Key Points
Question
What is the role of adjuvant therapy after curative-intent resection of ampullary adenocarcinoma?
Findings
In this cohort study that assessed 357 operative resections, neither fluorouracil-based nor gemcitabine-based regimens were associated with improved survival, even when assessed relative to intestinal or pancreatobiliary histologic subtype.
Meaning
The use of adjuvant chemotherapy, with or without radiotherapy, is not associated with improved long-term survival after curative-intent resection of ampullary adenocarcinoma.
Abstract
Importance
Ampullary adenocarcinoma is a rare malignant neoplasm that arises within the duodenal ampullary complex. The role of adjuvant therapy (AT) in the treatment of ampullary adenocarcinoma has not been clearly defined.
Objective
To determine if long-term survival after curative-intent resection of ampullary adenocarcinoma may be improved by selection of patients for AT directed by histologic subtype.
Design, Setting, and Participants
This multinational, retrospective cohort study was conducted at 12 institutions from April 1, 2000, to July 31, 2017, among 357 patients with resected, nonmetastatic ampullary adenocarcinoma receiving surgery alone or AT. Cox proportional hazards regression was used to identify covariates associated with overall survival. The surgery alone and AT cohorts were matched 1:1 by propensity scores based on the likelihood of receiving AT or by survival hazard from Cox modeling. Overall survival was compared with Kaplan-Meier estimates.
Exposures
Adjuvant chemotherapy (fluorouracil- or gemcitabine-based) with or without radiotherapy.
Main Outcomes and Measures
Overall survival.
Results
A total of 357 patients (156 women and 201 men; median age, 65.8 years [interquartile range, 58-74 years]) underwent curative-intent resection of ampullary adenocarcinoma. Patients with intestinal subtype had a longer median overall survival compared with those with pancreatobiliary subtype (77 vs 54 months; P = .05). Histologic subtype was not associated with AT administration (intestinal, 52.9% [101 of 191]; and pancreatobiliary, 59.5% [78 of 131]; P = .24). Patients with pancreatobiliary histologic subtype most commonly received gemcitabine-based regimens (71.0% [22 of 31]) or combinations of gemcitabine and fluorouracil (12.9% [4 of 31]), whereas treatment of those with intestinal histologic subtype was more varied (fluorouracil, 50.0% [17 of 34]; gemcitabine, 44.1% [15 of 34]; P = .01). In the propensity score–matched cohort, AT was not associated with a survival benefit for either histologic subtype (intestinal: hazard ratio, 1.21; 95% CI, 0.67-2.16; P = .53; pancreatobiliary: hazard ratio, 1.35; 95% CI, 0.66-2.76; P = .41).
Conclusions and Relevance
Adjuvant therapy was more frequently used in patients with poor prognostic factors but was not associated with demonstrable improvements in survival, regardless of tumor histologic subtype. The value of a multimodality regimen remains poorly defined.
This cohort study examines whether long-term survival after curative-intent resection of ampullary adenocarcinoma may be improved by selection of patients for adjuvant therapy directed by histologic subtype.
Introduction
Ampulla of Vater adenocarcinoma is a rare malignant neoplasm that arises within the duodenal ampullary complex.1,2 Although it is associated with a more favorable prognosis than adenocarcinomas of the adjacent pancreatic duct, common bile duct, or duodenum,3,4 poor rates of long-term cure with surgical resection alone make a compelling argument for a multimodality strategy for this disease.5,6
To date, at least 4 randomized clinical trials have assessed the use of adjuvant therapy (AT) in resected periampullary carcinoma,7,8,9,10 although rarity of the disease has prohibited prospective study of ampullary adenocarcinoma distinct from adjacent periampullary malignant neoplasms. Only the Japanese Study Group of Surgical Adjuvant Therapy for Carcinomas of the Pancreas and Biliary Tract randomized clinical trial, in which no survival benefit was observed for adjuvant mitomycin C and fluorouracil relative to surgery alone (SA), included a subset analysis of patients with ampullary adenocarcinoma.7 These trials aggregated several periampullary malignant neoplasms with plausibly heterogeneous biological responses to therapy, providing few prospective data from which to base treatment recommendations specific to ampullary cancers. Moreover, the ampulla represents the junction of the biliary, pancreatic, and digestive tracts; as such, 2 major histologic phenotypes of ampullary adenocarcinoma (intestinal and pancreatobiliary) have been identified.11,12 The interaction between histologic subtype and response to therapy is an underexplored topic.8
Drawing on the collective experience of 12 pancreatic surgical institutions from 3 countries, this study sought to examine contemporary factors associated with receipt of AT, the association of AT with survival, and stratification of any association based on the histologic phenotype of ampullary adenocarcinoma.
Methods
Data were accrued from April 1, 2000, to July 31, 2017, for patients who underwent surgical resection of ampullary adenocarcinoma performed at 12 institutions comprising the Ampullary Carcinoma Study Group. Prospectively maintained institutional databases were retrospectively supplemented to accrue a data list of patient demographics, perioperative outcomes, long-term recurrence, and survival. Pathology reports were rereviewed and carcinomas of the adjacent pancreas, bile duct, or duodenum were excluded. The study was approved by the University of Pennsylvania Institutional Review Board, who waived patient consent because only deidentified data were obtained.
The primary outcome, overall survival (OS), was defined as the interval between the date of surgery and date of death or last contact. Recurrence-free survival was defined as the interval between date of surgery and date of recurrence or last contact or death not secondary to recurrent malignant neoplasm.
Adjuvant chemotherapy (AC) was defined by receipt of either single-agent or multiagent chemotherapy initiated without palliative intent. When combined with radiotherapy, the regimen was classified as either adjuvant chemoradiotherapy (ACRT) when given concurrently or as AC+ACRT when a chemotherapeutic regimen was given temporally distinct from radiotherapy with a sensitizing chemotherapy agent. Radiotherapy alone was not used in this patient cohort. Tumor stage was evaluated by surgical pathologic findings and defined by the 8th edition of the American Joint Committee on Cancer’s Cancer Staging Manual.13
Histologic subtype was determined by institutional review of pathologic findings and did not necessarily include immunohistochemical staining characteristics (eg, CDX or MUC1) given the strong correlation between cytokeratin-immunohistochemistry and histologic classification.14 Given this study’s focus on the interaction between histologic characteristics and response to chemotherapy, patients with an unknown histologic subtype were excluded from analysis.
The Fistula Risk Score was calculated for each pancreatoduodenectomy (PD) based on the weighted influence of the following 4 risk factors: (1) pancreatic parenchyma, (2) pathologic characteristics of the disease, (3) duct size, and (4) intraoperative blood loss.15 Nonampullary adenocarcinoma PDs from the Pancreas Fistula Study Group were used for comparative study of fistula risk and occurrence.16 Clinically relevant postoperative pancreatic fistula (CR-POPF) was defined in accordance with updated 2016 International Study Group on Pancreatic Surgery consensus guidelines,17 and its occurrence and severity was evaluated through 90 days after the index operation. Complications were scored according to the Modified Accordion Severity Grading System and quantified using previously validated severity utility weights.18,19 Fistula mitigation strategies were used at the discretion of the operating surgeon. Prophylactic octreotide was defined by its use as a preventive strategy and was generally started at the time of operation, although certain surgeon practices included administration prior to the operation.
Statistical Analysis
Descriptive statistics are presented as frequencies and percentages for categorical variables and as mean and SD or median and interquartile range (IQR) for continuous variables. The Pearson χ2 test was used to analyze categorical and the Wilcoxon rank sum test was used to analyze continuous variables. Fixed-effects regression modeling with stepwise elimination (P ≤ .05 for entry and P > .10 for removal) was used to identify variables independently associated with AT. The influence of AT on OS was analyzed using Cox proportional hazards regression modeling with backward stepwise selection (P ≤ .05 for entry and P > .10 for removal).
Propensity score matching (PSM) is a method used to minimize treatment selection bias when estimating causal treatment outcomes in nonrandomized studies.20 The SA and AT sets were matched 1:1 according to propensity scores accounting for all factors associated with the use of AT or survival hazard on Cox modeling (ie, age, race/ethnicity, pT classification, nodal status, and perineural invasion) using the “greedy” nearest neighbor matching algorithm without replacement, with a caliper size of 0.1 × log(SD of the propensity score).21 After PSM, OS between the SA and AT groups was examined by Kaplan-Meier estimates.
Missing data were rare and were addressed by including a category titled “unknown” for nominal variables with missing data. Analyses were performed with SPSS, version 24.0 (IBM Corp). All P values were from 2-sided tests, and results were deemed statistically significant at P ≤ .05.
Results
Patient Demographics and Tumor Characteristics
A total of 756 patients with ampullary adenocarcinoma underwent curative-intent resection during the study period. Serial exclusion of patients with unknown histologic characteristics (n = 369), in situ disease as determined by final pathologic examination (n = 10), and inadequate follow-up (<30 days) (n = 14), as well as those who died within 90 days of surgery (n = 6), yielded a final study cohort of 357 patients.
Median patient age was 65.8 years (IQR, 58-74 years), 201 (56.3%) were men, 156 (43.7%) were women, and 311 (87.1%) were white (Table 1). Presenting symptoms included biliary obstruction (ie, jaundice or cholangitis; 249 [69.7%]), abdominal pain (64 [17.9%]), weight loss (48 [13.4%]), bleeding or anemia (14 [3.9%]), and pancreatitis (10 [2.8%]). A total of 28 patients (7.8%) reported no symptoms prior to diagnosis.
Table 1. Demographic and Clinicopathologic Characteristics of Overall Cohort.
| Characteristic | Patients, No. (%) (N = 357) |
|---|---|
| Female sex | 156 (43.7) |
| Race/ethnicitya | |
| White | 311 (87.1) |
| Black | 22 (6.2) |
| Asian or other | 8 (2.2) |
| Age, yb | |
| <65 | 171 (47.9) |
| 65-75 | 99 (27.7) |
| >75 | 74 (20.7) |
| BMIc | |
| <25 | 145 (40.6) |
| 25-30 | 135 (37.8) |
| >30 | 68 (19.0) |
| Procedure | |
| Pancreatoduodenectomy | 354 (99.2) |
| Total pancreatectomy | 1 (0.3) |
| Ampullectomy | 2 (0.6) |
| Pylorus preservation | 243 (68.1) |
| Reconstruction, PJ | 338 (94.7) |
| T classificationd | |
| T1 | 42 (11.8) |
| T2 | 124 (34.7) |
| T3 | 115 (32.2) |
| T4 | 75 (21.0) |
| N classificatione | |
| Negative | 134 (37.5) |
| Positive | 222 (62.2) |
| Pathologic stage | |
| I | 97 (27.2) |
| II | 26 (7.3) |
| III | 234 (65.5) |
| Histologic subtype | |
| Intestinal | 196 (54.9) |
| Pancreatobiliary | 138 (38.7) |
| Mixed intestinal and pancreatobiliary | 16 (4.5) |
| Other | 7 (2.0) |
| Differentiationf | |
| Well | 44 (12.3) |
| Moderate | 202 (56.6) |
| Poor | 99 (27.7) |
| Lymphovascular invasiong | |
| Yes | 227 (63.6) |
| No | 126 (35.3) |
| Perineural invasionh | |
| Yes | 173 (48.5) |
| No | 179 (50.1) |
| Resection margin | |
| R0 | 330 (92.4) |
| R1 | 27 (7.6) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); PJ, pancreaticojejunostomy.
Race/ethnicity data missing for 16 patients.
Age data missing for 13 patients.
BMI data missing for 9 patients.
T classification data missing for 1 patient.
N classification data missing for 1 patient.
Histologic grade data missing for 12 patients.
Lymphovascular invasion data missing for 4 patients.
Perineural invasion data missing for 5 patients.
Pancreatoduodenectomy (354 [99.2%]) was the predominant operation performed (Table 1). Most surgical procedures (243 [68.1%]) were pylorus preserving, and most patients (338 [94.7%]) underwent pancreaticojejunostomy reconstruction. Final pathologic examination revealed a mean (SD) tumor diameter of 2.4 (1.5) cm. Most patients (234 [65.5%]) had stage III disease. Adverse prognostic features, such as poor differentiation (99 [27.7%]), lymphovascular invasion (227 [63.6%]), and perineural invasion (173 [48.5%]), were frequently observed. The most common histologic subtype was intestinal ([196] 54.9%), followed by pancreatobiliary (138 [38.7%]) and mixed intestinal and pancreatobiliary variants ([16] 4.5%).
Perioperative Outcomes
Of the patients with ampullary adenocarcinoma with a known Fistula Risk Score who underwent PD (310 [86.8%]), the mean (SD) Fistula Risk Score was 4.3 (1.9), and most fistulas (218 of 310 [70.3%]) were in the moderate risk zone. In comparison, the mean (SD) Fistula Risk Score of 4722 PDs comprising the patients with nonampullary adenocarcinoma from the Pancreas Fistula Data Set16 was significantly lower (3.6 [2.2]; P < .001), with fewer cases of soft pancreatic texture (2499 of 4722 [52.9%] vs 229 of 310 [73.9%]; P < .001) and fewer cases within the moderate to high risk zone (3164 of 4722 [67.0%] vs 259 of 310 [83.5%]; P < .001) (eTable 1 in the Supplement). Accordingly, patients with ampullary adenocarcinoma were significantly more likely to experience a CR-POPF (83 of 310 [26.8%] vs 557 of 4722 [11.8%]; P < .001; odds ratio [OR], 2.73; 95% CI, 2.09-3.57) and have a longer hospital length of stay (9 days [IQR, 7-15 days] vs 8 days [IQR, 7-13 days]; P = .002). There was no difference in the mean (SD) fistula-specific accordion severity grade between patients with ampullary adenocarcinoma and those with nonampullary adenocarcinoma (2.6 [0.9] vs 2.8 [1.2]; P = .08), meaning that CR-POPFs were not more severe when they occurred.
Regarding fistula mitigation strategies, octreotide prophylaxis, biological sealants, and drain omission were more frequently used in the ampullary adenocarcinoma cohort. However, there was no association between CR-POPF and drain omission, octreotide prophylaxis, or biological sealant use in this cohort.
Use of Multimodality Therapies
Adjuvant therapy was administered to 200 patients (56.0%). Most of these 200 patients received AC (148 [74.0%]), with ACRT (21 [10.5%]) and AC+ACRT (31 [15.5%]) used less frequently. Of the patients who received AC, 112 of 199 (56.3%) received a single-agent regimen, 75 of 199 (37.7%) received a multiagent regimen, and 12 of 199 (6.0%) received an unknown regimen.
Univariate analysis demonstrated significant differences in patient and tumor characteristics between the SA and AT (AC, ACRT, or AC+ACRT) cohorts (ie, age, race/ethnicity, pT classification, nodal involvement, histologic grade, lymphovascular invasion, and perineural invasion). Histologic subtype (intestinal vs pancreatobiliary) was not associated with AT administration (101 of 191 [52.9%] vs 78 of 131 [59.5%]; P = .24).
Variables significantly associated with AT receipt on univariate analysis (P ≤ .05) were entered into a multivariable model to identify factors independently associated with use of AT. Node positivity (OR, 5.17; 95% CI, 3.01-8.87) and perineural invasion (OR, 2.74; 95% CI, 1.59-4.70) were independently associated with receipt of AT, whereas older age (age >75 years: OR, 0.27; 95% CI, 0.14-0.55 [reference: age, ≤65 years]) and black race (OR, 0.26; 95% CI, 0.09-0.76 [reference: white]) were associated with the omission of AT.
Influence of Adjuvant Therapy on Survival in Unmatched Cohort
The association of AT with OS was examined using Cox proportional hazards regression modeling incorporating variables significantly associated with OS by univariate analysis (ie, age, race/ethnicity, pT classification, nodal involvement, grade, lymphovascular invasion, perineural invasion, margin status, and the use of AC). Patients with intestinal subtype had an improved median OS compared with those with pancreatobiliary subtype (77 vs 54 months; P = .05). After adjusting for potential confounders and treating institution, the use of AT was not independently associated with risk of death (Table 2). Pathologic stage was associated with both the use of AT (stage I, 16 of 92 [17.4%]; stage II, 16 of 25 [64.0%]; and stage III, 168 of 227 [74.0%]; P < .001) and median OS (stage I, 137 months; stage II, 57 months; and stage III, 45 months; log-rank P < .001); however, AT was not associated with improved survival for any stage of disease (eFigure 1 in the Supplement). In the patients who received AT, the addition of radiotherapy was not associated with improvement in OS (ACRT: hazard ratio, 1.41; 95% CI, 0.78-2.55; AC+ACRT: hazard ratio, 1.20; 95% CI, 0.66-2.20).
Table 2. Multivariable Cox Proportional Hazards Regression Modeling for Overall Survival Among 357 Unmatched Patients.
| Characteristic | Hazard Ratio (95% CI)a | P Value |
|---|---|---|
| Age, y | ||
| <65 | 1 [Reference] | .004 |
| 65-75 | 1.41 (0.88-2.27) | .15 |
| >75 | 2.67 (1.58-4.51) | <.001 |
| Race/ethnicity | ||
| White | 1 [Reference] | <.001 |
| Black | 3.93 (1.95-7.93) | <.001 |
| Asian or other | 0.30 (0.04-2.28) | .25 |
| T classification | ||
| T1-2 | 1 [Reference] | NA |
| T3-4 | 2.48 (1.60-3.84) | <.001 |
| N classification | ||
| Node-negative | 1 [Reference] | NA |
| Node-positive | 2.52 (1.54-4.14) | <.001 |
| Grade | ||
| Well differentiated | 1 [Reference] | .24 |
| Moderately differentiated | NA | .60 |
| Poorly differentiated or undifferentiated | NA | .10 |
| Lymphovascular invasion | ||
| Absent | 1 [Reference] | NA |
| Present | NA | .53 |
| Perineural invasion | ||
| Absent | 1 [Reference] | NA |
| Present | NA | .05 |
| Resection margin | ||
| Margin negative (R0) | 1 [Reference] | NA |
| Margin positive (R1-R2) | NA | .06 |
| Adjuvant therapy | ||
| No | 1 [Reference] | NA |
| Yes | NA | .68 |
Abbreviation: NA, not applicable.
Hazard ratios indicate relative hazard for death and were adjusted for all variables included.
Efficacy of AT in the PSM Cohort
The PSM cohort comprised 162 patients: 81 (50.0%) in the SA group and 81 (50.0%) in the AT group. Previously observed differences between cohorts were successfully balanced after matching (eTable 2 in the Supplement). Most patients in the AT group received AC (64 [79.0%]) compared with ACRT (8 [9.9%]) or AC+ACRT (9 [11.1%]).
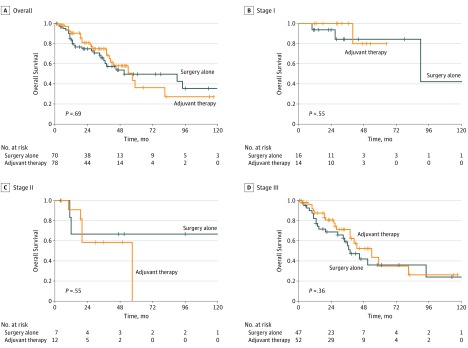
At a median follow-up of 34 (IQR, 22-53) months, median OS in the PSM cohort was 57 months (IQR, 28-138 months); the 1-year actuarial survival rate was 89.3%, and the 5-year actuarial survival rate was 45.9%. Overall survival was not significantly different for patients receiving AT relative to SA (hazard ratio, 0.90; 95% CI, 0.51-1.56; Figure 1A), including when they were stratified by stage (Figure 1B-D).
Figure 1. Association of Adjuvant Therapy and Overall Survival in the Propensity Score–Matched Cohort .
A, Overall cohort (n = 148). B, Stage I (n = 30). C, Stage II (n = 19). D, Stage III (n = 99).
Association of AT With OS Stratified by Tumor Histologic Subtype
There was no significant difference in median OS between the 2 major histologic variants (intestinal, 59 months vs pancreatobiliary, 51 months; log-rank P = .12). No survival benefit was associated with AT for either histologic subtype (intestinal: hazard ratio, 1.21; 95% CI, 0.67-2.16; P = .53; pancreatobiliary: hazard ratio, 1.35; 95% CI, 0.66-2.76; P = .41).
To explore the potential association between tumor histologic subtype and association with OS based on AT regimen used, the administered chemotherapies were dichotomized between fluorouracil-based regimens and gemcitabine-based regimens. Fluorouracil-based regimens included monotherapy (10 of 68 [14.7%]) and multiagent therapies when combined with oxaliplatin (eg, XELOX [capecitabine and oxaliplatin] or FOLFOX [leucovorin, fluorouracil, and oxaliplatin], 6 of 68 [8.8%]) or irinotecan (FOLFIRI [fluorouracil, leucovorin, and irinotecan], 7 of 68 [10.3%]). Gemcitabine-based regimens included monotherapy (31 of 68 [45.6%]) or multiagent therapy when combined with oxaliplatin (3 of 68 [4.4%]) or abraxane (3 of 68 [4.4%]). Several patients received both gemcitabine and fluorouracil (6 of 68 [8.8%]). Patients with the pancreatobiliary histologic subtype most commonly received gemcitabine-based regimens (22 of 31 [71.0%]) or combinations of gemcitabine and fluorouracil (4 of 31 [12.9%]), whereas treatment of the intestinal histologic subtype was more varied (fluorouracil, 17 of 34 [50.0%]; gemcitabine, 15 of 34 [44.1%]) (P = .01).
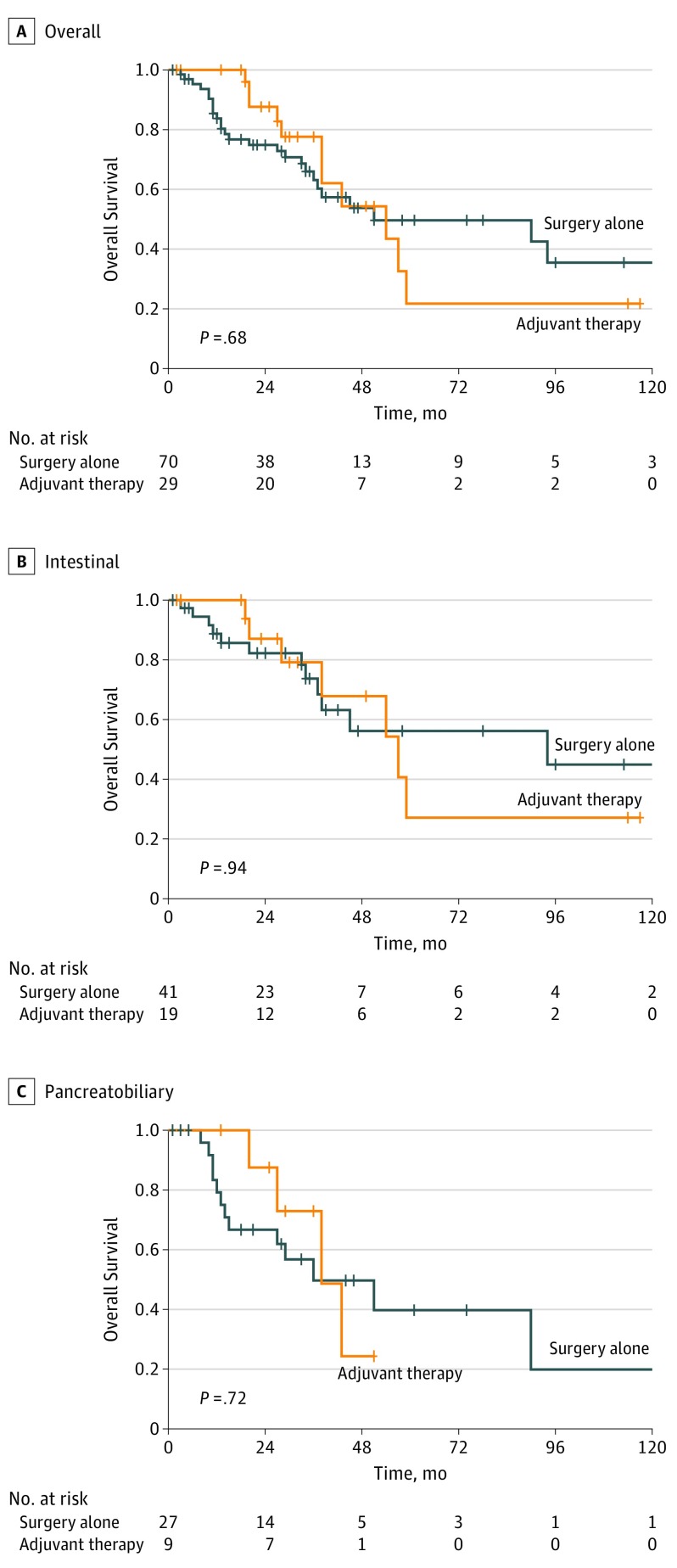
When limiting analysis to patients who received fluorouracil-based regimens, there was no association between the use of AT and OS (Figure 2A), including when patients were stratified by intestinal (Figure 2B) and pancreatobiliary (Figure 2C) histologic subtypes. There was no survival benefit associated with AT when limited to patients who received multiagent therapy.
Figure 2. Association of Fluorouracil-Based Chemotherapy and Overall Survival in the Propensity Score–Matched Cohort .
A, Overall cohort (n = 100). B, Intestinal histologic subtype (n = 61). C, Pancreatobiliary histologic subtype (n = 36).
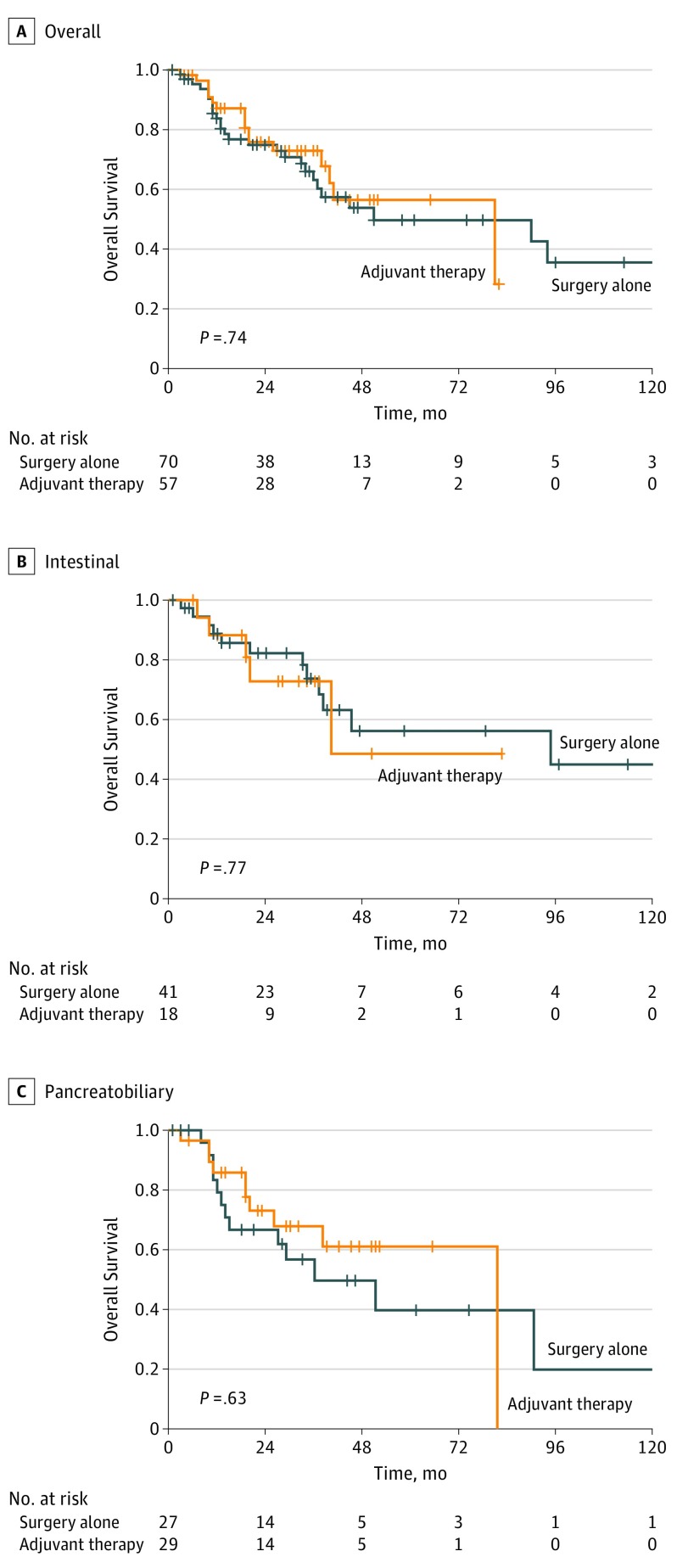
Likewise, use of gemcitabine-based regimens relative to SA was not associated with improved OS (Figure 3A), with no difference for the subsets of patients with intestinal (Figure 3B) or pancreatobiliary (Figure 3C) histologic subtype. There was no survival benefit associated with AT when limited to patients who received multiagent therapy.
Figure 3. Association of Gemcitabine-Based Chemotherapy and Overall Survival in the Propensity Score–Matched Cohort .
A, Overall cohort (n = 127). B, Intestinal histologic subtype (n = 59). C, Pancreatobiliary histologic subtype (n = 54).
Outcome of the Addition of Radiotherapy to AC Regimens
In the PSM cohort, 17 of 81 patients (21.0%) received radiotherapy as part of their AT regimen. Most patients (10 of 13 [76.9%]) received 50.4 Gy, and the remaining patients (3 of 13 [23.1%]) received 45.0 Gy. Radiotherapy-including regimens were not associated with improved OS relative to SA (hazard ratio, 0.77; 95% CI, 0.41-1.44; P = .41) or improvements relative to AC (hazard ratio, 1.58; 95% CI, 0.69-2.60; P = .28).
Data on recurrence were available for 144 of the 162 patients in the PSM cohort (88.9%). Of 58 patients (40.3%) who experienced a recurrence, the predominant pattern was distant (38 [65.5%]) rather than locoregional (11 [19.0%]) or both (5 [8.6%]). The addition of radiotherapy to chemotherapy regimens was not associated with improved recurrence-free survival relative to SA or AC (eFigure 2 in the Supplement).
Discussion
Ampullary carcinoma is a rare malignant neoplasm without established protocols for optimal multimodality management after curative-intent resection. Without published guidelines from the National Comprehensive Cancer Network or the European Society for Medical Oncology, practice patterns have been extrapolated from consensus guidelines for nearby periampullary malignant neoplasms. However, whether the histomolecular phenotype defines discrete prognostic groups or can be used to guide selection of AT has rarely been explored. To our knowledge, this multi-institutional study represents one of the largest reported series of surgically resected ampullary adenocarcinomas with complete histologic and AT details and highlights several important features regarding the overall management of these patients. In this study, AT was used equally for patients with intestinal and pancreatobiliary histologic subtypes. However, when AT was used, it was not associated with improvements in OS regardless of chemotherapeutic regimen or the addition of radiotherapy. Moreover, among patients receiving AT, there was no interaction between histologic subtype and long-term survival.
In this study, there was no survival benefit of AT in the multivariable model, which was also confirmed in the PSM cohort. These results are corroborated by a recent meta-analysis involving 14 studies and 1671 patients, in which no survival advantage was seen with the use of AC or chemoradiotherapy.22 That meta-analysis did not exclude periampullary malignant neoplasms (eg, distal bile duct or duodenum), and it did not have 3 of the 4 randomized clinical trials on this topic.8,9,10
The merits of additive radiotherapy were also explored in the PSM cohort, and no benefit was observed relative to SA or AC alone. That distant recurrence represents the most common pattern of disease relapse (>60%) may explain such findings. Published data on this topic are discordant. Two randomized clinical trials,9,10 including the European Organisation for Research and Treatment of Cancer Trial 40891 of fluorouracil and radiotherapy, failed to show a benefit, as did a PSM analysis of the Surveillance, Epidemiology, and End Results data set.23 On the other hand, a meta-analysis of 10 retrospective studies demonstrated a significant advantage with adjuvant chemoradiotherapy24; this meta-analysis included the positive study from the Johns Hopkins Hospital–Mayo Clinic collaboration.25 The Mayo Clinic recently published a follow-up, single-institution retrospective study of chemotherapy and/or chemoradiotherapy, which demonstrated an independent association between AT and improved long-term survival.26
Histomolecular phenotype has been cited as a factor independently associated with survival in patients with ampullary carcinoma. At least 2 studies12,27 have found a significant association between the pancreatobiliary histologic subtype and poorer long-term survival. In our unmatched cohort, there was also poorer medial OS in the pancreatobiliary subset (54 vs 77 months; P = .05). Histologic subtype was not included in the multivariable Cox proportional hazards regression because it failed to meet the predefined statistical threshold. Nevertheless, this seemingly different survival pattern has been cited to suggest that ampullary carcinoma represents more than 1 distinct disease, with potential implications for therapeutic strategies. However, in this study, there was no benefit for either fluorouracil-based or gemcitabine-based regimens and no observable interaction between chemotherapeutic agent and histologic subtype. In agreement with the European Study Group for Pancreatic Cancer-3 trial, in which no survival benefit was observed for either leucovorin-modulated fluorouracil or single-agent gemcitabine relative to SA,8 there was no benefit to either chemotherapy regimen in this study. Although histologic subtype was associated with type of chemotherapy administered in this series, there was no evidence that matching chemotherapeutic regimen to histologic subtype was deferentially associated with survival. Substratification in this 2-by-2 matrix likely left these analyses underpowered, although there were no trends for any such comparisons. A post hoc analysis of the European Study Group for Pancreatic Cancer-3 trial found no significant improvement in survival for either the intestinal or pancreatobiliary histologic subtypes, although further stratification by chemotherapeutic agent was not performed.8
Limitations
Several limitations warrant emphasis. Because of this study’s retrospective and nonrandomized design, demonstrated associations cannot be interpreted as causative. Regression modeling and PSM are statistical methods that can adjust for unbalanced covariates between study groups, although both are limited by the potential influence of unmeasured confounders. The exclusion of patients who died within 90 days of surgical resection was 1 method used to minimize the risk of the immortal time bias, a bias of observational cohort studies in which patients who die prior to initiation of therapy are categorized into the control group, which may exaggerate the apparent benefits seen in the treatment group. Second, while accounting for numerous granularities not otherwise available in cancer registries, such as specific chemotherapeutic agents and recurrence-free survival, there remain certain data omissions, such as treatment completion and related treatment toxic effects, which are an important consideration in the decision for the use and type of AT. Third, disease rarity may have limited the statistical power of certain analyses; however, given the relative scarcity of this pathologic subtype (13% of all PDs performed in the Pancreas Fistula Study Group), rigorous testing of these hypotheses may remain elusive. In particular, the subgroup analyses of multiagent chemotherapy may have been underpowered given that gemcitabine monotherapy was the most frequently used regimen in this series. The infrequent use of multiagent chemotherapy in this series may have limited the efficacy of this overall strategy. Fourth, these data may not apply to the use of systemic therapy in the neoadjuvant setting, a strategy not used in this series. The rate of CR-POPF was greater than 2-fold that observed among patients with nonampullary adenocarcinoma, likely driven by the higher rate of soft gland texture with this pathologic subtype, which may limit receipt of all requisite therapy for this high-risk disease. At least 1 study from the MD Anderson Cancer Center has demonstrated that neoadjuvant therapy can be safely used for ampullary adenocarcinoma with no association with perioperative morbidity or CR-POPF rates.28
Conclusions
By analysis of this multi-national series, the use of chemotherapy, with or without radiotherapy, after curative-intent resection of ampullary adenocarcinoma is not associated with improved long-term survival. Neither intestinal nor pancreatobiliary histologic subtypes treated with AT were associated with improved survival regardless of the chemotherapeutic agent used. Improvements in the care of patients with ampullary adenocarcinoma will require identification of novel therapeutics and/or sequencing strategies to improve long-term survival compared with the current agents.
eTable 1. Univariate Analysis of Operative Characteristics and Postoperative Outcomes of Pancreatoduodenectomy, Stratified by Disease Pathology (n = 5082)
eTable 2. Demographic and Clinicopathologic Variables of the Propensity Score-Matched Cohort, and Univariate Comparison Between Patients Receiving Surgery Alone or Adjuvant Therapy (n = 162)
eFigure 1. Association of Adjuvant therapy and Overall Survival in the Unmatched Cohort, Stratified by Pathologic Stage (stage I: n = 86; stage II: n = 21; stage III: n = 211)
eFigure 2. Association of Adjuvant Chemoradiotherapy Relative to Surgery Alone (left; n = 79) or Chemotherapy Alone (right; n = 75) on Recurrence-Free Survival in the Propensity Score-Matched Cohort
References
- 1.Goodman MT, Yamamoto J. Descriptive study of gallbladder, extrahepatic bile duct, and ampullary cancers in the United States, 1997-2002. Cancer Causes Control. 2007;18(4):415-422. doi: 10.1007/s10552-006-0109-4 [DOI] [PubMed] [Google Scholar]
- 2.Benhamiche AM, Jouve JL, Manfredi S, Prost P, Isambert N, Faivre J. Cancer of the ampulla of Vater: results of a 20-year population-based study. Eur J Gastroenterol Hepatol. 2000;12(1):75-79. doi: 10.1097/00042737-200012010-00014 [DOI] [PubMed] [Google Scholar]
- 3.Woo SM, Ryu JK, Lee SH, et al. . Recurrence and prognostic factors of ampullary carcinoma after radical resection: comparison with distal extrahepatic cholangiocarcinoma. Ann Surg Oncol. 2007;14(11):3195-3201. doi: 10.1245/s10434-007-9537-y [DOI] [PubMed] [Google Scholar]
- 4.Morris-Stiff G, Alabraba E, Tan YM, et al. . Assessment of survival advantage in ampullary carcinoma in relation to tumour biology and morphology. Eur J Surg Oncol. 2009;35(7):746-750. doi: 10.1016/j.ejso.2008.10.010 [DOI] [PubMed] [Google Scholar]
- 5.Chavez MT, Sharpe JP, O’Brien T, et al. . Management and outcomes following pancreaticoduodenectomy for ampullary adenocarcinoma. Am J Surg. 2017;214(5):856-861. doi: 10.1016/j.amjsurg.2017.01.029 [DOI] [PubMed] [Google Scholar]
- 6.Stiles ZE, Behrman SW, Deneve JL, et al. . Ampullary adenocarcinoma: defining predictors of survival and the impact of adjuvant therapy following surgical resection for stage I disease. J Surg Oncol. 2018;117(7):1500-1508. doi: 10.1002/jso.25021 [DOI] [PubMed] [Google Scholar]
- 7.Takada T, Amano H, Yasuda H, et al. ; Study Group of Surgical Adjuvant Therapy for Carcinomas of the Pancreas and Biliary Tract . Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? a phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95(8):1685-1695. doi: 10.1002/cncr.10831 [DOI] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Moore MJ, Cox TF, et al. ; European Study Group for Pancreatic Cancer . Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308(2):147-156. doi: 10.1001/jama.2012.7352 [DOI] [PubMed] [Google Scholar]
- 9.Morak MJ, van der Gaast A, Incrocci L, et al. . Adjuvant intra-arterial chemotherapy and radiotherapy versus surgery alone in resectable pancreatic and periampullary cancer: a prospective randomized controlled trial. Ann Surg. 2008;248(6):1031-1041. doi: 10.1097/SLA.0b013e318190c53e [DOI] [PubMed] [Google Scholar]
- 10.Smeenk HG, van Eijck CH, Hop WC, et al. . Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: long-term results of EORTC trial 40891. Ann Surg. 2007;246(5):734-740. doi: 10.1097/SLA.0b013e318156eef3 [DOI] [PubMed] [Google Scholar]
- 11.Robert PE, Leux C, Ouaissi M, et al. . Predictors of long-term survival following resection for ampullary carcinoma: a large retrospective French multicentric study. Pancreas. 2014;43(5):692-697. doi: 10.1097/MPA.0000000000000112 [DOI] [PubMed] [Google Scholar]
- 12.Chang DK, Jamieson NB, Johns AL, et al. . Histomolecular phenotypes and outcome in adenocarcinoma of the ampulla of Vater. J Clin Oncol. 2013;31(10):1348-1356. doi: 10.1200/JCO.2012.46.8868 [DOI] [PubMed] [Google Scholar]
- 13.Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: pancreas and hepatobiliary Cancers. Ann Surg Oncol. 2018;25(4):845-847. doi: 10.1245/s10434-017-6025-x [DOI] [PubMed] [Google Scholar]
- 14.Zhou H, Schaefer N, Wolff M, Fischer HP. Carcinoma of the ampulla of Vater: comparative histologic/immunohistochemical classification and follow-up. Am J Surg Pathol. 2004;28(7):875-882. doi: 10.1097/00000478-200407000-00005 [DOI] [PubMed] [Google Scholar]
- 15.Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM Jr. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216(1):1-14. doi: 10.1016/j.jamcollsurg.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 16.Ecker BL, McMillan MT, Maggino L, et al. . Pancreatogastrostomy vs. pancreatojejunostomy: a risk-stratified analysis of 5316 pancreatoduodenectomies. J Gastrointest Surg. 2018;22(1):68-76. doi: 10.1007/s11605-017-3547-2 [DOI] [PubMed] [Google Scholar]
- 17.Bassi C, Marchegiani G, Dervenis C, et al. ; International Study Group on Pancreatic Surgery (ISGPS) . The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161(3):584-591. doi: 10.1016/j.surg.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 18.Porembka MR, Hall BL, Hirbe M, Strasberg SM. Quantitative weighting of postoperative complications based on the accordion severity grading system: demonstration of potential impact using the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(3):286-298. doi: 10.1016/j.jamcollsurg.2009.12.004 [DOI] [PubMed] [Google Scholar]
- 19.Strasberg SM, Hall BL. Postoperative morbidity index: a quantitative measure of severity of postoperative complications. J Am Coll Surg. 2011;213(5):616-626. doi: 10.1016/j.jamcollsurg.2011.07.019 [DOI] [PubMed] [Google Scholar]
- 20.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150-161. doi: 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acharya A, Markar SR, Sodergren MH, et al. . Meta-analysis of adjuvant therapy following curative surgery for periampullary adenocarcinoma. Br J Surg. 2017;104(7):814-822. doi: 10.1002/bjs.10563 [DOI] [PubMed] [Google Scholar]
- 23.Miura JT, Jayakrishnan TT, Amini A, et al. . Defining the role of adjuvant external beam radiotherapy on resected adenocarcinoma of the ampulla of Vater. J Gastrointest Surg. 2014;18(11):2003-2008. doi: 10.1007/s11605-014-2629-7 [DOI] [PubMed] [Google Scholar]
- 24.Kwon J, Kim BH, Kim K, Chie EK, Ha SW. Survival benefit of adjuvant chemoradiotherapy in patients with ampulla of Vater cancer: a systematic review and meta-analysis. Ann Surg. 2015;262(1):47-52. doi: 10.1097/SLA.0000000000001182 [DOI] [PubMed] [Google Scholar]
- 25.Narang AK, Miller RC, Hsu CC, et al. . Evaluation of adjuvant chemoradiation therapy for ampullary adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Radiat Oncol. 2011;6:126. doi: 10.1186/1748-717X-6-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin Z, Hartgers ML, Sanhueza CT, et al. . Prognostic factors and benefits of adjuvant therapy after pancreatoduodenectomy for ampullary adenocarcinoma: Mayo Clinic experience. Eur J Surg Oncol. 2018;44(5):677-683. doi: 10.1016/j.ejso.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 27.Valsangkar NP, Ingkakul T, Correa-Gallego C, et al. . Survival in ampullary cancer: potential role of different KRAS mutations. Surgery. 2015;157(2):260-268. doi: 10.1016/j.surg.2014.08.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cloyd JM, Wang H, Overman M, et al. . Influence of preoperative therapy on short- and long-term outcomes of patients with adenocarcinoma of the ampulla of Vater. Ann Surg Oncol. 2017;24(7):2031-2039. doi: 10.1245/s10434-017-5777-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Univariate Analysis of Operative Characteristics and Postoperative Outcomes of Pancreatoduodenectomy, Stratified by Disease Pathology (n = 5082)
eTable 2. Demographic and Clinicopathologic Variables of the Propensity Score-Matched Cohort, and Univariate Comparison Between Patients Receiving Surgery Alone or Adjuvant Therapy (n = 162)
eFigure 1. Association of Adjuvant therapy and Overall Survival in the Unmatched Cohort, Stratified by Pathologic Stage (stage I: n = 86; stage II: n = 21; stage III: n = 211)
eFigure 2. Association of Adjuvant Chemoradiotherapy Relative to Surgery Alone (left; n = 79) or Chemotherapy Alone (right; n = 75) on Recurrence-Free Survival in the Propensity Score-Matched Cohort