Key Points
Question
Are there differences in performance between equations that estimate glomerular filtration rate in adults 65 years and older?
Findings
This single-center, cross-sectional study included 2247 French adults aged 65 to 90 years and older at a single referral center. When comparing 4 plasma creatinine–based glomerular filtration rate–estimating equations (Chronic Kidney Disease–Epidemiology Collaboration, Lund-Malmö Revised, full age spectrum, and Berlin Initiative Study) with the reference inulin-measuring method, there were no clinically significant differences in terms of bias, precision, or accuracy.
Meaning
There do not appear to be performance advantages for the use of any of these equations in persons 65 years and older.
This cross-sectional study compares 4 equations used to estimate glomerular filtration rate against measurement of inulin 2247 older French adults with varying degrees of kidney health.
Abstract
Importance
Estimating glomerular filtration rate (GFR) is useful in many clinical conditions. However, very few studies have evaluated the performance of GFR-estimating equations in older adults at various degrees of kidney impairment.
Objective
To determine the performance of plasma-creatinine-based equations Chronic Kidney Disease–Epidemiology Collaboration (CKD-EPI), Lund-Malmö Revised, (LMR), full age spectrum (FAS), and Berlin Initiative Study (BIS) 1 in older adults across a broad spectrum of GFRs.
Design, Setting, and Participants
Single-center cross-sectional study performed in France including 2247 participants aged 65 to 90 years who underwent inulin GFR measurements from July 1, 2003, to July 30, 2017, for suspected or established renal dysfunction, for renal risk, before kidney donation, or after kidney transplant.
Main Outcomes and Measures
The main outcome measure was GRF measured by inulin clearance. Equation performance criteria considered bias (difference between estimated and measured GFR), precision (interquartile range of the median difference), and accuracy P30 (percentage of estimated GFRs lying between [measured GFR – 30% of measured GFR] and [measured GFR + 30% of measured GFR]).
Results
The mean (SD) age of the 2247 participants was 71.5 (5) years and 1192 (53.0%) were male. The difference in median (95% CI) bias was significant between CKD-EPI vs LMR (−4.0 [–4.0 to –3.5 mL/min/1.73 m2; P < .001]) and CKD-EPI vs FAS (–2.0 [–3.5 to –2.5] mL/min/1.73 m2, P < .001) but not significant between CKD-EPI vs BIS 1 (0.0 [–1.5 to 0.5], P = .07, Mood test). In patients aged 65 to 74 years with measured GFR<45 mL/min/1.73 m2, the difference in median P30 (95% CI) was not significant between CKD-EPI vs LMR (P = .08) and CKD-EPI vs FAS (P = .48) but significant vs BIS 1 (P = .004, McNemar test). In subjects 75 years and older, with measured GFR less than 45 mL/min/1.73 m2, LMR and BIS 1 were more accurate than CKD-EPI and FAS (P30 = 74.5 [70.0-79.5] and 73.0 [68.0-78.0] vs 69.0 [64.5-74.0] and 69.0 [65.5-72.0]). In all patients, despite small statistical differences, the performance of CKD-EPI equation was not clinically different from that of LMR, FAS, or BIS 1.
Conclusions and Relevance
In a referral group of patients 65 years and older who had GFR estimated using CDK-EPI, LMR, BIS 1, and FAS equations, a comparison with renal inulin clearance found that none of the equations had a superior diagnostic performance. Each had limitations regarding accuracy.
Introduction
In persons older than 65 years with chronic kidney disease (CKD), accurate estimation of glomerular filtration rate (GFR) is important for correct CKD classification, management, and drug dosage.1,2,3,4 Determining the GFR by reference methods (eg, clearance of inulin, iohexol, or 51Cr-EDTA) is not possible in everyday practice; the CKD clinical guidelines from the National Kidney Foundation and Kidney Disease: Improving Global Outcomes1,2 have recommended using GFR-estimating equations as noninvasive alternatives.
In 2013, it was estimated that 47% of US adults 70 years and older met criteria for CKD.4 However, in this age group, an estimated GFR less than 60 mL/min/1.73 m2 without albuminuria was not associated with lower life expectancy.5,6,7 This has led researchers to propose a lower GFR value (<45 mL/min/1.73 m2) to define CKD in older adults.7 In addition, aging is associated with structural and physiological changes in the kidney and the muscle mass that may affect GFR estimation from plasma creatinine.5,6,7,8,9 Concerns about the accuracy of the Chronic Kidney Disease–Epidemiology Collaboration (CKD-EPI) equation in older adults has led to proposals for new equations10,11,12 and recent studies have reported that the CKD-EPI equation13 might be less reliable than newer equations in estimating GFR in this population.11,12,14
To clarify this issue, we evaluated the performance of 4 plasma creatinine–based equations: the classic CKD-EPI vs Full Age Spectrum (FAS), Lund-Malmö Revised (LMR), and Berlin Initiative Study (BIS 1). The study compared equation-estimated GFRs with measured GFRs (by inulin clearance) in older adults of different age groups and different CKD levels. We also assess the ability of these GFR estimates to detect CKD in older adults as defined by inulin GFR measurement less than 45 mL/min/1.73 m2.
Methods
Study Population and Setting
This retrospective cross-sectional study was planned to include all consecutive patients 65 to 90 years old who underwent a GFR measurement by either of 2 reference methods (urinary inulin or plasma iohexol clearance) between July 1, 2003, and July 30, 2017, at a single university hospital, Hôpital Edouard Herriot in Lyon, France, for suspected or established renal dysfunction, for renal risk, before kidney donation, or after kidney transplant. However, because these reference methods are not strictly comparable,15,16 the study had to exclude iohexol clearance measurements (n = 741) and keep only the first measurement of inulin clearance in 2247 patients (Figure 1).
Figure 1. Flowchart of the Study.
BIS 1 indicates Berlin Initiative Study 1 equation; CKD-EPI, chronic kidney disease epidemiology collaboration equation; FAS, full age spectrum equation; GFR, glomerular filtration rate; and LMR, Lund-Malmö revised equation.
All procedures were carried out in accordance with the ethical standards of the institutional and/or national research committee and with the 2013 Helsinki Declaration17 and its later amendments or with comparable ethical standards. Appropriate informed consent was obtained from each participant or his or her legal representative. The consent form included information on the procedure itself as well as on the possibility of later use of the data for research purposes.
According to the French law applicable at the time of the study, an observational study that did not change routine treatment of patients did not need to be declared or submitted to a research ethics board (Loi Huriet-Sérusclat 88-1138, December 20, 1988, and its subsequent amendments; text available at http://www.chu-toulouse.fr/IMG/pdf/loihuriet.pdf). Furthermore, according to the French Haute Autorité de Santé,18 no correction factor for race and ethnicity in the CKD-EPI equation should be applied in the European population; therefore, data concerning race and ethnicity were not collected and are not available.
Laboratory Assessments
Reference Method for GFR Measurement
Renal inulin clearance measurement was carried out only in patients who could empty their bladder easily and completely; this criterion excluded those who needed bladder catheterization.
The renal clearance of inulin used a polyfructosan-based method (Inutest, Fresenius Kabi). A standard technique was used by trained staff with a continuous infusion after a 30 mg/kg priming dose of polyfructosan. Water diuresis was induced by a first oral administration of 5 mL/kg of water followed by 3 mL/kg every 30 minutes combined with an intravenous infusion of 0.9% sodium chloride. Three to 4 urine samples were collected and a blood sample was drawn midway through each collection period. The retained clearance value was the mean of 3 or 4 values obtained by the usual UV/P formula (U, urinary concentration of polyfructosan; V, urine flow rate; and P, plasma concentration of polyfructosan). The measurements of plasma and urine polyfructosan were performed with the same enzymatic method that demonstrated good specificity and reproducibility (within-run precision, <1%; between-run precision, <3.5%).19 The results were expressed per 1.73 m2 body surface area according to the Dubois equation: body surface area = height0.725 × weight0.425 × 0.007184.
Plasma Creatinine Measurement
All plasma creatinine measurements were performed with methods traceable to the National Institute of Standards and Technology (isotope-dilution mass spectrometry-calibrated).
From June 8, 2003, to June 8, 2010, plasma creatinine concentration was measured using a kinetic colorimetric compensated Jaffé technique (Roche Modular) whose results were standardized against the concentrations obtained by liquid chromatography mass spectrometry by linear regression adjustment. The calibration equation was as follows: standardized plasma creatinine = 0.9395 × Jaffé compensated serum creatinine in μmol/L + 4.6964. The coefficient of correlation was 0.97.
From June 9, 2010, to July 30, 2017, all plasma creatinine values were obtained by an enzymatic technique. According to the Kidney Disease: Improving Global Outcomes guidelines, the 2 techniques are relatively similar.2 Plasma creatinine was expressed in μmol/L.
GFR-Estimating Equations
The equations used in the study population (with no correction for race and ethnicity) were the following (PCr indicates plasma creatinine):
Chronic Kidney Disease–Epidemiology Collaboration
Female, PCr ≤61.88: Estimated GFR = 144 × [PCr/61.88]–0.329 × [0.993]Age
Female, PCr >61.88: Estimated GFR = 144 × [PCr/61.88]–1.209 × [0.993]Age
Male, PCr ≤79.56: Estimated GFR = 141 × [PCr/79.56]–0.411 × [0.993]Age
Male, PCr >79.56: Estimated GFR = 141 × [PCr/79.56]–1.209 × [0.993]Age
Lund-Malmö Revised
Estimated GFR = e X–0.0158 × Age +0.438 × ln(Age)
Female, PCr <150: X = 2.50 + 0.0121 × (150 – PCr)
Female, PCr ≥150: X = 2.50 – 0.926 × ln(PCr/150)
Male, PCr <150: X = 2.56 + 0.00968 × (180 – PCr)
Male, PCr ≥180: X = 2.56 – 0.926 × ln(PCr/180)
Full Age Spectrum
Age ≥40 years: Estimated GFR = (107.3 × Q/PCr) × 0.988(Age-40) with Q = 80 μmol/L in men and 62 μmol/L in women
Berlin Initiative Study
Men: Estimated GFR = 3736 × PCr –0.87 × Age–0.95
Women: Estimated GFR = 3736 X PCr -0.87 × Age–0.95 × 0.82
Statistical Analysis
The study considered 3 criteria for performance: bias, precision, and accuracy. Bias was defined as the median difference between measured GFR and estimated GFR. Thus, a negative bias indicates that an equation overestimates the GFR and vice versa. Precision was defined as the interquartile range (IQR) of the differences between measured GFR and estimated GFR.
Accuracy was considered under 2 criteria: the root mean square error (RMSE), the square root of (log of measured GFR – log of estimated GFR)2; and P30, percentage of estimates within 30% of the measured value.20 A P30 greater than 90% qualifies an equation as satisfactory for clinical interpretation.1,2,15,21
The concordance correlation coefficient (CCC) was used to assess the strength of theoretical agreement between each estimated GFR and measured GFR (after logarithmic transformation of their values). The CCC ranges between −1 and 1; 1 denotes perfect agreement, greater than 0.990, almost perfect agreement; 0.950 to 0.990, substantial agreement; 0.900 to 0.949, moderate agreement; and less than 0.900, poor agreement.22
The 95% CIs around bias, precision, RMSE, and P30 values were calculated using a bootstrap method (2000 bootstraps).23 To assess and compare the 4 equations, the analysis was carried out in 2 separate age groups (65-74 years and ≥75 years)24 and 2 measured GFR categories (<45 and ≥45 mL/min/1.73 m2).7 Subanalyses were carried out on 2 other measured GFR categories (<60 and ≥60 mL/min/1.73 m2), obese patients, kidney transplant recipients, and patients with various categories of albuminuria.
The area under the receiver operating characteristic (ROC) curves (AUC) was used to determine the ability of the GFR-estimating equations to discriminate between elderly patients with and without CKD (defined as measured GFR <45 mL/min/1.73 m2).7 Median biases were compared using the Mood median test.25 P30 values were compared using Cochran Q with pairwise McNemar test and Holm-Bonferroni correction.26 Areas under the curve were compared using the Delong Clarke-Pearson method.27 Results from kidney transplant recipients were compared with those of nonrecipients using the Wilcoxon signed-rank test. Whenever necessary, the Holm-Bonferroni method was used to correct for multiple comparisons.
The sample size and the measurement precision in this study were high; thus, small changes in any variable could lead to small P values. In such conditions, the American Statistical Association recommends the use of P < .005.28 Differences between measured GFR and estimated GFR were considered clinically meaningful when 2 conditions were fulfilled: the RMSEs differed by more than 2% and the biases differed by more than 5 mL/min/1.73 m2.29
The database used for this study had no missing data. Statistical analyses were performed using R for Windows, version 3.4.4 (R-Cran project, http://cran.r-project.org/).
Results
Participant Characteristics
From an initial population of 3539 participants, 2247 participants met the study criteria (Figure 1). The sociodemographic and clinical characteristics of these participants are shown in Table 1. At inclusion the mean (SD) age of the participants was 71.5 (5.0) years. Among these participants, 1192 (53.0%) were male and 311 (14.0%) were kidney transplant recipients. The mean (SD) of measured GFR was 44.5 (21) mL/min/1.73 m2. Within the measured GFR range of 5 to 147 mL/min/1.73 m2, 43.5% of measurements had values less than 45 mL/min/1.73 m2. There was no significant difference in the mean measured GFR between kidney transplant recipients and the other participants.
Table 1. Sociodemographica and Clinical Characteristics of the 2247 Participants.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Whole Cohort | Patients With Measured GFR <45 mL/min/1.73 m2 | Patients With Measured GFR ≥45 mL/min/1.73 m2 | |
| Participants | 2247 (100) | 991 (43.5) | 1256 (56.5) |
| Age, mean (SD), y | 71.5 (5.0) | 72.0 (5.5) | 70.5 (4.5) |
| Age group, y | |||
| 65-74 | 1710 (76.0) | 670 (67.5) | 1040 (83.0) |
| ≥75 | 537 (24.0) | 321 (32.5) | 216 (17.0) |
| Sex | |||
| Male | 1192 (53.0) | 541 (54.5) | 651 (52.0) |
| Female | 1055 (47.0) | 450 (45.5) | 605 (48.0) |
| Potential kidney donors | 16 (7.0) | 0 | 16 (13.0) |
| Weight, mean (SD), kg | 71.5 (18.0) | 73.0 (17.5) | 68.5 (16.5) |
| Height, mean (SD), cm | 163.5 (9.5) | 163.5 (9.5) | 163.0 (9.0) |
| BSA, mean (SD), m2 | 1.75 (0.20) | 1.78 (0.20) | 1.73 (0.20) |
| Mean BMI | 26.5 (5.5) | 27.3 (6.0) | 25.7 (5.0) |
| BMI ≥30 | 466 (21.0) | 257 (26.0) | 209 (16.5) |
| PCr, median (IQR), mg/dL | 1.28 (0.97-1.64) | 1.70 (1.39-2.11) | 1.04 (0.84-1.26) |
| Measured GFR in all participants, mean (SD), mL/min/1.73 m2 | 44.5 (21.0) | 31.0 (9.0) | 64.0 (15.5) |
| Albuminuria, median (IQR), mg/g | 45 (15-225) | 95 (30-500) | 25 (10-100) |
| Albuminuria, mg/g | |||
| UACR <30 | 940 (42.0) | 255 (26.0) | 685 (54.5) |
| UACR 30-300 | 827 (37.0) | 427 (43.0) | 400 (32.0) |
| UACR >300 | 480 (21.0) | 309 (31.0) | 171 (13.5) |
| UACR >3000b | 79 (3.5) | 59 (5.9) | 20 (1.6) |
| Patient with transplanted kidney | 311 (14.0) | 156 (16.0) | 155 (12.5) |
| Measured GFR in patients with transplanted kidney, mean (SD) | 47.0 (17.0) | 34.0 (7.5) | 60.0 (14.0) |
| Measured GFR in patients without transplant, mean (SD) | 49.5 (21.5) | 30.0 (9.5) | 65.0 (15.5) |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; BSA, body surface area; GFR, glomerular filtration rate; IQR, interquartile range; PCr, plasma creatinine; UACR, urinary albumin/creatinine ratio.
SI conversion factors: To convert plasma creatinine to micromoles per liter, multiply by 88.4. For an approximate conversion of albumin to creatinine ratio from milligrams per gram to milligrams per mole, divide by 10.
According to the French Haute Autorité de Santé,18 no correction factor for race and ethnicity in the CKD-EPI equation should be applied in the European population; therefore, no data concerning race and ethnicity were collected.
UACR >3000 mg/g defines nephrotic albuminuria.
Performance According to Equation Type, CKD Class, and Age
In the whole cohort of participants, none of the 3 other equations demonstrated a clinically better performance than CKD-EPI. Nevertheless, several differences, although not clinically significant, were statistically significant.
Regarding bias, the median bias between CKD-EPI and each of LMR and FAS was significant (less bias in the latter) (–4.0 [–4.0 to –3.5] and –2.0 [–3.5 to –2.5] mL/min/1.73 m2; P < .001); the bias vs BIS 1 was not significant (0.0 [–1.5 to 0.5] mL/min/1.73 m2; P = .07) (Table 2).
Table 2. Pairwise Comparisons Between GFR-Estimating Equationsa.
| Pairwise Comparisons | Median (95% CI) Difference in Bias | P Values | Median (95% CI) Difference in P30 | P Value |
|---|---|---|---|---|
| Whole Cohort (N = 2247) | ||||
| CKD-EPI-LMR | −4.0 (−4.0 to −3.5) | <.001 | −3.5 (−6.0 to −1.5) | <.001 |
| CKD-EPI-FAS | −2.0 (−3.5 to −2.5) | <.001 | −2.0 (−4.5 to 0.5) | .07 |
| CKD-EPI-BIS 1 | 0.0 (−1.5 to 0.5) | .07 | 0.5 (0.0 to 0.5) | .48 |
| Patients Aged 65-74 Years (N = 1710) | ||||
| CKD-EPI-LMR | −4.0 (−4.5 to −3.5) | <.001 | −4.0 (−3.5 to −1.5) | <.001 |
| CKD-EPI-FAS | −2.0 (−2.5 to −1.0) | <.001 | −2.0 (−3.5 to −1.5) | .002 |
| CKD-EPI-BIS 1 | −1.0 (−1.5 to −0.5) | .004 | 0.5 (0.0 to 0.5) | .66 |
| Patients Aged ≥75 Years (N = 537) | ||||
| CKD-EPI-LMR | −4.0 (−4.5 to −3.5) | <.001 | −3.0 (−8.0 to 2.0) | .07 |
| CKD-EPI-FAS | −3.0 (−3.5 to −2.5) | .001 | −2.0 (−7.0 to 3.0) | .19 |
| CKD-EPI-BIS 1 | −1.0 (−1.5 to 0.5) | .46 | 1.0 (−0.5 to 0.5) | .68 |
Abbreviations: BIS 1, Berlin Initiative Study 1 equation; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration equation; FAS, full age spectrum equation; GFR, glomerular filtration rate; LMR, Lund-Malmö Revised equation.
Median difference in bias is the difference between equation biases (measured GFR minus estimated GFR; a negative median difference means that, on average, the second equation provides closer values to measured GFR by inulin clearance than the first equation). P30, percentage of estimates within 30% of the measured value; here too, a negative median difference means that, on average, more values from the second equation are close to measured GFR by inulin clearance than from the first equation). 95% CIs were calculated by a bootstrap method (2000 bootstraps) over all measure differences. The significance level was .005. 95% CI as calculated by a bootstrap method.
Regarding accuracy, LMR equation was the most accurate; it had the lowest RMSE (95% CI) 0.185 (0.178-0.190) (Table 3).
Table 3. Bias, Precision, and Accuracy of the 4 GFR-Estimating Equations.
| Patients and Criteria | CKD-EPI | LMR | FAS | BIS 1 |
|---|---|---|---|---|
| Whole Cohort (N = 2247)a | ||||
| Median bias (95% CI) | −2.0 (−3.0 to −1.0) | 2.0 (1.5 to 3.5) | 0.0 (−0.5 to 0.5) | −2.0 (−3.5 to −1.5) |
| IQR (95% CI) | 14.0 (13.0 to 15.5) | 13.0 (12.0 to 13.0) | 14.0 (13.0 to 15.0) | 14.0 (13.0 to 15.0) |
| P30 (95% CI) | 78.0 (76.0 to 80.0) | 81.5 (80.0 to 83.0) | 79.0 (78.0 to 82.0) | 77.5 (75.5 to 79.0) |
| RMSE (95% CI) | 0.195 (0.188 to 0.203) | 0.185 (0.178 to 0.190) | 0.188 (0.182 to 0.196) | 0.189 (0.183 to 0.196) |
| CCC (95% CI) | 0.832 (0.827 to 0.838) | 0.838 (0.832 to 0.843) | 0.826 (0.820 to 0.832) | 0.817 (0.812 to 0.823) |
| AUC (95% CI) | 0.921 (0.916 to 0.926) | 0.922 (0.917 to 0.927) | 0.919 (0.915 to 0.924) | 0.918 (0.914 to 0.924) |
| Patients Aged 65-74 Years (n = 1710)b | ||||
| Median bias (95% CI) | 2.0 (1.0 to 2.5) | −2.0 (−3.0 to −1.5) | 0.0 (−0.5 to 0.5) | 2.0 (−0.5 to 1.5) |
| IQR (95% CI) | 15.0 (14.5 to 17.0) | 14.0 (13.0 to 15.5) | 14.0 (12.5 to 15.0) | 15.0 (14.0 to 16.5) |
| P30 (95% CI) | 79.5 (76.0 to 83.0) | 82.0 (80.5 to 84.0) | 80.5 (78.5 to 82.0) | 78.0 (76.0 to 80.0) |
| RMSE (95% CI) | 0.202 (0.194 to 0.211) | 0.190 (0.182 to 0.198) | 0.196 (0.187 to 0.204) | 0.195 (0.188 to 0.204) |
| CCC (95% CI) | 0.808 (0.802 to 0.815) | 0.824 (0.818 to 0.830) | 0.807 (0.801 to 0.814) | 0.804 (0.797 to 0.810) |
| AUC (95% CI) | 0.917 (0.913 to 0.923) | 0.917 (0.911 to 0.923) | 0.914 (0.908 to 0.920) | 0.915 (0.909 to 0.921) |
| Patients Aged ≥75 Years (n = 537)c | ||||
| Median bias (95% CI) | 3.0 (2.5 to 5.0) | −2.0 (−3.5 to −1.5) | 0.0 (−1.0 to 0.5) | 2.0 (1.5 to 3.5) |
| IQR (95% CI) | 13.0 (12.0 to 15.0) | 12.0 (11.0 to 14.0) | 13.0 (12.0 to 15.0) | 13.0 (11.0 to 15.0) |
| P30 (95% CI) | 77.0 (73.0 to 80.0) | 80.0 (76.5 to 83.5) | 78.5 (75.0 to 82.0) | 76.0 (72.5 to 79.5) |
| RMSE (95% CI) | 0.173 (0.160 to 0.185) | 0.164 (0.152 to 0.176) | 0.166 (0.154 to 0.179) | 0.171 (0.159 to 0.183) |
| CCC (95% CI) | 0.791 (0.779 to 0.803) | 0.804 (0.792 to 0.815) | 0.783 (0.770 to 0.795) | 0.776 (0.763 to 0.788) |
| AUC (95% CI) | 0.920 (0.911 to 0.930) | 0.923 (0.913 to 0.932) | 0.922 (0.912 to 0.932) | 0.920 (0.910 to 0.930) |
Abbreviations: AUC, area under the receiver operating characteristic curve; BIS 1, Berlin Initiative Study 1 equation; CCC, concordance correlation coefficient according to Crawford et al, 2007; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration equation; FAS, full age spectrum equation; GFR, glomerular filtration rate; IQR, interquartile range of bias values; 95% CI as calculated by a bootstrap method. LMR, Lund-Malmö Revised equation; median bias, median of differences between measured GFRs by inulin clearance and estimated GFRs by a given formula; P30, percentage of estimates within 30% of the measured value; RMSE, root mean square error or square root of (log of measured GFR – log of estimated GFR)2.
Mean (SD) measured GFR in mL/min/1.73 m2: 44.5 (21.0).
Mean (SD) measured GFR in mL/min/1.73 m2: 52.0 (21.0).
Mean (SD) measured GFR in mL/min/1.73 m2: 41.0 (19.0).
In patients aged 65 to 74 years with measured GFR less than 45 mL/min/1.73 m2, the median (95% CI) differences in P30 between CKD-EPI and each of LMR and FAS were not significant (P = .08 and P = .48, respectively) but the median difference in P30 with BIS 1 was significant (P = .004) (eTable 1 in the Supplement).
There were some significant differences regarding accuracy between patients with measured GFR less than 45 mL/min/1.73 m2. In patients aged 65 to 74 years, precision criterion P30 was the highest with LMR equation (72.0 [69.0-76.0]) and the lowest with BIS 1 equation (59.0 [55.0-63.0]) (eTable 2 in the Supplement). In patients 75 years and older, LMR and BIS 1 were more accurate than CKD-EPI and FAS (74.5 [70.0-79.5] and 73.0 [68.0-78.0] vs 69.0 [64.5-74.0] and 69.0 [65.5-72.0]) (eTable 2 in the Supplement).
Concordance Correlation Coefficient
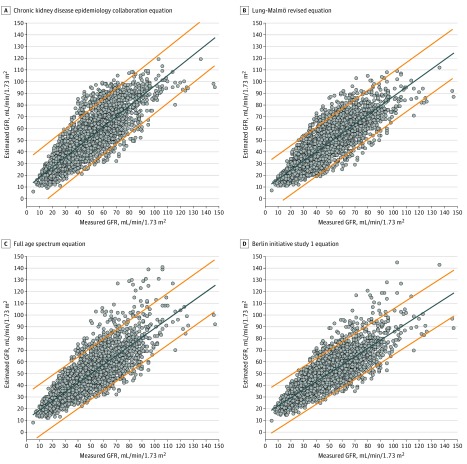
In the whole population and in all subgroups, no CCC between measured GFR and estimated GFR by any equation was greater than 0.900. BIS 1 had the lowest CCC in the whole cohort and in participants aged 65 to 75 years (Figure 2).
Figure 2. Chronic Kidney Disease Epidemiology Collaboration Equation Scatter Plots of Equation-Estimated Glomerular Filtration Rates in Function of Inulin-Measured Glomerular Filtration Rates in the Whole Population.
The solid line represents the regression line. The dotted lines represent the 95% limits of prediction. The concordance correlation coefficient (CCC) for panel A is 0.832 (95% CI, 0.827-0.838); B, 0.838 (95% CI, 0.832-0.843); C, 0.817 (95% CI, 0.812-0.823); D, 0.826 (95% CI, 0.820-0.832).
Area Under the Receiver Operating Curve
There were no significant differences in the abilities (AUCs) of the equations to detect a measured GFR less than 45 mL/min/1.73 m2; the AUCs (95% CI) were 0.921 (0.916-0.926) for CKD-EPI, 0.922 (0.917-0.927) for LMR, 0.919 (0.915-0.924) for FAS, and 0.918 (0.914-0.924) for BIS 1. There were also no significant differences in equation abilities to detect measured GFR less than 60 mL/min/1.73 m2 (eFigure in the Supplement).
Subgroup Analyses
Overall, the 4 equations did not show clinically significant differences between the various subgroups: obese vs nonobese (eTable 3 in the Supplement), transplant recipients vs non recipients (eTable 4 in the Supplement), albumin values in the normal range (<30 mg/g) vs other categories of albuminuria (eTable 5 in the Supplement), and patients with less than 60 vs patients with greater than 60 mL/min/1.73 m2 (eTable 6 in the Supplement).
Discussion
We found that the performance of the CKD-EPI equation in diagnosing CKD in persons older than 65 years was not significantly better or worse than the newer equations. None of the equations estimated a GFR that was 70% to 130% of the measured GFR more than 80% of the time.
This study demonstrated close performances of the 4 GFR-estimating equations between measured GFR groups in terms of bias, IQR, and RMSE. However, the decrease in P30 in the low-GFR group should be interpreted with caution, because small absolute errors may still indicate equation disagreement in those with lower GFRs.
As previously demonstrated, a GFR-estimating equation performs best in populations that resemble the population within which it was developed.15 For instance, the CKD-EPI equation is recommended for estimating GFR in adults of any age in North America, Europe, and Australia. It was developed in a North American and European population with a wide age range (mean [SD], 50 [15] years) and a mean measured GFR of 68.0 mL/min/1.73 m2; in addition, CKD-EPI equation takes into account age, sex, race, and ethnicity.13 Although the proportion of patients 65 years or older within the CKD-EPI development and internal validation data sets was 13.0%, the present study found adequate performance of CKD-EPI in older adults at different levels of measured GFR.4
The LMR equation was developed in a large cohort of patients referred for GFR measurement in a European population, predominantly (67%) from a single country (Sweden). In this population, 28% were 70 years or older10 and had a mean [SD] measured GFR of 55 (9-21) mL/min/1.73 m2. In similar populations, this equation performed better than CKD-EPI, especially in people 70 years and older, but this did not receive an external validation.14,30,31,32 In the present study, however, the performance of LMR equation was only slightly more accurate than the CKD-EPI equation (P30) in the subgroup of patients with measured GFR less than 45 mL/min/1.73 m2, which is close to the measured GFR in the development population.
The FAS equation was designed to estimate GFR across a broad range of ages, children to older adults.12 It was developed in a predominantly European population in a multicenter study that included 1764 patients 70 years and older (mean [SD] age, 77 [5.4] years) with mean [SD] measured GFR of 55.7 [20.6] mL/min/1.73 m2. In the present study, the FAS equation did not perform better than the other 3 equations.
The rare equations developed for GFR estimation in old subjects have reasonable performance, usually with poor accuracy in subjects with measured GFR 60 mL/min/1.73 m2 or greater.10,11,12,32,33,34,35,36,37 This is the case of BIS 111 that was developed in subjects 70 years and older (mean [range] age, 78.5 [9-121] years) and included 29.4% of healthy older adults with mean [range] measured GFR of 60.3 mL/min/1.73 m2. In comparison with plasma iohexol clearance, the performance of BIS 1 was excellent: absolute bias close to zero (0.11), good precision (IQR, 11.1) and accuracy (P30: 95.1%). The BIS 1 equation appeared then to be a good tool for estimating GFR in older adults.11 However, other studies that attempted to validate this equation reported conflicting results.10,12,14,31,32,35,36,37,38,39 In the present study, BIS 1 had a poor performance in persons aged 65 to 74 years with measured GFR less than 45 mL/min/1.73 m2. This is in agreement with other results37,40 and is probably owing to differences between study and development cohorts.
In older adults, late referrals for CKD management may result in suboptimal outcomes, including increased mortality, higher rates of hospitalization, increased referrals for kidney transplant, and higher rates of catheter use for dialysis. Although plasma creatinine is the most used marker to estimate GFR, the method has many limitations; it is influenced by muscle mass and diet, especially in older adults, women, and children. Cystatin C, a low-molecular-weight protein, does not present such limitations and could be a better marker; this led to equations based on cystatin C or cystatin C plus plasma creatinine. Up to now, several studies have reported that cystatin C was more accurate than creatinine in detecting CKD in older adults at both cutoffs of 45 and 60 mL/min/1.73 m2.8,14,36 A recent study reported that the addition of cystatin C improved all creatinine-based equations.32 Thus, the Kidney Disease: Improving Global Outcomes recommended the recourse to GFR estimation by cystatin C whenever the estimated GFR by creatinine is below 60 mL/min/1.73 m2 and when a confirmation of CKD is required.2 However, cystatin C is not always available, as in this study.
The strengths of the present study are that it used a unique data set regarding size, age range, measured GFR range, use of plasma creatinine assays calibrated on standardized values, use of a reference method for GFR measurement (ie, inulin clearance),16 and rigorous statistical techniques.
Limitations
This study had several limitations. First, it was a single-center study that included potential kidney donors and patients referred for suspected or confirmed renal disease. Second, the data set is devoid of information about race and ethnicity and therefore did not allow us to assess the effect of this characteristic, which is common to other European studies (LMR, FAS, and BIS 1). However, recent studies have reported that GFR is independent of race and ethnicity.41 Third, the performance of the equations in persons with measured GFR less than 30 mL/min/1.73 m2 could not be examined because of the small number of participants with severe CKD. Fourth, the use of plasma creatinine alone as endogenous marker (without cystatin C) has some well-known limitations, especially in older participants with sarcopenia.9,42,43
This study was conducted in a single institution on a referral population, which might have introduced a selection bias, both in terms of demographic and clinical characteristics. Thus, the overall result of “no differences between the four equations” should be investigated in populations with more complex mixes of races and ethnicities and in populations with lower suspicion of moderate to severe CKD.
Conclusions
Among a referral group of patients 65 years and older who had GFR estimated by CDK-EPI, LMR, BIS 1, and FAS, a comparison with renal inulin clearance found that none of the equations had a superior diagnostic performance. Each equation had limitations regarding accuracy. Thus, any of the 4 equations may be used to estimate GRF in adults 65 years and older, depending on local clinical, technical, or practical criteria.
eTable 1. Median Bias and P30 Comparisons Between GFR-Estimating Equations
eTable 2. Bias, Precision, and Accuracy of the Four GFR-Estimating Equations According to Measured GFR
eTable 3. Performance Criteria of the Four GFR-Estimating Equations in 466 Obese Patients (BMI≥30 kg/m2)
eTable 4. Performance Criteria of the Four GFR-Estimating Equations in 311 Kidney Transplanted Patients
eTable 5. Performance Criteria of the Four GFR-Estimating Equations According to Categories of Albuminuria
eTable 6. Performance Criteria of the Four GFR-Estimating Equations According to Renal Function
eFigure. ROC Curve Analysis of Diagnostic Accuracy of Calculated Clearance From the CKD-EPI, LMR, FAS and BIS 1 Equations
References
- 1.Levey AS, Coresh J, Balk E, et al. ; National Kidney Foundation . National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137-147. doi: 10.7326/0003-4819-139-2-200307150-00013 [DOI] [PubMed] [Google Scholar]
- 2.Group KDIGO. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1-150. [DOI] [PubMed] [Google Scholar]
- 3.Eckardt KU, Coresh J, Devuyst O, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382(9887):158-169. doi: 10.1016/S0140-6736(13)60439-0 [DOI] [PubMed] [Google Scholar]
- 4.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260-272. doi: 10.1016/S0140-6736(13)60687-X [DOI] [PubMed] [Google Scholar]
- 5.Garasto S, Fusco S, Corica F, et al. Estimating glomerular filtration rate in older people. Biomed Res Int. 2014;2014:916542. doi: 10.1155/2014/916542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallan SI, Matsushita K, Sang Y, et al. ; Chronic Kidney Disease Prognosis Consortium . Age and association of kidney measures with mortality and end-stage renal disease. JAMA. 2012;308(22):2349-2360. doi: 10.1001/jama.2012.16817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glassock R, Delanaye P, El Nahas M. An age-calibrated classification of chronic kidney disease. JAMA. 2015;314(6):559-560. doi: 10.1001/jama.2015.6731 [DOI] [PubMed] [Google Scholar]
- 8.Bevc S, Hojs N, Hojs R, Ekart R, Gorenjak M, Puklavec L. Estimation of glomerular filtration rate in elderly chronic kidney disease patients: comparison of three novel sophisticated equations and simple cystatin C equation. Ther Apher Dial. 2017;21(2):126-132. doi: 10.1111/1744-9987.12523 [DOI] [PubMed] [Google Scholar]
- 9.Glassock RJ, Rule AD. The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney Int. 2012;82(3):270-277. doi: 10.1038/ki.2012.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Björk J, Grubb A, Sterner G, Nyman U. Revised equations for estimating glomerular filtration rate based on the Lund-Malmö Study cohort. Scand J Clin Lab Invest. 2011;71(3):232-239. doi: 10.3109/00365513.2011.557086 [DOI] [PubMed] [Google Scholar]
- 11.Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012;157(7):471-481. doi: 10.7326/0003-4819-157-7-201210020-00003 [DOI] [PubMed] [Google Scholar]
- 12.Pottel H, Hoste L, Dubourg L, et al. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant. 2016;31(5):798-806. doi: 10.1093/ndt/gfv454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Björk J, Bäck SE, Ebert N, et al. GFR estimation based on standardized creatinine and cystatin C: a European multicenter analysis in older adults. Clin Chem Lab Med. 2018;56(3):422-435. doi: 10.1515/cclm-2017-0563 [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Inker LA. Assessment of glomerular filtration rate in health and disease: a state of the art review. Clin Pharmacol Ther. 2017;102(3):405-419. doi: 10.1002/cpt.729 [DOI] [PubMed] [Google Scholar]
- 16.Soveri I, Berg UB, Björk J, et al. ; SBU GFR Review Group . Measuring GFR: a systematic review. Am J Kidney Dis. 2014;64(3):411-424. doi: 10.1053/j.ajkd.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 18.Haute Autorité de Santé Evaluation du débit de filtration glomérulaire et du dosage de la créatininémie dans le diagnostic de la maladie rénale chronique chez l’adulte. https://www.has-sante.fr/portail/upload/docs/application/pdf/2011-12/rapport_dfg_creatininemie.pdf. Accessed September 12, 2018.
- 19.Selistre L, De Souza V, Cochat P, et al. GFR estimation in adolescents and young adults. J Am Soc Nephrol. 2012;23(6):989-996. doi: 10.1681/ASN.2011070705 [DOI] [PubMed] [Google Scholar]
- 20.Stevens LA, Zhang Y, Schmid CH. Evaluating the performance of GFR estimating equations. J Nephrol. 2008;21(6):797-807. [PMC free article] [PubMed] [Google Scholar]
- 21.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2)(suppl 1):S1-S266. [PubMed] [Google Scholar]
- 22.Crawford SB, Kosinski AS, Lin HM, Williamson JM, Barnhart HX. Computer programs for the concordance correlation coefficient. Comput Methods Programs Biomed. 2007;88(1):62-74. doi: 10.1016/j.cmpb.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 23.Bland JM, Altman DG. Statistics notes: bootstrap resampling methods. BMJ. 2015;350:h2622. doi: 10.1136/bmj.h2622 [DOI] [PubMed] [Google Scholar]
- 24.Adams PE, Martinez ME, Vickerie JL, Kirzinger WK. Summary health statistics for the U.S. population: National Health Interview Survey, 2010. Vital Health Stat 10. 2011;(251):1-117. . [PubMed] [Google Scholar]
- 25.Richter SJ, McCann MH. Multiple comparison of medians using permutation tests. J Mod Appl Stat Methods. 2007;6(2):399-412. doi: 10.22237/jmasm/1193889900 [DOI] [Google Scholar]
- 26.Cohen JF, Chalumeau M, Cohen R, Korevaar DA, Khoshnood B, Bossuyt PM. Cochran’s Q test was useful to assess heterogeneity in likelihood ratios in studies of diagnostic accuracy. J Clin Epidemiol. 2015;68(3):299-306. doi: 10.1016/j.jclinepi.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 27.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ioannidis JPA. The proposal to lower P value thresholds to. 005. JAMA. 2018;319(14):1429-1430. doi: 10.1001/jama.2018.1536 [DOI] [PubMed] [Google Scholar]
- 29.Stevens LA, Schmid CH, Zhang YL, et al. Development and validation of GFR-estimating equations using diabetes, transplant and weight. Nephrol Dial Transplant. 2010;25(2):449-457. doi: 10.1093/ndt/gfp510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyman U, Grubb A, Larsson A, et al. The revised Lund-Malmö GFR estimating equation outperforms MDRD and CKD-EPI across GFR, age and BMI intervals in a large Swedish population. Clin Chem Lab Med. 2014;52(6):815-824. doi: 10.1515/cclm-2013-0741 [DOI] [PubMed] [Google Scholar]
- 31.Björk J, Jones I, Nyman U, Sjöström P. Validation of the Lund-Malmö, Chronic Kidney Disease Epidemiology (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) equations to estimate glomerular filtration rate in a large Swedish clinical population. Scand J Urol Nephrol. 2012;46(3):212-222. doi: 10.3109/00365599.2011.644859 [DOI] [PubMed] [Google Scholar]
- 32.Björk J, Grubb A, Gudnason V, et al. Comparison of glomerular filtration rate estimating equations derived from creatinine and cystatin C: validation in the Age, Gene/Environment Susceptibility-Reykjavik elderly cohort. Nephrol Dial Transplant. 2018;33(8):1380-1388. doi: 10.1093/ndt/gfx272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevens LA, Schmid CH, Greene T, et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis. 2010;56(3):486-495. doi: 10.1053/j.ajkd.2010.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kilbride HS, Stevens PE, Eaglestone G, et al. Accuracy of the MDRD (Modification of Diet in Renal Disease) study and CKD-EPI (CKD Epidemiology Collaboration) equations for estimation of GFR in the elderly. Am J Kidney Dis. 2013;61(1):57-66. doi: 10.1053/j.ajkd.2012.06.016 [DOI] [PubMed] [Google Scholar]
- 35.Lopes MB, Araújo LQ, Passos MT, et al. Estimation of glomerular filtration rate from serum creatinine and cystatin C in octogenarians and nonagenarians. BMC Nephrol. 2013;14:265. doi: 10.1186/1471-2369-14-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan L, Levey AS, Gudnason V, et al. Comparing GFR estimating equations using Cystatin C and creatinine in elderly individuals. J Am Soc Nephrol. 2015;26(8):1982-1989. doi: 10.1681/ASN.2014060607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koppe L, Klich A, Dubourg L, Ecochard R, Hadj-Aissa A. Performance of creatinine-based equations compared in older patients. J Nephrol. 2013;26(4):716-723. doi: 10.5301/jn.5000297 [DOI] [PubMed] [Google Scholar]
- 38.Alshaer IM, Kilbride HS, Stevens PE, et al. External validation of the Berlin equations for estimation of GFR in the elderly. Am J Kidney Dis. 2014;63(5):862-865. doi: 10.1053/j.ajkd.2014.01.013 [DOI] [PubMed] [Google Scholar]
- 39.Vidal-Petiot E, Haymann JP, Letavernier E, et al. External validation of the BIS (Berlin Initiative Study)-1 GFR estimating equation in the elderly. Am J Kidney Dis. 2014;63(5):865-867. doi: 10.1053/j.ajkd.2014.01.421 [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Cheng MH, Shi CG, et al. Variability of glomerular filtration rate estimation equations in elderly Chinese patients with chronic kidney disease. Clin Interv Aging. 2012;7:409-415. doi: 10.2147/CIA.S36152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yayo E, Ayé M, Yao C, et al. Measured (and estimated) glomerular filtration rate: reference values in West Africa. Nephrol Dial Transplant. 2018;33(7):1176-1180. doi: 10.1093/ndt/gfx244 [DOI] [PubMed] [Google Scholar]
- 42.Epstein M. Aging and the kidney. J Am Soc Nephrol. 1996;7(8):1106-1122. [DOI] [PubMed] [Google Scholar]
- 43.Baxmann AC, Ahmed MS, Marques NC, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3(2):348-354. doi: 10.2215/CJN.02870707 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Median Bias and P30 Comparisons Between GFR-Estimating Equations
eTable 2. Bias, Precision, and Accuracy of the Four GFR-Estimating Equations According to Measured GFR
eTable 3. Performance Criteria of the Four GFR-Estimating Equations in 466 Obese Patients (BMI≥30 kg/m2)
eTable 4. Performance Criteria of the Four GFR-Estimating Equations in 311 Kidney Transplanted Patients
eTable 5. Performance Criteria of the Four GFR-Estimating Equations According to Categories of Albuminuria
eTable 6. Performance Criteria of the Four GFR-Estimating Equations According to Renal Function
eFigure. ROC Curve Analysis of Diagnostic Accuracy of Calculated Clearance From the CKD-EPI, LMR, FAS and BIS 1 Equations