Abstract
Human urinary DNA adducts may be useful surrogate biomarkers to estimate carcinogen exposure and activation, particularly if such adducts are of high selectivity from a specific carcinogen source. In this report, we provided evidence supporting tobacco use and 4- (methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) being the dominant source for 3-methyladenine (3-mA), while nontobacco factors contribute significantly to 7-methylguanine and 1-methyladenine in the urine. Upon confirmation in human urine samples from larger populations in the future, urinary 3-mA may be used to estimate NNK bioactivation in smokers and to facilitate the development of a chemopreventive agent against NNK-induced carcinogenesis.
Graphical Abstract

Human urine has been widely used for disease diagnosis,1 drug abuse detection,2 environmental toxin exposure estimation,3 and other applications, because of its enrichment of metabolites from the whole body over a period of time, the collective biological information on the subjects, and the relative easy access to samples.4 Among various urinary metabolites, DNA base adducts derive from spontaneous depurination of labile DNA damages, which may be generated from the endogenous processes, upon environmental carcinogen exposure/bioactivation, or their combinations. Because of the challenge to access target tissues, especially for large population-based studies, urinary DNA adducts may be of great potential to estimate the levels of DNA damage in the body. For urinary DNA adducts to be useful for assessing carcinogen exposure/bioactivation, it is important to understand the source for such DNA adducts—whether they can be formed from the endogenous metabolism or environmental carcinogens and their relative contributions. With such knowledge, the DNA adducts may also be used as surrogate biomarkers to monitor the efficacy of potential interventions in clinical settings.
Tobacco use is one major cause for a wide range of human diseases, including a number of cancers.5 Tobacco smoke contains over 60 chemical carcinogens.5 Reactive parent compounds or bioactivated intermediates of these carcinogens can react with different nucleophilic functional groups on DNA to generate a range of modifications. The most extensively characterized modifications are those on the nucleobases. Some of these modifications are labile, such as modifications on the N7 position of guanosine, and are prone to depurinate spontaneously to produce base adducts.6 The base adducts would be excreted into the urine. Although such labile DNA modifications are less likely to be carcinogenic, the excreted DNA adducts have the potential to estimate both levels of carcinogen exposure and bioactivation as discussed.
Since tobacco smoke contains some carcinogens that are tobacco specific, such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), tobacco exposure may present a unique opportunity to characterize the causality relationship between tobacco carcinogen exposure and certain urinary DNA adducts, particularly those that may derive from NNK. In addition, tobacco use is the major source of nicotine exposure among smokers. The sum of seven nicotine metabolites in the urine (nicotine-N-oxide, free and glucuronidated forms of nicotine, cotinine, 3-hydroxycotinine), which has been termed total nicotine equivalent (TNE), can be used to estimate the amount of tobacco use.7 Although nicotine does not directly contribute to DNA adduct formation, the correlation of urinary DNA adducts to TNE in the same urine samples offers another unique angle to explore whether tobacco use may be the dominant source for a particular DNA adduct.
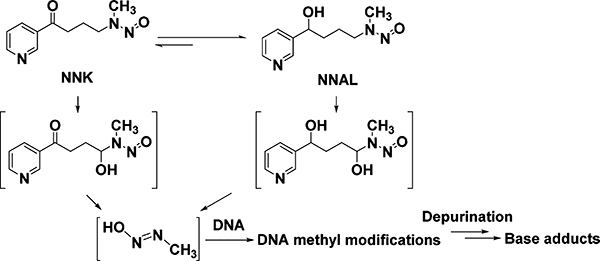
In this study, we explored this concept using three urinary DNA base adducts, 7-methylguanine (7-mG), 3-methyladenine (3-mA), and 1-methyladenine (1-mA), as the model system. These adducts were selected for evaluation because NNK and its major metabolite, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), can generate reactive intermediates to methylate DNA (Scheme 1), while DNA methylation may occur endogenously or upon exposure to other therapeutic agents/carcinogens. Indeed there have been some conflicting reports on this.8–11 Urinary total NNAL (the sum of free NNAL and NNAL glucuronide conjugates) has also been used as a biomarker to assess human exposure to tobacco.5 We therefore quantified these adducts and total NNAL in the urine samples from nonsmokers and smokers. Their correlations with TNE were explored as well. Lastly, NNK exposure in A/J mice was used to substantiate the observations in humans.
Scheme 1.
DNA Methyl Adducts Derived from NNK
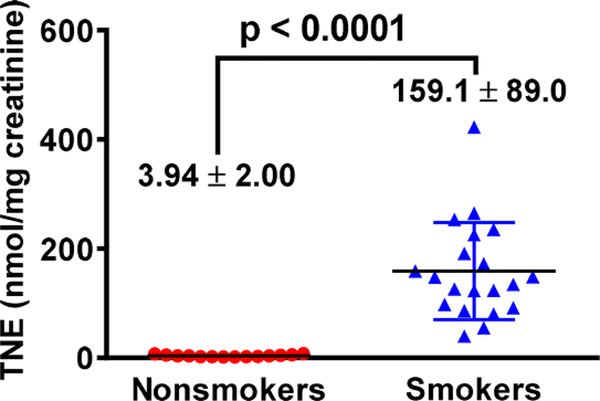
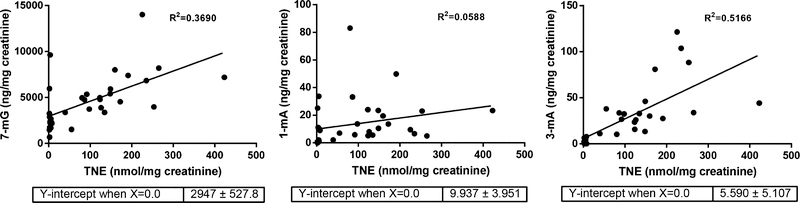
We first quantified TNE in urine samples from 15 nonsmokers and 20 smokers (demographic information in Table S1) to confirm their smoking status following the established method.12 One sample from a nonsmoker had a significant level of TNE, comparable to the average value among smokers, which was excluded for future analysis (Figure 1). The same set of urine samples were analyzed with an isotope dilution tandem mass spectrometry method for 7-mG, 1-mA, and 3-mA (Figures 2, S1–2; Table S2). The average levels of these three urinary methyl adducts were all higher in smokers than nonsmokers, suggesting that tobacco use may contribute to their formations. However, 3-mA was 11.3 times higher in smokers than nonsmokers, while 7-mG and 1-mA were only 1.9 and 2.7 times higher in smokers than nonsmokers, respectively. These data suggest that tobacco use is potentially the dominant source for 3-mA formation in smokers, while endogenous processes or other environmental factors contribute significantly to the formation of 7-mG and 1-mA. This was further supported by the correlation results of their levels with TNE (Figure 3). First of all, 3-mA had a decent positive correlation with TNE (R2 = 0.5166), 7-mG had a moderate positive correlation (R2 = 0.3690), while 1-mA had nearly no positive correlation (R2 = 0.0588). These correlations suggest that the amount of urinary 3-mA is more dependent on the amount of tobacco use relative to 7-mG and 1-mA. Second, the relative amount of 3-mA approached zero when the TNE level was extrapolated to zero, while there would be substantial amounts of 7-mG and 1-mA when TNE approached zero (Figure 3). In addition, the levels of 7-mG and 1-mA among a number of nonsmokers were comparable or higher than some smokers (Figure 2). On the other hand, the levels of 3-mA among all nonsmokers were lower than the lowest level detected among the smokers (Figure 2). These results overall suggest that urinary 3-mA is a better surrogate biomarker for tobacco exposure relative to 7-mG and 1-mA. Correlations of urinary total NNAL to three methyl base adducts and TNE are summarized in Table S3. Urinary total NNAL had a decent positive correlation with TNE (R2 = 0.4025), consistent with previous reports that it could serve as a biomarker for tobacco smoke exposure. On the other hand, urinary total NNAL had a weak positive correlation with methyl base adducts, potentially because it did not reflect the bioactivation (α-hydroxylation) of NNK and NNAL.
Figure 1.
Urinary TNE among nonsmokers (n = 14) and smokers (n = 20).
Figure 2.
Three urinary methyl DNA adducts among nonsmokers (n = 14) and smokers (n = 20) (two-tailed t test).
Figure 3.
Linear correlations of three methyl DNA adducts with TNE in urine among nonsmokers and smokers.
Since NNK is the best-known carcinogen in tobacco for DNA methylation and likely plays an important role for human carcinogenesis, particularly lung cancer, it is important to determine whether the significant increase in urinary 3-mA among smokers may be due to their exposure to NNK. To explore this, we tested whether these three methyl adducts could be detected in the mouse urine when A/J mice were exposed to NNK. Briefly, A/J mice were given a single dose of NNK at 100 mg/kg bodyweight via i.p. injection with urine samples collected at different time points (0.5, 8, 24, 48, and 96 h after NNK administration) and analyzed (Figure 4, n = 2–7). A/J mice without NNK treatment served as the control. The dose of NNK used herein has been widely employed to characterize NNK- induced DNA damage and tumor burden in the A/J mouse lung carcinogenesis model.13
Figure 4.
Levels of 7-mG and 3-mA in the urine samples of A/J mice. The mice were given a single dose of NNK at a dose of 100 mg/kg bodyweight via i.p. injection (n = 2–7). Comparison was made with the control group by Dunnett’s test when one-way ANOVA was statistically significant. *,p < 0.05; **,p < 0.01; ***,p < 0.001; ****,p < 0.0001.
Similar to observations in human nonsmoker urine, a significant amount of 7-mG was detected in the control mouse urine, demonstrating that 7-mG can be formed without NNK exposure (Figure 4). For NNK-treated mice, there was no increase in urinary 7-mG 0.5 h after NNK exposure, but there were significant increases 8 and 24 h later, which returned to the baseline level 96 h after NNK exposure. The highest level of 7-mG was at the 8 h time point, and it was 1.89 times of the level in the control mice. The level of 1-mA in all mice urine, irrespective of NNK treatment or not, was below the limit of detection (66 pg/μL urine, Table S4). On the other hand, the urinary levels of 3-mA at 0.5, 8, and 24 h after NNK exposure were significantly higher than that in the control group. Its peak level was at the 8 h time point, which was 315 times of the level in the control group. Even at 48 h, the level of 3-mA was 7.4 times of the level in the control group; the lack of statistical significance was likely due to the limited number of samples at 48 h (n = 2).
In summary, three methyl DNA adducts were quantified in urine from human smokers and nonsmokers and A/J mice with/ without NNK exposure. Both human and mouse data suggest that to bacco use and NNK are the major source of urinary 3-mA. Tobacco use and NNK exposure also contribute to urinary 7-mG, while a significant portion of 7-mG derives from other processes. On the other hand, 1-mA appeared to be less dependent on tobacco use and likely independent of NNK exposure. 3-mA therefore maybe a promising urinary biomarker candidate for tobacco carcinogen exposure and likely NNK bioactivation. Confirmation with larger sample sets will be needed in the future, which may pave the path for its clinical application.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Jordan Paladino and Vickie Nguyen for the help of sample collection and preparation.
Funding
The work was part-funded by R01CA193286 (C.X.), University of Minnesota Masonic Cancer pilot program (N.F. and C.X.), University of Florida Medicinal Chemistry Mass Spectrometry Support (C.X.), College of Pharmacy Frank Duckworth Endowment (C.X.), and University of Florida Health Cancer Center Startup fund (C.X.).
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.chemrestox.8b00155.
Experimental procedures and data (PDF)
REFERENCES
- (1).Wu D, Ni J, Beretov J, Cozzi P, Willcox M, Wasinger V, Walsh B, Graham P, and Li Y (2017) Urinary biomarkers in prostate cancer detection and monitoring progression. Crit Rev. Oncol Hematol 118, 15. [DOI] [PubMed] [Google Scholar]
- (2).Diao X, and Huestis MA (2017) Approaches, Challenges, and Advances in Metabolism of New Synthetic Cannabinoids and Identification of Optimal Urinary Marker Metabolites. Clin. Pharmacol. Ther 101, 239. [DOI] [PubMed] [Google Scholar]
- (3).Turner PC, Flannery B, Isitt C, Ali M, and Pestka J (2012) The role of biomarkers in evaluating human health concerns from fungal contaminants in food. Nutr. Res. Rev 25, 162. [DOI] [PubMed] [Google Scholar]
- (4).Finn WF, and Porter GA (1999) Urinary biomarkers: recommendations of the Joint European/United States Workshop for future research. Renal Failure 21, 445. [DOI] [PubMed] [Google Scholar]
- (5).Hecht SS (2003) Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer 3, 733. [DOI] [PubMed] [Google Scholar]
- (6).Gates KS, Nooner T, and Dutta S (2004) Biologically relevant chemical reactions of N7-alkylguanine residues in DNA. Chem. Res. Toxicol 17, 839. [DOI] [PubMed] [Google Scholar]
- (7).Murphy SE (2017) Nicotine Metabolism and Smoking: Ethnic Differences in the Role of P450 2A6. Chem. Res. Toxicol 30, 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Shuker DE, and Farmer PB (1992) Relevance of urinary DNA adducts as markers of carcinogen exposure. Chem. Res. Toxicol 5, 450. [DOI] [PubMed] [Google Scholar]
- (9).Hu CW, Hsu YW, Chen JL, Tam LM, and Chao MR (2014) Direct analysis of tobacco-specific nitrosamine NNK and its metabolite NNAL in human urine by LC-MS/MS: evidence of linkage to methylated DNA lesions. Arch. Toxicol 88, 291. [DOI] [PubMed] [Google Scholar]
- (10).Fay LB, Leaf CD, Gremaud E, Aeschlimann JM, Steen C, Shuker DE, and Turesky RJ (1997) Urinary excretion of 3- methyladenine after consumption of fish containing high levels of dimethylamine. Carcinogenesis 18, 1039. [DOI] [PubMed] [Google Scholar]
- (11).Prevost V, and Shuker DE (1996) Cigarette smoking and urinary 3-alkyladenine excretion in man. Chem. Res. Toxicol. 9, 439. [DOI] [PubMed] [Google Scholar]
- (12).Murphy SE, Park SS, Thompson EF, Wilkens LR, Patel Y, Stram DO, and Le Marchand L (2014) Nicotine N- glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis 35, 2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Hecht SS (1998) Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol 11, 559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.