Abstract
This study describes point-of-sale prices for orally administered anticancer drugs offered through Medicare Part D and out-of-pocket changes in spending as a result of decreasing coinsurance and price changes between 2010 and 2019.
For Medicare beneficiaries, Part D provides access to prescribed drugs. However, access to high-priced specialty medications covered by Part D may be limited by increasing drug prices and Part D benefit designs requiring patients to pay a percentage of the drug’s price with every fill through coinsurance. To improve affordability of drugs for patients, the Affordable Care Act has gradually reduced the required coinsurance for branded drugs in the coverage gap (ie, doughnut hole) from 100% in 2010 to 25% in 2019.1 However, price increases over that same period might have offset expected savings for patients needing specialty drugs, including anticancer drugs.2,3,4,5 We assessed point-of-sale prices from 2010 to 2018 for orally administered anticancer drugs offered through Medicare Part D and estimated how out-of-pocket spending changed from 2010 to 2019 benefit designs as a result of decreasing coinsurance as the Part D coverage gap closed.
Methods
We used Medicare formulary and pricing files for the fourth quarter of 2010 through the fourth quarter of 2018 to describe point-of-sale prices (ie, excluding rebates and discounts) for a single fill of each anticancer medication. We compared prices in 2010 (or the first year a newer product was observed in the data) and 2018. We also calculated the percentage price increase or decrease for monthly fills over this period. We estimated price changes net of inflation, adjusting drug prices and out-of-pocket spending to 2018 US dollars using the Consumer Price Index.
Next, we calculated expected out-of-pocket spending for Medicare beneficiaries under the standard benefit design in 2010 and 2019 for the 13 drugs available during both years. This standard benefit design used coinsurance rather than copayments (98% of Part D plans required coinsurance for all drugs/years studied). We assumed beneficiaries used 12 months of therapy and no other medications for comparisons.
Results
In 2010, 13 anticancer medications were covered by Part D and 54 in 2018. The mean price per fill in 2010 was $7438 vs $13 992 in 2018. In 2018, 48 of 54 medications had monthly prices exceeding $10 000 per fill and 21 had prices exceeding $15 000 per fill (Table). Across all drugs and varying years of approval (pre-2010 through 2018), mean prices rose by 5.8% per year above inflation. Changes in mean per-fill price from the first observed fill year to 2018 was 40.4% overall, ranging from a reduction of 44% for generic imatinib ($8570 in 2016 vs $4822 in 2018) to an increase of 306% for gefitinib ($1960 in 2010 vs $7960 in 2018).
Table. Mean Point-of-Sale Prices and Price Changes for Orally Administered Anticancer Drugs Under Medicare Part Da.
| Drug Name | Brand/Generic | Approval Year | First Year Observed | Mean Price for 1-mo Fill, $ | % Change | ||
|---|---|---|---|---|---|---|---|
| First Year | In 2018 | First vs Last Year | Mean Annual | ||||
| Imatinib | Brand | 2001 | 2010 | 5143 | 10 620 | 106 | 11.0 |
| Gefitinib | Brand | 2003 | 2010 | 1960 | 7960 | 306 | 16.2 |
| Thalidomide | Brand | 2003 | 2010 | 7135 | 8583 | 20 | 2.0 |
| Erlotinib | Brand | 2004 | 2010 | 4911 | 8655 | 76 | 6.8 |
| Lenalidomide | Brand | 2005 | 2010 | 11 832 | 21 412 | 81 | 8.4 |
| Sorafenib | Brand | 2005 | 2010 | 8508 | 19 118 | 125 | 10.9 |
| Dasatinib | Brand | 2006 | 2010 | 8902 | 13 194 | 48 | 5.7 |
| Sunitinib | Brand | 2006 | 2010 | 9595 | 18 706 | 95 | 8.2 |
| Vorinostat | Brand | 2006 | 2010 | 10 259 | 15 415 | 50 | 5.3 |
| Lapatinib | Brand | 2007 | 2010 | 4147 | 7659 | 85 | 8.1 |
| Nilotinib | Brand | 2007 | 2010 | 9279 | 10 487 | 13 | 3.0 |
| Everolimus | Brand | 2009 | 2010 | 7726 | 15 965 | 107 | 9.5 |
| Pazopanib | Brand | 2009 | 2010 | 6311 | 12 392 | 96 | 9.1 |
| Abiraterone | Brand | 2011 | 2011 | 5439 | 10 437 | 92 | 8.1 |
| Crizotinib | Brand | 2011 | 2012 | 11 121 | 16 470 | 48 | 6.5 |
| Ruxolitinib | Brand | 2011 | 2012 | 8238 | 12 942 | 57 | 7.1 |
| Vemurafenib | Brand | 2011 | 2012 | 11 451 | 11 137 | −3 | −0.1 |
| Axitinib | Brand | 2012 | 2012 | 9849 | 14 756 | 50 | 7.3 |
| Vismodegib | Brand | 2012 | 2012 | 8986 | 11 862 | 32 | 5.0 |
| Bosutinib | Brand | 2012 | 2013 | 9003 | 14 636 | 63 | 9.5 |
| Cabozantiniba | Brand | 2012 | 2013 | 11 440 | 16 606 | 45 | 7.5 |
| Enzalutamide | Brand | 2012 | 2013 | 8684 | 11 144 | 28 | 5.3 |
| Regorafenib | Brand | 2012 | 2013 | 11 613 | 17 284 | 49 | 7.5 |
| Ponatinib | Brand | 2012 | 2016 | 17 476 | 17 249 | −1 | −0.6 |
| Dabrafenib | Brand | 2013 | 2013 | 8360 | 10 179 | 22 | 3.6 |
| Pomalidomide | Brand | 2013 | 2013 | 16 410 | 24 406 | 49 | 8.8 |
| Trametinib | Brand | 2013 | 2013 | 9568 | 11 032 | 15 | 2.2 |
| Afatinib | Brand | 2013 | 2014 | 6450 | 8378 | 30 | 6.6 |
| Ibrutinib | Brand | 2013 | 2014 | 12 588 | 13 496 | 7 | 2.0 |
| Ceritinib | Brand | 2014 | 2014 | 14 515 | 17 115 | 18 | 4.7 |
| Idelalisib | Brand | 2014 | 2015 | 8520 | 10 503 | 23 | 6.2 |
| Olaparib | Brand | 2014 | 2016 | 13 568 | 14 374 | 6 | 2.8 |
| Palbociclib | Brand | 2015 | 2015 | 15 150 | 16 539 | 9 | 2.9 |
| Panobinostat | Brand | 2015 | 2015 | 14 772 | 16 639 | 13 | 3.5 |
| Alectinib | Brand | 2015 | 2016 | 13 024 | 14 887 | 14 | 6.8 |
| Cobimetinib | Brand | 2015 | 2016 | 9150 | 10 295 | 13 | 5.9 |
| Imatinib | Generic | 2015 | 2016 | 8570 | 4822 | −44 | −28.1 |
| Ixazomib | Brand | 2015 | 2016 | 12 704 | 13 627 | 7 | 3.6 |
| Lenvatinib | Brand | 2015 | 2016 | 13 676 | 17 651 | 29 | 13.1 |
| Osimertinib | Brand | 2015 | 2016 | 14 280 | 15 024 | 5 | 2.6 |
| Sonidegib | Brand | 2015 | 2016 | 10 629 | 11 021 | 4 | 2.1 |
| Trifluridine | Brand | 2015 | 2016 | 12 051 | 12 853 | 7 | 3.4 |
| Cabozantinibb | Brand | 2016 | 2016 | 15 256 | 17 924 | 17 | 8.1 |
| Venetoclax | Brand | 2016 | 2016 | 10 100 | 11 495 | 14 | 6.5 |
| Rucaparib | Brand | 2016 | 2017 | 14 285 | 15 300 | 7 | 6.9 |
| Brigatinib | Brand | 2017 | 2017 | 14 802 | 16 911 | 14 | 13.3 |
| Midostaurin | Brand | 2017 | 2017 | 16 676 | 17 709 | 6 | 6.0 |
| Niraparib | Brand | 2017 | 2017 | 10 212 | 11 867 | 16 | 15.0 |
| Ribociclib | Brand | 2017 | 2017 | 19 515 | 21 348 | 9 | 9.0 |
| Plan-Drug Observations, No. | Price per Fill in First Year, Mean | Price Per Fill in 2018, SD | Mean % Change for First vs Last Year | Mean Annual Change, SD | |||
| Drugs available since 2010 (n = 13) | 150 500 | 7438 | 2651 | 12 883 | 4935 | 93.0 | 8.0 |
| All drugs (N = 54) | 31 635 | 11 404 | 4158 | 13 992 | 4341 | 40.4 | 5.8 |
Five branded drugs had observed data only for 2018, with mean prices for 1-month fills as follows: abemaciclib, $12 576; acalabrutinib, 14 630; enasidenib, $25 868; neratinib, $11 910; and apalutamide, $11 369.
Drug is available under 2 branded drug names: Cometriq (approved in 2012) and C, abometyx (approved in 2016). Prices represent the reimbursed amount at the point of sale, including both Medicare and patient contributions. First-year and last-year prices were compared against external references to ensure dosing changes were not driving the findings. In such cases, monthly prices were adjusted (n = 11) to more closely match the expected prices at launch (https://www.mskcc.org/sites/default/files/node/25097/documents/111516-drug-costs-table.pdf) and in 2018 (Medicare Part D Plan Finder [https://www.medicare.gov/find-a-plan/questions/home.aspx] and GoodRx [https://www.goodrx.com/]).
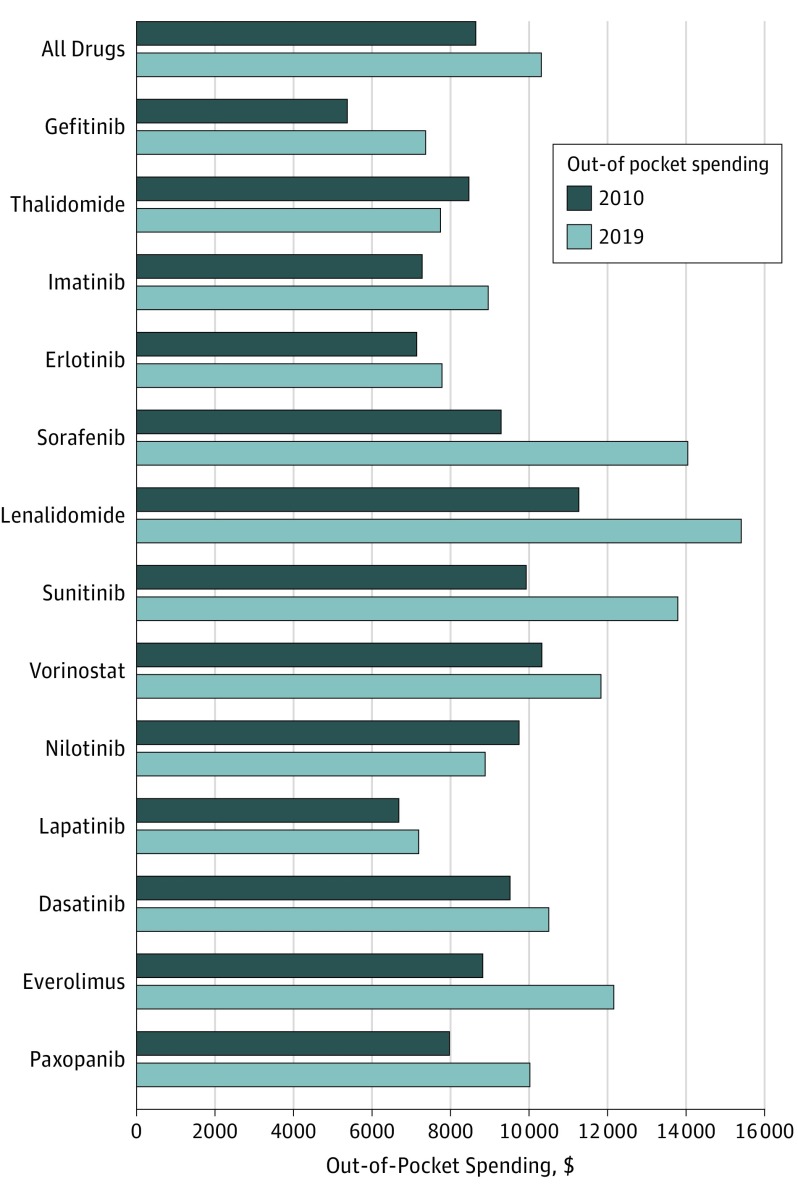
Despite efforts to close the coverage gap between 2010 and 2019, mean expected out-of-pocket spending in 2019 benefit designs increased for 12 of 13 orally administered anticancer drugs available in both years (mean 12-month out-of-pocket spending in 2010 was $8794 and in 2019 is expected to be $10 470; mean increase, $1676) (Figure). Estimated annual out-of-pocket spending in 2019 is expected to be lowest for lapatinib ($7220) and highest for lenalidomide ($15 472).
Figure. Expected Annual Patient Out-of-Pocket Spending for Anticancer Medications in 2010 and 2019 Benefit Designs.
The graph shows expected out-of-pocket spending for Medicare beneficiaries, assuming 12 months of therapy and the mean point-of-sale price for a 30-day or typical supply from the fourth quarter of 2010 and 2018, respectively. We also assumed that beneficiaries had no other medication use and used the standard benefit design (approximately 98% of plans required coinsurance for all drugs and years studied). Median prices resulted in similar spending and are not shown.
Discussion
The number of orally administered anticancer medications covered under Part D has increased since 2010, with mean monthly point-of-sale prices for anticancer drugs reaching nearly $14 000 in 2018. Anticancer drug prices have increased beyond inflation between 2010 and 2018, resulting in higher out-of-pocket spending for patients despite the Part D coverage gap closing.
Limitations of this study include use of point-of-sale prices, which do not reflect rebates or discounts. However, out-of-pocket spending for patients facing coinsurance is based on the point-of-sale price. Moreover, rebates are likely to be limited for anticancer drugs on Part D because they lack head-to-head competitors in most instances. Because anticancer drugs are part of a protected class in Part D, these findings may not generalize to other drugs.
Savings expected through closing the Part D coverage gap or through other policy changes, such as point-of-sale rebates, will be unlikely to offer financial protections to patients needing anticancer drugs. Moreover, because beneficiaries pay a percentage of the drug’s price and have no out-of-pocket spending limits on Part D, even large price decreases may not provide sufficient financial relief to patients requiring long-term anticancer drug use. Efforts to reduce drug prices and limit beneficiary out-of-pocket spending are needed to improve access to high-cost drugs.
Section Editor: Jody W. Zylke, MD, Deputy Editor.
References
- 1.Medicare Payment Advisory Commission Report to the Congress: Medicare payment policy. http://www.medpac.gov/docs/default-source/reports/mar18_medpac_entirereport_sec.pdf. Published March 2018. Accessed April 11, 2019.
- 2.Mailankody S, Prasad V. Five years of cancer drug approvals: innovation, efficacy, and costs. JAMA Oncol. 2015;1(4):539-540. doi: 10.1001/jamaoncol.2015.0373 [DOI] [PubMed] [Google Scholar]
- 3.Workman P, Draetta GF, Schellens JHM, Bernards R. How much longer will we put up with $100,000 cancer drugs? Cell. 2017;168(4):579-583. doi: 10.1016/j.cell.2017.01.034 [DOI] [PubMed] [Google Scholar]
- 4.Howard DH, Bach PB, Berndt ER, Conti RM. Pricing in the market for anticancer drugs. J Econ Perspect. 2015;29(1):139-162. doi: 10.1257/jep.29.1.139 [DOI] [PubMed] [Google Scholar]
- 5.Light DW, Kantarjian H. Market spiral pricing of cancer drugs. Cancer. 2013;119(22):3900-3902. doi: 10.1002/cncr.28321 [DOI] [PubMed] [Google Scholar]