This study examines the trends in incidence rates of pediatric thyroid cancer in the United States from 1973 to 2013 by demographic and tumor characteristics.
Key Points
Question
What are the trends in US pediatric thyroid cancer incidence rates?
Findings
In this cross-sectional study of 1806 pediatric patients with thyroid cancer diagnosed from 1973 to 2013, the annual percent change in pediatric thyroid cancer incidence increased from 1.1% per year from 1973 to 2006 to 9.5% per year from 2006 to 2013.
Meaning
The findings suggest that the marked increase in pediatric thyroid cancer between 2006 and 2013 was not solely attributable to enhanced detection.
Abstract
Importance
The incidence of thyroid cancer is increasing by 3% annually. This increase is often thought to be attributable to overdiagnosis in adults. A previous study reported a 1.1% annual increase in the incidence of pediatric thyroid cancer. However, the analysis was limited to the period from 1973 to 2004 and was performed in a linear fashion, which does not account for changes in incidence trends over time.
Objective
To analyze trends in pediatric thyroid cancer incidence based on demographic and tumor characteristics at diagnosis.
Design, Setting, and Participants
This cross-sectional study included individuals younger than 20 years who had a diagnosis of thyroid cancer in the Surveillance, Epidemiology, and End Results (SEER) 9 database from 1973 to 2013. Cases of thyroid cancer were identified using the International Classification of Diseases for Oncology, Third Edition and were categorized by histologic type, stage, and tumor size.
Main Outcomes and Measures
Annual percent change (APC) in the incidence rates was calculated using joinpoint regression analysis.
Results
Among 1806 patients included in the analysis, 1454 (80.5%) were female and 1503 (83.2%) were white; most patients were aged 15 to 19 years. The overall incidence rates of thyroid cancer increased annually from 0.48 per 100 000 person-years in 1973 to 1.14 per 100 000 person-years in 2013. Incidence rates gradually increased from 1973 to 2006 (APC, 1.11%; 95% CI, 0.56%-1.67%) and then markedly increased from 2006 to 2013 (APC, 9.56%; 95% CI, 5.09%-14.22%). The incidence rates of large tumors (>20 mm) gradually increased from 1983 to 2006 (APC, 2.23%; 95% CI, 0.93%-3.54%) and then markedly increased from 2006 to 2013 (APC, 8.84%; 95% CI, 3.20%-14.79%); these rates were not significantly different from incidence rates of small (1-20 mm) tumors. The incidence rates of regionally extended thyroid cancer gradually increased from 1973 to 2006 (APC, 1.44%; 95% CI, 0.68%-2.21%) and then markedly increased from 2006 to 2013 (APC, 11.16%; 95% CI, 5.26%-17.40%); these rates were not significantly different from the incidence rates of localized disease.
Conclusions and Relevance
The incidence rates of pediatric thyroid cancer increased more rapidly from 2006 to 2013 than from 1973 to 2006. The findings suggest that there may be a co-occurring increase in thyroid cancer in the pediatric population in addition to enhanced detection.
Introduction
Thyroid cancer is the most rapidly increasing cancer in the United States.1 The incidence rates increased by 3% annually from 1974 to 2013, from 4.6 per 100 000 person-years from 1974 to 1977 to 14.4 per 100 000 person-years from 2010 to 2013.2 In adults, it has been hypothesized that overdiagnosis, attributable to the increased ability to detect and diagnose small, indolent tumors that would remain asymptomatic and otherwise not require treatment, is responsible for this phenomenon.3,4,5,6,7 However, others hypothesize that increased thyroid cancer incidence-based mortality rates since the late 1980s and increased incidence rates of large tumors and advanced-stage disease demonstrate a true increase in occurrence of thyroid cancer.2,8,9
Thyroid cancer is the most common endocrine cancer in the pediatric population.10 Beginning in 2002, there were specific initiatives to limit children’s exposure to ionizing radiation to decrease the risk of later development of cancer.11,12 Consequently, children are less likely than adults to undergo imaging studies, potentially limiting the association of overdiagnosis with thyroid cancer incidence trends. Few studies to date have examined incidence trends of pediatric thyroid cancer in the United States. One study reported an incidence rate increase of 1.1% per year.13 However, this study analyzed incidence trends in a linear fashion, which does not account for variation in trends over time. Moreover, the analysis was limited to the period from 1973 to 2004, and it is unknown whether the incidence rates have increased since 2004. The goals of our study were to analyze pediatric thyroid cancer incidence trends by demographic and tumor characteristics in the United States from 1973 to 2013 and pediatric thyroid cancer incidence-based mortality from 1983 to 2013.
Methods
This study was deemed to be exempt from human subjects review by the Stanford University Institutional Review Board because it used a deidentified, public use research file, the Surveillance, Epidemiology, and End Results (SEER) 9 cancer incidence file maintained by the National Cancer Institute. This file includes information from 9 high-quality, population-based registries accounting for approximately 10% of the US population: California (San Francisco and Oakland), Connecticut, Georgia (Atlanta), Hawaii, Iowa, Michigan (Detroit), New Mexico, Utah, and Washington (Seattle and Puget Sound region). Age-adjusted incidence rates of thyroid cancer among patients younger than 20 years were calculated for the years 1973 through 2013. The incidence-based mortality analysis was restricted to deaths that occurred between 1983 and 2013 to prevent underestimation of mortality rates in the earlier years.
Thyroid cancer cases between 1973 and 2013 were identified by International Classification of Diseases for Oncology (ICD-O), Third Edition topography code C73. Cases were classified according to histologic type (ICD-O histologic codes) as papillary thyroid cancer (8050, 8260, 8340-8344, 8350, and 8450-8460), follicular thyroid cancer (8290, 8330-8335), medullary thyroid cancer (8345, 8510-8513), and anaplastic thyroid cancer (8020-8035). Stage at diagnosis was recorded in SEER from 1973 to 2013, by SEER Historic Stage A, as localized (limited to the thyroid gland), regional (tumor extension beyond the limits of the thyroid gland or spread by more than 1 lymphatic or vascular supply route), distant (extracervical metastasis), and unknown. Tumor size has been recorded in SEER since 1983 using 3 different schemes: Extent of Disease–4 codes from 1983 to 1987, Extent of Disease–10 codes from 1988 to 2003, and Collaborative Staging codes from 2004 to 2013. These codes were combined to categorize cases into small tumors (1-20 mm) and large tumors (>21 mm).14
Statistical Analysis
The SEER computer software (SEER*Stat, version 8.3.4 [National Cancer Institute; Information Management Services]) was used to calculate incidence and mortality rates. All rates were age adjusted to the 2000 US standard population and expressed per 100 000 person-years. Incidence-based mortality rates were calculated as the number of deaths from thyroid cancer among cases diagnosed in the SEER-9 registries over person-time at risk among individuals in the SEER areas. A joinpoint regression analysis program (Joinpoint Regression Program, version 4.4.0.0 [National Cancer Institute]) was used to calculate annual percentage change (APC) and 95% CIs. t Tests were used to determine whether APCs were statistically significantly different from zero. The best-fitting log-linear regression model was selected to identify calendar years (joinpoints) when APCs changed significantly.15 An estimate was considered to be statistically significant at α = .05, and tests were 2-tailed.
Results
We identified 1806 cases of thyroid cancer in residents of the SEER-9 areas who were younger than 20 years from 1973 to 2013. The case counts and incidence rates according to demographic characteristics are shown in Table 1. Female patients (1454 [80.5%]), white patients (1503 [83.2%]), and patients aged 15 to 19 years (1383 [76.6%]) comprised most cases.
Table 1. Demographic Characteristics and Incidence Rates of Pediatric Thyroid Cancer per SEER Data, 1973-2013.
| Characteristic | Cases, No. (%) | Incidence Rate, per 100 000 Person-Years (95% CI) |
|---|---|---|
| Overall | 1806 (100) | 0.60 (0.44-0.81) |
| Sex | ||
| Male | 352 (19.5) | 0.23 (0.11-0.44) |
| Female | 1454 (80.5) | 0.99 (0.69-1.37) |
| Race | ||
| White | 1503 (83.2) | 0.66 (0.47-0.91) |
| Black | 87 (4.8) | 0.21 (0.04-0.75) |
| Other | 189 (10.5) | 0.59 (0.19-1.48) |
| Unknown | 27 (1.5) | NA |
| Age, y | ||
| 0-9 | 75 (4.1) | 0.05 (0.01-0.20) |
| 10-14 | 348 (19.3) | 0.46 (0.21-0.90) |
| 15-19 | 1383 (76.6) | 1.81 (1.26-2.53) |
Abbreviations: NA, not applicable; SEER, Surveillance, Epidemiology, and End Results.
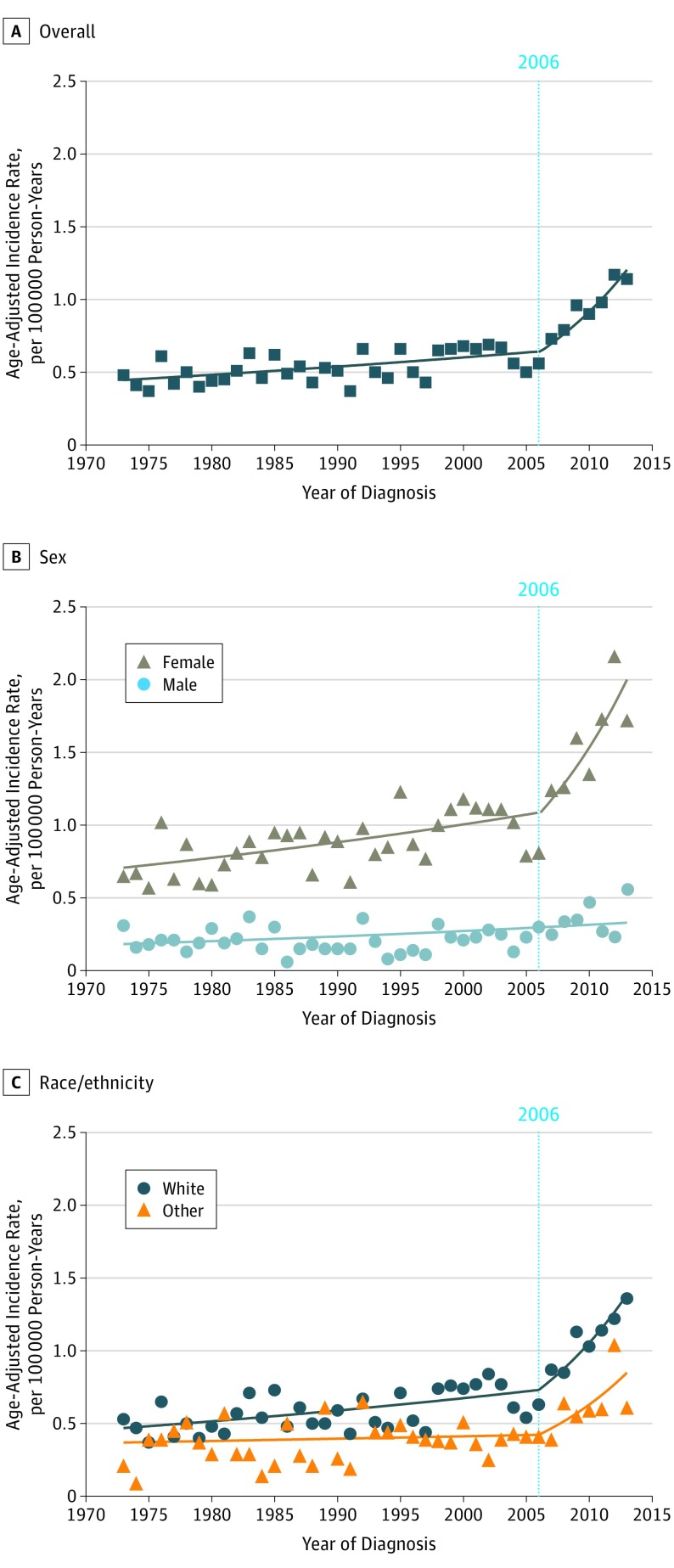
Overall, the incidence of pediatric thyroid cancer increased from 0.48 per 100 000 person-years (95% CI, 0.32-0.68 per 100 000 person-years) in 1973 to 1.14 per 100 000 person-years (95% CI, 0.91-1.4 per 100 000 person-years) in 2013 (mean APC, 2.54; 95% CI, 1.69-3.40). The results of the joinpoint regression analyses are shown in Table 2. Pediatric thyroid cancer incidence rates gradually increased from 1973 to 2006 (APC, 1.11%; 95% CI, 0.56%-1.67%) and then markedly increased from 2006 to 2013 (APC, 9.56%; 95% CI, 5.09%-14.22%) (Figure 1A). Incidence rates among females gradually increased from 1973 to 2006 (APC, 1.31%; 95% CI, 0.67%-1.95%) and then markedly increased from 2006 to 2013 (APC, 9.29%; 95% CI, 4.20% -14.62%), whereas the incidence rates among males gradually increased from 1973 to 2013 (APC, 1.48%; 95% CI, 0.52%-2.45%) with no joinpoints (Figure 1B). The incidence rates in the white population gradually increased from 1973 to 2006 (APC, 1.35%; 95% CI, 0.69%-2.02%) and then markedly increased from 2006 to 2013 (APC, 9.56%; 95% CI, 4.22%-15.18%). The incidence rates in black and other racial/ethnic groups were too low to individually perform joinpoint analysis, but when combined, the incidence rates in the nonwhite population increased gradually from 1973 to 2006 (APC, 2.10%; 95% CI, 0.28%-2.13%) and then markedly increased from 2006 to 2013 (APC, 10.49%; 95% CI, 1.29%-20.51%) (Figure 1C). The incidence rates of thyroid cancer in the population aged 10 to 14 years increased gradually from 1973 to 2013 (APC, 1.20%; 95% CI. 0.28%-3.96%) with no joinpoints, whereas the rates in the population aged 15 to 19 years gradually increased from 1973 to 2006 (APC, 1.24%; 95% CI, 0.60%-1.89%) and then markedly increased from 2006 to 2013 (APC, 10.54%; 95% CI, 2.51%-15.81%). The incidence rate for thyroid cancer among patients younger than 10 years was too low to perform joinpoint analysis.
Table 2. Pediatric Thyroid Cancer Incidence by Demographic Factors and Tumor Characteristics.
| Characteristic | Overall 1973-2013, Mean APC, % (95% CI) | Period 1 | Period 2 | ||
|---|---|---|---|---|---|
| Years | APC, % (95% CI) | Years | APC, % (95% CI) | ||
| Overall | 2.54 (1.69 to 3.40) | 1973-2006 | 1.11 (0.56 to 1.67) | 2006-2013 | 9.56 (5.09 to 14.22) |
| Sex | |||||
| Male | 1.48 (0.52 to 2.45) | 1973-2013 | 1.48 (0.52 to 2.45) | NA | NA |
| Female | 2.66 (1.69 to 6.64) | 1973-2006 | 1.31 (0.67 to 1.95) | 2006-2013 | 9.29 (4.20 to 14.62) |
| Race | |||||
| White | 2.74 (1.73 to 3.77) | 1973-2006 | 1.35 (0.69 to 2.02) | 2006-2013 | 9.56 (4.22 to 15.18) |
| Nonwhite | 2.10 (0.28 to 3.96) | 1973-2006 | 0.41 (−0.90 to 1.73) | 2006-2013 | 10.49 (1.29 to 20.51) |
| Age, y | |||||
| 0-9 | NA | NA | NA | NA | NA |
| 10-14 | 1.20 (0.28 to 2.13) | 1973-2013 | 1.20 (0.28 to 2.13) | NA | NA |
| 15-19 | 2.81 (1.85 to 3.78) | 1973-2006 | 1.24 (0.60 to 1.89) | 2006-2013 | 10.54 (2.51 to 15.81) |
| Histologic type | |||||
| Papillary | 2.99 (2.12 to 3.87) | 1973-2006 | 1.39 (0.80 to 1.97) | 2006-2013 | 10.92 (6.35 to 15.70) |
| Follicular | −0.15 (−1.31 to 1.03) | 1973-2013 | −0.15 (−1.31 to 1.03) | NA | NA |
| Other | NA | NA | NA | NA | NA |
| SEER Historic Stage A | |||||
| Localized | 2.33 (1.10 to 3.58) | 1973-2005 | 0.92 (0.04 to 1.81) | 2005-2013 | 8.81 (2.73 to 13.92) |
| Regional | 3.08 (1.95 to 4.22) | 1973-2006 | 1.44 (0.68 to 2.21) | 2006-2013 | 11.16 (5.26 to 17.40) |
| Distant | NA | NA | NA | NA | NA |
| Unknown | NA | NA | NA | NA | NA |
| Tumor size, mm | |||||
| 1-20 | 3.63 (2.08 to 5.21) | 1983-2005 | 1.53 (0.11 to 2.97) | 2005-2013 | 9.66 (4.81 to 17.73) |
| >20 | 3.74 (2.18 to 5.31) | 1983-2006 | 2.23 (0.93 to 3.54) | 2006-2013 | 8.84 (3.20 to 14.79) |
Abbreviations: APC, annual percent change; NA, not applicable (not enough data for analysis); SEER, Surveillance, Epidemiology, and End Results.
Figure 1. Incidence Rate of Pediatric Thyroid Cancer From 1973 to 2013 Overall and by Demographic Characteristics.
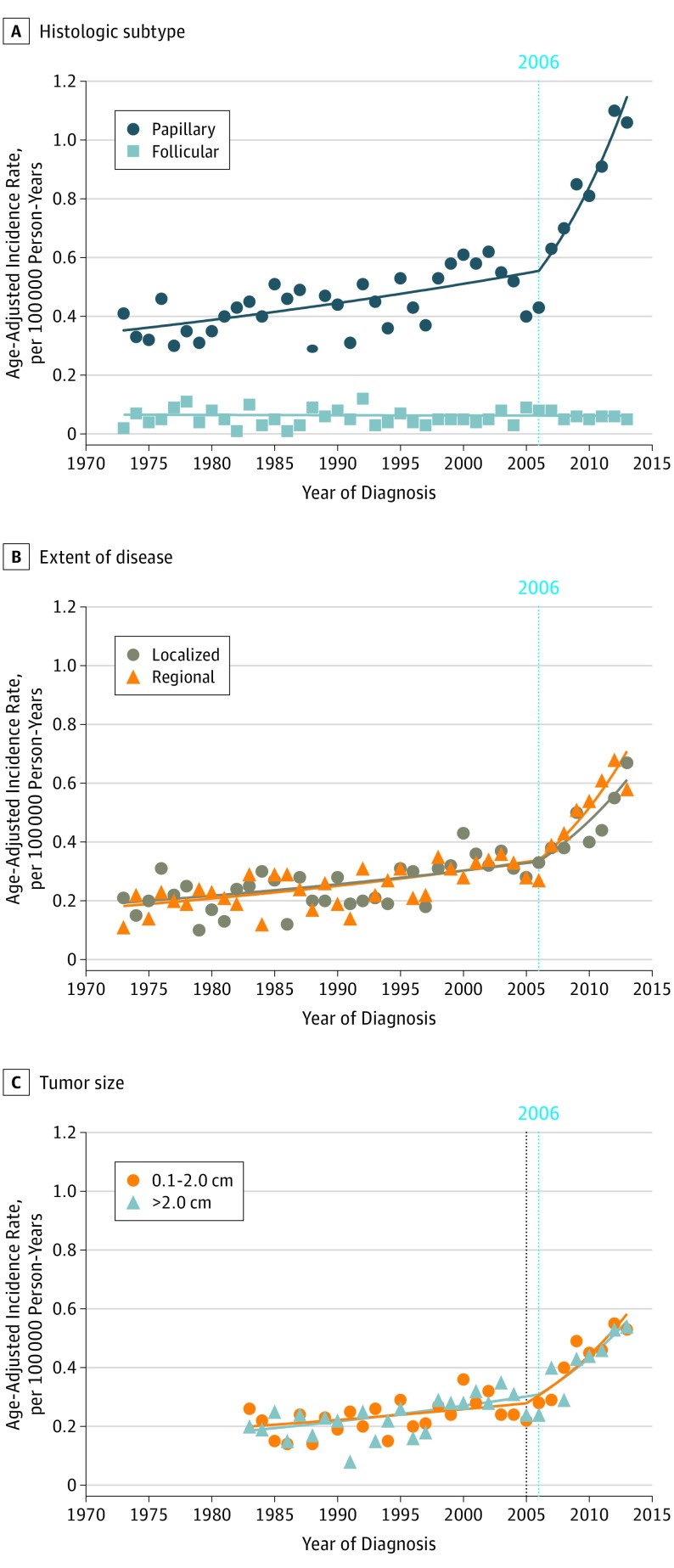
Incidence trends by clinical factors and tumor characteristics are also shown in Table 2. The incidence trends varied by histologic type. The incidence rates of papillary thyroid cancer gradually increased from 1973 to 2006 (APC, 1.39%; 95% CI, 0.80%-1.97%) and then markedly increased from 2006 to 2013 (APC, 10.92%; 95% CI, 6.35%-15.70%), whereas the incidence rate of follicular thyroid cancer remained unchanged from 1973 to 2013 (APC, −0.15%; 95% CI, −1.31% to 1.03%) (Figure 2A). The incidence rates of medullary and anaplastic thyroid cancer were too low for joinpoint analysis. The incidence trends did not significantly vary by extent of disease as defined by SEER Historic Stage A (Figure 2B). The incidence rates of localized thyroid cancer gradually increased from 1973 to 2005 (APC, 0.92%; 95% CI, 0.04%-1.81%) and then markedly increased from 2005 to 2013 (APC, 8.81%; 95% CI, 2.73%-13.92%), whereas the incidence rates of regionally extended thyroid cancer gradually increased from 1973 to 2006 (APC, 1.44%; 95% CI, 0.68%-2.21%) and then markedly increased from 2006 to 2013 (APC, 11.16%; 95% CI, 5.26%-17.40%). The incidence rate of distantly spread thyroid cancer was too low for joinpoint analysis. Information on tumor size was available for cases diagnosed between 1983 and 2013. The incidence trends of pediatric thyroid cancer did not significantly vary by size at diagnosis (Figure 2C). The incidence rate of small tumors (1-20 mm) gradually increased from 1983 to 2005 (APC, 1.53%; 95% CI, 0.11%-2.97%) and then markedly increased from 2005 to 2013 (APC, 9.66%; 95% CI, 4.81%-17.73%). Similarly, the incidence rate of large tumors gradually increased from 1983 to 2006 (APC, 2.23%; 95% CI, 0.93%-3.54%) and then markedly increased from 2006 to 2013 (APC, 8.84%; 95% CI, 3.20%-14.79%).
Figure 2. Incidence Rate of Pediatric Thyroid Cancer From 1973 to 2013 by Tumor Characteristics.
Mortality due to thyroid cancer among individuals younger than 20 years was low, with 11 cases identified from 1983 to 2013. Two cases each were reported in 2002, with every other year having 1 or no mortalities. The incidence-based mortality rates ranged from 0 to 6.35 × 10−4 per 100 000 person-years and were too low for joinpoint analysis.
Discussion
To our knowledge, the current study was the first to systematically compare trends in pediatric thyroid cancer incidence by tumor characteristics. The main finding of this study is that incidence rates of pediatric thyroid cancer gradually increased 1.1% per year from 1973 to 2006 and markedly increased 9.6% per year after 2006. The increase in incidence rates after 2006 was similarly mirrored by increases in incidence rates for papillary histologic subtype, female sex, and adolescent age. Pediatric thyroid cancer incidence trends did not vary by tumor size or extent of disease. Incidence-based mortality rates were low.
Thyroid cancer is relatively uncommon in the pediatric population; however, the overall incidence has been increasing. A previous SEER database study13 analyzed incidence trends of pediatric thyroid cancer and showed that the rate of pediatric thyroid cancer increased 1.1% per year from 1973 to 2004. In that study, incidence trends were analyzed in a linear fashion and did not account for changes in incidence rates over time. In the current study, joinpoint analysis was used to account for changes in incidence rates over time, revealing that the previously reported incidence rate increase of 1.1% per year extended to 2006, with an increase of 9.6% per year from 2006 to 2013. This marked increase in pediatric thyroid cancer incidence rate was associated with increases in cancers with the papillary histologic subtype, whereas the rate of medullary carcinoma remained unchanged throughout the study period, reflecting trends found in adult studies.2
Overdiagnosis appeared to be associated with much of the increase in thyroid cancer incidence in previous studies.3,4,5,6,7 Higher rates of imaging, more sensitive imaging, and higher rates of fine-needle aspiration biopsies have enhanced the detection of small, indolent tumors. The rates of imaging have been increasing in children’s hospitals and emergency departments.16,17 Although improved technology and increased availability of imaging services have yielded benefits, overutilization has become a concerning phenomenon, with contributing factors including lack of evidence-based appropriateness criteria for imaging, defensive practice behaviors, and financial incentives within current payment mechanisms.18 When considering these factors along with the increased availability of point-of-care ultrasonography, the number of physicians performing ultrasonography, and use of cross-sectional imaging in pediatric hospitals,17,19 overutilization of medical imaging can be hypothesized to be associated with overdiagnosis of pediatric thyroid cancer.
A growing body of work, however, contradicts the overdiagnosis hypothesis. Chen et al9 showed that the incidence rates of differentiated thyroid cancers of all sizes (<1.0 cm, 1.0-2.9 cm, 3.0-3.9 cm, and >4 cm) increased among men and women from 1988 to 2005. Subsequently, Lim et al2 demonstrated that papillary thyroid cancer incidence increased for all SEER stages at diagnosis (4.6% per year for localized, 4.3% per year for regional, 2.4% per year for distant, and 1.8% per year for unknown) from 1974 to 2013 and that incidence-based mortality due to papillary thyroid cancer increased 1.1% per year from 1994 to 2013. Increased incidence rates of nodal disease may in part be attributable to stage migration. Stage migration occurs when newer diagnostic technology results in upstaging of tumors. In this situation, many cases that would have been classified in the lower stage are then classified in the higher stage. Because the prognosis of cancer that migrated from the lower stage to the higher stage is worse than that of lower-stage cancer but better than that of higher-stage cancer, survival rates may be increased in both groups.20 Although stage migration may have contributed to increased incidence rates of regional disease, it does not explain the increased rates of incidence-based mortality demonstrated in the study by Lim et al.2
Our study showed a marked increase in pediatric thyroid cancer incidence rates after 2006. This increase may have been associated with overdiagnosis in children or represents a true increase in thyroid cancer incidence associated with environmental exposures. In 2006, the American Thyroid Association published general guidelines that mention pediatric thyroid nodules for the first time, recommending a similar diagnostic and therapeutic approach in children as in adults.21 These recommendations may have led to increased diagnosis of thyroid cancer owing to increasing use of diagnostic ultrasonography and ultrasonography-guided biopsies in the pediatric population. However, the significant increase in large tumors at a rate of 8.84% per year suggests that enhanced diagnosis of small, indolent tumors is not solely responsible for this trend. This finding is consistent with a growing body of work in adult thyroid cancer that challenges the prevailing notion that the increased incidence rate of thyroid cancer is solely attributable to overdiagnosis.2,3,5 A combination of enhanced diagnoses and an actual increase in pediatric thyroid cancer incidence, likely associated with changes in environmental risk factors, may be the main contributors to this trend.19
One potential environmental factor associated with an increase in pediatric thyroid cancer rates is increased exposure to medical radiation. The association between ionizing radiation and development of childhood thyroid cancer has been demonstrated in survivors of the Chernobyl accident and the nuclear bombings in Hiroshima and Nagasaki.22,23 Children are at greater risk than adults of developing cancer from radiation,24 and there was a 600% per capita increase in medical radiation in the United States between 1982 and 2006.25 Sigurdson et al26 showed that in survivors of malignant disease in childhood who received radiotherapy of the head, neck, or upper chest, a dose-dependent relationship existed between radiation exposure up to 30 Gy and risk of subsequent primary thyroid cancer. Lubin et al27 performed a pooled analysis of 9 cohorts of children with radiation exposure (2 cohorts treated for malignant diseases, 6 cohorts treated for benign diseases, and 1 cohort of atomic bomb survivors) and also found that a dose-dependent relationship existed between low-dose radiation exposure and subsequent childhood thyroid cancer.
Increased exposure to ionizing radiation from imaging studies may also have contributed to the increased incidence of pediatric thyroid cancer. Mathews et al28 showed that computed tomography (CT) during childhood was associated with increased incidence of cancer in a longitudinal, population-based study using Australian Medicare records. Since the introduction of CT in the 1970s, recent advances have increased its accessibility and have resulted in an increase in CT use, with 62 million CT scans obtained in 2006 compared with 3 million in 1980 in the United States.29 Miglioretti et al30 showed that CT use doubled among children younger than 5 years and tripled among children aged 5 to 14 years from 1996 to 2005, stabilized from 2005 to 2007, and declined from 2007 to 2011, likely owing to recent efforts to reduce exposure to ionization radiation. The mean latency between childhood radiation exposure and subsequent thyroid cancer is between 8.5 and 10 years,26,31 which is consistent with the increased prevalence of pediatric thyroid cancer in the 15- to 19-year age group in our study. However, Ohtsuru et al32 showed that large-scale mass ultrasonography screening of children and young adults within 5 years of the 2011 Fukushima Daiichi nuclear power station accident identified many subclinical thyroid cancers in an age-dependent manner (29 cases per 100 000 person-years for ages 15-17 years, 48 cases per 100 000 person-years for ages 18-20 years, and 64 cases per 100 000 person-years for ages 21-22 years). These high incidence rates represent prevalent disease rather than effects of radiation because all diagnoses were made before the effects of radiation could manifest according to epidemiologic evidence for postradiation-exposure thyroid cancer in Japan. This finding would support overdiagnosis rather than radiation exposure being associated with the increasing incidence of pediatric thyroid cancer. Furthermore, although pediatric thyroid cancer is more likely to carry a radiation signature (RET/PTC [rearranged in transformation/papillary thyroid carcinomas]), there has been an overall decline in the proportion of thyroid cancer with radiation signatures; however, the proportion of mutations that are less likely to have a radiation origin (BRAF and RAS point mutations) has increased.33,34,35,36
Strengths and Limitations
The main strength of our study is its large sample size and diverse patient characteristics. Using the SEER database allowed us to analyze a large and diverse population with quality control. The catchment areas used in the SEER database were selected for their ability to maintain a high-quality cancer reporting system and for demographic characteristics that are representative of the US population as a whole. We limited our analysis to the SEER-9 database because it includes the initial 9 cancer registries that joined the SEER program between 1973 and 1975. Another strength of this study is the use of joinpoint regression analysis as the statistical model. Joinpoint analysis reduces bias compared with traditional linear regression by accounting for variation over time rather than assuming that the association is constant within the observation period.37 These results should be interpreted like other studies that used joinpoint regression in an identical fashion to assess trends in thyroid cancer incidence in adults.2,9
This study is primarily limited by its descriptive nature. Furthermore, the SEER database does not capture data about environmental exposures and risk factors that are associated with thyroid cancer. It also does not provide information on method of cancer detection, whether clinically apparent or incidentally discovered on imaging. Consequently, we can only speculate on the potential reasons for the observed incidence trends.
Conclusions
The incidence rates of pediatric thyroid cancer increased more rapidly from 2006 to 2013 than from 1973 to 2006. This increase was largely associated with increases in the incidence rates for papillary histologic subtype, female sex, and adolescent age. More aggressive workup of thyroid nodules in the pediatric population may be contributing to these recent trends. However, the similar marked increases in the incidence rates of large tumors and advanced-stage disease suggest a true increase in the occurrence of pediatric thyroid cancer in the United States rather than enhanced detection of early-stage tumors. Future studies are needed to investigate the causes and recent trajectory of the increasing incidence of thyroid cancer.
References
- 1.American Cancer Society Cancer Facts & Figures 2017. Atlanta, GA: American Cancer Society; 2017
- 2.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017;317(13):1338-1348. doi: 10.1001/jama.2017.2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295(18):2164-2167. doi: 10.1001/jama.295.18.2164 [DOI] [PubMed] [Google Scholar]
- 4.Kent WDT, Hall SF, Isotalo PA, Houlden RL, George RL, Groome PA. Increased incidence of differentiated thyroid carcinoma and detection of subclinical disease. CMAJ. 2007;177(11):1357-1361. doi: 10.1503/cmaj.061730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317-322. doi: 10.1001/jamaoto.2014.1 [DOI] [PubMed] [Google Scholar]
- 6.Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic? the increasing impact of overdiagnosis. N Engl J Med. 2016;375(7):614-617. doi: 10.1056/NEJMp1604412 [DOI] [PubMed] [Google Scholar]
- 7.Morris LGT, Tuttle RM, Davies L. Changing trends in the incidence of thyroid cancer in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142(7):709-711. doi: 10.1001/jamaoto.2016.0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiol Biomarkers Prev. 2009;18(3):784-791. doi: 10.1158/1055-9965.EPI-08-0960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988-2005. Cancer. 2009;115(16):3801-3807. doi: 10.1002/cncr.24416 [DOI] [PubMed] [Google Scholar]
- 10.Dinauer CA, Breuer C, Rivkees SA. Differentiated thyroid cancer in children: diagnosis and management. Curr Opin Oncol. 2008;20(1):59-65. doi: 10.1097/CCO.0b013e3282f30220 [DOI] [PubMed] [Google Scholar]
- 11.The ALARA concept in pediatric CT intelligent dose reduction. Pediatr Radiol. 2002;32:219-220. doi: 10.1007/s00247-002-0665-z [DOI] [PubMed] [Google Scholar]
- 12.Zacharias C, Alessio AM, Otto RK, et al. Pediatric CT: strategies to lower radiation dose. AJR Am J Roentgenol. 2013;200(5):950-956. doi: 10.2214/AJR.12.9026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogan AR, Zhuge Y, Perez EA, Koniaris LG, Lew JI, Sola JE. Pediatric thyroid carcinoma: incidence and outcomes in 1753 patients. J Surg Res. 2009;156(1):167-172. doi: 10.1016/j.jss.2009.03.098 [DOI] [PubMed] [Google Scholar]
- 14.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi: 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H-J, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335-351. doi: [DOI] [PubMed] [Google Scholar]
- 16.Parker MW, Shah SS, Hall M, Fieldston ES, Coley BD, Morse RB. Computed tomography and shifts to alternate imaging modalities in hospitalized children. Pediatrics. 2015;136(3):e573-e581. doi: 10.1542/peds.2015-0995 [DOI] [PubMed] [Google Scholar]
- 17.Broder J, Fordham LA, Warshauer DM. Increasing utilization of computed tomography in the pediatric emergency department, 2000-2006. Emerg Radiol. 2007;14(4):227-232. doi: 10.1007/s10140-007-0618-9 [DOI] [PubMed] [Google Scholar]
- 18.Hendee WR, Becker GJ, Borgstede JP, et al. Addressing overutilization in medical imaging. Radiology. 2010;257(1):240-245. doi: 10.1148/radiol.10100063 [DOI] [PubMed] [Google Scholar]
- 19.Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12(11):646-653. doi: 10.1038/nrendo.2016.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon: stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312(25):1604-1608. doi: 10.1056/NEJM198506203122504 [DOI] [PubMed] [Google Scholar]
- 21.Cooper DS, Doherty GM, Haugen BR, et al. ; American Thyroid Association Guidelines Taskforce . Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16(2):109-142. doi: 10.1089/thy.2006.16.109 [DOI] [PubMed] [Google Scholar]
- 22.Tuttle RM, Becker DV. The Chernobyl accident and its consequences: update at the millennium. Semin Nucl Med. 2000;30(2):133-140. doi: 10.1053/nm.2000.5412 [DOI] [PubMed] [Google Scholar]
- 23.Furukawa K, Preston D, Funamoto S, et al. Long-term trend of thyroid cancer risk among Japanese atomic-bomb survivors: 60 years after exposure. Int J Cancer. 2013;132(5):1222-1226. doi: 10.1002/ijc.27749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kutanzi KR, Lumen A, Koturbash I, Miousse IR. Pediatric exposures to ionizing radiation: carcinogenic considerations. Int J Environ Res Public Health. 2016;13(11):E1057. doi: 10.3390/ijerph13111057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mettler FA Jr, Thomadsen BR, Bhargavan M, et al. Medical radiation exposure in the U.S. in 2006: preliminary results. Health Phys. 2008;95(5):502-507. doi: 10.1097/01.HP.0000326333.42287.a2 [DOI] [PubMed] [Google Scholar]
- 26.Sigurdson AJ, Ronckers CM, Mertens AC, et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): a nested case-control study. Lancet. 2005;365(9476):2014-2023. doi: 10.1016/S0140-6736(05)66695-0 [DOI] [PubMed] [Google Scholar]
- 27.Lubin JH, Adams MJ, Shore R, et al. Thyroid cancer following childhood low-dose radiation exposure: a pooled analysis of nine cohorts. J Clin Endocrinol Metab. 2017;102(7):2575-2583. doi: 10.1210/jc.2016-3529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346(7910):f2360. doi: 10.1136/bmj.f2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284. doi: 10.1056/NEJMra072149 [DOI] [PubMed] [Google Scholar]
- 30.Miglioretti DL, Johnson E, Williams A, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013;167(8):700-707. doi: 10.1001/jamapediatrics.2013.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winship T, Rosvoll RV. Cancer of the thyroid in children. Proc Natl Cancer Conf. 1970;6:677-681. http://www.ncbi.nlm.nih.gov/pubmed/5458135. Accessed December 21, 2018. [PubMed] [Google Scholar]
- 32.Ohtsuru A, Midorikawa S, Ohira T, et al. Incidence of thyroid cancer among children and young adults in Fukushima, Japan, screened with 2 rounds of ultrasonography within 5 years of the 2011 Fukushima Daiichi nuclear power station accident. JAMA Otolaryngol Head Neck Surg. 2019;145(1):4-11. doi: 10.1001/jamaoto.2018.3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romei C, Fugazzola L, Puxeddu E, et al. Modifications in the papillary thyroid cancer gene profile over the last 15 years. J Clin Endocrinol Metab. 2012;97(9):E1758-E1765. doi: 10.1210/jc.2012-1269 [DOI] [PubMed] [Google Scholar]
- 34.Mathur A, Moses W, Rahbari R, et al. Higher rate of BRAF mutation in papillary thyroid cancer over time: a single-institution study. Cancer. 2011;117(19):4390-4395. doi: 10.1002/cncr.26072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung CK, Little MP, Lubin JH, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab. 2014;99(2):E276-E285. doi: 10.1210/jc.2013-2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikiforov YE, Rowland JM, Bove KE, Monforte-Munoz H, Fagin JA. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 1997;57(9):1690-1694. http://www.ncbi.nlm.nih.gov/pubmed/9135009. Accessed January 15, 2019. [PubMed] [Google Scholar]
- 37.Rea F, Pagan E, Compagnoni MM, et al. Joinpoint regression analysis with time-on-study as time-scale: application to three Italian population-based cohort studies. Epidemiol Biostat Public Health. 2017;14(3):e12616-1-e12616-7. doi: 10.2427/12616 [DOI] [Google Scholar]