Key Points
Question
How does the safety, tolerability, and pharmacokinetics (PK) of PF-06801591, an anti–PD-1 (programmed cell death 1) monoclonal antibody, administered subcutaneously (SC) compare with intravenous (IV) administration in patients with advanced solid tumors?
Findings
In this phase 1 dose-escalation trial of 40 patients with advanced or metastatic solid tumors, the safety and tolerability of both administration methods appears to be comparable. Exposure following SC administration was within the expected efficacious dose range, and objective responses were seen with both IV and SC dosing.
Meaning
Subcutaneous administration of an anti–PD-1 antibody in patients with advanced solid tumors appears to be feasible.
This phase 1 dose-escalation trial compares the safety, tolerability, and pharmacokinetics of PF-06801591, a monoclonal antibody, administered subcutaneously vs intravenously in patients with advanced or metastatic solid tumors.
Abstract
Importance
We assessed feasibility of monthly subcutaneous administration of PF-06801591, a humanized immunoglobulin G4 monoclonal antibody that binds to the programmed cell death (PD-1) receptor and blocks its interaction with PD-1 ligands.
Objective
To evaluate the safety, efficacy, and pharmacokinetics of PF-06801591 administered intravenously vs subcutaneously.
Design, Setting, and Participants
Ongoing phase 1, open-label, multicenter, dose-escalation study of 40 patients, 18 years or older, with locally advanced or metastatic solid tumors, enrolled between March 8, 2016, and March 5, 2018, from 4 US medical centers.
Interventions
An intravenous dose of 0.5, 1, 3, or 10 mg/kg of PF-06801591 was administered every 3 weeks or a subcutaneous dose of 300 mg was administered every 4 weeks. Dose escalation occurred after 2 to 4 patients were enrolled per dose level, with additional patients enrolled in each cohort for further assessment.
Main Outcomes and Measures
The primary end points were dose-limiting toxic effects and safety. Secondary end points included pharmacokinetics, immunogenicity, PD-1 receptor occupancy, and efficacy.
Results
Of 40 enrolled patients (12 men and 28 women; mean [SD] age, 61 [13] years) in this phase 1 dose-escalation trial, 25 received PF-06801591 intravenously at escalating dose levels (0.5, 1, 3, or 10 mg/kg) and 15 patients received the monoclonal antibody subcutaneously at a single dose level. No dose-limiting toxic effects were observed. Grade 3 or higher treatment-related adverse events occurred in 4 (16%) patients treated intravenously and 1 (6.7%) patient treated subcutaneously. Immune-related adverse events occurred in 10 (40%) patients treated intravenously and 3 (20%) treated subcutaneously. No dose–adverse event associations were observed during intravenous dose escalation, and no serious skin toxic effects occurred with subcutaneous delivery. Responses were seen in 5 patients receiving PF-06801591 intravenously and in 2 patients treated subcutaneously for an overall objective response rate of 18.4%. Median overall survival was not reached with intravenous dosing vs 10.7 months with subcutaneous administration. Exposure to PF-06801591 increased in a dose-proportional manner over the range of intravenous doses. Median time to maximum observed serum concentration was 8 days after subcutaneous administration. Full PD-1 receptor occupancy was seen in all dose cohorts.
Conclusions and Relevance
Anti–PD-1 antibody PF-06801591 was tolerable and showed antitumor activity in a variety of tumor types across all dose levels of intravenous and subcutaneous administration. Monthly subcutaneous administration of PF-06801591 offers a convenient, effective alternative to currently available intravenously administered checkpoint inhibitors.
Trial Registration
ClinicalTrials.gov identifier: NCT02573259
Introduction
Novel antitumor therapies that target the PD-1 (programmed cell death 1) receptor and its ligands (PD-L1 and PD-L2) can reverse cancer-mediated immune evasion.1 The PD-1 receptor is upregulated on activated effector T lymphocytes. Expression of PD-L1 is triggered on tumor cells and other immune cells upon cytokine release by activated T cells, and PD-L2 is mainly expressed on macrophages and dendritic cells.2,3,4 Binding of the PD-1 receptor to its ligands negatively regulates the antitumor immune response by inhibiting T-cell proliferation, cytokine production, and cytotoxic functions.5,6
Antibodies that block PD-1/PD-L1 have been approved for multiple tumor indications.7,8,9,10,11 PF-06801591 is a humanized immunoglobulin G4 monoclonal antibody that binds PD-1 to block its interaction with PD-L1 and PD-L2. This monoclonal antibody has been shown to induce T-cell proliferation and proinflammatory cytokine secretion in human activated CD8+ T cells in vitro.12
To date, all approved checkpoint inhibitors are administered intravenously (IV). However, as patients receiving these agents achieve durable responses and long-term survival, the cumulative time required for repetitive IV infusions in clinic may result in lost work productivity and personal time for patients and accumulating health care costs. Furthermore, as ongoing experimental efforts on combination regimens of anti–PD-1 with other IV medications increase, patients are spending more time in the clinic. The potential benefit associated with subcutaneous (SC) administration led to the evaluation of PF-06801591.
Studies of other SC-administered monoclonal antibodies for treatment of cancer have demonstrated noninferior efficacy with an improved or similar safety profile and/or tolerability to IV administration.13,14,15,16,17,18,19 Furthermore, SC administration is associated with greater patient satisfaction, lower cost, and lower resource use compared with IV administration.18,20,21,22,23,24,25 Unlike some other approved SC-administered monoclonal antibodies in oncology, which require a recombinant human hyaluronidase to enhance permeation,13,14 PF-06801591 does not require any modification or combination to enable SC delivery.
We report on the dose-escalation part of a phase 1 study of IV and SC administration of PF-06801591, which is, to our knowledge, the first study to demonstrate the feasibility of SC administration of an anti-PD-1 antibody in patients.
Methods
This ongoing, open-label, multicenter phase 1 study (NCT02573259) evaluates the safety, tolerability, pharmacokinetics (PK), immunogenicity, and efficacy of PF-06801591 after IV or SC administration in patients with locally advanced or metastatic solid tumors. The trial protocol is available in Supplement 1. The PD-1 receptor occupancy and baseline PD-L1 expression were also assessed. The study consists of 2 parts: dose escalation (part 1), which is reported herein, and dose expansion (part 2).
Patient Population
In part 1 (dose escalation) of the trial, adults (age, ≥18 years) with a diagnosis of locally advanced or metastatic solid tumors and 1 or more measurable lesions as defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 were enrolled between March 8, 2016, and March 5, 2018. Tumor types included in this study were melanoma, squamous cell carcinoma of head and neck, ovarian cancer, sarcoma, non–small cell lung cancer (NSCLC), urothelial carcinoma, and other tumors with reported clinical evidence of response to anti–PD-1/PD-L1 agents. Patients had progressed on 1 or more prior lines of therapy or refused standard-of-care therapy, were not previously treated with anti–PD-1/PD-L1 agents, and had adequate renal, bone marrow, liver, and cardiac function, with Eastern Cooperative Oncology Group performance status 0 or 1.
Patients with active brain or leptomeningeal metastases, choroidal (ocular) melanoma, active known or suspected autoimmune disease, or systemic treatment with corticosteroids or other immunosuppressive medications within 14 days of study drug administration as well as those experiencing prior grade 3 or higher immune-mediated adverse events (AEs) related to prior immune-modulatory therapy were excluded.
Study Design and Treatment
This study was approved by the IntegReview Ethical Review Board, Western Institutional Review Board, the University of California Los Angeles Institutional Review Board, and the University of North Carolina Institutional Review Board and was conducted in accordance with the protocol, International Conference on Harmonization Good Clinical Practice guidelines, and applicable local regulatory requirements and laws. Written patient informed consent was provided by all patients prior to study initiation.
Patients received PF-06801591 IV doses of 0.5, 1, 3, or 10 mg/kg every 3 weeks or a SC dose of 300 mg once monthly. The SC doses were administered into abdominal skin as 3 separate injections of 100 mg (2 mL of 50 mg/mL) at each dosing. The SC 300-mg once monthly dose was selected with considerations of nonclinical safety, clinical safety and tolerability data from the IV cohorts in the current study, and feasibility, in terms of injection volume, with the current formulation. The population was divided into 2 cohorts: safety and pharmacodynamics. The safety cohort enrolled 2 to 4 patients per dose level. The pharmacodynamic cohort enrolled 2 to 5 patients per dose level for IV injection and 11 patients for SC administration. Subcutaneous administration of 300 mg of PF-06801591 was initiated only after all patients in the IV 3 mg/kg safety cohort safely completed the 21-day observation period for dose-limiting toxic effects (DLTEs). Forty patients were enrolled in part 1 of the study. Treatment will continue for up to 2 years or until disease progression, unacceptable toxic effects, or withdrawal of consent.
Part 2 (dose expansion) of this trial, which is ongoing with results not yet available, will further evaluate the SC 300-mg once monthly dose in about 100 patients with advanced/metastatic NSCLC or urothelial carcinoma who have not been previously treated with an anti–PD-1/PD-L1 agent.
Dose-Limiting Toxic Effects
The following drug-related AEs that occurred during the first cycle of treatment (21 days for IV, 28 days for SC) were classified as DLTEs: grade 5 AE; any grade 4 hematologic AE, except grade 4 neutropenia lasting more than 5 days from initiation of granulocyte colony-stimulating factor; grade 4 thrombocytopenia associated with bleeding, platelet transfusion requirement, or platelet count less than 10,000/µL; nonhematologic grade 4 AE or grade 3 AE lasting more than 7 days despite optimal supportive care; grade 3 central nervous system AE, regardless of duration; and drug-induced liver injury.
Safety and Efficacy Assessments
Assessment of AEs included type, incidence, severity (graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, v4.03), timing, seriousness, and relatedness to the study drug. Other safety assessments included vital signs; physical examination; 12-lead electrocardiogram; local injection site tolerability (SC cohort only); laboratory tests, including pregnancy tests; and verification of concomitant treatments.
Tumor assessments by computed tomography or magnetic resonance imaging scans were conducted at baseline, during treatment (every 6 weeks for IV administration or 8 weeks for SC administration until confirmed disease progression by immune-related RECIST [irRECIST], death, withdrawal of consent, or subsequent administration of an anti-cancer therapy agent), whenever disease progression was suspected, and at the end of treatment (EOT).
Pharmacokinetics and Immunogenicity Assessments
Blood samples were collected for PK analysis of PF-06801591 in patients treated with IV administration at predose and 1 and 24 hours after the start of first infusion and days 3, 8, and 15 postdose cycle 1; predose and 1 hour postdose cycle 2; predose cycle 3; predose, 1 hour postdose, and days 8 and 15 postdose cycle 4; predose cycles 6, 8, and 10; and at the EOT. In the SC cohort, blood samples were collected as previously described, but also on day 21 of cycles 1 and 4 and predose cycle 5. The PF-06801591 serum concentrations were quantified using a validated electrochemiluminescence assay (lower limit of quantification ≥48 ng/mL). Standard PK parameters, including maximum serum concentration (Cmax), time to maximum serum concentration (Tmax), and area under the serum concentration vs time curve over the dosing interval (AUCτ) were estimated using noncompartmental analysis.
Blood samples for the determination of antidrug antibodies (ADAs) and neutralizing antibodies (NAbs) were collected predose at cycles 1, 2, 4, 6, 8, and 10, and at the EOT. Samples were tested for ADA using an internally developed and fully validated electrochemiluminescence bridging assay. Samples positive for ADA were further evaluated for the presence of NAbs using a validated cell-based assay.
Biomarker Assessments
Blood samples for PD-1 receptor occupancy were collected at predose, after 24 hours, and days 8, 15, and 21 (for SC only) of cycle 1; predose cycles 2 and 3; predose and days 15 and 21 (for SC only) of cycle 4; predose cycle 5; and at the EOT. The expression of free PD-1 receptor was measured on peripheral blood T-cell subsets by flow cytometry. The anti–PD-1 clone used was unable to bind in the presence of PF-06801591, hence a reduction in free PD-1 after dosing reflects that the PD-1 receptor is occupied by the drug.
Archived tissue samples were collected from all patients at screening (not required if a fresh tumor sample was obtained at screening). Expression of PD-L1 (clone SP-263; Ventana) was evaluated in available tumor biopsies by immunohistochemistry and pathologist scoring.
Statistical Analysis
The primary end points in part 1 of this study included DLTEs, AEs, and abnormal laboratory results. Secondary end points included PK; incidence of ADA and NAb; PD-1 receptor occupancy; and efficacy end points, including objective response rate (ORR) according to RECIST v1.126 and irRECIST,27 progression-free survival (PFS), duration of stable disease, and duration of response.
A modified toxicity probability interval method28 targeting a DLTE rate of 27.5% and an acceptable DLTE interval (22.5%-32.5%) was used for dose-escalation decisions with IV administration of PF-06801591. Safety data were summarized descriptively and included all enrolled patients who received 1 dose or more (n = 40).
Efficacy was summarized descriptively and analyzed separately in the intent-to-treat (ITT) population, which included all enrolled patients, and in the modified ITT (mITT) population, which included all enrolled patients who received 1 dose or more of PF-06801591, with measurable disease at baseline assessment (within 28 days prior to study entry) and 1 or more postbaseline assessment or disease progression or death before the first tumor assessment.
Results
Patients
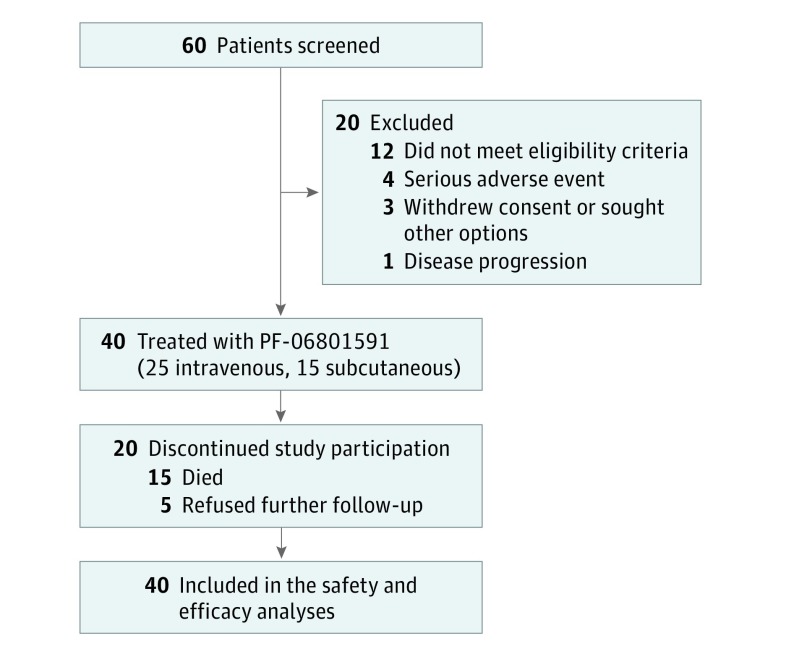
Between March 8, 2016, and March 5, 2018 (data cutoff), 40 patients (12 men and 28 women; mean [SD] age, 61 [13] years) were enrolled from 4 US medical centers and treated in the dose-escalation phase, with 25 patients in the IV (0.5 mg/kg, n = 2; 1 mg/kg, n = 8; 3 mg/kg, n = 8; 10 mg/kg, n = 7) cohorts and 15 patients in the 300 mg SC cohort (Figure 1). At data cutoff with a median follow-up period of 14.7 and 7.4 months in the IV and SC cohorts, respectively, 34 patients discontinued treatment (median [range] duration of treatment was 2.8 [0.03-20.96] months) because of either death (n = 3), AEs (n = 3), global deterioration of health (n = 5), disease progression or relapse (n = 20), or refusal to continue treatment for other reasons (n = 3), whereas 6 patients were still receiving treatment (median [range] duration of treatment was 14.5 [8.3-23.1] months). Patient demographic and baseline characteristics are summarized in the Table.
Figure 1. CONSORT Flow Diagram.
Table. Patient Demographic and Baseline Characteristics, by Cohort Arm.
| Characteristic | No. (%)a | ||
|---|---|---|---|
| All IV Doses (n = 25) |
300 mg SC Dose (n = 15) |
Total (N = 40) | |
| Sex | |||
| Male | 8 (32) | 4 (27) | 12 (30) |
| Female | 17 (68) | 11 (73) | 28 (70) |
| Age, mean (SD) [range], y | 61 (14) [26-87] | 61 (9) [50-77] | 61 (13) [26-87] |
| ECOG PS | |||
| 0 | 14 (56) | 6 (40) | 20 (50) |
| 1 | 11 (44) | 9 (60) | 20 (50) |
| Primary cancer diagnosis | |||
| Ovarian | 11 (44) | 4 (27) | 15 (38) |
| Sarcoma | 6 (24) | 0 | 6 (15) |
| SCCHN | 5 (20) | 2 (13) | 7 (18) |
| SCLC | 1 (4) | 2 (13) | 3 (8) |
| Peritoneal neoplasm | 1 (4) | 1 (7) | 2 (5) |
| Esophageal | 0 | 2 (13) | 2 (5) |
| Endometrial | 0 | 1 (7) | 1 (3) |
| Melanoma | 1 (4) | 0 | 1 (3) |
| NSCLC | 0 | 1 (7) | 1 (3) |
| RCC | 0 | 1 (7) | 1 (3) |
| Salivary gland | 0 | 1 (7) | 1 (3) |
| No. of prior therapiesb | |||
| Mean (range) | 3.0 (0-8) | 2.7 (0-8) | 2.9 (0-8) |
| Chemotherapy | 22 (88) | 13 (87) | 35 (88) |
| Targeted therapy | 12 (48) | 5 (33) | 17 (43) |
| Immuno-oncology (OX-40 or CTLA-4) | 2 (8) | 0 | 2 (5) |
| Baseline PD-L1 expressionc | |||
| High | 7 (28) | 3 (20) | 10 (25) |
| Low | 12 (48) | 10 (67) | 22 (55) |
| Not determinedd | 6 (24) | 2 (13) | 8 (20) |
Abbreviations: CTLA-4, cytotoxic T-lymphocyte–associated antigen 4; ECOG PS, Eastern Cooperative Oncology Group performance status; IV, intravenous; NSCLC, non–small cell lung cancer; PD-L1, programmed cell death ligand 1; RCC, renal cell carcinoma; SC, subcutaneous; SCCHN, squamous cell carcinoma of the head and neck; SCLC, small cell lung cancer.
The number and percentage of patients are listed unless otherwise specified.
Groups of types of prior therapy are not mutually exclusive.
High expression is greater than or equal to 1% of tumor cells expressing PD-L1 (programmed cell death ligand 1), and low expression is less than 1% of tumor cells expressing PD-L1. The percentage is based on the total number of patients.
Reflects patients with no available tumor tissue for PD-L1 determination.
Safety
No DLTEs were observed nor was a maximum tolerated dose reached. Median (range) duration of treatment was 5.6 (0.03-23.09) months with IV administration of PF-06801591 and 2.1 (0.03-8.77) months with SC administration. Treatment-emergent AEs across all cycles occurred in 24 (96%) patients treated with PF-06801591 IV and in 15 (100%) treated with SC administration of the drug. Grade 3 or higher AEs occurred in 11 (44%) and 6 (40%) patients treated with PF-06801591 IV and SC, respectively. The most common all-causality and treatment-related AEs are reported in eTable 1 in Supplement 2. In 4 (10%) patients, treatment-related grade 3 AEs occurred, including fatigue (0.5 mg/kg IV), dermatitis bullous (1 mg/kg IV), pancreatic failure and peripheral sensory neuropathy (3 mg/kg IV), and anemia (300 mg SC). Treatment-related grade 4 AE (hyperglycemia) occurred in 1 patient (10 mg/kg IV). No treatment-related grade 5 AEs occurred. Ten (40.0%) and 3 (20.0%) patients treated with PF-06801591 IV and SC, respectively, had immune-related AEs (eTable 1 in Supplement 2).
Serious AEs (all causality, all cycles) occurred in 9 (36.6%) patients treated with IV PF-06801591: disease progression (n = 5); upper gastrointestinal hemorrhage (n = 2); and atrial fibrillation, infected neoplasm, intestinal obstruction, and pelvic fracture (n = 1 each). Serious AEs (all causality, all cycles) occurred in 6 (40%) patients treated with SC administration: neoplasm progression (n = 2) and dysphagia, lung infection, pneumonia, pneumonitis, respiratory failure, sepsis, small intestinal obstruction, and vomiting (n = 1 each). Treatment-related serious AE (grade 2 pneumonitis) occurred in 1 patient treated with SC PF-06801591 who received steroid therapy and recovered. The only reported SC injection-site reaction was 1 instance of grade 1 injection-site pain. Treatment discontinuations owing to treatment-emergent AEs occurred in 7 (28.0%) patients who received IV PF-06801591 and 1 (6.7%) patient who received SC PF-06801591. Dose interruptions owing to treatment-emergent AEs occurred in 10 (40.0%) and 3 (20.0%) patients treated with PF-06801591 IV and SC, respectively (eTable 1 in Supplement 2).
Efficacy
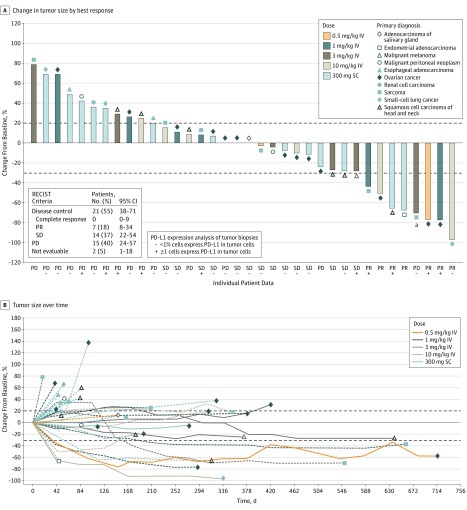
Two patients discontinued the study without any postbaseline tumor scan for reasons other than death or disease progression and were not included in the mITT population. In the mITT population (n = 38), ORR was achieved in 7 (18.4%; n = 5 IV, n = 2 SC) patients according to both RECIST and irRECIST (Figure 2A and eTable 2 in Supplement 2). Partial responses were observed for ovarian cancer, squamous cell carcinoma of head and neck, sarcoma, small cell lung cancer, and microsatellite instability-high endometrial adenocarcinoma. One patient with alveolar soft-part sarcoma treated with 3 mg/kg PF-06801591 IV had a 30% or more reduction in target lesion tumor burden, but a new measurable lesion outside of the field of the initial baseline computed tomography scan was identified, and the patient was reported as having progressive disease. A retrospective assessment revealed that the new measurable lesion had been noted on scans prior to study screening and later scans showed that it regressed 40% on treatment. Disease control (responses plus stable disease) was achieved in 21 (55.3%; n = 14 IV, n = 7 SC) and 24 (63.2%; n = 16 IV, n = 8 SC) patients by RECIST and irRECIST, respectively (Figure 2A and eTable 2 in Supplement 2). Median (range) duration of response was 7 (3-12) months and 8 (3-15) months according to RECIST and irRECIST, respectively. Median (range) duration of stable disease was 6 (2-21) months and 6 (1-21) months, according to RECIST and irRECIST, respectively. Tumor response to therapy over time is shown in Figure 2B.
Figure 2. Percent Change in Tumor Size in the Modified Intent-to-Treat Population of 38 Patients.
aPatient had 30% or more reduction in target lesion tumor burden, but a new measurable lesion outside of field of the initial baseline CT scans was identified, and the patient was reported as having progressive disease (PD). A retrospective assessment showed the new measurable lesion had been noted on scans prior to study screening, and later scans showed that it regressed 40% on treatment. B, Solid lines represent patients still on treatment, and dashed lines represent patients who have discontinued treatment. IV indicates intravenous; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors version 1.1; SC, subcutaneous; SD, stable disease.
Based on RECIST criteria, median (95% CI) investigator-assessed PFS was 4.0 (1.6-5.6) months in the mITT population, 4.7 (1.4-8.8) months in the IV cohort, and 3.0 (1.6-6.0) months in the SC cohort (eFigure 1 in Supplement 2).
For ovarian cancer, the highest enrolled tumor type (n = 15), 3 (20.0%) patients achieved partial response and 6 (40.0%) had stable disease (Figure 2A), with median PFS of 5.3 (95% CI, 1.3-8.3) months and median overall survival not reached (95% CI, 9.1-not reached) months.
Pharmacokinetics and Immunogenicity
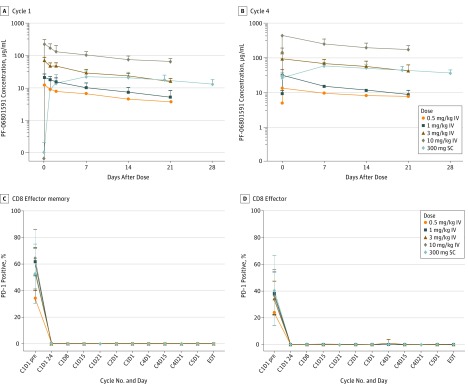
PF-06801591 demonstrated linear PK over the dose range of 0.5-10 mg/kg IV, with dose-dependent increases in the mean Cmax and AUCτ observed in cycle 1 (eTable 3 in Supplement 2 and Figure 3A). The coefficient of variation values for Cmax and AUCτ were 24% to 35% and 22% to 36%, respectively (n = 2-8 per dose level), following the first IV dose. After SC administration, PF-06801591 was slowly absorbed, with a median Tmax of about 8 days (n = 15). Steady state exposure after repeated SC 300-mg once monthly dosing fell within the range observed following IV dosing of 1 mg/kg and 3 mg/kg every 3 weeks, suggesting approximately 60% SC bioavailability (eTable 3 in Supplement 2 and Figure 3B).
Figure 3. Concentration-Time Profiles of PF-06801591 After Intravenous or Subcutaneous Administration and PD-1 Receptor Occupancy by PF-06801591.
Concentration-time profiles (A and B) were assayed using serum and the PD-1 (programmed cell death 1) receptor occupancy (C and D), as measured by reduction in free cell surface PD-1 postdosing, was assayed using whole blood taken at the identified time points from patients treated with PF-06801591. Representative plots are shown for PD-1 receptor occupancy. Values represent mean ± SD. C indicates cycle; D, day; EOT, end of treatment; IV, intravenous; pre, predose; SC, subcutaneous.
Preliminary immunogenicity assessment included all patients treated with PF-06801591 who had 1 or more valid ADA results. Of 35 evaluable patients, 3 (8.6%) were positive for treatment-emergent ADAs following IV (n = 21) or SC (n = 14) administration of the drug: 1 each in 0.5-mg/kg IV, 1-mg/kg IV, and 300-mg SC cohorts, with onset at day 1 of cycles 2, 6, and 4, respectively. The presence of ADAs was not associated with hypersensitivity or infusion reactions and did not significantly affect PF-06801591 clearance. No NAbs against PF-06801591 were detected in patients with ADAs.
Biomarker Assessments
Full PD-1 receptor occupancy was seen on peripheral CD8+ T cells across patients in all dose level cohorts irrespective of baseline PD-1 expression levels (Figure 3C). The ORR was higher in patients with high (≥1%) PD-L1 expression compared with patients with low (<1%) PD-L1 expression or the total population (eTable 2 in the Supplement).
Discussion
In the dose-escalation portion of this phase 1 study, PF-06801591 IV administration of 0.5 to 10 mg/kg every 3 weeks or SC administration of 300 mg once monthly was well tolerated and showed antitumor activity in patients with locally advanced or metastatic solid tumors. To our knowledge, this is the first study to demonstrate the feasibility of SC administration of a PD-1 inhibitor. In terms of safety, no DLTEs were observed and most treatment-related AEs were grade 1 or 2. There was no dose-AE association with IV dose escalation and no significant injection-site toxic effects with SC administration. The most commonly occurring treatment-related AEs in this study were consistent with those previously reported for other checkpoint inhibitors in patients with solid tumors.29,30,31,32,33,34
Although all currently available immune checkpoint inhibitors are administered via the IV route, studies have reported a consistent patient preference for SC vs IV drug administration.23,24 Most IV therapies require more frequent clinic visits and longer time spent in the clinic for drug infusion; hence, SC administration not only can reduce time spent in the clinic but can also decrease medical costs and make more efficient use of resources while simultaneously improving patient experience and satisfaction.21,22,35
We observed that PF-06801591 had efficacy similar to that of published data on other PD-1 checkpoint inhibitors. Objective responses were observed in patients across almost all IV and SC cohorts. The ORR was 18.4% (20% in 15 patients with ovarian cancer) with duration of response ranging from 2.7 to more than 12.4 months, which compares favorably with ORR previously reported in patients with similar solid tumor subtypes, and specifically ovarian cancer (5.9%-15%) treated with PD-1 blockade.11,30,33
The pharmacokinetics of PF-06801591 following IV administration were similar to those observed for other anti–PD-1 antibodies.34,36 Steady state exposure following SC administration of 300 mg of PF-06801591 once monthly fell within the range observed with IV dosing at 1 mg/kg and 3 mg/kg every 3 weeks, which is consistent with the dose range for anti–PD-1 antibodies as a class where additional efficacy benefit is not gained by higher doses.37,38 Despite different routes of administration, preliminary data did not suggest any differences in the incidence of immunogenicity of PF-06801591 administered IV vs SC, which is consistent with observation of other therapeutic proteins that have been evaluated by both routes.39 Tolerability, PK, PD-1 receptor occupancy, feasibility, and preliminary antitumor activity observed in part 1, in addition to prior knowledge from other anti–PD-1 antibodies, appear to support the appropriateness of the SC regimen explored in this study. Thus, SC administration of 300 mg of PF-06801591 once a month was selected for further evaluation in part 2 dose expansion.
In ongoing studies, the anti–PD-1 antibodies pembrolizumab (NCT03665597) and nivolumab (NCT03656718) and anti–PD-L1 antibodies atezolizumab (NCT03735121) and KN03540 are also being tested subcutaneously either alone or in combination with recombinant human hyaluronidase with dose intervals of 1 to 3 weeks.
Conclusions
PF-06801591 appears to be safe and tolerable and demonstrated antitumor activity in a variety of tumor types with both IV and SC routes of administration. No modifications to PF-06801591 or additions such as hyaluronidase were required to enable SC administration. Further characterization of SC administration of the drug at 300 mg once monthly in NSCLC and urothelial carcinoma, where the activity of anti-PD1 is well-known,8,10,11,41,42,43 is ongoing.
Trial Protocol
eFigure 1. Kaplan-Meier Estimate of Progression-Free Survival Based on Investigator Assessment by IV Combined versus Subcutaneous (RECIST), mITT
eTable 1. Treatment-Emergent Adverse Events–All Cycles, by PF-06801591 Arm
eTable 2. Summary of Tumor Assessments, mITT Analysis
eTable 3. PK Parameters of PF-06801591 After IV or SC Administration
Data Sharing Statement
References
- 1.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707-723. doi: 10.1016/j.cell.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537-1544. doi: 10.1182/blood-2008-12-195792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350-1355. doi: 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2(2):116-126. doi: 10.1038/nri727 [DOI] [PubMed] [Google Scholar]
- 5.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704. doi: 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069-1086. doi: 10.1158/2159-8290.CD-18-0367 [DOI] [PubMed] [Google Scholar]
- 7.Imfinzi (durvalumab) injection for intravenous use [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP. 2017. https://www.azpicentral.com/imfinzi/imfinzi.pdf#page=1. Accessed September 10, 2018.
- 8.OPDivo (nivolumab) solution, intravenous infusion [package insert]. Princeton, NJ: Bristol-Myers Squibb Co. 2017. https://packageinserts.bms.com/pi/pi_opdivo.pdf. Accessed September 10, 2018.
- 9.Bavencio (avelumab) for intravenous use [package insert]. Rockland, MD: Serono EMD. 2017. http://www.emdserono.com/bavencioPrescribingInformation. Accessed September 10, 2018.
- 10.Tecentriq (atezolizumab) for intravenous infusion [package insert]. San Francisco, CA: Genentech. 2017. https://www.gene.com/download/pdf/tecentriq_prescribing.pdf. Accessed September 10, 2018.
- 11.Keytruda (pembrolizumab) lyophilized powder; intravenous infusion [package insert]. County Cork, Ireland: Merck & Co 2017. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf. Accessed September 10, 2018.
- 12.Youssef S, Abdiche Y, Nguyen H, et al. In vitro properties and pre-clinical activity of PF-06801591, a high-affinity engineered anti-human PD-1 [abstract]. Cancer Res. 2017;77(13)(suppl):Abstract nr 2667. [Google Scholar]
- 13.Davies A, Merli F, Mihaljević B, et al. Efficacy and safety of subcutaneous rituximab versus intravenous rituximab for first-line treatment of follicular lymphoma (SABRINA): a randomised, open-label, phase 3 trial. Lancet Haematol. 2017;4(6):e272-e282. doi: 10.1016/S2352-3026(17)30078-9 [DOI] [PubMed] [Google Scholar]
- 14.Ismael G, Hegg R, Muehlbauer S, et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012;13(9):869-878. doi: 10.1016/S1470-2045(12)70329-7 [DOI] [PubMed] [Google Scholar]
- 15.Lugtenburg P, Avivi I, Berenschot H, et al. Efficacy and safety of subcutaneous and intravenous rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in first-line diffuse large B-cell lymphoma: the randomized MabEase study. Haematologica. 2017;102(11):1913-1922. doi: 10.3324/haematol.2017.173583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merz M, Salwender H, Haenel M, et al. Subcutaneous versus intravenous bortezomib in two different induction therapies for newly diagnosed multiple myeloma: an interim analysis from the prospective GMMG-MM5 trial. Haematologica. 2015;100(7):964-969. doi: 10.3324/haematol.2015.124347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minarik J, Pavlicek P, Pour L, et al. ; Czech Myeloma Group . Subcutaneous bortezomib in multiple myeloma patients induces similar therapeutic response rates as intravenous application but it does not reduce the incidence of peripheral neuropathy. PLoS One. 2015;10(4):e0123866. doi: 10.1371/journal.pone.0123866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12(5):431-440. doi: 10.1016/S1470-2045(11)70081-X [DOI] [PubMed] [Google Scholar]
- 19.Wu S, Zheng C, Chen S, et al. Subcutaneous administration of bortezomib in combination with thalidomide and dexamethasone for treatment of newly diagnosed multiple myeloma patients. Biomed Res Int. 2015;2015:927105. doi: 10.1155/2015/927105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franken MG, Kanters TA, Coenen JL, et al. Potential cost savings owing to the route of administration of oncology drugs: a microcosting study of intravenous and subcutaneous administration of trastuzumab and rituximab in the Netherlands. Anticancer Drugs. 2018;29(8):791-801. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Vivanco G, Salvador J, Diez R, et al. Cost minimization analysis of treatment with intravenous or subcutaneous trastuzumab in patients with HER2-positive breast cancer in Spain. Clin Transl Oncol. 2017;19(12):1454-1461. doi: 10.1007/s12094-017-1684-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olofsson S, Norrlid H, Karlsson E, Wilking U, Ragnarson Tennvall G. Societal cost of subcutaneous and intravenous trastuzumab for HER2-positive breast cancer—an observational study prospectively recording resource utilization in a Swedish healthcare setting. Breast. 2016;29:140-146. doi: 10.1016/j.breast.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 23.Pivot X, Gligorov J, Müller V, et al. ; PrefHer Study Group . Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol. 2013;14(10):962-970. doi: 10.1016/S1470-2045(13)70383-8 [DOI] [PubMed] [Google Scholar]
- 24.Rummel M, Kim TM, Aversa F, et al. Preference for subcutaneous or intravenous administration of rituximab among patients with untreated CD20+ diffuse large B-cell lymphoma or follicular lymphoma: results from a prospective, randomized, open-label, crossover study (PrefMab). Ann Oncol. 2017;28(4):836-842. [DOI] [PubMed] [Google Scholar]
- 25.Ponzetti C, Canciani M, Farina M, Era S, Walzer S. Potential resource and cost saving analysis of subcutaneous versus intravenous administration for rituximab in non-Hodgkin’s lymphoma and for trastuzumab in breast cancer in 17 Italian hospitals based on a systematic survey. Clinicoecon Outcomes Res. 2016;8:227-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 27.Seymour L, Bogaerts J, Perrone A, et al. ; RECIST working group . iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143-e152. doi: 10.1016/S1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji Y, Liu P, Li Y, Bekele BN. A modified toxicity probability interval method for dose-finding trials. Clin Trials. 2010;7(6):653-663. doi: 10.1177/1740774510382799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an anti-programmed death-ligand 1 antibody, In patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol. 2017;35(19):2117-2124. doi: 10.1200/JCO.2016.71.6795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455-2465. doi: 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856-1867. doi: 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gulley JL, Rajan A, Spigel DR, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol. 2017;18(5):599-610. doi: 10.1016/S1470-2045(17)30240-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015-4022. doi: 10.1200/JCO.2015.62.3397 [DOI] [PubMed] [Google Scholar]
- 34.Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21(19):4286-4293. doi: 10.1158/1078-0432.CCR-14-2607 [DOI] [PubMed] [Google Scholar]
- 35.North RT, Harvey VJ, Cox LC, Ryan SN. Medical resource utilization for administration of trastuzumab in a New Zealand oncology outpatient setting: a time and motion study. Clinicoecon Outcomes Res. 2015;7:423-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto N, Nokihara H, Yamada Y, et al. Phase I study of nivolumab, an anti-PD-1 antibody, in patients with malignant solid tumors. Invest New Drugs. 2017;35(2):207-216. doi: 10.1007/s10637-016-0411-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chatterjee M, Turner DC, Felip E, et al. Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann Oncol. 2016;27(7):1291-1298. doi: 10.1093/annonc/mdw174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Feng Y, Bajaj G, et al. Quantitative characterization of the exposure-response relationship for cancer immunotherapy: a case study of nivolumab in patients with advanced melanoma. CPT Pharmacometrics Syst Pharmacol. 2017;6(1):40-48. doi: 10.1002/psp4.12133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamuro L, Kijanka G, Kinderman F, et al. Perspectives on subcutaneous route of administration as an immunogenicity risk factor for therapeutic proteins. J Pharm Sci. 2017;106(10):2946-2954. doi: 10.1016/j.xphs.2017.05.030 [DOI] [PubMed] [Google Scholar]
- 40.Papadopoulos KPH, Harb W, Lu N, et al. Phase I study of KN035: a novel fusion anti-PD-L1 antibody administered subcutaneously in patients with advanced solid tumors in the USA. Ann Oncol. 2018;29(suppl 8). doi: 10.1093/annonc/mdy288.013 [DOI] [Google Scholar]
- 41.Balar AV, Galsky MD, Rosenberg JE, et al. ; IMvigor210 Study Group . Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67-76. doi: 10.1016/S0140-6736(16)32455-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909-1920. doi: 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312-322. doi: 10.1016/S1470-2045(17)30065-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Kaplan-Meier Estimate of Progression-Free Survival Based on Investigator Assessment by IV Combined versus Subcutaneous (RECIST), mITT
eTable 1. Treatment-Emergent Adverse Events–All Cycles, by PF-06801591 Arm
eTable 2. Summary of Tumor Assessments, mITT Analysis
eTable 3. PK Parameters of PF-06801591 After IV or SC Administration
Data Sharing Statement