Key Points
Question
Does structured, short-term formula supplementation for at-risk term neonates affect the proportion still breastfeeding at 6 and 12 months?
Findings
In this randomized clinical trial that enrolled 164 mother-newborn dyads with newborn weight loss at or above the 75th percentile for hour of age, structured, short-term neonatal formula supplementation did not affect breastfeeding prevalence at 6 months.
Meaning
Using neonatal formula supplementation in a structured, short-term manner did not affect breastfeeding through 6 months; further research is needed to determine its effect on breastfeeding through the recommended duration of 12 months.
This randomized clinical trial assigns mother-newborn dyads to exclusive breastfeeding or breastfeeding supplemented with small amounts formula in the first week of life to evaluate the effect of formula supplementation on duration of breastfeeding at 6 and 12 months.
Abstract
Importance
Breastfeeding through 6 and 12 months are 2 goals of the Centers for Disease Control and Prevention Healthy People 2020 initiative, but the 6-month goal is met for only 52% of US infants and the 12-month goal for 30% of US infants.
Objective
To determine whether structured, short-term formula supplementation for at-risk neonates affects the proportion still breastfeeding at 6 and 12 months.
Design, Setting, and Participants
This randomized clinical trial conducted at 2 US academic medical centers enrolled 164 exclusively breastfeeding mother-infant dyads of mothers who were not yet producing copious milk and infants who were 24 to 72 hours old with newborn weight loss at or above the 75th percentile for age. Participants were enrolled from January 2015 through September 2016.
Interventions
Early Limited Formula (ELF), a structured formula supplementation protocol (10 mL formula fed after each breastfeeding until mothers produced copious milk), compared with control dyads, who continued exclusive breastfeeding and received a safety teaching intervention.
Main Outcomes and Measures
The study’s primary outcome was any breastfeeding at 6 months. Secondary outcomes included age at breastfeeding cessation and any breastfeeding at 12 months. All outcomes were assessed by maternal phone survey.
Results
Eighty-two newborns were randomized to ELF and 82 to the control group. Mean (SD) maternal age was 31.4 (5.9) years, and 114 (69.5%) self-identified as non-Hispanic white; 20 (12.2%), Hispanic; 17 (10.4%), Asian; 5 (3.0%), non-Hispanic black; and 7 (4.3%), other. Compared with controls, mothers randomized to ELF were less likely to be married (n = 53 [64.6%] vs n = 66 [80.5%]; P = .03) and had shorter mean (SD) intended duration of breastfeeding (8.6 [3.4] vs 9.9 [4.4] months; P = .049). Median (interquartile range) duration of breastfeeding in the cohort was 9 (6-12) months. At 6 months, 47 (65%) infants randomized to ELF were breastfeeding, compared with 60 (77%) of the control infants (absolute difference, –12%; 95% CI, –26% to 3%; P = .12). At 12 months, 21 of the 71 ELF infants available for analysis (29.6%) were breastfeeding, compared with 37 of the available 77 (48.1%) control infants (risk difference, –18%; 95% CI, –34% to –3%). Marital status and intended breastfeeding duration were both associated with breastfeeding duration; models adjusting for these found a hazard ratio for time-to-event of breastfeeding cessation through 12 months of 0.74 (95% CI, 0.48-1.14) for ELF infants compared with infants in the control group.
Conclusions and Relevance
In this cohort with high breastfeeding prevalence, ELF was not associated with any improvement in breastfeeding duration. Future research should examine the effect of ELF in populations at higher risk of early cessation.
Trial Registration
ClinicalTrials.gov identifier: NCT02313181
Introduction
Numerous epidemiologic and observational1,2,3,4 studies have found that duration of breastfeeding is shorter for infants who receive formula in the early newborn period.5,6,7,8 This observed association has informed clinical guidelines and public health initiatives that discourage the use of formula during the birth hospitalization.9,10,11,12 However, the causal relationship between early formula and reduced breastfeeding duration is uncertain, because 3 randomized clinical trials have not demonstrated a deleterious effect of early supplementation on breastfeeding duration.13,14,15 Because formula may ameliorate neonatal hyperbilirubemia and dehydration, exclusively breastfed infants may be at increased risk of early morbidity from these sources.
A structured, short-term formula supplementation protocol called Early Limited Formula (ELF), which delivers 10 mL of formula by syringe after each breastfeeding until the onset of copious maternal milk, improved the prevalence of breastfeeding and of exclusive breastfeeding at 3 months for newborns enrolled in a small pilot randomized trial conducted from 2009 through 2011.15 To examine whether ELF might improve breastfeeding duration through 6 and 12 months in a larger cohort, we conducted the study of Early Limited Formula for Treating Lactation Concerns (ELF-TLC). Initial ELF-TLC findings revealed no effect of ELF on breastfeeding or intestinal microbiota through 1 month of age; a trend toward reduced readmission was also noted.16 Below, we report the effect of ELF in ELF-TLC on breastfeeding at 6 and 12 months of age.
Methods
Trial Design, Participants, and Setting
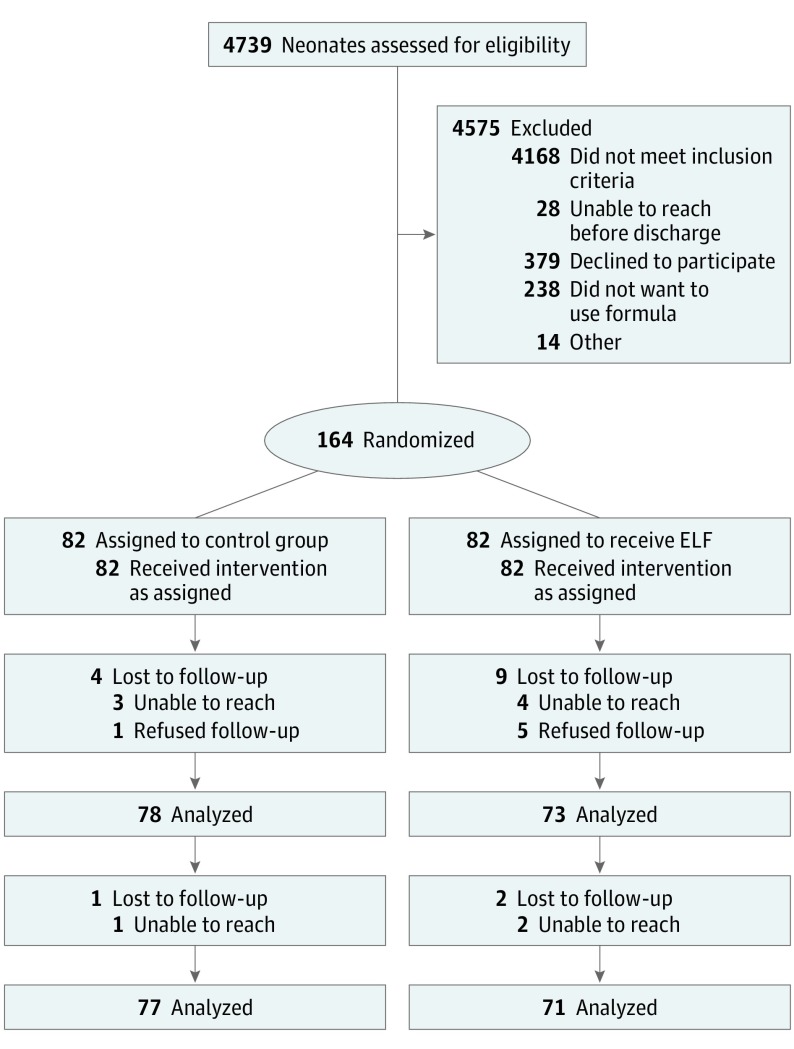
This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. Early Limited Formula for Treating Lactation Concerns enrolled 164 healthy, exclusively breastfeeding term (≥37 weeks) singletons born at the University of California, San Francisco Medical Center (San Francisco) and at Penn State Milton S. Hershey Medical Center (Hershey, Pennsylvania) between January 2015 and September 2016 (Figure 1). Infants were included if they were 24 to 48 hours old and had weight loss at or above the 75th percentile on the Newborn Weight Tool (http://www.newbornweight.org) and mothers who had not yet begun copious milk production.17 Weight measurement to determine eligibility for enrollment was obtained during routine hospital care. Infants were excluded if birth weight was less than 2500 g, the clinical team had recommended against breastfeeding, they had received formula, required a greater level of care than a level 1 nursery, had mothers who were aged younger than 18 years or could not speak English, were not expected to be discharged home with their parents, or were being observed for narcotic abstinence syndrome. Infants were also excluded if they had already lost 10% or more of their birth weight, because such infants commonly received supplementation in both enrolling hospitals. Before recruiting the first participant, ELF-TLC was approved by the University of California San Francisco Committee on Human Research and the Human Subjects Protection Office at Penn State College of Medicine. A study nurse obtained informed consent from mothers for both mother and infant prior to enrollment (Trial Protocol in Supplement 1).
Figure 1. CONSORT diagram for the Early Limited Formula for Treating Lactation Concerns (ELF-TLC) Randomized Clinical Trial.
Recruitment and Randomization
A randomized allocation sequence was generated by an independent biostatistician using a password-encoded Excel spreadsheet with randomly permuted blocks of 2 and 4 participants stratified on location (University of California, San Francisco or Pennsylvania State University) and on method of delivery (vaginal or cesarean). After obtaining informed consent, the study nurse accessed this randomized allocation sequence to determine treatment assignment. Newborns enrolled in ELF-TLC were randomly assigned in a 1:1 ratio either to breastfeeding with ELF (intervention) or to continue exclusive breastfeeding with a safety intervention (control). Recruitment was stopped when target enrollment was achieved.
Interventions
For dyads randomly assigned to the ELF intervention group, study nurses supported mothers in breastfeeding their infant and then taught mothers to use a feeding syringe to deliver 10 mL of formula to their infants immediately after each time they breastfed before the onset of copious breast milk. This ELF intervention was taught using extensively hydrolyzed formula (Nutramigen; Mead Johnson, Inc), both because of the potential benefits of extensively hydrolyzed formula18 and because of its successful use in previous work.15 Mothers were instructed to discontinue using ELF once they had begun copious milk production.
For dyads randomly assigned to the control group, study nurses supported mothers in breastfeeding their infant and then instructed mothers to breastfeed exclusively unless directed by a health care practitioner. To control for the time and attention that study nurses gave to mothers of ELF infants while teaching the ELF technique, study nurses taught infant safety techniques (including household water temperature, car seat position, and safe infant sleep environment) to mothers in the control group for 15 minutes. Both groups received a single nurse visit.
For both groups, at the time of enrollment, the study nurse completed a survey querying mothers regarding demographic and clinical characteristics as well as previous breastfeeding experience and intention. After the study nurse completed this initial assessment and teaching, no additional clinical management was provided by ELF-TLC, and enrolled infants resumed usual care with their clinicians. Before beginning enrollment and again at the midpoint of enrollment, the principal investigator (V.J.F.) trained all study nurses in breastfeeding support, use of ELF, and delivery of the safety control intervention.
Measures
All outcomes were assessed by phone survey of mothers. Our institutional review board–approved protocol’s primary outcome, breastfeeding at 6 months, was assessed by a research assistant blinded to treatment allocation with the item, “Has your baby breastfed or received any breast milk in the past 24 hours?” This item was also used to assess breastfeeding duration through 12 months. At the first assessment at which a mother responded “no” to the item “Has your baby breastfed or received any breast milk in the past 24 hours?,” age at breastfeeding cessation was assessed using the item, “How old was [name of child] when he/she completely stopped breastfeeding or being fed breast milk?” Exclusive breastfeeding was assessed using the following items: “How old was [name of child] when he/she was first fed formula?”; “How old was [name of child] when he/she was first fed anything other than breast milk or formula?”; “In the past 7 days, how often was your baby fed formula?”; and “In the past 7 days, how often was your baby fed something other than breast milk or formula?” Infants who were fed either formula or something other than breast milk or formula were defined as not exclusively breastfeeding. Intended breastfeeding duration was assessed at enrollment using the item, “For how long are you planning to breastfeed?”
Statistical Analysis
Estimating 10% dropout by the time of primary outcome assessment at 6 months, a sample size of 164 newborns was selected to achieve outcomes for 148 infants at 6 months and thereby achieve 90% power (α = .05) to detect a relative risk of 1.4 for ELF vs control with respect to the primary outcome of breastfeeding prevalence at 6 months of age, which was estimated at 60%.5,8,19,20,21,22,23,24,25 χ2 Testing was used to compare the ELF and control groups with respect to this primary outcome as well as with respect to other clinically meaningful dichotomous variables. The Fisher exact test was used to compare ELF and control data in prespecified subgroups of household income levels. Relative risks were calculated for the primary outcome and risk differences were calculated for both primary and secondary outcomes. Wald-based CIs (calculated on the log scale for relative risks) were also given. With respect to the secondary outcome of time-to-event of breastfeeding cessation, Cox proportional hazards analysis was used to compare the ELF and control groups and to compare clinically meaningful dichotomous predictor variables.
Our approach to multivariable analysis was as follows. For clinically meaningful variables that demonstrated an association with both treatment assignment and breastfeeding status and were therefore potential confounding variables, we assessed whether adjustment for the potential confounding variable attenuated the relationship between treatment assignment and breastfeeding and whether there was no interaction between treatment assignment and the potential confounding variable. For potential confounding variables that had no interaction with treatment assignment, we included the potential confounding variable in multivariable analysis of the effect of treatment assignment on breastfeeding without adding an interaction term. Multivariable logistic regression was used to compare dichotomous outcomes and multivariable Cox proportional hazards analysis was used to compare time-to-event outcomes between the ELF and control groups while adjusting for clinically meaningful potential confounding variables and stratification variables. For clinically meaningful dichotomous predictor variables that varied significantly between the ELF and control groups and for site of enrollment, we also analyzed the effect of ELF on breastfeeding prevalence at 6 and 12 months by subgroup using χ2 testing.
All analyses of the effect of random assignment to ELF were by intention to treat and were conducted in Stata version 14.1 (Stata Corp). In addition to the above intention-to-treat analyses of the effect of ELF on outcomes, we also explored, independent of treatment assignment, the relationship between formula use at 1 week of age and breastfeeding practices using Wald CIs and Cox proportional hazards analysis. All tests for statistical significance were 2 sided and P<.05 indicated statistical significance.
Results
Of 571 eligible mother-infant dyads, ELF-TLC enrolled 164 (28.7%); of 407 (71.3%) who were not enrolled, 238 (58.5%) stated they did not want to use formula. Enrolled infants had a mean (SD) gestational age of 39.4 (1.1) weeks and chronological age of 35.9 (9.2) hours (Table 1). Enrolled mothers had a mean (SD) age of 31.4 (5.9) years; 110 (67.1%) had completed college, 112 of 150 who responded to income questions (74.6%) reported a household income greater than $50 000, and 114 (69.5%) self-identified as non-Hispanic white. Compared with controls, mothers of infants randomly assigned to ELF were less likely to be married (53 of 80 [66.0%] vs 66 of 81 [81.5%]; P = .03) and had a mean (SD) intended duration of breastfeeding that was shorter (8.6 [3.4] vs 9.9 [4.4] months; P = .049). Median (interquartile range [IQR]) duration of ELF use after treatment assignment was 2 (1.5-3.0) days. At 12 months post-partum, 149 mothers (90.8%) responded to the final survey; attrition did not differ by treatment assignment.
Table 1. Baseline Demographic and Clinical Characteristics by Treatment Assignment to Early Limited Formula or Continued Exclusive Breastfeeding.
| Characteristic | No. (%) | |
|---|---|---|
| Early Limited Formula Group (Intervention) (n = 82) | Continued Exclusive Breastfeeding (Control) (n = 82) | |
| Gestational age, mean (SD), wk | 39.4 (1.2) | 39.4 (1.1) |
| Vaginal delivery | 61 (74.4) | 60 (73.1) |
| Infant age at enrollment, mean (SD), h | 35.7 (9.5) | 36.1 (9.0) |
| Maternal age, mean (SD), y | 31.3 (5.6) | 31.6 (6.2) |
| Enrolled at Pennsylvania State University | 40 (48.8) | 40 (48.8) |
| Maternal race/ethnicity | ||
| Non-Hispanic white | 57 (69.5) | 57 (69.5) |
| Non-Hispanic black | 3 (3.6) | 2 (2.4) |
| Hispanic | 10 (12.2) | 10 (12.2) |
| Asian | 9 (11.0) | 8 (9.8) |
| Other | 2 (2.4) | 5 (6.1) |
| Maternal educational attainment | ||
| 8th grade or less | 1 (1.2) | 0 (0) |
| Some high school | 2 (2.4) | 2 (2.4) |
| High school graduate | 4 (4.9) | 9 (11.0) |
| Some college or technical school | 21 (25.6) | 12 (14.6) |
| Completed college | 33 (40.2) | 31 (37.8) |
| Postgraduate training | 19 (23.2) | 27 (32.9) |
| Marrieda | 53 (66.0) | 66 (81.5) |
| Primiparousb | 51 (63) | 48 (58.5) |
| % Weight loss at enrollment, mean (SD) | 6.3 (1.6) | 6.4 (1.5) |
| Birth weight, mean (SD), g | 3372 (670) | 3398 (453) |
| US born | 66 (80.5) | 66 (82) |
| Mother with breastfeeding experience | 32 (39.0) | 31 (37.8) |
| Intended breastfeeding duration, mean (SD), mo | 8.6 (3.4) | 9.9 (4.4) |
| Household annual income, $ | ||
| <10 000 | 3 (3.7) | 1 (1.2) |
| 10 000-24 999 | 8 (9.8) | 7 (8.5) |
| 25 000-49 999 | 10 (12.2) | 9 (11.0) |
| 50 000-74 999 | 12 (14.6) | 4 (4.9) |
| 75 000-100 000 | 4 (4.9) | 11 (13.4) |
| >100 000 | 37 (45.1) | 44 (53.7) |
| Unknown/declined | 8 (9.8) | 6 (7.3) |
Calculated from denominator 80 for the Early Limited Formula group and 81 for control group.
Calculated from denominator of 81 for the Early Limited Formula group.
Effect of Treatment Assignment on Breastfeeding Duration
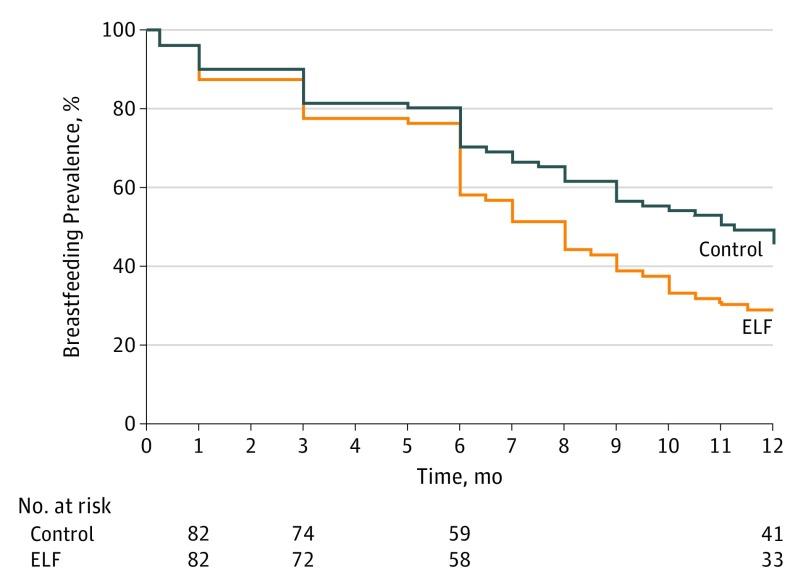
The relative risk for ELF compared with control with respect to the outcome of breastfeeding prevalence at 6 months was 0.85 (95% CI, 0.69-1.04) (Table 2). Treatment assignment also did not affect the prevalence of exclusive breastfeeding at 6 months. Breastfeeding prevalence at 12 months was lower for those randomly assigned to ELF at birth. In bivariate Cox proportional hazards analysis, infants in the ELF group had a shorter time to breastfeeding cessation through 12 months than infants in the control group (hazard ratio [HR], 0.65 [95% CI, 0.43-0.97] months) (Figure 2). No beneficial or detrimental effect of ELF on breastfeeding at 6 months was demonstrated for any subgroup, including subgroups of household income, maternal marital status, maternal educational attainment, and site of enrollment; at 12 months, breastfeeding prevalence was lower for infants assigned to ELF in the subgroup of high-income mothers and the subgroup enrolled at the University of California, San Francisco (eTable 1 in Supplement 2).
Table 2. Adjusted and Unadjusted Analysis of Interval Breastfeeding Prevalence by Treatment Assignment.
| Outcome(s) | No. (%) | Unadjusted (95% CI) | Adjusted (95% CI)a | |||
|---|---|---|---|---|---|---|
| Early Limited Formula | Control | Absolute Risk Difference | Hazard Ratio | Odds Ratio | Hazard Ratio | |
| Primary | ||||||
| Breastfeeding at 6 mo | 47 (65) | 60 (79) | −12% (−26 to 3) | NA | 0.60 (0.24 to 1.38) | NA |
| Secondary | ||||||
| Exclusive breastfeeding at 6 mo | 25 (35) | 36 (46) | −11% (−27 to 4) | NA | 0.69 (0.33 to 1.44) | NA |
| Breastfeeding at 12 mo | 21 (30) | 37 (48) | −18% (−34 to −3) | NA | 0.70 (0.30 to 1.59) | NA |
| Time-to-event of breastfeeding cessation | NA | NA | NA | 0.65 (0.43 to 0.97) | 0.74 (0.48 to 1.14) | |
Abbreviation: NA, not applicable.
Adjusted for site of enrollment, method of delivery and maternal marital status and intended duration of breastfeeding.
Figure 2. Breastfeeding Cessation Through 12 Months of Age by Treatment Assignment for Infants.
Data were only collected at 1, 3, 6, and 12 months. ELF indicates Early Limited Formula.
Effect of Treatment Assignment on Outcomes Adjusting for Potential Confounding Variables
Intended duration of breastfeeding and maternal marital status were unevenly distributed between the 2 groups at baseline by chance and were also associated in bivariate analysis with breastfeeding at 6 and 12 months and with time-to-event of breastfeeding cessation (eTable 2 in Supplement 2). In multivariable logistic regression also adjusting for stratification variables, adjusting for marital status attenuated the odds ratio for the effect of ELF on breastfeeding at 6 months from 0.56 (95% CI, 0.28-1.15) to 0.61 (95% CI, 0.27-1.40) and attenuated the odds ratio for the effect of ELF on breastfeeding at 12 months from 0.45 (95% CI, 0.23-0.89) to 0.55 (95% CI, 0.25-1.20). There was no interaction between treatment assignment and marital status, or between treatment assignment and planned duration of breastfeeding. Multivariable proportional hazards analysis adjusting for marital status and intended duration of breastfeeding as well as for the stratification variables found that the HR for ELF compared with control was 0.74 (95% CI, 0.48-1.14) with respect to time-to-event of breastfeeding cessation through 12 months. Maternal age, education, income, and site of enrollment were all strongly associated with time to breastfeeding cessation but did not differ by treatment assignment.
Relationship of Formula Feeding at 1 Week to Subsequent Breastfeeding Practices
Among 127 enrolled infants who responded to assessment of breastfeeding at 1 week of age, 38 of 60 infants in the ELF intervention group (63.3%) and 25 of 67 infants in the control group (78%) had breastfed without formula for the past 24 hours (P = .08). The receipt of formula at 1 week of age was very strongly associated with all subsequent breastfeeding outcomes. At ages 6 and 12 months, respectively, 11 (31.4%) and 2 (5.7%) of 35 infants who had received formula in the past 24 hours at age 1 week were still breastfeeding, compared with 75 (85.2%) and 47 (53.4%) of the 88 who had not received formula in the past 24 hours at age 1 week. Thus, the risk differences for the effect of receiving formula at 1 week on the outcomes of breastfeeding prevalence at 6 months was –53.8 (95% CI, –70.9 to –36.7) and for 12 months, it was –46.9 (95% CI, –60.0 to –33.9). Receipt of formula at age 1 week was also associated with time-to-event of breastfeeding cessation (HR, 4.84; 95% CI, 3.00-7.78); this effect was noted among infants randomly assigned to ELF (HR, 3.37; 95% CI, 1.77-6.44) and even more pronounced among those randomly assigned to control (HR, 7.16; 95% CI, 3.51-14.6).
Discussion
Using ELF in the first days after birth did not alter the prevalence of breastfeeding or formula use at 6 months of age in the ELF-TLC cohort of infants likely to breastfeed successfully, who were born in US academic institutions with strong breastfeeding support, and had a median breastfeeding duration of 9 months. However, infants randomly assigned to ELF had a lower prevalence of breastfeeding at 12 months of age than those assigned to the control intervention in unadjusted analyses. Owing to the uneven distribution of major predictors of breastfeeding cessation that occurred by chance at enrollment, it is unclear whether this observed deleterious association between ELF and breastfeeding at 12 months may be attributable to confounding. Nevertheless, our results do not provide evidence that ELF should be used for the goal of improving breastfeeding duration through 12 months in populations similar to those studied in ELF-TLC. It is unknown whether the effect of ELF on long-term breastfeeding prevalence might differ for populations at higher risk of early cessation.
Our finding that ELF did not affect breastfeeding prevalence through age 6 months but may have decreased breastfeeding prevalence at 12 months raises an important question regarding the mechanism of action for these observed relationships between early feeding and subsequent breastfeeding duration. Previous studies of the deleterious association between early formula and subsequent breastfeeding duration have suggested that the causal mechanism for this association related to increased infant satiety from receipt of formula and concomitant decreased infant breastfeeding demand, increased maternal milk stasis, and decreased maternal milk production.5,6,7,26 However, this causal mechanism is not supported by the results from our trial, which demonstrated a relationship between ELF and breastfeeding at 12 months but not at earlier time points. The substantial majority of infants randomly assigned to ELF had discontinued ELF by age 1 week and were exclusively breastfeeding at that time. It is unlikely that the behavior of 6-month-old infants who received ELF briefly after birth followed by resumption of exclusive breastfeeding differed from the behavior of 6-month-old infants exclusively breastfed since birth. As a result, any effect of ELF on long-term breastfeeding could be attributable to an alteration in maternal or family behavior associated with the early use of formula. It is possible that using ELF in the newborn period reduces maternal or other family commitment to avoiding formula in later infancy, perhaps especially once complementary feeding has been initiated, and that greater use of formula in later infancy increases the risk of breastfeeding cessation before 12 months of age.
The mechanism by which ELF may have had a delayed, detrimental effect on long-term breastfeeding duration is important because for some infants, small volumes of formula can be beneficial in the neonatal period for ameliorating or precluding early morbidity such as hyperbilirubinemia or dehydration. One potential mechanism for a delayed deleterious effect of ELF on breastfeeding might be exposure of the parents to clinical counseling strategies in early infancy that assert the importance of a dichotomous, “virgin”27 state with respect to formula, rather than a more nuanced approach to supporting breastfeeding. Although exposure to specific clinical counseling strategies was not assessed in ELF-TLC, it is known that 58% of those who declined to enroll stated that they did not want to use formula for their infants; this suggests that the target population had been exposed to counseling against formula use. For mothers who either do choose or are required to use small volumes of formula in early infancy, previous exposure to a starkly dichotomous counseling strategy during routine clinical care could potentially dampen subsequent commitment to avoiding formula during later infancy and could therefore be a mechanism by which the early use of small volumes of formula leads to premature cessation of breastfeeding.
However, starkly dichotomous counseling regarding early formula use is not evidence based. Although many studies have shown a deleterious association between any formula use and subsequent breastfeeding outcomes,28,29,30,31,32 the timing and the actual volume used may be a factor. Only a few studies have separated the effect of small volumes of formula from that of large volumes of formula,2,15,16 and no studies that have specifically examined the effect of small volumes of formula have demonstrated any association with deleterious outcomes.33 Our study’s results are consistent with the possibility that ELF may have clinically significant benefits as well as risks for subsequent breastfeeding behavior. Counseling that implies a deleterious effect of small volumes of formula in the neonatal period is inaccurate and could be detrimental to long-term breastfeeding success.
When weighing the risks and benefits of formula use in early infancy, clinicians and parents may wish to consider ELF-TLC’s results highlighting a potentially important role for formula use at age 1 week. Although an effect of ELF on breastfeeding at age 6 months was not demonstrated, the use of formula at 1 week had a negative association with breastfeeding outcomes at both 6 and 12 months. If formula is used in the first few days after birth to ameliorate hyperbilirubinemia or dehydration, the risks and benefits of formula use should be discussed, and if formula is used, it should be discontinued as soon as possible. Ongoing formula use at 1 week of age indicates a mother at high risk of early breastfeeding cessation.
Limitations
Our study has several important limitations. First, ELF-TLC enrolled newborns with weight loss at or above the 75th percentile but excluded newborns with weight loss of 10% or greater. Second, enrolled participants delivered in US academic medical centers with strong breastfeeding support, and although 67.9% of mothers were college graduates, 15% were younger than 25 years. Further research is needed to identify supplementation strategies supportive of breastfeeding for infants with weight loss of 10% or greater and in other settings. Third, 1 in 4 eligible mothers consented to enroll in ELF-TLC. Mothers who chose to enroll may have been ambivalent about whether formula would be beneficial to their infant, and so may not be representative of all mothers of newborns with pronounced weight loss.
Conclusions
Breastfeeding prevalence at 6 months of age was not altered in our study population by the use of ELF in the newborn period. Given results previously released by the ELF trial suggesting that ELF may ameliorate neonatal morbidity, these new findings indicate that in settings with strong breastfeeding support and long duration of breastfeeding, ELF should be used in the early newborn period only when the beneficial effect on neonatal morbidity is expected to exceed any potential deleterious effect on long-term breastfeeding. Our findings also confirm that to minimize any potential deleterious effect on long-term breastfeeding, any formula initiated in the first few days should be discontinued as soon as possible and before 1 week of age. The results of ELF-TLC may also reassure mothers and clinicians that most newborns who use formula in the first few days are able to discontinue its use by 1 week. Future research is needed to identify specific populations likely to receive net benefits from targeted early supplementation and to develop evidence-based criteria for the precise timing and amount of supplementation that will minimize newborn morbidity while improving long-term breastfeeding duration. Such criteria could potentially allow clinicians, parents, and hospitals to work together to optimize overall health outcomes during early infancy and beyond.
Trial Protocol
eTable 1. Breastfeeding Prevalence at 6 and 12 Months Within Subgroups Unevenly Distributed at Baseline or Pre-specified and Within Each Site, by Treatment Assignment
eTable 2. Univariate and Multivariable Hazard Ratios (HR) for Demographic and Clinical Predictors Present at Birth, of Time to Breastfeeding Cessation Through Age 12 Months
Data Sharing Statement
References
- 1.Kuzniewicz MW, Greene DN, Walsh EM, McCulloch CE, Newman TB. Association between laboratory calibration of a serum bilirubin assay, neonatal bilirubin levels, and phototherapy use. JAMA Pediatr. 2016;170(6):557-661. doi: 10.1001/jamapediatrics.2015.4944 [DOI] [PubMed] [Google Scholar]
- 2.Wickremasinghe AC, Kuzniewicz MW, McCulloch CE, Newman TB. Efficacy of subthreshold newborn phototherapy during the birth hospitalization in preventing readmission for phototherapy. JAMA Pediatr. 2018;172(4):378-385. doi: 10.1001/jamapediatrics.2017.5630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flaherman VJ, Schaefer EW, Kuzniewicz MW, Li SX, Walsh EM, Paul IM. Early weight loss nomograms for exclusively breastfed newborns. Pediatrics. 2015;135(1):e16-e23. doi: 10.1542/peds.2014-1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller JR, Flaherman VJ, Schaefer EW, et al. Early weight loss nomograms for formula fed newborns. Hosp Pediatr. 2015;5(5):263-268. doi: 10.1542/hpeds.2014-0143 [DOI] [PubMed] [Google Scholar]
- 5.Holmes AV, Auinger P, Howard CR. Combination feeding of breast milk and formula: evidence for shorter breast-feeding duration from the National Health and Nutrition Examination Survey. J Pediatr. 2011;159(2):186-191. doi: 10.1016/j.jpeds.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 6.Chantry CJ, Dewey KG, Peerson JM, Wagner EA, Nommsen-Rivers LA. In-hospital formula use increases early breastfeeding cessation among first-time mothers intending to exclusively breastfeed. J Pediatr. 2014;164(6):1339-1345.e1335. doi: 10.1016/j.jpeds.2013.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolton TA, Chow T, Benton PA, Olson BH. Characteristics associated with longer breastfeeding duration: an analysis of a peer counseling support program. J Hum Lact. 2009;25(1):18-27. doi: 10.1177/0890334408325985 [DOI] [PubMed] [Google Scholar]
- 8.Petrova A, Hegyi T, Mehta R. Maternal race/ethnicity and one-month exclusive breastfeeding in association with the in-hospital feeding modality. Breastfeed Med. 2007;2(2):92-98. doi: 10.1089/bfm.2006.0030 [DOI] [PubMed] [Google Scholar]
- 9.Section on Breastfeeding . Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827-e841. doi: 10.1542/peds.2011-3552 [DOI] [PubMed] [Google Scholar]
- 10.Kellams A, Harrel C, Omage S, Gregory C, Rosen-Carole C. ABM Clinical Protocol #3: Supplementary Feedings in the Healthy Term Breastfed Neonate, Revised 2017. Breastfeed Med. 2017;12:188-198. doi: 10.1089/bfm.2017.29038.ajk [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . Nutrition: exclusive breastfeeding. https://www.who.int/nutrition/topics/exclusive_breastfeeding/en/. Accessed May 19, 2018.
- 12.Centers for Disease Control and Prevention . Maternal, infant, and child health. https://www.healthypeople.gov/2020/topics-objectives/topic/maternal-infant-and-child-health/objectives. Accessed May 20, 2018.
- 13.Gray-Donald K, Kramer MS, Munday S, Leduc DG. Effect of formula supplementation in the hospital on the duration of breast-feeding: a controlled clinical trial. Pediatrics. 1985;75(3):514-518. [PubMed] [Google Scholar]
- 14.Schubiger G, Schwarz U, Tönz O. UNICEF/WHO baby-friendly hospital initiative: does the use of bottles and pacifiers in the neonatal nursery prevent successful breastfeeding? Neonatal Study Group. Eur J Pediatr. 1997;156(11):874-877. doi: 10.1007/s004310050734 [DOI] [PubMed] [Google Scholar]
- 15.Flaherman VJ, Aby J, Burgos AE, Lee KA, Cabana MD, Newman TB. Effect of early limited formula on duration and exclusivity of breastfeeding in at-risk infants: an RCT. Pediatrics. 2013;131(6):1059-1065. doi: 10.1542/peds.2012-2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flaherman VJ, Narayan NR, Hartigan-O’Connor D, Cabana MD, McCulloch CE, Paul IM. The effect of early limited formula on breastfeeding, readmission, and intestinal microbiota: a randomized clinical trial. J Pediatr. 2018;196:84-90.e81. doi: 10.1016/j.jpeds.2017.12.073 [DOI] [PubMed] [Google Scholar]
- 17.Chapman DJ, Pérez-Escamilla R. Maternal perception of the onset of lactation is a valid, public health indicator of lactogenesis stage II. J Nutr. 2000;130(12):2972-2980. doi: 10.1093/jn/130.12.2972 [DOI] [PubMed] [Google Scholar]
- 18.Gourley GR, Kreamer B, Arend R. The effect of diet on feces and jaundice during the first 3 weeks of life. Gastroenterology. 1992;103(2):660-667. doi: 10.1016/0016-5085(92)90862-S [DOI] [PubMed] [Google Scholar]
- 19.Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012;8(8):CD003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blomquist HK, Jonsbo F, Serenius F, Persson LA. Supplementary feeding in the maternity ward shortens the duration of breast feeding. Acta Paediatr. 1994;83(11):1122-1126. doi: 10.1111/j.1651-2227.1994.tb18263.x [DOI] [PubMed] [Google Scholar]
- 21.Adlerberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta Paediatr. 2009;98(2):229-238. doi: 10.1111/j.1651-2227.2008.01060.x [DOI] [PubMed] [Google Scholar]
- 22.Ahrné S, Lönnermark E, Wold AE, et al. Lactobacilli in the intestinal microbiota of Swedish infants. Microbes Infect. 2005;7(11-12):1256-1262. doi: 10.1016/j.micinf.2005.04.011 [DOI] [PubMed] [Google Scholar]
- 23.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352(6285):539-544. doi: 10.1126/science.aad9378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guaraldi F, Salvatori G. Effect of breast and formula feeding on gut microbiota shaping in newborns. Front Cell Infect Microbiol. 2012;2:94. doi: 10.3389/fcimb.2012.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houghteling PD, Walker WA. Why is initial bacterial colonization of the intestine important to infants’ and children’s health? J Pediatr Gastroenterol Nutr. 2015;60(3):294-307. doi: 10.1097/MPG.0000000000000597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawkins SS, Stern AD, Baum CF, Gillman MW. Compliance with the Baby-Friendly Hospital Initiative and impact on breastfeeding rates. Arch Dis Child Fetal Neonatal Ed. 2014;99(2):F138-F143. doi: 10.1136/archdischild-2013-304842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merewood A. Of elves and ethics: first, do no harm. J Hum Lact. 2013;29(4):443. doi: 10.1177/0890334413505281 [DOI] [PubMed] [Google Scholar]
- 28.Oddy WH. Breastfeeding, childhood asthma, and allergic disease. Ann Nutr Metab. 2017;70(suppl 2):26-36. doi: 10.1159/000457920 [DOI] [PubMed] [Google Scholar]
- 29.Azad MB, Vehling L, Lu Z, et al. ; CHILD Study Investigators . Breastfeeding, maternal asthma and wheezing in the first year of life: a longitudinal birth cohort study. Eur Respir J. 2017;49(5):1602019. doi: 10.1183/13993003.02019-2016 [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention . Vital signs: hospital practices to support breastfeeding—United States, 2007 and 2009. MMWR Morb Mortal Wkly Rep. 2011;60(30):1020-1025. [PubMed] [Google Scholar]
- 31.Bentley JP, Simpson JM, Bowen JR, Morris JM, Roberts CL, Nassar N. Gestational age, mode of birth and breastmilk feeding all influence acute early childhood gastroenteritis: a record-linkage cohort study. BMC Pediatr. 2016;16:55. doi: 10.1186/s12887-016-0591-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quigley MA, Carson C, Sacker A, Kelly Y. Exclusive breastfeeding duration and infant infection. Eur J Clin Nutr. 2016;70(12):1420-1427. doi: 10.1038/ejcn.2016.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith HA, Becker GE. Early additional food and fluids for healthy breastfed full-term infants. Cochrane Database Syst Rev. 2016;(8):CD006462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Breastfeeding Prevalence at 6 and 12 Months Within Subgroups Unevenly Distributed at Baseline or Pre-specified and Within Each Site, by Treatment Assignment
eTable 2. Univariate and Multivariable Hazard Ratios (HR) for Demographic and Clinical Predictors Present at Birth, of Time to Breastfeeding Cessation Through Age 12 Months
Data Sharing Statement