Key Points
Question
Is black race associated with worse prostate cancer outcomes after controlling for known prognostic variables and access to care?
Findings
In this multiple-cohort study of 306 100 patients with prostate cancer, black race was not associated with inferior prostate cancer–specific mortality in cohorts from Veterans Affairs health system and National Cancer Institute–sponsored randomized clinical trials. Black race was associated with increased prostate cancer–specific mortality within the Surveillance, Epidemiology, and End Results US population registry, but not in the high-risk subgroup after baseline covariable adjustment, although black race remained associated with increased other-cause mortality.
Meaning
With similar access to care and standardized treatment, black men with nonmetastatic prostate cancer appeared to have comparable stage-for-stage prostate cancer–specific mortality to white men.
This multiple-cohort study quantifies the association of black race with long-term survival outcomes after controlling for known prognostic variables and access to care among men with prostate cancer.
Abstract
Importance
Black men are more likely to die of prostate cancer than white men. In men with similar stages of disease, the contribution of biological vs nonbiological differences to this observed disparity is unclear.
Objective
To quantify the association of black race with long-term survival outcomes after controlling for known prognostic variables and access to care among men with prostate cancer.
Design, Setting, and Participants
This multiple-cohort study included updated individual patient-level data of men with clinical T1-4N0-1M0 prostate cancer from the following 3 cohorts: Surveillance, Epidemiology, and End Results (SEER [n = 296 273]); 5 equal-access regional medical centers within the Veterans Affairs health system (VA [n = 3972]); and 4 pooled National Cancer Institute–sponsored Radiation Therapy Oncology Group phase 3 randomized clinical trials (RCTs [n = 5854]). Data were collected in the 3 cohorts from January 1, 1992, through December 31, 2013, and analyzed from April 27, 2017, through April 13, 2019.
Exposures
In the VA and RCT cohorts, all patients received surgery and radiotherapy, respectively, with curative intent. In SEER, radical treatment, hormone therapy, or conservative management were received.
Main Outcomes and Measures
Prostate cancer–specific mortality (PCSM). Secondary measures included other-cause mortality (OCM). To adjust for demographic-, cancer-, and treatment-related baseline differences, inverse probability weighting (IPW) was performed.
Results
Among the 306 100 participants included in the analysis (mean [SD] age, 64.9 [8.9] years), black men constituted 52 840 patients (17.8%) in the SEER cohort, 1513 (38.1%) in the VA cohort, and 1129 (19.3%) in the RCT cohort. Black race was associated with an increased age-adjusted PCSM hazard (subdistribution hazard ratio [sHR], 1.30; 95% CI, 1.23-1.37; P < .001) within the SEER cohort. After IPW adjustment, black race was associated with a 0.5% (95% CI, 0.2%-0.9%) increase in PCSM at 10 years after diagnosis (sHR, 1.09; 95% CI, 1.04-1.15; P < .001), with no significant difference for high-risk men (sHR, 1.04; 95% CI, 0.97-1.12; P = .29). No significant differences in PCSM were found in the VA IPW cohort (sHR, 0.85; 95% CI, 0.56-1.30; P = .46), and black men had a significantly lower hazard in the RCT IPW cohort (sHR, 0.81; 95% CI, 0.66-0.99; P = .04). Black men had a significantly increased hazard of OCM in the SEER (sHR, 1.30; 95% CI, 1.27-1.34; P < .001) and RCT (sHR, 1.17; 95% CI, 1.06-1.29; P = .002) IPW cohorts.
Conclusions and Relevance
In this study, after adjustment for nonbiological differences, notably access to care and standardized treatment, black race did not appear to be associated with inferior stage-for-stage PCSM. A large disparity remained in OCM for black men with nonmetastatic prostate cancer.
Introduction
Population-based estimates demonstrate that black men are more likely to be diagnosed with prostate cancer, more likely to present with distant metastases, and nearly 2.5 times more likely die of the disease when compared with non-Hispanic white men.1 Black men are also more likely to have comorbid illness such as cardiovascular disease2 and diabetes,3 diagnoses that are negatively associated with survival outcomes with or without prostate cancer. These disparities are a major health concern and an area of ongoing research and national funding.4 Each year, the Surveillance, Epidemiology, and End Results (SEER) database reports age-adjusted prostate cancer–specific mortality (PCSM) rates. However, fully adjusting for measurable and unmeasurable confounders within these registries is difficult.5,6,7 Black men with prostate cancer have lower socioeconomic status,8 are diagnosed at a later stage,9 and receive less guideline-concordant care.10,11
Therefore, whether black race intrinsically confers a stage-for-stage increased biological risk of PCSM or whether the observed disparity is driven by race as a complex societal construct is uncertain.12 Given the limitations of US population-based data to fully address this question, complementary and diverse systems that provide improved access to cancer care and curative therapy would help to quantify any residual disparity that remains in PCSM after controlling for known prognostic variables.
We therefore sought to assess PCSM, other-cause mortality (OCM), and all-cause mortality (ACM) differences between black and white men using 3 cohorts of patients diagnosed with nondistant metastatic prostate cancer. We analyzed an updated contemporary US population-based cohort using the SEER program to quantify current population-based estimates of disparities. We compared these estimates with those in 2 additional cohorts: a multicenter cohort from 5 equal-access regional hospitals within the Veterans Affairs (VA) health system and an individual patient-level cohort of men treated in 4 National Cancer Institute–sponsored Radiation Therapy Oncology Group (RTOG) randomized clinical trials (RCTs) with mature follow-up. We hypothesized that population registry–estimated disparities, even after statistical adjustment, may persist, and that estimated disparities would be lower in the 2 cohorts with standardized access to care or a standardized approach to treatment and follow-up in cooperative group trials.
Methods
SEER Cohort
We used the population-based SEER program to identify men diagnosed with American Joint Commission on Cancer Staging clinical cT1-4N0-1M0 prostate cancer from January 1, 2004, through December 31, 2013. The inclusion period began in 2004, which was the first year in which complete T-stage, Gleason score, and prostate-specific antigen (PSA) information was available; notably, all PSA values underwent quality assurance by SEER for the entire study period.13 Furthermore, these data were linked to the first-ever release of a validated socioeconomic status variable by SEER.14 Men with metastatic disease and a PSA level greater than 98 ng/mL (to convert to micrograms per liter, multiply by 1.0) (details are unavailable above this level) were excluded from our analysis. Institutional review board approval was obtained from the Dana-Farber Cancer Institute, and waiver of informed consent was granted, as all data were deidentified.
Shared Equal-Access Regional Cancer Hospital (VA) Cohort
The VA cohort is an institutional review board–approved database including men treated with radical prostatectomy at 5 equal-access medical centers within the VA Medical Centers system (West Los Angeles, Palo Alto, and San Diego, California; Augusta, Georgia; and Durham and Asheville, North Carolina). Institutional review board approval was received from each institution to abstract and combine data, and waiver of informed consent was granted, as all data were deidentified. All patients in the surgical cohort were diagnosed with cT1-4N0-1M0 prostate cancer from January 1, 1992, through December 31, 2013. Patients treated with preoperative androgen deprivation therapy or radiotherapy and those with PSA levels of greater than 150 ng/mL were excluded from our analysis.
RCT Cohort
Most clinical trials do not accrue a significant proportion of black men.15 The RTOG-led cooperative group trials are a notable exception. All patients from the 4 trials included men with cT1-4N0-1M0 prostate cancer treated in phase 3 RCTs within the RTOG that completed enrollment from January 1, 1992, through December 31, 2004, to allow for long-term follow-up. These inclusion criteria resulted in 4 trials with finalized publication: RTOG 92-02,16 94-08,17 94-13,18 and 99-10.19 These trials accrued patients at more than 150 centers primarily in the United States and Canada. Individual patient–level data were acquired via data sharing agreement, and each trial’s principal investigator was invited to participate in the present analysis. Inclusion criteria varied per individual trial; however, all trials excluded patients with metastatic disease and PSA levels greater than 150 ng/mL (eTable 1 in the Supplement). Each trial received institutional review board approval from the participating centers, and all patients provided written informed consent.
Outcomes
The primary outcome was the cumulative incidence of PCSM. Within the SEER cohort, PCSM was defined by algorithmic analysis of death certificates. Within the VA cohort, PCSM was defined as metastatic or castration-resistant prostate cancer at time of death without an alternative cause in the medical record. For the RCT cohort, PCSM was defined per each individual RCT, most commonly as death attributed to prostate cancer or an unknown cause of death with metastatic prostate cancer, which was reviewed by the trial investigators based on death certificates. Secondary outcomes were the cumulative incidence of OCM (non-PCSM) and ACM.
Statistical Analysis
Data were analyzed from April 27, 2017, through April 13, 2019. For each cohort (SEER, VA, and RCT), race was classified as black or white as reported by the data source. Other races and ethnicities were excluded to minimize heterogeneity. Inverse probability weighting (IPW) via a propensity model was used to adjust for imbalances by race.20 In the propensity model, for all cohorts, ages at diagnosis and pretreatment PSA levels were included as continuous variables, and biopsy Gleason score and clinical T and N stages as categorical variables. Within the RCT cohort, Eastern Cooperative Oncology Group performance status and study treatment were included as categorical variables. Within the SEER cohort, the type of therapy received (radical prostatectomy, radiotherapy [external beam radiotherapy and/or brachytherapy], or no definitive treatment [active surveillance, watchful waiting, and primary androgen deprivation therapy]) was included as a categorical variable. Yost socioeconomic status was included as a continuous variable (the first validated composite index of 7 indicators of income, occupation, housing, and education in SEER).21 Insurance status, documented in SEER beginning in 2007, was included as a categorical variable (insured, Medicaid, uninsured, or unknown). Missing data by race are summarized in eTable 2 in the Supplement, and imputations were not performed.
Hazard ratios (HRs) were calculated for black men compared with white men (reference category). Our primary analytic method of comparison was with Fine-Gray subdistribution HR (sHR) models.22 To ensure our results were not solely due to differences in the hazard of competing risks between races, we also performed cause-specific proportional HR analysis. Competing risk analyses are included in the main text, and cause-specific analyses are in the eTable 6 in the Supplement.
In sensitivity analysis to understand the association of baseline characteristic differences with outcomes, stepwise IPW models were developed as follows: (1) age-adjusted IPW, which only weights imbalances in age; (2) age- and stage-adjusted IPW, which weights imbalances in age and cancer-specific prognostic variables; and (3) fully adjusted IPW, which includes the aforementioned variables plus socioeconomic variables (for the SEER cohort), performance status (for the RCT cohort), and treatment variables (for the SEER and RCT cohorts). Exploratory subgroup analyses were analyzed by age and National Comprehensive Cancer Network (NCCN) risk group.23 Two-sided P < .05 was considered statistically significant. Analyses were performed with R, version 3.4.2 (R Foundation for Statistical Computing) and SAS, version 9.4 (SAS Institute, Inc). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for observational studies.
Results
Baseline Characteristics
Among the 306 100 participants (mean [SD] age, 64.9 [8.9] years; median age, 65 years [interquartile range (IQR), 59-71 years]), median follow-up was 75 months (IQR, 49-104 months) for the SEER cohort, 97 months (IQR, 59-144 months) for the VA cohort, and 104 months (IQR, 68-132 months) for the RCT cohort (Table). Black men were similarly represented in the SEER and RCT cohorts with 52 840 of 296 273 patients (17.8%) and 1129 of 5854 patients (19.3%), respectively. By comparison, black men had increased representation in the VA cohort at 1513 of 3972 patients (38.1%). The median age of black men was 2 to 3 years younger than that of white men in each cohort (SEER cohort, 62 [IQR, 56-69] vs 65 [IQR, 59-71] years; VA cohort, 61 [IQR, 56-65] vs 64 [IQR, 60-68] years; RCT cohort, 69 [IQR, 64-73] vs 71 [IQR, 66-74] years [P < .001]). The SEER and VA cohorts contained similar percentages of NCCN-defined high-risk disease (65 414 [22.1%] and 746 [18.8%], respectively), whereas the RCT cohort was comparatively enriched (2570 [43.9%]). Within all 3 cohorts, black men had greater percentages of NCCN-defined high-risk disease (1.8% for the SEER cohort; 0.5% for the VA cohort; and 5.2% for the RCT cohort), although absolute differences were small. Black men in the SEER cohort had a lower socioeconomic status (lowest quintile, 29.5% vs 17.7%) and lower rates of non-Medicaid insurance (61.9% vs 64.6%) and curative local treatment (76.3% vs 79.1%) (P < .001) (eTable 3 in the Supplement).
Table. Summary of Unadjusted Cohort Characteristics by Race.
| Variable | Cohorta | |||||
|---|---|---|---|---|---|---|
| SEER (n = 296 273) | VA (n = 3972) | RCT (n = 5854) | ||||
| White | Black | White | Black | White | Black | |
| All | 243 433 (82.1) | 52 840 (17.8) | 2459 (61.9) | 1513 (38.1) | 4725 (80.7) | 1129 (19.3) |
| Follow-up, median (IQR), mob | 76 (49-105) | 70 (45-100) | 98 (61-145) | 94 (55-142) | 105 (71-132) | 101 (60-131) |
| Age, median (IQR), y | 65 (59-71) | 62 (56-69) | 64 (60-68) | 61 (56-65) | 71 (66-74) | 69 (64-73) |
| Gleason biopsy scorec | ||||||
| ≤6 | 115 709 (47.5) | 23 304 (44.1) | 1214 (49.4) | 724 (47.9) | 1926 (40.8) | 494 (43.8) |
| 7 | 95 810 (39.4) | 22 515 (42.6) | 896 (36.4) | 600 (39.7) | 1978 (41.9) | 424 (37.6) |
| 8 | 18 440 (7.6) | 4498 (8.5) | 248 (10.1) | 142 (9.4) | 512 (10.8) | 140 (12.4) |
| 9-10 | 13 474 (5.5) | 2523 (4.8) | 101 (4.1) | 47 (3.1) | 309 (6.5) | 71 (6.3) |
| T stage | ||||||
| T1-T2a | 109 467 (45.0) | 28 634 (54.2) | 2040 (83.0) | 1339 (88.5) | 2306 (48.8) | 619 (54.8) |
| T2b-T2c | 108 214 (44.5) | 19 878 (37.6) | 398 (16.2) | 171 (11.3) | 1393 (29.5) | 307 (27.2) |
| T3-T4 | 25 752 (10.6) | 4328 (8.2) | 21 (0.9) | 3 (0.2) | 1026 (21.7) | 203 (18.0) |
| N stage | ||||||
| N0 | 237 646 (97.6) | 51 727 (97.9) | 2459 (100) | 1513 (100) | 2802 (59.3) | 657 (58.2) |
| N1-N2 | 5787 (2.4) | 1113 (2.1) | 0 | 0 | 59 (1.2) | 12 (1.1) |
| NX | 0 | 0 | 0 | 0 | 1864 (39.4) | 460 (40.7) |
| Pretreatment PSA level, ng/mL | ||||||
| Median (IQR) | 6.0 (4.5-8.9) | 6.6 (4.8-10.8) | 6.2 (4.6-9.2) | 7.0 (4.9-10.7) | 11.5 (7.2-17.7) | 13.7 (7.9-22.2) |
| <10 | 194 149 (79.8) | 38 086 (72.1) | 1944 (79.1) | 1071 (70.8) | 1991 (42.1) | 404 (35.8) |
| ≥10 to 19 | 34 055 (14.0) | 9278 (17.6) | 392 (15.9) | 319 (21.1) | 1599 (33.8) | 347 (30.7) |
| ≥20 | 15 229 (6.3) | 5476 (10.4) | 123 (5.0) | 123 (8.1) | 1135 (24.0) | 378 (33.5) |
| Risk groupd | ||||||
| Low | 54 305 (22.3) | 11 574 (21.9) | 868 (35.3) | 474 (31.3) | 524 (11.1) | 130 (11.5) |
| Intermediate | 130 387 (53.6) | 27 693 (52.4) | 1134 (46.1) | 750 (49.6) | 2115 (44.8) | 444 (39.3) |
| High | 52 954 (21.8) | 12 460 (23.6) | 457 (18.6) | 289 (19.1) | 2027 (42.9) | 543 (48.1) |
| Node positive | 5787 (2.4) | 1113 (2.1) | 0 | 0 | 59 (1.2) | 12 (1.1) |
Abbreviations: IQR, interquartile range; PSA, prostate specific antigen; RCT, randomized control trial; SEER, Surveillance, Epidemiology, and End Results; VA, Veterans Affairs.
SI conversion factor: To convert PSA to micrograms per liter, multiply by 0.001.
Cohorts are derived from the SEER database, shared equal-access VA regional hospital cohort, and Radiation Therapy Oncology Group RCTs. Unless otherwise indicated, data are expressed as number (percentage) of patients. Percentages have been rounded and may not total 100.
Follow-up for the SEER cohort began at time of diagnosis, whereas follow-up for the VA and RCT cohorts began at time of radical treatment.
Higher scores indicate higher grade.
Defined per the National Comprehensive Cancer Network.
After fully adjusted IPW, baseline characteristics were balanced in the 3 cohorts between black and white men. Owing to the SEER cohort’s size, small differences remained statistically significant in the baseline variables such as PSA level (median, 6.4 [IQR, 4.8-9.7] vs 6.0 [IQR, 4.5-9.0] ng/mL) and socioeconomic status (quintile 1, 19.4% vs 20.3%) (eTable 4 and eFigure 1 in the Supplement).
Prostate Cancer–Specific Mortality
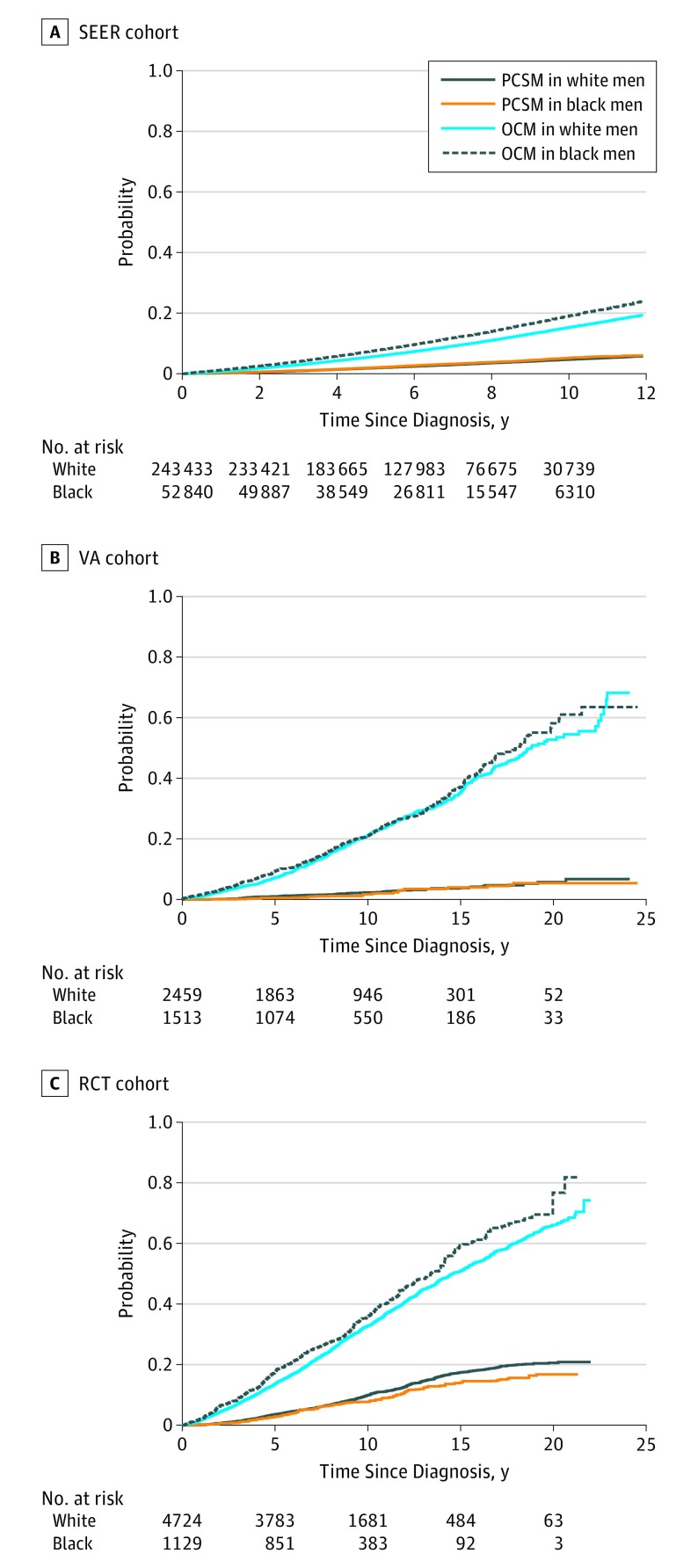
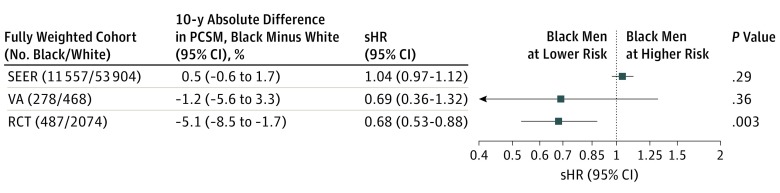
The cumulative incidence of PCSM for the fully adjusted IPW SEER, VA, and RCT cohorts are summarized in Figure 1. Stepwise introduction of IPW variables are seen in Figure 2. Within the SEER cohort, black men had a 30% relative increased subdistribution PCSM hazard in the age-adjusted IPW model (sHR, 1.30; 95% CI, 1.23-1.37; P < .001). In the age- and stage-adjusted IPW model, the sHR decreased to 1.17 (95% CI, 1.11-1.24; P < .001); and in the fully adjusted IPW model, the sHR decreased further to 1.09 (95% CI, 1.04-1.15; P < .001). Black men within the fully adjusted IPW SEER cohort had an absolute increased PCSM rate of only 0.5% compared with white men at 10 years after diagnosis (95% CI, 0.2%-0.9%). In the subgroup analysis, the increased PCSM hazard in black men was present in those with low- and intermediate-risk disease, but not in those with high-risk disease (sHR, 1.04; 95% CI, 0.97-1.12; P = .29) (Figure 3).
Figure 1. Cumulative Incidence of Prostate Cancer–Specific Mortality (PCSM) and Other-Cause Mortality (OCM) After Inverse Probability Weighting.
Cohorts are derived from the Surveillance, Epidemiology, and End Results database (SEER), a shared equal-access Veterans Affairs regional hospital cohort (VA), and Radiation Therapy Oncology Group randomized clinical trials (RCT).
Figure 2. Forest Plot of Fine-Gray Competing-Risk Subdistribution Hazard Ratios (sHRs) of Prostate Cancer–Specific Mortality (PCSM).
Cohorts are derived from the Surveillance, Epidemiology, and End Results database (SEER), a shared equal-access Veterans Affairs regional hospital cohort (VA), and Radiation Therapy Oncology Group randomized clinical trials (RCT).
Figure 3. Forest Plot of Fine-Gray Competing-Risk Subdistribution Hazard Ratios (sHRs) of Prostate Cancer–Specific Mortality (PCSM) by National Comprehensive Cancer Network High-Risk Subgroup.
Cohorts are derived from the Surveillance, Epidemiology, and End Results database (SEER), a shared equal-access Veterans Affairs regional hospital cohort (VA), and Radiation Therapy Oncology Group randomized clinical trials (RCT).
In contrast to the SEER cohort, the VA and RCT cohorts demonstrated no significant difference in PCSM sHR by race in the age-adjusted IPW models. Furthermore, no statistically significant difference in PCSM by race was found in the fully adjusted IPW VA cohort (sHR, 0.85; 95% CI, 0.56-1.30; P = .46). Within the fully adjusted IPW RCT cohort, black men had a lower PCSM sHR (0.81; 95% CI, 0.66-0.99; P = .04) and had an absolute 2.2% (95% CI, 0.1%-4.3%) lower rate of PCSM at 10 years compared with white men. In the fully adjusted IPW RCT high-risk subgroup, black men also had a lower PCSM sHR (0.68; 95% CI, 0.53-0.68; P = .003).
Other-Cause and All-Cause Mortality
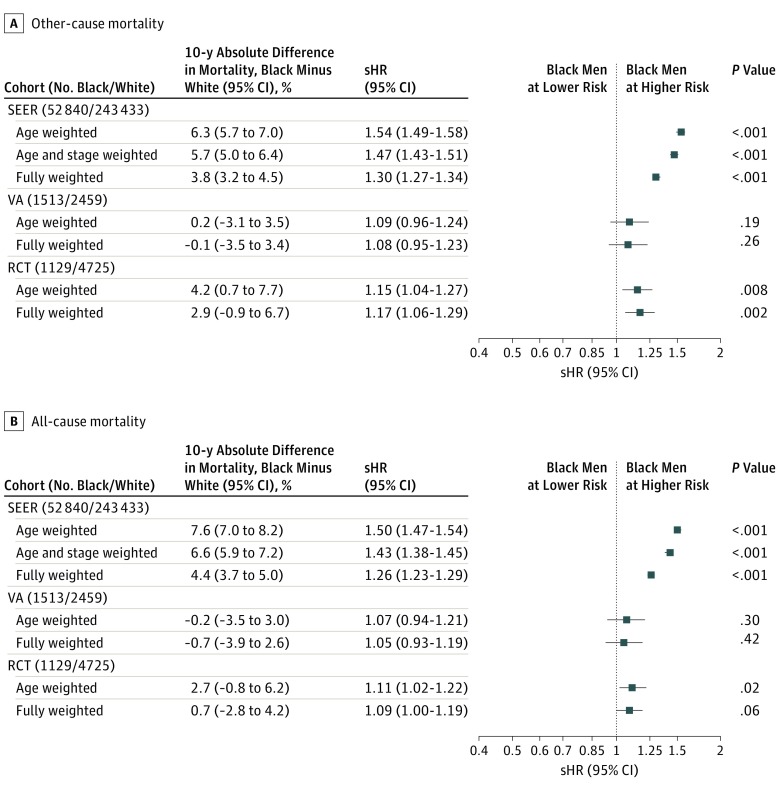
Similar step-wise IPW models for OCM and ACM are seen in the forest plots in Figure 4. As with PCSM, the sHR of OCM in SEER decreased as more variables were included in the propensity weight. Black men had an increased sHR of OCM in the fully adjusted IPW SEER (1.30; 95% CI, 1.27-1.34; P < .001) and RCT (1.17; 95% CI, 1.06-1.29; P = .002) cohorts, but the sHR was not significantly increased in the fully adjusted IPW VA cohort (1.08; 95% CI, 0.95-1.23; P = .26). At 10 years, black men had an absolute increased OCM rate of 3.8% (95% CI, 3.2%-4.5%) and 2.9% (95% CI, −0.9% to 6.7%) in the SEER and RCT cohorts, respectively.
Figure 4. Forest Plot of Fine-Gray Competing-Risk Subdistribution Hazard Ratios (sHRs) of Other-Cause Mortality and HRs of All-Cause Mortality.
Cohorts are derived from the Surveillance, Epidemiology, and End Results database (SEER), a shared equal-access Veterans Affairs regional hospital cohort (VA), and Radiation Therapy Oncology Group randomized clinical trials (RCT).
Black men had an increased hazard of ACM within the SEER cohort, even after full IPW adjustment (HR, 1.26; 95% CI, 1.23-1.29; P < .001). The absolute ACM rate was 4.4% (95% CI, 3.7%-5.0%) higher for black men at 10 years, driven by their increased OCM. Black men also had an increased hazard of ACM in the IPW RCT cohort (HR, 1.09; 95% CI, 1.00-1.19; P = .06). As shown in eTable 6 in the Supplement, the HR estimates for PCSM and OCM were very similar when analyzed with and without competing risk adjustments for all cohorts and subset analyses by risk group.
Discussion
To our knowledge, this study represents the most comprehensive analysis to date of the association of black race with outcomes in nonmetastatic prostate cancer among men diagnosed with similar stage disease. Furthermore, to our knowledge and to date, this analysis is the largest individual patient-level analysis of black men with prostate cancer treated in RCTs with long-term follow-up consisting of more than 1100 black men. More than 1500 surgically treated black men in an equal-access VA cohort and more than 50 000 black men within the SEER cohort were also included. The diverse nature of these cohorts, including a range of risk groups treated and varying treatments (eg, surgery or radiotherapy), strengthens our study and broadens its generalizability.
Within SEER, after fully adjusted IPW, we found that black race was associated with a 0.5% absolute increased rate of PCSM at 10 years (sHR, 1.09), with no statistically significant difference evident in the high-risk subgroup. Black race was also not associated with inferior PCSM outcomes in men treated within systems with equal access to care (VA cohort) or within the standardized treatment approach and follow-up of a cooperative group trial (RCT cohort), with or without propensity adjustment. In fact, the sHR estimates of the VA and RCT cohorts favored better outcomes for black men, with this association reaching statistical significance within the RCT cohort (sHR, 0.81; P = .04). These findings have several important implications.
First, we demonstrate that significant disparities remain at the population level for black men with prostate cancer. The age-adjusted sHR (1.30) and age- and stage-adjusted sHR (1.17) for PCSM within SEER were both substantially higher than the fully adjusted sHR (1.09). In the unadjusted cohort, black men had higher rates of socioeconomic barriers to access of timely, high-quality medical care including lower composite scores of income, housing, occupation, and educational attainment, along with lower rates of insurance. Black race remains associated with many factors that negatively affect outcomes, and disparities persist at the population level. Continued efforts are needed to address this clear racial health inequity driven by modifiable nonbiological risk factors.
Second, our approach highlights the challenges of interpreting population-based data.24 We adjusted for age, insurance, and a newly released validated socioeconomic status variable. Moreover, we adjusted for cancer- and treatment-related confounders, including the newly released quality-assured PSA values, which were a significant limitation in prior SEER analyses.25 Inclusion of these crucial prognostic factors substantially decreased the estimated age-only PCSM hazard for black men, but we still came to a slightly different conclusion with respect to the unexplained significant association of black race when using SEER population data compared with the VA and RCT cohorts. Residual group imbalances,26 unmeasured confounders,5 cause of death attribution bias,27 and issues with coding treatment-related variables28 may all contribute to the difficulty in interpreting outcomes from population registries.6 We included several cohorts for comparison with explicit stepwise adjustment, and the overall findings provide evidence against an increased biological risk associated with black race in men with new nonmetastatic prostate cancer diagnosed at a similar stage. However, even if we assume our IPW SEER analysis provides a more accurate reflection of the unexplained residual association of black race with lower PCSM, the absolute difference is less than 1% at 10 years after diagnosis at the population level.
Analysis of a small study29 that included 4 earlier RTOG trials conducted from 1975 through 1992 similarly concluded that race is not independently associated with outcome. This earlier data set was limited to 133 black men, and treatment predated the PSA era for 80% of patients.29 Our cohort was completely independent and included nearly 10-fold the number of black men in the RCT cohort alone, all treated in the contemporary PSA era. Our results are also in line with data suggesting similar outcomes between black and white men with advanced prostate cancer treated in clinical trials,30 with recent suggestions that black men may actually have comparatively better outcomes.31 Taken together, these data should encourage efforts to improve access to high-quality, standardized, evidence-based treatment and further work to understand why black men may in fact have improved cancer-specific outcomes.
Third, within the SEER cohort, black race was associated with increased hazard of OCM, which was substantially higher than the increased PCSM hazard by nearly 3-fold. An increased OCM hazard was also evident in the RCT cohort. Bach et al32 similarly noted that survival differences in advanced prostate cancer were associated with an increased risk of OCM. Although the competing risk of death has long been highlighted in men with low-risk cancer treated conservatively,33,34 our study suggests we must focus not only on the prostate cancer diagnosis but also on improving general health status, even in those with high-risk disease. Cardiovascular and cerebrovascular disease, along with diabetes, accounted for one-third of deaths in patients with cancer in 2012,1 with death rates in black patients exceeding those in white patients. Moreover, treatments for prostate cancer such as androgen deprivation therapy can worsen comorbid conditions, further exacerbating disparities.35 Without addressing these comorbidities, differences will persist in black men with prostate cancer, even if PCSM outcomes are equivalent.
We have presented estimates using cumulative incidence curves and sHRs from Fine-Gray regression models. These methods allow us to summarize how PCSM differs between black and white men in the presence of the competing risk of OCM. Differences in the cumulative incidence of PCSM between race groups could be due to differences in the competing risk of OCM between race groups.36 However, as shown in eTable 6 in the Supplement, the cause-specific sHRs (which are not affected by the differences in OCM) are very similar to the sHRs with competing risks, thus negating this point.
Limitations
Owing to data availability and eligibility criteria, PSA levels were slightly different in the SEER cohort (PSA level, <98 ng/mL) compared with the RCT and VA cohorts (PSA levels <150 ng/mL). This difference did not significantly affect the cohort composition because patients in the VA and RCT cohorts with PSA levels greater than 98 ng/mL numbered less than 1%. We did not have detailed comorbidity information for the VA and RCT cohorts, which could affect sHR estimates if known. Even within an RCT and despite IPW techniques, residual confounding may exist. However, a data set with less confounding than that from large multicenter trials is unlikely to exist. We would ideally have access to randomized data from multiple surgery cohorts as we did with the radiotherapy-treated patients. These data, unfortunately, are not available. Black men represented fewer than 1% of participants in the Prostate Testing for Cancer and Treatment trial37 and 13% in the Prostate Cancer Intervention vs Observation trial.38 Percentages were not reported in the Scandinavian Prostate Cancer Group Study Number 4 trial.39 Our data do not address the biological propensity to develop an incident prostate cancer, nor the likelihood of having higher-risk disease or metastatic prostate cancer at diagnosis, both of which may contribute to the higher rate ratio of prostate cancer death in black men. Prostate cancer has a long natural history, and continued follow-up is important to confirm our findings. Finally, even though outcomes were similar, our study does not address whether biology is the same or whether sensitivities to specific treatments are similar for black and white men.40
Conclusions
In our study, black race was not associated with worse PCSM outcomes in men with newly diagnosed nonmetastatic prostate cancer treated within health care systems with standardized access or within a standardized treatment approach and follow-up. In contrast, within the SEER cohort, black race was associated with multiple socioeconomic barriers to quality care. After adjustment for these factors, the differences by race in PCSM for men with high-risk disease were no longer substantially different. These results suggest that efforts are needed to address the modifiable social factors contributing to racial disparity in prostate cancer. Black race was consistently associated with increased OCM, and we suggest that future research efforts are needed to identify and address these competing causes of death.
eTable 1. Randomized Clinical Trial Details
eTable 2. Exclusions and Missing Variables in the SEER, VA, and RCT Data Sets
eTable 3. Description of Baseline Characteristics by Race (All Variables)
eTable 4. Description of Baseline Characteristics by Race After Inverse Probability Weighting (All Variables)
eTable 5. Inverse Probability Weighted 5- and 10-y estimates of Prostate Cancer–Specific Mortality, Other-Cause Mortality, and All-Cause Mortality
eTable 6. Summary of Prostate Cancer–Specific Mortality, Other-Cause Mortality, and All-Cause Mortality Models
eFigure 1. Absolute Standard Percentage Differences in the Variables by Race Within the Weighted and Unweighted Data Sets for SEER (A), VA (B), and RCT (C)
eFigure 2. Subgroup Forest Plots of Hazard of Prostate Cancer Specific Mortality for SEER Cohort (A), SEER Cohort With Fine-Gray Competing Risks (B), VA Cohort (C), VA Cohort With Fine-Gray Competing Risks (D), RCT Cohort (E), and RCT Cohort With Fine-Gray Competing Risks (F)
eFigure 3. Cumulative Incidence of Prostate Cancer–Specific Mortality and Other-Cause Mortality for the Unadjusted and Unweighted SEER (A), VA (B), and RCT (C) Cohorts
eFigure 4. Cumulative Incidence of All-Cause Mortality for the Inverse Probability Weighted SEER (A), VA (B), and RCT (C) Cohorts
eFigure 5. Cumulative Incidence of All-Cause Mortality for the Unadjusted SEER (A), VA (B), and RCT (C) Cohorts
References
- 1.DeSantis CE, Siegel RL, Sauer AG, et al. . Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016;66(4):290-308. doi: 10.3322/caac.21340 [DOI] [PubMed] [Google Scholar]
- 2.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233-1241. doi: 10.1161/01.CIR.0000158136.76824.04 [DOI] [PubMed] [Google Scholar]
- 3.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283(17):2253-2259. doi: 10.1001/jama.283.17.2253 [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institute NCI Center to Reduce Cancer Health Disparities (CRCHD). https://www.cancer.gov/about-nci/organization/crchd. Published April 5, 2018. Accessed July 6, 2018.
- 5.Giordano SH, Kuo YF, Duan Z, Hortobagyi GN, Freeman J, Goodwin JS. Limits of observational data in determining outcomes from cancer therapy. Cancer. 2008;112(11):2456-2466. doi: 10.1002/cncr.23452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGale P, Cutter D, Darby SC, Henson KE, Jagsi R, Taylor CW. Can observational data replace randomized trials. J Clin Oncol. 2016;34(27):3355-3357. doi: 10.1200/JCO.2016.68.8879 [DOI] [PubMed] [Google Scholar]
- 7.Park HS, Lloyd S, Decker RH, Wilson LD, Yu JB. Limitations and biases of the Surveillance, Epidemiology, and End Results database. Curr Probl Cancer. 2012;36(4):216-224. doi: 10.1016/j.currproblcancer.2012.03.011 [DOI] [PubMed] [Google Scholar]
- 8.Ward E, Jemal A, Cokkinides V, et al. . Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. doi: 10.3322/canjclin.54.2.78 [DOI] [PubMed] [Google Scholar]
- 9.Hoffman RM, Gilliland FD, Eley JW, et al. . Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2001;93(5):388-395. doi: 10.1093/jnci/93.5.388 [DOI] [PubMed] [Google Scholar]
- 10.Mahal BA, Aizer AA, Ziehr DR, et al. . Trends in disparate treatment of African American men with localized prostate cancer across National Comprehensive Cancer Network risk groups. Urology. 2014;84(2):386-392. doi: 10.1016/j.urology.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 11.Barocas DA, Penson DF. Racial variation in the pattern and quality of care for prostate cancer in the USA: mind the gap. BJU Int. 2010;106(3):322-328. doi: 10.1111/j.1464-410X.2010.09467.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontanarosa PB, Bauchner H. Race, ancestry, and medical research. JAMA. 2018;320(15):1539-1540. doi: 10.1001/jama.2018.14438 [DOI] [PubMed] [Google Scholar]
- 13.National Cancer Institute PSA values and SEER data. https://seer.cancer.gov/data/psa-values.html. Updated April 14, 2017. Accessed July 6, 2018.
- 14.National Cancer Institute Census tract-level SES database. https://seer.cancer.gov/seerstat/databases/census-tract/index.html. Accessed July 18, 2018.
- 15.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720-2726. doi: 10.1001/jama.291.22.2720 [DOI] [PubMed] [Google Scholar]
- 16.Goserelin, flutamine, and radiation therapy in treating patients with locally advanced prostate cancer. ClinicalTrials.gov identifier: NCT00767286. https://clinicaltrials.gov/ct2/show/NCT00767286. Updated January 28, 2014. Accessed April 22, 2019.
- 17.Radiation therapy with or without antiandrogen therapy in treating patients with stage I or stage II prostate cancer. ClinicalTrials.gov identifier: NCT00002597. https://clinicaltrials.gov/ct2/show/NCT00002597. Updated June 14, 2018. Accessed April 22, 2019.
- 18.Radiation therapy and hormone therapy in treating patients with prostate cancer. ClinicalTrials.gov identifier: NCT00769548. https://www.clinicaltrials.gov/ct2/show/NCT00769548. Updated January 4, 2017. Accessed April 22, 2019.
- 19.Hormone therapy and radiation therapy in treating patients with prostate cancer. ClinicalTrials.gov identifier: NCT00005044. https://clinicaltrials.gov/ct2/show/NCT00005044. Updated October 31, 2017. Accessed April 22, 2019.
- 20.Austin PC, Schuster T. The performance of different propensity score methods for estimating absolute effects of treatments on survival outcomes: a simulation study. Stat Methods Med Res. 2016;25(5):2214-2237. doi: 10.1177/0962280213519716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703-711. doi: 10.1023/A:1011240019516 [DOI] [PubMed] [Google Scholar]
- 22.Latouche A, Allignol A, Beyersmann J, Labopin M, Fine JP. A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol. 2013;66(6):648-653. doi: 10.1016/j.jclinepi.2012.09.017 [DOI] [PubMed] [Google Scholar]
- 23.Mohler J, Antonorakis E, Armstrong A NCCN Clinical Practice Guideline in Oncology: prostate cancer, version 2.2017. 2017. https://www.nccn.org/professionals/physician_gls/default.aspx#prostate. Accessed March 25, 2017.
- 24.Soni PD, Hartman HE, Dess RT, et al. . Comparison of population-based observational studies with randomized trials in oncology. J Clin Oncol. Published online March 21, 2019. doi: 10.1200/JCO.18.01074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furlow B. US National Cancer Institute investigates PSA coding errors. Lancet Oncol. 2015;16(6):614. doi: 10.1016/S1470-2045(15)70196-8 [DOI] [PubMed] [Google Scholar]
- 26.D’Agostino RB Jr, D’Agostino RB Sr. Estimating treatment effects using observational data. JAMA. 2007;297(3):314-316. doi: 10.1001/jama.297.3.314 [DOI] [PubMed] [Google Scholar]
- 27.Feuer EJ, Merrill RM, Hankey BF. Cancer surveillance series: interpreting trends in prostate cancer—part II: cause of death misclassification and the recent rise and fall in prostate cancer mortality. J Natl Cancer Inst. 1999;91(12):1025-1032. doi: 10.1093/jnci/91.12.1025 [DOI] [PubMed] [Google Scholar]
- 28.Jagsi R, Abrahamse P, Hawley ST, Graff JJ, Hamilton AS, Katz SJ. Underascertainment of radiotherapy receipt in Surveillance, Epidemiology, and End Results registry data. Cancer. 2012;118(2):333-341. doi: 10.1002/cncr.26295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roach M III, Lu J, Pilepich MV, Asbell SO, Mohiuddin M, Grignon D. Race and survival of men treated for prostate cancer on radiation therapy oncology group phase III randomized trials. J Urol. 2003;169(1):245-250. doi: 10.1016/S0022-5347(05)64078-5 [DOI] [PubMed] [Google Scholar]
- 30.Spratt DE, Chen YW, Mahal BA, et al. . Individual patient data analysis of randomized clinical trials: impact of black race on castration-resistant prostate cancer outcomes. Eur Urol Focus. 2016;2(5):532-539. doi: 10.1016/j.euf.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 31.Halabi S, Dutta S, Tangen CM, et al. . Overall survival of black and white men with metastatic castration-resistant prostate cancer treated with docetaxel. 2019;37(5):403-410. doi: 10.1200/JCO.18.01279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287(16):2106-2113. doi: 10.1001/jama.287.16.2106 [DOI] [PubMed] [Google Scholar]
- 33.Albertsen PC, Hanley JA, Fine J. 20-Year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293(17):2095-2101. doi: 10.1001/jama.293.17.2095 [DOI] [PubMed] [Google Scholar]
- 34.Albertsen PC, Moore DF, Shih W, Lin Y, Li H, Lu-Yao GL. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol. 2011;29(10):1335-1341. doi: 10.1200/JCO.2010.31.2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen PL, Alibhai SM, Basaria S, et al. . Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67(5):825-836. doi: 10.1016/j.eururo.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 36.Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18(8):2301-2308. doi: 10.1158/1078-0432.CCR-11-2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamdy FC, Donovan JL, Lane JA, et al. ; ProtecT Study Group . 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415-1424. doi: 10.1056/NEJMoa1606220 [DOI] [PubMed] [Google Scholar]
- 38.Wilt TJ, Brawer MK, Jones KM, et al. ; Prostate Cancer Intervention versus Observation Trial (PIVOT) Study Group . Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367(3):203-213. doi: 10.1056/NEJMoa1113162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bill-Axelson A, Holmberg L, Garmo H, et al. . Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370(10):932-942. doi: 10.1056/NEJMoa1311593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.George DJ, Heath EI, Sartor AO, et al. . Abi Race: a prospective, multicenter study of black (B) and white (W) patients (pts) with metastatic castrate resistant prostate cancer (mCRPC) treated with abiraterone acetate and prednisone (AAP). J Clin Oncol. 2018;36(18)(suppl). doi: 10.1200/JCO.2018.36.18_suppl.LBA5009 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Randomized Clinical Trial Details
eTable 2. Exclusions and Missing Variables in the SEER, VA, and RCT Data Sets
eTable 3. Description of Baseline Characteristics by Race (All Variables)
eTable 4. Description of Baseline Characteristics by Race After Inverse Probability Weighting (All Variables)
eTable 5. Inverse Probability Weighted 5- and 10-y estimates of Prostate Cancer–Specific Mortality, Other-Cause Mortality, and All-Cause Mortality
eTable 6. Summary of Prostate Cancer–Specific Mortality, Other-Cause Mortality, and All-Cause Mortality Models
eFigure 1. Absolute Standard Percentage Differences in the Variables by Race Within the Weighted and Unweighted Data Sets for SEER (A), VA (B), and RCT (C)
eFigure 2. Subgroup Forest Plots of Hazard of Prostate Cancer Specific Mortality for SEER Cohort (A), SEER Cohort With Fine-Gray Competing Risks (B), VA Cohort (C), VA Cohort With Fine-Gray Competing Risks (D), RCT Cohort (E), and RCT Cohort With Fine-Gray Competing Risks (F)
eFigure 3. Cumulative Incidence of Prostate Cancer–Specific Mortality and Other-Cause Mortality for the Unadjusted and Unweighted SEER (A), VA (B), and RCT (C) Cohorts
eFigure 4. Cumulative Incidence of All-Cause Mortality for the Inverse Probability Weighted SEER (A), VA (B), and RCT (C) Cohorts
eFigure 5. Cumulative Incidence of All-Cause Mortality for the Unadjusted SEER (A), VA (B), and RCT (C) Cohorts