Key Points
Question
What is the prevalence of age disparities among participants in randomized clinical trials in oncology, and what factors are associated with heightened age disparities?
Findings
In an analysis of 302 randomized clinical trials collectively comprising 262 354 participants, trial participants were significantly younger than the population by disease site. Industry-funded trials and trials testing a targeted therapy had larger age disparities; age imbalances among trial participants appear to be widening over time.
Meaning
Age disparities among cancer trial participants are pervasive, worsening, and associated with industry sponsorship; future strategies must address these inequalities to ensure generalizability of trial results as well as trial access equity.
Abstract
Importance
Seminal investigation 2 decades ago alerted the oncology community to age disparities in participation in cooperative group trials; less is known about whether these disparities persist in industry-funded research.
Objective
To characterize the age disparities among trial enrollees on randomized clinical trials (RCTs) of common cancers in clinical oncology and identify factors associated with wider age imbalances.
Data Sources
Phase 3 clinical oncology RCTs were identified through ClinicalTrials.gov.
Study Selection
Multiarm RCTs assessing a therapeutic intervention for patients with breast, prostate, colorectal, or lung cancer (the 4 most common cancer disease sites) were included.
Data Extraction and Synthesis
Trial data were extracted from ClinicalTrials.gov. Trial screening and parameter identification were independently performed by 2 individuals. Data were analyzed in 2018.
Main Outcomes and Measures
The difference in median age (DMA) between the trial participant median age and the population-based disease-site-specific median age was determined for each trial.
Results
Three hundred two trials met inclusion criteria. The trials collectively enrolled 262 354 participants; 249 trials (82.5%) were industry-funded. For all trials, the trial median age of trial participants was a mean of 6.49 years younger than the population median age (95% CI, −7.17 to −5.81 years; P < .001). Age disparities were heightened among industry-funded trials compared with non–industry-funded trials (mean DMA, −6.84 vs −4.72 years; P = .002). Enrollment criteria restrictions based on performance status or age cutoffs were associated with age disparities; however, industry-funded trials were not more likely to use these enrollment restrictions than non–industry-funded trials. Age disparities were also larger among trials that evaluated a targeted systemic therapy and among lung cancer trials. Linear regression modeling revealed a widening gap between trial and population median ages over time at a rate of −0.19 years annually (95% CI, −0.37 to −0.01 years; P = .04).
Conclusions and Relevance
Age disparities between trial participants and the incident disease population are pervasive across trials and appear to be increasing over time. Industry sponsorship of trials is associated with heightened age imbalances among trial participants. With an increasing role of industry funding among cancer trials, efforts to understand and address age disparities are necessary to ensure generalizability of trial results as well as equity in trial access.
This study characterizes the age disparities among trial enrollees in randomized clinical trials of common cancers (breast, prostate, colorectal, and lung cancer) in US population-based median age and identifies factors associated with age imbalances.
Introduction
As the population in the United States ages, cancer incidence is expected to increase; by 2030 it is estimated that 70% of all new cancer diagnoses will be in patients 65 years and older, emphasizing the increasing relevance of geriatric populations in oncology.1 Seminal data from 15 to 20 years ago demonstrated marked underrepresentation of older patients with cancer enrolled on clinical trials funded by the National Cancer Institute (NCI).2,3,4 Randomized clinical trials (RCTs) often establish the standard of care; with underrepresentation of older patients, concerns regarding the applicability of trial results persist.5,6,7 We hypothesized that age disparities continue to exist for patients with cancer enrolled in clinical trials. Therefore, we examined age disparities among modern oncologic RCTs for breast, prostate, colorectal, and lung cancer (the 4 most common disease sites), characterizing the differences between trial participants and the population by disease site. We identified specific trial-related factors associated with heightened age disparities (including industry sponsorship) and asked whether age disparities were improving with time.
Methods
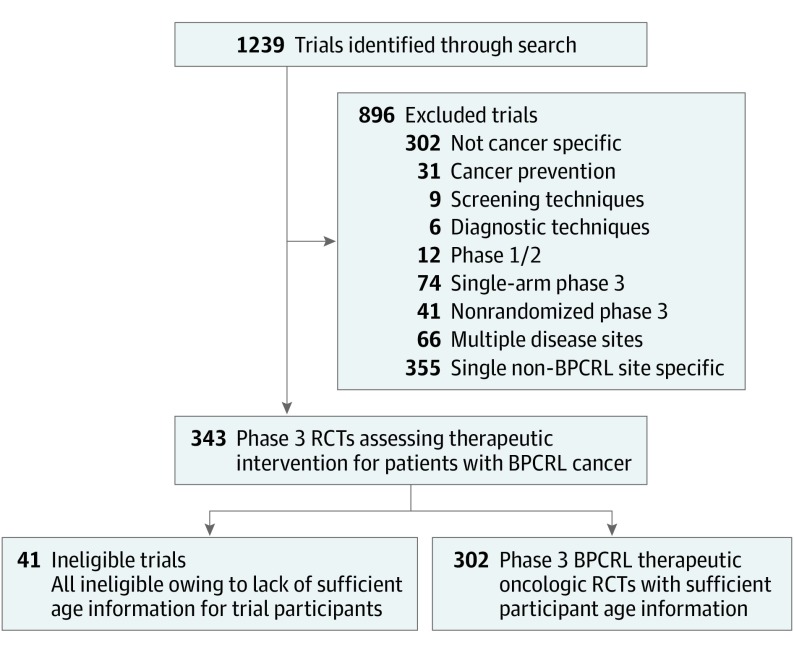
No institutional review board approval was required from our home institution (the University of Texas MD Anderson Cancer Center Institutional Review Board) and was therefore waived. Informed consent was not obtained because all data were trial-level data publicly available. The ClinicalTrials.gov website was queried on November 19, 2017, to identify oncologic RCTs. The following “Advanced” search parameters were used: other terms: “cancer”; study type: “All Studies”; status: excluded “Not yet recruiting”; phase: phase 3; and study results: “With Results.” This yielded 1239 trials. Trials were then screened for cancer-specific phase 3 randomized multiarm trials addressing a therapeutic intervention (Figure). Only trials addressing single disease sites of breast, prostate, colorectal, or lung cancer were included. Trials that did not provide the median age of enrollees were ineligible. Trial screening and parameter identification were independently performed by 2 individuals. For each trial, the trial median age was compared with the median age for the relevant disease site based on the NCI Surveillance, Epidemiology, and End Results (SEER) database.8 The SEER median age by disease site was also matched to the time of trial enrollment. Statistical analyses included independent-samples Mann-Whitney U and Kruskal-Wallis tests, as well as linear regression modeling. χ2 T testing was used to compare proportions across groups. The P value level of significance was specified a priori at .05, and all P values were 2-sided. Analyses were performed using SPSS, version 22.0 (IBM).9
Figure. Flowchart of Clinical Trial Screening, Eligibility, and Inclusion.
BPCRL indicates breast/prostate/colorectal/lung cancer; RCTs, randomized clinical trials.
Results
Three hundred two trials met inclusion criteria (Figure); these trials collectively enrolled a total of 262 354 patients, with years of enrollment initiation from 1994 to 2015. For each trial, the difference in median age (DMA) was calculated, representing the trial median age minus the population median age. For all trials, the mean DMA was −6.49 years (95% CI, −7.17 to −5.81 years, P < .001; Table). There were wider age differences among industry-funded trials, with a mean DMA of −6.84 years for industry-sponsored trials compared with −4.72 years for non–industry-sponsored trials (P = .002; Table). Trials with enrollment criteria restricting the upper age limit of participants (26 of 302 trials; 8.6%) or restricting enrollees to those with Eastern Cooperative Oncology Group performance status 0-1 were associated with larger DMA (Table). There was no association between industry funding and either upper age limit (upper age limit for 8.4% of industry-funded trials [21 of 249 trials] vs 9.4% of non–industry-funded trials [5 of 53 trials]; P = .75) or Eastern Cooperative Oncology Group performance status enrollment restrictions (performance status restriction for 53.5% of industry-funded trials [99 of 185 trials] vs 50.0% of non–industry-funded trials [17 of 34 trials]; P = .71). We then identified trials restricted to patients with a molecular subtype associated with a younger patient population than that of the disease site more generally (such as HER2-overexpressing breast cancers or EGFR-mutant non–small cell lung cancers). These molecular-subtype-restricted studies, accounting for 14.9% of trials (45 of 302), were more likely to be industry funded than unrestricted trials (93.3% [42 of 45 trials] vs 81.2% [207 of 257 trials]; P = .045) and were more likely to enroll younger patients (Table). Even excluding these subtype-restricted trials, the remaining trials still enrolled younger patients than the population (mean DMA, −5.88 years; 95% CI −6.62 to −5.13 years; P < .001) and continued to demonstrate an association between industry funding and heightened age disparities (−6.18 vs −4.45 years; P = .01). Among systemic therapy trials, those that tested a targeted therapy (ie, monoclonal antibody or small molecule inhibitor) were associated with a larger DMA than those that tested cytotoxic chemotherapy (−7.72 vs −5.30 years; P = .01; Table). Targeted therapy trials were more likely to be industry funded than cytotoxic chemotherapy trials (93.8% vs 79.8%; P = .001); when we restricted analysis to targeted therapy trials only, industry sponsorship remained associated with a larger DMA (−7.94 vs −4.33 years; P = .01).Finally, examining all trials (N = 302), the DMA was analyzed by year of enrollment initiation. Linear regression modeling revealed an estimated annual change of −0.19 years in the DMA (95% CI, −0.37 to −0.01 years; P = .04), suggesting widening age disparities over time.
Table. Trial Factors Associated With Age Disparities.
| Trial Factor | No. of Trials | Mean DMA (SE), years | P Value |
|---|---|---|---|
| All included trials | 302 | −6.49 (0.34) | <.001a |
| Industry funding of trial | |||
| Yes | 249 | −6.84 (0.38) | .002 |
| No | 53 | −4.72 (0.79) | |
| Cooperative group trial | |||
| Yes | 74 | −6.24 (0.73) | .34 |
| No | 228 | −6.57 (0.39) | |
| Age restriction enrollment criterionb | |||
| Yes | 26 | −10.20 (1.46) | .001 |
| No | 276 | −5.71 (0.34) | |
| ECOG PS restriction criterion | |||
| Yes (restricted to PS 0-1) | 117 | −7.40 (0.42) | .01 |
| No (not restricted to PS 0-1) | 103 | −5.35 (0.66) | |
| Molecular profile restriction criterionc | |||
| Yes | 45 | −9.99 (0.60) | <.001 |
| No | 257 | −5.88 (0.38) | |
| Disease site | |||
| Breast | 107 | −7.76 (0.52) | .001 |
| Colorectal | 36 | −6.96 (0.57) | |
| Lung | 105 | −8.98 (0.35) | |
| Prostate | 54 | 2.66 (0.48) | |
| Modality | |||
| Systemic therapy | 247 | −6.89 (0.38) | .04 |
| Radiotherapy | 7 | −3.64 (1.88) | |
| Surgery | 2 | −12.10 (11.10) | |
| Supportive care | 45 | −4.76 (0.83) | |
| Systemic therapy subgroup | |||
| Cytotoxic chemotherapy | 85 | −5.30 (0.78) | .01 |
| Targeted therapy | 162 | −7.72 (0.39) | |
| Trial success (PEP met) | |||
| Yes | 130 | −6.40 (0.58) | .73 |
| No | 120 | −6.67 (0.49) | |
Abbreviations: DMA, difference in median age (trial minus population); ECOG, Eastern Cooperative Oncology Group; PEP, primary end point; PS, performance status.
For all included trials (N = 302), the P value provided represents the results of a 1-sample t test comparing the mean DMA for all trials against a hypothetical population average DMA of 0. All other P values provided reflect Mann-Whitney U tests or Kruskal-Wallis tests for each trial factor listed.
Trials that included a restriction on the upper limit of enrollees were classified as having an “age restriction” enrollment criterion.
“Molecular Profile Restriction” trials were those that included an enrollment criterion that selected for younger patients based on the molecular profile of patients’ tumors; this included trials specifically for patients with triple-negative breast cancer, HER2-overexpressing breast cancer, ALK-rearranged non–small-cell lung cancer, or EGFR-mutant non–small-cell lung cancer, all molecular subtypes of each disease site associated with a younger patient population. Modality addressed the primary intervention as part of the randomization in the trial. Systemic therapy modality trials included those testing chemotherapy, targeted systemic agents, immunotherapy, and others; these trials all used systemic therapies to improve disease-related outcomes such as overall and disease-free survival. Supportive care trials included those where the intervention aimed to decrease or prevent disease-related or treatment-related toxicity as the primary end point (rather than survival/disease-control outcomes as discussed previously). Systemic therapy trials were further subdivided into cytotoxic chemotherapy and targeted therapy; the latter includes monoclonal antibodies, small molecule inhibitors, and similar agents. Fifty-two trials were excluded from analysis of trial success (whether the PEP was met) because the primary end point had not been published for these trials at time of analysis.
Discussion
Across all trials, we observed a substantial difference between the median age of trial participants and the population median age by disease site. Historically, the federal government has addressed cancer clinical trial enrollment disparities by primarily focusing on sex and race/ethnicity.2,4,10 While prior reports have shown some successes in addressing those imbalances,2 our data demonstrate that age disparities remain a persistent and worsening problem for oncology trials. Furthermore, industry-funded trials were associated with wider age disparities, and government initiatives generally do not extend to that setting. The role of industry sponsorship is particularly noteworthy given the increasing proportion of industry-funded oncologic RCTs.11,12 Concerns regarding industry sponsorship leading to bias in results reporting have been expressed previously,13 but to our knowledge, no prior studies have demonstrated demographic disparities among trial participants as a function of industry funding. A potential contributing factor could be that industry-funded trials might be more available at centers treating a greater proportion of younger patients; notably, we identified no association between industry funding and increased use of age-based or performance status–based enrollment criteria. Understanding the basis for the observed association between industry sponsorship and age disparities is critical to address this challenge.
Limitations
While the disease sites included in this study represent the leading causes of cancer incidence and mortality, these sites may not be representative of the overall cancer trials landscape. This is mitigated by the fact that trial enrollees from these 4 sites account for most NCI trial participants.2 Additionally, population median ages by disease site were based on domestic SEER data. Most included trials (232 of 302; 76.8%) were multinational, and 26 trials (8.6%) enrolled exclusively in a single country outside the United States. Consequently, our study is limited by extrapolation of US demographics to other countries; differential screening practices for these disease sites in particular may affect country-specific age demographics.14 Moreover, SEER captures patients with the relevant diagnosis, not only those who received treatment; SEER population median ages could therefore be older than the ages of patients who received therapy.15 The SEER median age may also disproportionately exclude older patients with cancer owing to several possible factors (including patient/family preferences, patient comorbidities, and physician bias). This analysis may therefore underestimate the extent of trial participant age disparities based on the limitations of SEER median age.15 Lastly, the median age as a metric gives limited information regarding the exact proportion of older patients enrolled on a given study. The median was chosen owing to the heterogeneity of reporting age distribution across trials and served as a common measure across trials for comparison.
Conclusions
Age disparities among participants in oncologic RCTs are pervasive, worsening, and associated with industry sponsorship. Future efforts must focus on understanding the basis for these imbalances and addressing them; as the cancer population continues to age, such efforts are necessary to ensure both generalizability of trial results and trial access equity.
References
- 1.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27(17):1769-1773. doi: 10.1200/JCO.2008.20.8983 [DOI] [PubMed] [Google Scholar]
- 2.Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061-2067. doi: 10.1056/NEJM199912303412706 [DOI] [PubMed] [Google Scholar]
- 3.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21(7):1383-1389. doi: 10.1200/JCO.2003.08.010 [DOI] [PubMed] [Google Scholar]
- 4.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720-2726. doi: 10.1001/jama.291.22.2720 [DOI] [PubMed] [Google Scholar]
- 5.Pang HH, Wang X, Stinchcombe TE, et al. Enrollment trends and disparity among patients with lung cancer in national clinical trials, 1990 to 2012. J Clin Oncol. 2016;34(33):3992-3999. doi: 10.1200/JCO.2016.67.7088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freedman RA, Foster JC, Seisler DK, et al. Accrual of older patients with breast cancer to alliance systemic therapy trials over time: protocol A151527. J Clin Oncol. 2017;35(4):421-431. doi: 10.1200/JCO.2016.69.4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stinchcombe TE, Zhang Y, Vokes EE, et al. Pooled analysis of individual patient data on concurrent chemoradiotherapy for stage III non-small-cell lung cancer in elderly patients compared with younger patients who participated in US national cancer institute cooperative group studies. J Clin Oncol. 2017;35(25):2885-2892. doi: 10.1200/JCO.2016.71.4758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institutes of Health, National Cancer Institute, Surveillance Epidemiology, and End Results Cancer Statistics SEER Cancer Statistics Review 1975-2015: Table 12, median age of cancer patients at diagnosis, 2011-2015, by primary cancer site, race and sex. https://seer.cancer.gov/csr/1975_2015/browse_csr.php?sectionSEL=1&pageSEL=sect_01_table.12. Accessed: Febraury 5, 2019.
- 9.Corp IBM. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp. [Google Scholar]
- 10.NIH Revitalization Act, S 1, 103rd Cong (1993).
- 11.Booth CM, Cescon DW, Wang L, Tannock IF, Krzyzanowska MK. Evolution of the randomized controlled trial in oncology over three decades. J Clin Oncol. 2008;26(33):5458-5464. doi: 10.1200/JCO.2008.16.5456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun GH, Steinberg JD, Jagsi R. The calculus of national medical research policy: the United States versus Asia. N Engl J Med. 2012;367(8):687-690. doi: 10.1056/NEJMp1206643 [DOI] [PubMed] [Google Scholar]
- 13.Moy B, Jagsi R, Gaynor RB, Ratain MJ. The impact of industry on oncology research and practice. Am Soc Clin Oncol Educ Book. 2015:130-137. doi: 10.14694/EdBook_AM.2015.35.130 [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Yang L, Du C, Fang X, Wang N, Gu J. Characteristics and comparison of colorectal cancer incidence in Beijing with other regions in the world. Oncotarget. 2017;8(15):24593-24603. doi: 10.18632/oncotarget.15598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol. 2007;25(35):5570-5577. doi: 10.1200/JCO.2007.12.5435 [DOI] [PubMed] [Google Scholar]