, TC and ST, in habituating the perceived magnitude of, perception, and negative emotional reactions to, tinnitus. The trial was conducted in US Air Force, Navy, and integrated Department of Defense Medical Centers to take advantage of the likely increased prevalence of tinnitus in members of the military.20 We enrolled active-duty and retired military personnel and their dependents. Eligible study participants had subjective distressing tinnitus of at least 1 year’s duration and no evidence of a medical cause as determined by study-certified physicians. All participants had functionally adequate hearing sensitivity that did not routinely require amplification, ensuring that SG output would be audible. Other eligibility criteria included no treatment for tinnitus within the past year and a score of 40 or more on the Tinnitus Questionnaire (TQ),21 indicating a moderately severe effect of tinnitus on quality of life (for detailed eligibility criteria, please see the trial protocol in Supplement 1).22 The TRTT received ethical approval from institutional review boards at the University of Alabama, the Johns Hopkins Bloomberg School of Public Health, and participating clinical sites. No study participant was enrolled in the study before informed consent was obtained and documented by a signed informed consent statement.
Before randomization, participants were categorized according to TRT category as follows: 0 indicated no hyperacusis, no prolonged effect of noise exposure, and subjective hearing loss irrelevant; I indicated no hyperacusis, no prolonged effect of noise exposure, and no subjective hearing loss; II indicated no hyperacusis, no prolonged effect of noise exposure, and significant subjective hearing loss; III indicated hyperacusis, no prolonged effect of noise exposure, and subjective hearing loss irrelevant; and IV indicated hyperacusis, prolonged effect of noise exposure, and subjective hearing loss irrelevant.
Randomization
Participants were enrolled and randomly assigned to 1 of 3 groups: TRT, including TC and ST implemented with ear-level SGs; partial TRT, including TC and placebo SGs; or standard of care (SoC), a patient-centered counseling protocol that aligned with current military care and recommended practice guidelines.23 These 3 groups enabled us to compare TRT vs SoC, and the contributions of ST (comparing partial TRT with TRT) and TC (comparing partial TRT with SoC).
We generated the randomization schedule using a computer-generated random permutation with random blocking and stratification by clinical center. After eligibility was confirmed, clinic staff accessed the TRTT website to obtain a randomization assignment, designated as either TRT or Standard of Care. Audiologists ordered SGs directly from the manufacturer, General Hearing Instruments Inc. Each SG was identified as conventional or placebo by serial number in a schedule prepared prior to trial initiation and provided to General Hearing Instruments Inc. At randomization, the order form provided only the serial number of the assigned device to ensure blinding to SG type.
Interventions
Both TC and the fitting of the SGs for ST were implemented based on the general principles and methods for TRT described by Jastreboff and Hazell.24 Conventional and placebo SGs were identical in appearance and operation during the first 40 minutes of use, after which the placebo output was attenuated at a rate of 1 dB per minute. This decay took advantage of the natural perceptual adaptation to SG noise, a concept reinforced during counseling for all SGs. Also, the output reset to the original volume within 3 seconds after removal of placebo SGs from the ears, facilitating double-blinding of participant and audiologist. All SGs were fitted bilaterally and matched for loudness at a volume setting judged by the participant to be just below the point at which the tinnitus and SG noise blended. Initially, behind-the-ear SGs were provided to participants, but functional problems associated with sensor technology used to track SG use on the ear necessitated a switch to in-the-ear devices early in the study. Audiologists instructed participants to wear SGs as much as possible, at least 8 hours a day, and encouraged them to enrich their sound environment at all times, including with and without SG use. Participants reported number of hours per day, on average, that they wore devices since the previous study visit.
Tinnitus-specific educational counseling, the counseling arm of TRT, followed a structured, scripted protocol designed to initiate habituation of the patient to negative reactions to tinnitus. This counseling introduced treatment goals, reviewed the participant’s audiometric results, described the anatomy and physiology of the auditory system and pathways, and introduced concepts relevant to understanding tinnitus and the habituation process using the neurophysiological model of Jastreboff.25
The TRTT SoC protocol was consistent with information typically provided to patients with tinnitus at participating military medical centers and with professional guidelines for tinnitus management described in the American Speech-Language-Hearing Association’s preferred practice patterns in audiology.23 Standard of care, a patient-centered tinnitus approach, focused on the individual participant’s symptoms and aimed to reduce negative cognitive, affective, physical, and behavioral reactions to tinnitus. Participants in the SoC group also were encouraged to use environmental sound enrichment whenever possible, but not from SGs.
Audiologists were certified prior to providing counseling for both TC and SoC in the trial to minimize counselor-specific effects. To ensure blinding, each clinic certified at least 2 audiologists, 1 to provide treatment and the second (blinded) to measure follow-up outcomes. To facilitate and monitor treatment adherence, audiologists used treatment-specific visual aids, completed a checklist covering all salient counseling points, and audiotaped all counseling sessions for review by protocol monitors. Initial counseling took place at a treatment visit after randomization. Follow-up visits occurred at 3, 6, 12, and 18 months after the initial treatment visit.
Outcomes
The primary outcome was mean change in TQ score from baseline to follow-up, assessed longitudinally at 3, 6, 12, and 18 months of follow-up. End-of-treatment and longitudinal changes in scores on the subscales of the TQ were secondary outcomes. The TQ, a widely respected questionnaire with excellent validity and generalizability for quantifying tinnitus symptoms, was developed by Hallam,21 who early on advocated habituation-based tinnitus treatment.26 The 52-item TQ uses a 3-point response scale for each item and includes 5 subscales: psychological distress, intrusiveness, hearing difficulties, sleep disturbances, and somatic symptoms. To score the TQ, we added values for all questions, for a total of 102 points, with higher scores indicating a greater effect of tinnitus on the individual.
Other secondary outcomes included total and subscale scores of the Tinnitus Functional Index (TFI) and the Tinnitus Handicap Inventory (THI). The TFI is based on 25 items and 8 subscales (intrusiveness, reduced sense of control, cognitive interference, sleep disturbance, auditory difficulties, relaxation interference, reduced quality of life, and emotional distress).27 Each question has a 10-point response scale (ranging from 0, for no effect, to 10, for extreme effect), with the total score ranging from 0 to 100. The THI consists of 25 items and has 3 subscales (functional, emotional, and catastrophic); each question is evaluated using a 3-point response scale, with higher scores indicating a larger effect of tinnitus).28 The TRTT also measured the overall treatment effect using the 10-point visual analog scale (VAS) from the TRT Interview Form.29 Study participants ranked the level of their tinnitus “problem” from “not at all” to “as much as you can imagine.” Higher scores indicated a greater problem.
Additional outcomes included change in the Digit Symbol Substitution Test,30 the Beck Depression Inventory–Fast Screen31 as a screening questionnaire for depression, the Positive and Negative Affect Schedule (PANAS),32 the State-Trait Anxiety Index (STAI),33 and the Hearing Handicap Inventory.34 Audiologic outcomes assessed pure-tone audiometric thresholds, speech recognition thresholds, tinnitus pitch, and loudness match.
Statistical Analysis
The TRTT sample size was based on the difference in TQ scores between baseline and 18 months of follow-up, using a difference of 10 points on the TQ for the primary comparison (between TRT and SoC) and 7 points for comparisons of the separate contributions of ST and TC, α of 0.05, 80% power, SD of 12.5, and a 2-sided test. We divided α among the 3 comparisons using Bonferroni correction. Assuming 10% attrition, we estimated that 228 study participants (76 participants in each of the 3 groups) would be necessary for 80% power to detect a 7-point difference for analyses of ST and TC (Dupont and Plummer power and sample size calculations).35 This sample size provided greater than 95% power for the comparison of TRT vs SoC.
We used means, SDs, medians, and proportions to describe baseline characteristics of the study participants after examining the distributions of continuous variables for symmetry. We conducted longitudinal data analyses for the primary analysis of change in TQ score; mean changes in TQ scores were compared between treatment groups using repeated-measures generalized estimating equations (GEE) with exchangeable covariance structure. Prespecified factors considered for adjustment included clinical center and scores on the PANAS and STAI. Other factors considered were age, sex, TRT category, and type of SG (behind-the-ear or in-the-ear). Similar models were used to assess secondary objectives (TQ subscales, global and subscale scores of the TFI and THI, and VAS). We performed all analyses based on intention to treat. The only participants not included in the analyses were those with no follow-up visits. Missing values were managed using multiple imputation, which was implemented by SAS GENMOD with 50 iterations.36
For the secondary analyses, we compared the difference between TQ, TFI, and THI scores at baseline and at 18-month follow-up among treatment groups, using a similar GEE model. We also calculated the Cohen effect size for total and subscale values for the TQ, TFI, and THI, and for the 10-point VAS. Values of Cohen d of 0.20 to 0.49 represented small effect sizes, 0.50 to 0.79 represented medium effect sizes, and 0.80 or greater represented large effect sizes.37 We calculated the proportion of participants with clinically meaningful improvement, defined as a reduction of 13 points on the TFI27 and a reduction of 7 points on the THI.38 For the TQ, we used a difference of 7 points, as presented in the sample size estimates, and for the VAS we used a difference of 2 points, which is equivalent to a moderate to large treatment effect. P < .05 was considered significant, except for tests of the primary outcome, where P < .025 was considered significant.
Results
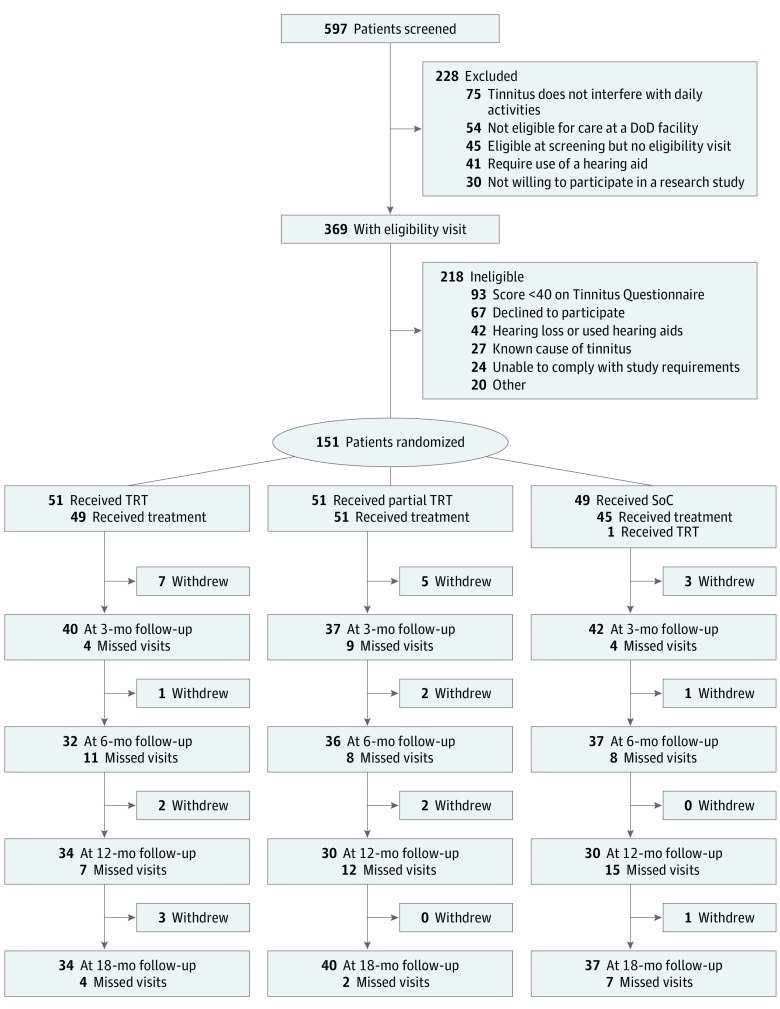
From August 4, 2011, to June 20, 2017, 597 individuals were screened for the TRTT. Of these, 151 study participants were randomized in the TRTT, 51 to TRT, 51 to partial TRT, and 49 to SoC (Figure; eTable 1 in Supplement 2). Enrollment in the trial was stopped in May 2015 because of slow recruitment, primarily due to difficulties previously described in conducting a multicenter randomized trial within US military hospitals.39 Twenty-seven participants withdrew or were lost to follow-up (5 in SoC, 9 in partial TRT, and 13 in TRT). There were no apparent differences in baseline characteristics of participants who withdrew compared with those who remained.
Figure. CONSORT Flow Diagram.
Numbers of study participants approached, enrolled, and followed up through 18 months. Ineligibility may be due to more than 1 reason. DoD indicates Department of Defense; SoC, standard of care; and TRT, tinnitus retraining therapy.
Mean (SD) participant age was 50.6 (11.3) years, 44 were women (29.1%), 36 (23.8%) reported belonging to a minority group, and 17 (11.3%) were of Hispanic or Latino origin (Table 1). Hearing sensitivity was within the audiometric normal to mild range of loss through 8000 Hz; a few participants had loudness discomfort levels consistent with hyperacusis (Table 1; eFigures 2 and 3 in Supplement 2). Participants had experienced tinnitus for a median of 8.3 years (range, 1-45 years). Almost half of the participants (68 [45.0%]) reported the tinnitus had been a significant problem for more than 5 years. Demographics and characteristics of tinnitus were similar across treatment groups (Table 1). Adherence to the treatment protocol was similar across all groups (eTable 2 in Supplement 2). The tinnitus affected study participants’ lives across most domains; baseline total and subscale scores on the TQ, TFI, and THI, as well as the VAS scores, were similar across treatment groups. All measures showed a reduction in the effect of tinnitus over time (Table 2; eFigure 3 in Supplement 2). Compared with baseline, at 18 months we observed a reduction of 7 points or more on the TQ in 86 of 111 participants (77.5%; 95% CI, 60.7%-86.2%), 13 points or more on the TFI in 52 of 111 participants (46.8%; 95% CI, 37.6%-56.1%), 7 points or more on the THI in 63 of 111 participants (56.8%; 95% CI, 47.5%-66.0%), and 2 points or more on the VAS in 45 of 93 participants (48.4%; 95% CI, 38.2%-58.5%). No differences were observed across groups in the Digit Symbol Substitution Test, STAI, PANAS, or Hearing Handicap Inventory (eTables 3-6 in Supplement 2).
Table 1. Demographic and Tinnitus Characteristics of TRTT Study Participants by Treatment Group.
| Characteristic | SoC (n = 49) | Partial TRT (n = 51) | TRT (n = 51) | Total (N = 151) |
|---|---|---|---|---|
| Age, mean (SD), y | 49.9 (10.0) | 50.9 (11.2) | 51.1 (12.6) | 50.6 (11.3) |
| Sex, No. (%) | ||||
| Male | 36 (73.5) | 37 (72.5) | 34 (66.7) | 107 (70.9) |
| Female | 13 (26.5) | 14 (27.5) | 17 (33.3) | 44 (29.1) |
| Race, No. (%) | ||||
| White | 35 (71.4) | 39 (76.5) | 36 (70.6) | 110 (72.9) |
| Black or African-American | 7 (14.3) | 6 (11.8) | 5 (9.8) | 18 (11.9) |
| Asian | 2 (4.1) | 4 (7.8) | 3 (5.9) | 9 (6.0) |
| Native Hawaiian or Pacific Islander | 0 | 1 (2.0) | 1 (2.0) | 2 (1.3) |
| Other | 3 (6.1) | 0 | 4 (7.8) | 7 (4.6) |
| Unknown or not reported | 2 (4.1) | 1 (2.0) | 2 (3.9) | 5 (3.3) |
| Ethnicity, No. (%) | ||||
| Hispanic or Latino | 5 (10.2) | 5 (9.8) | 7 (13.7) | 17 (11.3) |
| Marital status, No. (%) | ||||
| Married or living with partner | 41 (83.7) | 41 (80.4) | 39 (76.5) | 121 (80.1) |
| Widowed | 1 (2.0) | 0 | 0 | 1 (0.7) |
| Divorced or separated | 4 (8.2) | 4 (7.8) | 7 (13.7) | 15 (9.9) |
| Single or never married | 3 (6.1) | 6 (11.8) | 4 (7.8) | 13 (8.6) |
| Other or unknown | 0 | 0 | 1 (2.0) | 1 (0.7) |
| Tinnitus duration, mean (SD), y | 13.0 (11.4) | 11.7 (11.1) | 10.9 (8.9) | 11.8 (10.5) |
| Time during which tinnitus has been a significant problem, No. (%) | ||||
| <1 y | 3 (6.1) | 3 (5.9) | 3 (5.9) | 9 (6.0) |
| 1 to <2 y | 3 (6.1) | 8 (15.7) | 9 (17.7) | 20 (13.3) |
| 2 to <5 y | 20 (40.8) | 17 (33.3) | 15 (29.4) | 52 (34.4) |
| ≥5 y | 22 (44.9) | 22 (43.1) | 24 (47.1) | 68 (45.0) |
| Unknown | 1 (2.0) | 1 (2.0) | 0 | 2 (1.3) |
| Onset of tinnitus, No. (%) | ||||
| Sudden | 12 (24.5) | 17 (33.3) | 13 (25.5) | 42 (27.8) |
| Gradual | 32 (65.3) | 28 (54.9) | 30 (58.8) | 90 (59.6) |
| Unknown | 5 (10.2) | 6 (11.8) | 8 (15.7) | 19 (12.6) |
| Type of sound heard, No. (%) | ||||
| Tonal | 47 (95.9) | 47 (92.2) | 47 (92.2) | 141 (93.4) |
| Low-frequency noise | 1 (2.0) | 2 (3.9) | 4 (7.8) | 7 (4.6) |
| High-frequency noise | 0 | 1 (2.0) | 0 | 1 (0.7) |
| Crickets | 0 | 1 (2.0) | 0 | 1 (0.7) |
| Missing data | 1 (2.0) | 0 | 0 | 1 (0.7) |
| Accidentally exposed to sudden intense noise, No. (%) | 31 (63.3) | 26 (51.0) | 23 (45.1) | 80 (53.0) |
| Exposure to environmental hazards or other factors associated with tinnitus, No. (%) | 14 (28.6) | 15 (29.4) | 14 (27.5) | 43 (28.5) |
| Location of tinnitus, No. (%) | ||||
| 1 Ear | 8 (16.3) | 6 (11.8) | 10 (19.6) | 24 (15.9) |
| Both ears | 16 (32.7) | 23 (45.1) | 23 (45.1) | 62 (41.1) |
| In the head only | 11 (22.4) | 12 (23.5) | 6 (11.8) | 29 (19.2) |
| ≥1 Ear and in the head | 14 (28.6) | 10 (19.6) | 12 (21.6) | 36 (23.8) |
| Frequency match, most troublesome tinnitus, median (IQR), kHz | ||||
| Left ear (n = 81) | 8 (4.9-11.8) | 8 (6.0-9.3) | 8 (3.6-8.0) | 8 (4.9-9.3) |
| Right ear (n = 84) | 8 (6.0-12.0) | 8 (4.0-9.3) | 6 (6.0-8.0) | 7 (6.0-9.3) |
| Loudness match, most troublesome tinnitus, median (IQR), dB HL | ||||
| Left ear (n = 81) | 38 (28-44) | 40 (27-57) | 46 (34-60) | 40 (28-56) |
| Right ear (n = 84) | 35 (28-47) | 38 (26-49) | 44 (22-61) | 39 (26-49) |
| Loudness discomfort level at tinnitus pitch match frequency, median (IQR), dB HL | ||||
| Left ear | 95 (89-103) | 93 (86-100) | 95 (90-105) | 95 (88-105) |
| Right ear | 96 (90-105) | 95 (85-100) | 92 (87-105) | 95 (87-105) |
| TRT category | ||||
| 0 | 9 (18.4) | 11 (21.6) | 12 (23.5) | 32 (21.1) |
| I | 15 (30.5) | 12 (23.5) | 10 (19.6) | 37 (24.5) |
| II | 5 (10.2) | 3 (5.9) | 8 (15.7) | 16 (10.6) |
| III | 15 (30.6) | 17 (33.3) | 17 (33.3) | 49 (32.5) |
| IV | 4 (8.2) | 8 (15.7) | 4 (7.8) | 16 (10.6) |
| Missing | 1 (2.0) | 0 | 0 | 1 (0.7) |
Abbreviations: HL, hearing level; IQR, interquartile range; SoC, standard of care; TRT, tinnitus retraining therapy; TRTT, Tinnitus Retraining Therapy Trial.
Table 2. Global and Subscale Scores by Treatment Group and Visit.
| Treatment Group | Mean (SD) Value | Effect Size (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Baseline | At 3 mo | At 6 mo | At 12 mo | At 18 mo | Difference From Baseline to 18 mo | ||
| Standard of care, No. | 49 | 42 | 38 | 30 | 37 | 37 | NA |
| Partial TRT, No. | 51 | 37 | 35 | 30 | 40 | 40 | NA |
| TRT, No. | 51 | 40 | 32 | 34 | 34 | 34 | NA |
| Tinnitus Questionnaire | |||||||
| Total score (0-102) | |||||||
| Standard of care | 54.6 (11.2) | 43.5 (16.7) | 40.4 (14.8) | 35.8 (14.7) | 37.3 (16.8) | −16.5 (16.3) | −1.01 (−1.41 to −0.61) |
| Partial TRT | 54.4 (11.5) | 38.9 (16.7) | 37.1 (14.6) | 33.2 (15.2) | 35.9 (15.3) | −19.0 (15.9) | −1.16 (−1.56 to −0.76) |
| TRT | 56.4 (11.9) | 39.4 (16.6) | 34.8 (16.5) | 35.7 (16.9) | 39.0 (19.2) | −18.2 (15.1) | −1.32 (−1.78 to −0.85) |
| Emotional distress subscale (0-38) | |||||||
| Standard of care | 15.8 (5.5) | 11.1 (7.1) | 9.7 (7.1) | 7.7 (5.4) | 8.1 (6.3) | −7.4 (6.3) | −1.18 (−1.59 to −0.75) |
| Partial TRT | 17.0 (6.6) | 10.5 (7.1) | 9.5 (6.5) | 7.5 (6.5) | 8.6 (6.5) | −8.7 (7.9) | −1.10 (−1.49 to −0.70) |
| TRT | 17.2 (6.2) | 10.1 (7.0) | 8.5 (6.7) | 8.6 (7.0) | 9.2 (8.0) | −8.1 (6.3) | −1.28 (−1.73 to −0.82) |
| Intrusiveness subscale (0-14) | |||||||
| Standard of care | 11.3 (2.1) | 9.8 (2.7) | 9.2 (3.4) | 8.7 (3.3) | 8.6 (3.6) | −2.4 (3.3) | −0.73 (−1.09 to −0.36) |
| Partial TRT | 11.5 (2.4) | 9.5 (2.9) | 9.0 (3.3) | 8.2 (3.5) | 8.8 (3.0) | −2.4 (2.9) | −0.83 (−1.18 to −0.46) |
| TRT | 11.5 (2.5) | 9.1 (3.1) | 8.0 (3.0) | 8.1 (3.6) | 8.5 (3.3) | −2.7 (3.0) | −0.90 (−1.30 to −0.50) |
| Auditory perceptual difficulties (0-14) | |||||||
| Standard of care | 7.7 (3.7) | 6.7 (3.5) | 5.6 (3.7) | 4.9 (3.4) | 5.0 (3.7) | −2.4 (3.2) | −0.74 (−1.10 to −0.37) |
| Partial TRT | 7.1 (3.0) | 5.0 (3.5) | 5.0 (2.9) | 5.1 (2.8) | 4.8 (3.1) | −2.0 (3.2) | −0.63 (−0.97 to −0.29) |
| TRT | 7.2 (2.8) | 5.0 (3.2) | 4.9 (3.4) | 5.1 (3.1) | 5.7 (3.6) | −2.0 (3.0) | −0.67 (−1.04 to −0.30) |
| Sleep disturbance subscale (0-8) | |||||||
| Standard of care | 4.8 (2.4) | 3.7 (2.6) | 3.7 (2.6) | 3.4 (2.6) | 3.7 (2.6) | −1.2 (2.1) | −0.56 (−0.90 to −0.21) |
| Partial TRT | 4.3 (2.2) | 2.5 (2.2) | 2.5 (2.1) | 2.3 (2.2) | 2.2 (3.2) | −2.2 (2.0) | −1.06 (−1.45 to −0.67) |
| TRT | 4.9 (2.3) | 3.6 (2.3) | 3.2 (2.8) | 3.0 (2.2) | 3.3 (2.6) | −1.6 (2.3) | −0.68 (−1.05 to −0.30) |
| Somatic symptom subscale (0-8) | |||||||
| Standard of care | 4.9 (1.7) | 3.8 (2.2) | 3.4 (1.5) | 3.0 (1.9) | 3.6 (2.0) | −1.3 (2.1) | −0.61 (−0.96 to −0.26) |
| Partial TRT | 4.5 (1.8) | 3.4 (2.3) | 3.0 (1.6) | 2.8 (1.9) | 3.3 (2.1) | −1.5 (2.1) | −0.69 (−1.03 to −0.34) |
| TRT | 5.3 (1.6) | 3.6 (2.0) | 3.1 (2.1) | 3.4 (1.8) | 3.8 (2.3) | −1.7 (1.9) | −0.93 (−1.33 to −0.52) |
| Tinnitus Functional Index | |||||||
| Total score (0-100) | |||||||
| Standard of care | 53.5 (17.3) | 49.8 (17.6) | 44.7 (19.1) | 37.6 (20.9) | 41.6 (23.4) | −10.3 (21.9) | −0.47 (−0.81 to −0.13) |
| Partial TRT | 50.3 (17.1) | 40.6 (19.4) | 42.4 (19.7) | 35.6 (19.6) | 37.9 (18.0) | −14.4 (17.2) | −0.85 (−1.21 to −0.48) |
| TRT | 48.1 (17.6) | 43.1 (17.6) | 38.6 (18.3) | 38.6 (17.4) | 40.1 (19.6) | −6.7 (18.5) | −0.37 (−0.71 to −0.02) |
| Emotional distress subscale | |||||||
| Standard of care | 34.4 (23.6) | 26.9 (22.1) | 24.5 (23.6) | 19.3 (17.0) | 26.0 (25.5) | −8.8 (28.6) | −0.31 (−0.64 to 0.02) |
| Partial TRT | 31.8 (26.1) | 22.0 (23.7) | 22.8 (23.2) | 16.1 (20.7) | 18.1 (21.5) | −15.9 (23.5) | −0.68 (−1.02 to −0.33) |
| TRT | 31.6 (24.6) | 23.6 (21.1) | 18.9 (24.0) | 22.3 (19.8) | 24.0 (25.2) | −7.5 (25.7) | −0.29 (−0.63 to 0.06) |
| Intrusiveness subscale | |||||||
| Standard of care | 70.7 (17.3) | 68.0 (17.9) | 60.6 (21.0) | 51.9 (22.4) | 57.9 (24.5) | −11.4 (26.4) | −0.43 (−0.77 to −0.09) |
| Partial TRT | 67.5 (18.0) | 62.2 (21.6) | 59.7 (20.8) | 50.9 (21.3) | 57.1 (19.6) | −10.1 (20.6) | −0.49 (−0.81 to −0.16) |
| TRT | 63.3 (23.2) | 59.1 (20.3) | 52.7 (19.0) | 53.3 (17.7) | 52.1 (21.8) | −6.8 (27.8) | −0.24 (−0.58 to 0.10) |
| Reduced sense of control subscale | |||||||
| Standard of care | 67.8 (19.5) | 58.4 (20.1) | 54.3 (21.5) | 44.0 (25.2) | 46.3 (26.7) | −20.5 (27.6) | −0.74 (−1.11 to −0.38) |
| Partial TRT | 63.7 (16.7) | 55.5 (21.1) | 57.3 (18.6) | 45.2 (20.9) | 53.8 (20.6) | −12.0 (24.0) | −0.50 (−0.83 to −0.17) |
| TRT | 58.6 (20.9) | 56.2 (21.3) | 51.8 (17.9) | 49.3 (20.9) | 47.7 (20.0) | −9.02 (21.5) | −0.42 (−0.77 to −0.07) |
| Cognitive interference subscale | |||||||
| Standard of care | 47.8 (24.5) | 45.9 (23.7) | 41.8 (23.9) | 36.3 (25.9) | 37.3 (26.5) | −8.3 (29.2) | −0.28 (−0.61 to 0.05) |
| Partial TRT | 51.0 (22.1) | 39.3 (25.8) | 41.1 (25.7) | 36.4 (23.4) | 39.3 (23.8) | −14.7 (21.2) | −0.69 (−1.03 to −0.34) |
| TRT | 42.7 (21.2) | 38.5 (22.8) | 32.9 (22.7) | 36.9 (20.7) | 39.9 (24.2) | −1.08 (22.2) | −0.48 (−0.38 to 0.29) |
| Sleep disturbance subscale | |||||||
| Standard of care | 61.0 (30.2) | 57.9 (28.8) | 48.5 (30.2) | 44.2 (35.2) | 49.6 (32.3) | −10.0 (24.3) | −0.41 (−0.74 to −0.07) |
| Partial TRT | 50.3 (28.1) | 34.5 (24.7) | 38.0 (29.8) | 33.8 (29.3) | 31.6 (24.7) | −20.2 (23.4) | −0.86 (−1.22 to −0.49) |
| TRT | 55.4 (30.8) | 45.1 (29.6) | 42.5 (29.9) | 39.0 (26.1) | 42.0 (30.4) | −11.1 (31.4) | −0.35 (−0.70 to −0.00) |
| Auditory difficulties subscale | |||||||
| Standard of care | 46.6 (25.7) | 48.2 (23.4) | 45.0 (29.5) | 35.4 (24.5) | 40.5 (29.9) | −2.7 (29.2) | −0.09 (−0.41 to 0.02) |
| Partial TRT | 45.0 (27.2) | 40.4 (26.9) | 46.2 (25.3) | 36.9 (24.4) | 41.3 (26.7) | −6.6 (22.3) | −0.30 (−0.61 to 0.02) |
| TRT | 45.1 (27.2) | 47.4 (24.5) | 41.9 (28.3) | 40.6 (22.4) | 42.0 (24.2) | −7.1 (21.1) | −0.33 (−0.68 to 0.01) |
| Relaxation interference subscale | |||||||
| Standard of care | 68.2 (24.4) | 63.8 (24.2) | 58.0 (25.8) | 49.8 (30.7) | 51.2 (33.0) | −15.6 (33.1) | −0.47 (−0.81. −0.13) |
| Partial TRT | 61.9 (27.2) | 51.2 (28.4) | 51.8 (30.0) | 46.9 (29.5) | 45.2 (27.6) | −18.6 (30.2) | −0.62 (−0.95 to −0.27) |
| TRT | 63.9 (23.6) | 53.6 (24.5) | 51.7 (23.1) | 44.6 (23.5) | 47.3 (26.2) | −13.3 (26.7) | −0.50 (−0.85 to −0.14) |
| Reduced quality of life subscale | |||||||
| Standard of care | 37.0 (25.4) | 34.0 (23.3) | 29.8 (22.5) | 24.3 (19.8) | 28.2 (26.1) | −6.4 (24.4) | −0.26(−0.59 to 0.07) |
| Partial TRT | 35.7 (23.1) | 24.9 (22.4) | 27.2 (22.7) | 22.9 (24.3) | 22.1 (19.9) | −16.6 (20.9) | −0.79 (−1.14 to −0.43) |
| TRT | 30.1 (22.7) | 26.8 (20.4) | 20.6 (22.7) | 27.1 (20.5) | 29.5 (27.6) | −0.3 (23.0) | −0.01 (−0.34 to 0.32) |
| Tinnitus Handicap Inventory | |||||||
| Total score (0-100) | |||||||
| Standard of care | 38.6 (18.7) | 36.3 (17.7) | 33.4 (18.4) | 26.4 (14.6) | 29.2 (17.2) | −9.4 (17.7) | −0.53 (−0.87 to −0.18) |
| Partial TRT | 42.3 (20.7) | 30.4 (20.7) | 31.6 (18.6) | 27.2 (19.1) | 29.5 (17.0) | −12.6 (17.1) | −0.74 (−1.09 to −0.38) |
| TRT | 37.8 (13.0) | 30.5 (17.0) | 25.8 (16.4) | 29.2 (17.4) | 31.5 (20.5) | −6.1 (18.0) | −0.34 (−0.69 to 0.02) |
| Functional subscale (0-44) | |||||||
| Standard of care | 20.5 (9.2) | 21.2 (9.5) | 19.3 (9.5) | 16.0 (8.6) | 17.8 (10.0) | −2.4 (9.7) | −0.25 (−0.58 to 0.08) |
| Partial TRT | 22.5 (10.5) | 16.8 (10.4) | 18.3 (10.3) | 16.1 (10.4) | 17.1 (9.1) | −5.0 (7.0) | −0.71 (−1.06 to −0.36) |
| TRT | 19.8 (6.8) | 17.4 (8.7) | 14.9 (8.4) | 16.5 (8.2) | 18.1 (10.5) | −1.5 (8.7) | −0.17 (−0.52 to 0.17) |
| Emotional subscale (0-36) | |||||||
| Standard of care | 10.1 (6.4) | 8.8 (6.3) | 7.9 (6.7) | 5.1 (4.9) | 6.2 (5.6) | −4.3 (6.2) | −0.69 (−1.04 to −0.33) |
| Partial TRT | 11.0 (7.8) | 7.8 (7.2) | 7.3 (7.1) | 6.2 (7.1) | 7.1 (6.7) | −4.4 (7.7) | −0.57 (−0.90 to −0.23) |
| TRT | 9.8 (5.4) | 6.6 (6.2) | 5.6 (6.1) | 6.5 (6.4) | 7.0 (6.8) | −2.8 (8.0) | −0.36 (−0.71 to −0.00) |
| Catastrophic subscale (0-20) | |||||||
| Standard of care | 8.0 (3.5) | 6.3 (3.9) | 6.2 (4.2) | 5.3 (3.4) | 5.2 (3.8) | −2.7 (4.3) | −0.62 (−0.97 to −0.27) |
| Partial TRT | 8.7 (4.2) | 5.7 (4.4) | 6.0 (3.6) | 4.9 (3.6) | 5.4 (3.8) | −3.2 (4.4) | −0.73 (−1.08 to −0.37) |
| TRT | 8.2 (3.4) | 6.5 (3.8) | 5.3 (3.7) | 6.1 (4.1) | 6.5 (4.7) | −1.7 (3.8) | −0.44 (−0.80 to −0.08) |
| 10-Point VAS “How Much of a Problem Is Your Tinnitus?” | |||||||
| Score (0-10) | |||||||
| Standard of care | 6.3 (2.1) | NA | 5.0 (2.4) | 4.5 (2.5) | 4.3 (3.0) | −1.8 (2.8) | −0.64 (−1.01 to −0.26) |
| Partial TRT | 6.4 (2.2) | NA | 5.2 (2.1) | 3.8 (2.5) | 4.2 (2.5) | –2.1 (2.4) | −0.85 (−1.26 to −0.43) |
| TRT | 6.2 (2.2) | NA | 4.5 (2.4) | 4.1 (2.1) | 4.4 (2.4) | –1.8 (3.0) | −0.58 (−0.97 to −0.18) |
Abbreviations: NA, not applicable; TRT, tinnitus retraining therapy; VAS, visual analog scale.
Models across treatment groups used repeated-measures GEE, including adjustment for baseline instrument score, visit, clinical center, age, PANAS and STAI scores, and sex. We tested for audiologist, type of SG (behind-the-ear or in-the-ear), and TRT category and found no significant effect in the model for any of these factors. We found no difference in comparisons between either the partial TRT or TRT group and the SoC group on global TQ, TFI, or THI scores or for any subscale, other than a significant effect of partial TRT on the sleep subscale of both the TQ and TFI and the functional subscale of the THI (Table 3). In a similar comparison of the TRT group with the partial TRT group, we again found no difference between TQ, TFI, and THI total and subscale scores.
Table 3. Comparison of Overall Change in Scores on Tinnitus Questionnaire, Tinnitus Functional Index, and Tinnitus Handicap Inventory and Subscale Scores in Partial TRT and TRT Groups With Overall Change in SoC Group.
| Scale | Estimate (95% CI)a | |
|---|---|---|
| Partial TRT | TRT | |
| Tinnitus Questionnaire | ||
| Total score | −2.81 (−8.27 to 2.65) | −2.35 (−8.77 to 4.07) |
| Emotional distress | −0.60 (−3.16 to 1.96) | −0.39 (−3.08 to 2.29) |
| Intrusiveness | −0.12 (−1.14 to 0.90) | −0.36 (−1.38 to 0.67) |
| Auditory perceptual difficulties | −0.36 (−1.35 to 0.64) | 0.38 (−1.37 to 0.61) |
| Sleep disturbance | −0.87 (−1.56 to −0.17) | −0.10 (−0.74 to 0.53) |
| Somatic complaint | −0.20 (−0.86 to 0.46) | −0.07 (−0.83 to 0.69) |
| Tinnitus Functional Index | ||
| Total score | 2.74 (−2.21 to 7.70) | −0.14 (−5.30 to 5.02) |
| Emotional distress | −3.02 (−9.43 to 3.40) | −0.13 (−6.78 to 6.52) |
| Intrusiveness | −0.91 (−6.75 to 4.93) | −3.19 (−9.27 to 2.88) |
| Reduced sense of control | 3.29 (−3.00 to 9.54) | 3.12 (−3.44 to 9.68) |
| Cognitive interference | 0.94 (−5.44 to 7.32) | 1.41 (−5.20 to 8.01) |
| Sleep disturbance | −13.70 (−22.02 to −5.38) | −3.53 (−12.30 to 5.23) |
| Auditory difficulties | −0.23 (−7.72 to 7.26) | 3.71 (−3.96 to 11.38) |
| Relaxation interference | −4.46 (−11.48 to 2.55) | −1.85 (−9.07 to 5.38) |
| Reduced quality of life | −3.52 (−9.26 to 2.22) | 1.24 (−4.67 to 7.14) |
| Tinnitus Handicap Inventory | ||
| Total score | −4.51 (−9.15 to 0.13) | −0.05 (−4.90 to 4.81) |
| Functional subscale | −2.10 (−5.21 to −0.41) | −0.82 (−3.30 to 1.66) |
| Emotional subscale | −1.04 (−2.83 to 0.75) | 0.34 (−1.54 to 2.23) |
| Catastrophic subscale | −0.62 (−1.75 to 0.52) | 0.61 (−0.55 to 1.77) |
Abbreviations: SoC, standard of care; TRT, tinnitus retraining therapy.
Values representing mean difference in change in score over time compared with that of the SoC group were estimated using generalized estimating equations models adjusted for visit; clinical center; age group; sex; and baseline scores on the State-Trait Anxiety Inventory and Positive and Negative Affect Schedule, with multiple imputation for missing values.
Our secondary prespecified analysis compared changes from baseline mean scores with scores at the 18-month visit using GEE, adjusted using the same baseline factors as were used in the primary analysis. This analysis also showed no statistical difference between treatment groups. Compared with the SoC group, the difference in mean change over time in the TRT group was −0.61 (95% CI, −7.24 to 6.02) and −1.40 (95% CI, −7.62 to 4.83) in the partial TRT group.
Results of comparisons of effect size between baseline and 18-month follow-up scores for each of these instruments and corresponding subscales are shown in Table 2. Tinnitus Questionnaire total and subscale scores and the VAS score showed significant moderate to large effect sizes. Significant improvement was observed at 18 months in all treatment groups on TQ scores for TRT (effect size, −1.32; 95% CI, −1.78 to −0.85), partial TRT (effect size, −1.16; 95% CI, −1.56 to −0.76), and SoC (effect size, −1.01; 95% CI, −1.41 to −0.61). The effect sizes of the total and subscales values for the TFI and THI were also moderate, but some were not significant, as indicated by the 95% CI crossing zero.
In the TRT group, 24 of 34 participants (70.6%; 95% CI, 55.3%-86.9%) had a minimal clinically significant change in the reduction of the effect of tinnitus as measured on the TQ; the same was true for 16 of 34 participants (47.1%; 95% CI, 30.3%-63.8%) on the TFI, 18 of 34 participants (52.9%; 95% CI, 36.2%-69.7%) on the THI, and 11 of 29 participants (37.9%; 95% CI, 20.3%-55.6%) on the VAS (Table 4). In the partial TRT group, 33 of 40 participants (82.5%; 95% CI, 70.7%-94.3%) had a minimal clinically significant change in the reduction of the effect of tinnitus as measured on the TQ; the same was true for 21 of 40 participants (52.5%; 95% CI, 37.0%-68.0%) on the TFI, 25 of 40 participants (62.5%; 95% CI, 47.5%-77.5%) on the THI, and 18 of 31 participants (58.1%; 95% CI, 40.7%-75.4%) on the VAS. In the SoC group, 29 of 37 participants (78.4%; 95% CI, 65.1%-91.6%) had a minimal clinically significant change in the reduction of the effect of tinnitus as measured on the TQ; the same was true for 15 of 37 participants (40.5%; 95% CI, 24.7%-56.4%) on the TFI, 20 of 37 participants (54.1%; 95% CI, 38.0%-70.1%) on the THI, and 16 of 33 participants (48.5%; 31.4%-65.5%) on the VAS.
Table 4. Data on Participants With Minimal Clinically Significant Change Between Baseline and 18 Months by Treatment Group.
| Scale | Treatment Group, No./Total No. (%) [95% CI] | |||
|---|---|---|---|---|
| SoC (n = 37) | Partial TRT (n = 40) | TRT (n = 34) | Total (N = 111) | |
| Tinnitus Questionnaire (7-point difference) | 29/37 (78.4) [65.1-91.6] | 33/40 (82.5) [70.7-94.3] | 24/34 (70.6) [55.3-86.9] | 86/111 (77.5) [60.7-86.2] |
| Tinnitus Functional Index (13-point difference) | 15/37 (40.5) [24.7-56.4] | 21/40 (52.5) [37.0-68.0] | 16/34 (47.1) [30.3-63.8] | 52/111 (46.8) [37.6-56.1] |
| Tinnitus Handicap Inventory (7-point difference) | 20/37 (54.1) [38.0-70.1] | 25/40 (62.5) [47.5-77.5] | 18/34 (52.9) [36.2-69.7] | 63/111 (56.8) [47.5-66.0] |
| Visual analog scale (2-point difference) | 16/33(48.5) [31.4-65.5] | 18/31 (58.1) [40.7-75.4] | 11/29 (37.9) [20.3-55.6] | 45/93 (48.4) [38.2-58.5] |
Abbreviations: SoC, standard of care; TRT, tinnitus retraining therapy.
There were 4 serious adverse events, all in the partial TRT group, none of which were related to treatment. One participant in the TRT group had pain and swollen ear canal walls from SG wear. Thirty individuals (8 in the TRT group, 12 in the partial TRT group, and 10 in the SoC group) experienced depression, as measured by a score of 4 or more on the Beck Depression Inventory–Fast Screen, indicating suicidal ideation (eTable 7 in Supplement 2).
Discussion
In the TRTT, we found no difference between treatment groups when analyzed either as simple change between baseline and 18-month follow-up or across all follow-up visits as change on the TQ, TFI, THI, or VAS. Thus, there were no meaningful differences between TRT and SoC (our primary comparison) or between partial TRT and SoC or TRT (our secondary comparisons). With the exception of some sleep subscales, there were no clinically meaningful differences among treatment groups. About half of TRTT participants showed a significant reduction in the effect of tinnitus on their daily lives. This finding was evident across the TQ, TFI, THI, and VAS, highlighting the robustness of these findings. Our findings appear inconsistent with those of other studies reporting differences between TRT and other interventions,15,16,19 but similar to those of Westin et al,17 suggesting that a well-conducted comparison treatment can achieve an effect at least equivalent, if not superior, to TRT. One could reasonably argue that our control treatment (SoC) was more rigorous than existing care provided in the US military. However, it is consistent with American Speech-Language-Hearing Association practice guidelines and information typically provided in the military setting.
We found no additional effect from the SGs when we compared the TRT and partial TRT groups. This result suggests that TC alone is sufficient for achieving TRT treatment efficacy when an enriched sound background is encouraged throughout treatment for a functionally normal-hearing population. Tinnitus retraining therapy as practiced in the clinical community typically offers options for ST, including the use of enriched environmental sound, device-aided or not, and/or the use of SGs.8,9 The TRTT study participants had no or minimal hearing loss, and all groups were encouraged to use enriched environmental sound to reduce the contrast between the background sound and the tinnitus. The use of enriched environmental sound also was encouraged for the SoC group, which achieved a result similar to that in the partial TRT group, who effectively received only TC with environmental sound when using placebo SGs.
Limitations
This study had some limitations. We had a larger than expected number of missed visits and withdrawals, mostly in the TRT and partial TRT groups. Possibly the latter may be explained by participant frustration with early SG problems. Some attrition was also likely attributable to routine military operations (eg, deployment and changes in posting) and was unavoidable.39 We found no differences in demographics or other baseline characteristics between the participants who completed the study vs those who did not. However, the missed visits and attrition contributed to a lack of precision in our results, which, together with our smaller than planned sample size, may have masked any true differences between treatment groups, or masked an effect when one was present. As a cross-check, we conducted a sensitivity analysis by including only participants who completed all study visits (eTable 8 in Supplement 2) and still found no difference across treatment groups. Thus, we are reasonably confident in the validity of our results, notwithstanding the above concerns.
An unavoidable limitation of this trial was lack of study clinician expertise in providing TRT at study outset. None of the study clinicians was initially proficient with, or routinely provided, TRT; most study clinicians treated only a few participants during the trial. However, all clinicians showed excellent compliance with the protocol during the study.40 This lack of experience may explain the smaller treatment effects we found compared with other studies.15,16,19 The larger effects in some of these prior studies may also be ascribed to their single-site designs with small samples and little diversity. Also, in studies with hearing-impaired participants, aided treatment could have confounded any tinnitus treatment effect by improving participants’ communication abilities.
Another possible limitation of this study is that the TRTT included active-duty military personnel and their dependents, so a question might be raised regarding the applicability of these results to the civilian population. In general, there is little difference between individuals with tinnitus in the US civilian population and those serving in the military, as shown by the demographic characteristics for the TRTT population and previous measures of hearing-related quality of life.41,42 Nonetheless, we would expect military personnel to have experienced greater exposure to armament sound than civilians, which may explain the highly tonal quality of the tinnitus among the TRTT participants (Table 1).
Conclusions
We found few differences among SoC, TRT, or partial TRT in the treatment of tinnitus. Each of these treatments for debilitating tinnitus resulted in clinically significant improvement in most treated individuals.
Trial Protocol
eTable 1. Treatment Assignment by Clinical Center
eTable 2. Treatment Adherence by Treatment Group
eFigure 1. Hearing Thresholds
eFigure 2. Loudness Discomfort Levels
eFigure 3. Tinnitus Questionnaire, Tinnitus Functional Index, Tinnitus Handicap Inventory, and 10-Point VAS Scores by Visit and Treatment Group
eTable 3. Digital Symbol Substitution Test by Visit and Treatment Group
eTable 4. Hearing Handicap Inventory by Visit and Treatment Group
eTable 5. State-Trait Inventory by Visit and Treatment Group
eTable 6. Positive and Negative Affect Scale by Visit and Treatment Group
eTable 7. Beck Depression Inventory by Visit and Treatment Group
eTable 8. Comparison of Overall Change in TQ, TFI, and THI Total and Subscale Scores in Partial TRT and TRT Groups With Overall Change in SoC Group Using Full Case Population
Data Sharing Statement
References
- 1.McCormack A, Edmondson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016;337:70-79. doi: 10.1016/j.heares.2016.05.009 [DOI] [PubMed] [Google Scholar]
- 2.Kochkin S, Tyler R, Born J. MarkeTrak VIII: the prevalence of tinnitus in the United States and the self-reported efficacy of various treatments. Hear Rev. 2011;18(12):10-26. [Google Scholar]
- 3.American Tinnitus Association . Understanding the facts: demographics. https://www.ata.org/understanding-facts/demographics. Accessed December 17, 2018.
- 4.Dobie RA. Clinical trials and drug therapy for tinnitus. In: Snow J, ed. Tinnitus: Theory and Management. Hamilton, Ontario, Canada: BC Decker; 2004:266-277. [Google Scholar]
- 5.Parnes SM. Current concepts in the clinical management of patients with tinnitus. Eur Arch Otorhinolaryngol. 1997;254(9-10):406-409. doi: 10.1007/BF02439968 [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010;(9):CD005233. [DOI] [PubMed] [Google Scholar]
- 7.Jastreboff PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res. 1990;8(4):221-254. doi: 10.1016/0168-0102(90)90031-9 [DOI] [PubMed] [Google Scholar]
- 8.Gold SL, Formby C, Gray WC. Celebrating a decade of evaluation and treatment: the University of Maryland Tinnitus & Hyperacusis Center. Am J Audiol. 2000;9(2):69-74. doi: 10.1044/1059-0889(2000/014) [DOI] [PubMed] [Google Scholar]
- 9.Jastreboff PJ, Hazell JWP. Treatment of tinnitus based on a neurophysiological model. In: Vernon J, ed. Tinnitus and Relief. Boston, MA: Allyn & Bacon; 1998:201-217. [Google Scholar]
- 10.Bartnick G, Fabijanska A, Rogowski M. Our experience in treatment of patients with tinnitus and/or hyperacusis using the habituation method. Presented at: Proceedings of the 6th International Tinnitus Seminar. London, UK: The Tinnitus and Hyperacusis Centre; 1999:415-417. [Google Scholar]
- 11.Heitzmann T, Rubio L, Cardenas MR, Zofio E. The importance of continuity in TRT patients: results at 18 months. Presented at: Proceedings of the 6th International Tinnitus Seminar; 1999; London, UK. [Google Scholar]
- 12.Herraiz C, Hernandez FJ, Machado A, Lucas P, Tapia MC. Tinnitus retraining therapy: our experience. Presented at: Proceedings of the 6th International Tinnitus Seminar; 1999; London, UK. [Google Scholar]
- 13.McKinney CJH, Hazell JWP, Graham RL. An evaluation of the TRT method. Presented at: Proceedings of the 6th International Tinnitus Seminar. London, UK: The Tinnitus and Hyperacusis Centre; 1999: 415-417. [Google Scholar]
- 14.Sheldrake JB, Hazell JWP, Graham RL. Results of tinnitus retraining therapy. Presented at: Proceedings of the 6th International Tinnitus Seminar; 1999; London, UK: The Tinnitus and Hyperacusis Centre; 1999:292-296. [Google Scholar]
- 15.Henry JA, Schechter MA, Zaugg TL, et al. Outcomes of clinical trial: tinnitus masking versus tinnitus retraining therapy. J Am Acad Audiol. 2006;17(2):104-132. doi: 10.3766/jaaa.17.2.4 [DOI] [PubMed] [Google Scholar]
- 16.Bauer CA, Brozoski TJ. Effect of tinnitus retraining therapy on the loudness and annoyance of tinnitus: a controlled trial. Ear Hear. 2011;32(2):145-155. doi: 10.1097/AUD.0b013e3181f5374f [DOI] [PubMed] [Google Scholar]
- 17.Westin VZ, Schulin M, Hesser H, et al. Acceptance and commitment therapy versus tinnitus retraining therapy in the treatment of tinnitus: a randomised controlled trial. Behav Res Ther. 2011;49(11):737-747. doi: 10.1016/j.brat.2011.08.001 [DOI] [PubMed] [Google Scholar]
- 18.Tyler RS, Noble W, Coelho CB, Ji H. Tinnitus retraining therapy: mixing point and total masking are equally effective. Ear Hear. 2012;33(5):588-594. doi: 10.1097/AUD.0b013e31824f2a6e [DOI] [PubMed] [Google Scholar]
- 19.Bauer CA, Berry JL, Brozoski TJ. The effect of tinnitus retraining therapy on chronic tinnitus: a controlled trial. Laryngoscope Investig Otolaryngol. 2017;2(4):166-177. doi: 10.1002/lio2.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Formby C, Scherer R; TRTT Study Group . Rationale for the Tinnitus Retraining Therapy Trial. Noise Health. 2013;15(63):134-142. doi: 10.4103/1463-1741.110299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallam RS. Manual of the Tinnitus Questionnaire (TQ). London, UK: Psychological Corporation; 1996. [Google Scholar]
- 22.Scherer RW, Formby C, Gold S, et al. ; Tinnitus Retraining Therapy Trial Research Group . The Tinnitus Retraining Therapy Trial (TRTT): study protocol for a randomized controlled trial. Trials. 2014;15:396. doi: 10.1186/1745-6215-15-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Speech-Language-Hearing Association . Preferred practice patterns for the profession of audiology. http://asha.org/policy/PP2006-00274/. Accessed April 18, 2019. [PubMed]
- 24.Jastreboff PJ, Hazell JWP. Tinnitus retraining therapy (TRT): clinical implementation of the model. In: Tinnitus Retraining Therapy: Implementing the Neurophysiological Model. Cambridge, UK: Cambridge University Press; 2004:63-144. doi: 10.1017/CBO9780511544989.004 [DOI] [Google Scholar]
- 25.Jastreboff PJ. The neurophysiological model of tinnitus. In: Snow JBJ, ed. Tinnitus: Theory and Management. Hamilton, Ontario, Canada: BC Decker; 2004:96-106. [Google Scholar]
- 26.Hallam RS. Psychological approaches to the evaluation and management of tinnitus distress. In: Hazell JWP, ed. Tinnitus. Edinburgh, Scotland: Churchill Livingstone; 1987. [Google Scholar]
- 27.Meikle MB, Henry JA, Griest SE, et al. The Tinnitus Functional Index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 2012;33(2):153-176. doi: 10.1097/AUD.0b013e31822f67c0 [DOI] [PubMed] [Google Scholar]
- 28.Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1996;122(2):143-148. doi: 10.1001/archotol.1996.01890140029007 [DOI] [PubMed] [Google Scholar]
- 29.Henry JA, Jastreboff MM, Jastreboff PJ, Schechter MA, Fausti SA. Guide to conducting tinnitus retraining therapy initial and follow-up interviews. J Rehabil Res Dev. 2003;40(2):157-177. doi: 10.1682/JRRD.2003.03.0159 [DOI] [PubMed] [Google Scholar]
- 30.Wechsler D. The Wechsler Adult Intelligence Scale–Revised. San Antonio, TX: The Psychological Corporation; 1981. [Google Scholar]
- 31.Beck A, Steer R, Brown G. BDI-II Fast Screen for Medical Patients Manual. London, UK: The Psychological Corporation; 2000. [Google Scholar]
- 32.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063-1070. doi: 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- 33.Speilberger CD, Gorsuch RL, Lushene RL, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y). Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 34.Newman CW, Weinstein BE, Jacobson GP, Hug GA. Test-retest reliability of the Hearing Handicap Inventory for adults. Ear Hear. 1991;12(5):355-357. doi: 10.1097/00003446-199110000-00009 [DOI] [PubMed] [Google Scholar]
- 35.Dupont WD, Plummer WD Jr. Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11(2):116-128. doi: 10.1016/0197-2456(90)90005-M [DOI] [PubMed] [Google Scholar]
- 36.SAS Institute Inc . The MI procedure. SAS/STAT 14.1 User’s Guide. Cary, NC: SAS Institute Inc; 2015. [Google Scholar]
- 37.Cohen J. A power primer. Psychol Bull. 1992;112(1):155-159. doi: 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- 38.Zeman F, Koller M, Figueiredo R, et al. Tinnitus handicap inventory for evaluating treatment effects: which changes are clinically relevant? Otolaryngol Head Neck Surg. 2011;145(2):282-287. doi: 10.1177/0194599811403882 [DOI] [PubMed] [Google Scholar]
- 39.Scherer RW, Sensinger LD, Sierra-Irizarry B, Formby C; TRTT Research Group . Lessons learned conducting a multi-center trial with a military population: the Tinnitus Retraining Therapy Trial. Clin Trials. 2018;15(5):429-435. doi: 10.1177/1740774518777709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scherer RW, Erdman SA, Gold S, Formby C; TRTT Research Group . Treatment adherence in the Tinnitus Retraining Therapy Trial. Clin Trials. 2018;15(suppl 2):66. doi: 10.1177/1740774518790846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erdman SA, Demorest ME. Adjustment to hearing impairment: II, audiological and demographic correlates. J Speech Lang Hear Res. 1998;41(1):123-136. doi: 10.1044/jslhr.4101.123 [DOI] [PubMed] [Google Scholar]
- 42.Erdman SA, Demorest ME. Adjustment to hearing impairment: I, description of a heterogeneous clinical population. J Speech Lang Hear Res. 1998;41(1):107-122. doi: 10.1044/jslhr.4101.107 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Treatment Assignment by Clinical Center
eTable 2. Treatment Adherence by Treatment Group
eFigure 1. Hearing Thresholds
eFigure 2. Loudness Discomfort Levels
eFigure 3. Tinnitus Questionnaire, Tinnitus Functional Index, Tinnitus Handicap Inventory, and 10-Point VAS Scores by Visit and Treatment Group
eTable 3. Digital Symbol Substitution Test by Visit and Treatment Group
eTable 4. Hearing Handicap Inventory by Visit and Treatment Group
eTable 5. State-Trait Inventory by Visit and Treatment Group
eTable 6. Positive and Negative Affect Scale by Visit and Treatment Group
eTable 7. Beck Depression Inventory by Visit and Treatment Group
eTable 8. Comparison of Overall Change in TQ, TFI, and THI Total and Subscale Scores in Partial TRT and TRT Groups With Overall Change in SoC Group Using Full Case Population
Data Sharing Statement