Abstract
Cryptogenic organizing pneumonia (COP) is characterized by good response to corticosteroids, but frequent relapses after reduction or cessation of treatment are noted. The incidence, risk factors of relapse, and long-term outcomes of patients with COP remain undetermined. Patients with COP from September 2010 to December 2017 were enrolled. Hospital and office records were used as data sources. Clinical information, lab examinations, chest radiographs, treatment courses, and follow-up data were collected. Relapse group was defined as worsening of clinical manifestations in combination with progression of radiographic abnormalities in the absence of identified causes. Eighty-seven patients with COP were enrolled. Of them, 73 patients were treated with corticosteroids with relapse rate yielding 31.5% (23 of 73). Eleven patients were treated with macrolides and none of them relapsed. Fever was more common (65.2% vs. 32.0%, p = 0.004), C-reactive protein (CRP) was higher (31.5 ± 39.4 mg/L vs. 17.5 ± 32.2 mg/L, p = 0.038), and diffusion capacity for carbon monoxide (DLCO) % predicted was lower (45.9 ± 14.2% vs. 57.6 ± 18.5%, p = 0.050) in relapse group compared to nonrelapse group. Four patients who presented with organizing pneumonia (OP) as the first manifestation were ultimately diagnosed with OP secondary to autoimmune disease in follow-up. We showed relapse was common in COP patients treated with corticosteroids, but the prognosis was favorable. Fever, elevated CRP, and a reduced DLCO were related to relapse. As OP may not always be cryptogenic, a careful follow-up should be programmed to diagnose the underlying systemic disease.
Keywords: Cryptogenic organizing pneumonia, corticosteroids, relapse, outcome, risk factor
Introduction
Cryptogenic organizing pneumonia (COP) is a distinct clinicopathological entity with unknown etiology. Pathologically, it is characterized by plugs of granulation tissue lying within small airways, alveolar ducts, and alveoli.1 Corticosteroids remain the first line of treatment and usually are effective.2–4 However, relapse is common following the reduction or discontinuation of corticosteroid treatment.3,5 Steroids sparing agents such as macrolides and cyclophosphamide are also reported effective to COP.6–8
Few studies have reported the clinical features and long-term outcomes of patients with COP. Furthermore, the incidence and risk factors for relapse remain undetermined. Hence, we conducted this retrospective study in a Chinese center experienced in interstitial lung diseases to evaluate the clinical features and outcomes and to assess the potential risk factors related to the relapse in patients with biopsy-proven COP in a 7-year period.
Methods
Patients
Patients with biopsy-proven organizing pneumonia (OP) in our center from September 2010 to December 2017 were enrolled in the registry. Hospital and office records were used as data sources. The diagnosis of COP was established when clinical manifestations and radiological findings were compatible with OP, confirmed by the presence of histopathological pattern showing organization within alveolar ducts, alveoli, and bronchioles. A poor response to antibiotics and a good response to corticosteroids supported the diagnosis.1 OP associated with identified causes, such as connective tissue diseases (CTDs), infections, drugs, and environment exposure, were excluded.9–11 Patients with incomplete data were also excluded. This study was approved by the Ethics Committee of Nanjing Drum Tower Hospital. The requirement for consent was waived because the review of the patients’ data was anonymous.
Clinical information collection
Clinical information at admission was collected including demographics, smoking history, clinical symptoms, signs, and duration from onset of symptoms to diagnosis. The lab data included C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and the presence of serological autoantibodies. Pulmonary function tests included forced vital capacity (FVC), FVC% predicted, diffusion capacity for carbon monoxide (DLCO), and DLCO% predicted. Chest high-resolution computerized tomography (HRCT) in supine position was performed for all patients at initial diagnosis. A dedicated chest radiologist (KZ) reviewed all HRCTs and evaluated the main radiographic findings. The main HRCT abnormalities were documented as the presence of consolidation, ground-glass opacity (GGO), nodule, reticulation, and honeycombing according to guideline.1 The radiographic distribution patterns were evaluated. Treatment information included the doses and durations.
The decision to initiate systemic corticosteroid therapy was made by consulting with an experienced specialist (HC) in interstitial lung disease field. According to previous recommendations,12 corticosteroid treatment was initiated from 0.75 mg/kg/day prednisone for 1 month, then 0.5 mg/kg/day for 1–2 months, then 20 mg/day for 2–3 months, and then the dosage was tapered to 5–15 mg/day for 6–12 months. The total treatment duration was at least 1 year. Patients who could not tolerate side effects or refused corticosteroids were treated with macrolides or no drugs.
Patients were classified as corticosteroids group and steroids sparing group based on the therapeutic records of being treated with systemic corticosteroids for at least 6 months. Relapse group was defined as worsening of clinical manifestations in combination with progression of radiographic abnormalities in the absence of identified causes such as infections, heart failure, or pulmonary embolism. Nonrelapse group was defined as remission of clinical manifestations accompanied by resolution or retaining of radiographic abnormalities when discontinuation of corticosteroids was undertaken for at least 3 months. A histopathological evidence of COP was not essential in the diagnosis of relapse.13 All patients were followed up every 3–4 months. At each visit, patients were clinically examined, and the chest radiographs and lab data including biochemical parameters and serum antibodies were routinely obtained as clinically indicated. When corticosteroids were weaned off, patients were followed up at least 6 months. Special attention was paid to corticosteroid doses and treatment duration when relapse occurred. The deadline follow-up time was December 2018. In order to assess the factors that increased the likelihood of relapse, we compared clinical symptoms, signs, lab data, HRCT findings, corticosteroid doses, and treatment durations between relapse and nonrelapse groups.
Statistical analysis
Data were presented as mean with standard deviation for continuous variables or frequencies with percentages when variables were categorical. t-Test or the Mann–Whitney U test was used for continuous variables. The χ2 test was used for categorical variables. The value of p < 0.05 was considered statistically significant. All statistical analysis was conducted through IBM SPSS Statistics version 23.0.
Results
Patient inclusion
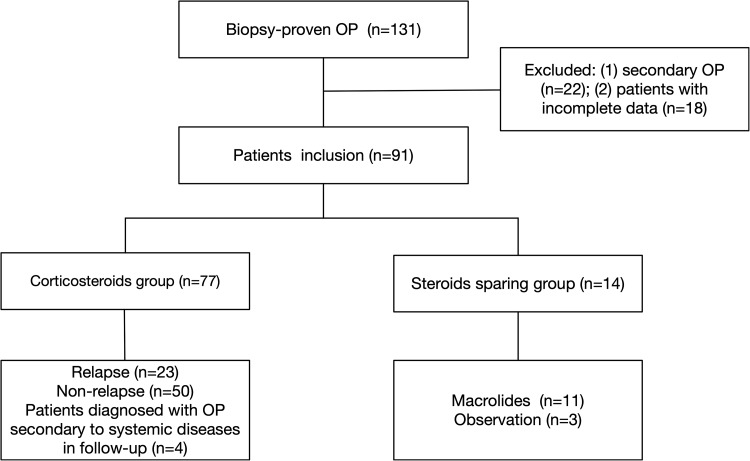
In total, there were 131 patients with biopsy-proven OP in the registry from September 2010 to December 2017. Twenty-two patients with identified causes and 18 patients with incomplete data were excluded. Four patients were diagnosed with OP associated with CTDs in follow-up. Hence, the present study included 87 patients with final diagnosis of COP (Figure 1). They were 45 males and 42 females with a mean age of 56.1 ± 10.4 years old (range 31–75 years old). Twenty-four patients (27.6%) reported having a smoking history. The mean duration from onset of symptoms to diagnosis was 3.0 ± 7.4 months (range 0.25–9 months). The average follow-up time was 50.3 ± 25.9 months (range 9.3–96.4 months). Pathological diagnosis was based on transbronchial lung biopsy in 58 (66.7%) patients, percutaneous lung biopsy in 28 (32.2%) patients, and surgical lung biopsy in one (1.1%) patient. Cough (73 patients, 83.9%), progressive dyspnea (54 patients, 62.1%), and fever (40 patients, 46.0%) were the most common symptoms, followed by weight loss (9 patients, 10.3%), hemoptysis (5 patients, 5.7%), and chest pain (4 patients, 4.6%). Crackles were found in 41 (47.1%) patients. Consolidation (54 patients, 62.1%) and GGO (36 patients, 41.4%) were the two most common abnormalities on HRCT.
Figure 1.
Flow chart of patients’ selection. OP: organizing pneumonia
Corticosteroids group
There were 73 patients who received corticosteroid treatment at diagnosis. The initial doses were 20–200 mg/day prednisone or its equivalent. Of these patients, the mean follow-up time was 50.3 ± 26.8 months (range 9.3–96.4 months). Fifty patients (68.5%) did not relapse with the mean treatment duration of 16.8 ± 16.0 months (range 6.0–70.0 months). Twenty-three patients (31.5%) developed relapses following treatment reduction or discontinuation. Twenty patients relapsed once, two patients relapsed twice and one patient suffered from four relapses. The majority of patients (13/23, 56.5%) developed relapses after the treatment cessation. Eight of them were within 3 months post withdrawal, three patients within 6 months, one patient within 1 year, and delayed relapse was also observed in one patient within 2 years. Ten patients (43.5%) developed relapses when the doses of corticosteroids were reduced. When relapses occurred, the dose was 20 mg/day in one patient, 10 mg/day in six patients, 7.5 mg/day in one patient, 5 mg/day in one patient, and 2.5 mg/day in one patient.
Comparison of clinical features between relapse and nonrelapse groups in COP patients treated with corticosteroids
As presented in Table 1, fever was more common in patients who relapsed than those who did not (65.2% vs. 32.0%, p = 0.004). The level of serum CRP was significantly higher (31.5 ± 39.4 mg/L vs. 17.5 ± 32.2 mg/L, p = 0.038), whereas the DLCO was significantly lower in relapse group compared to nonrelapse group (45.9 ± 14.2% vs. 57.6 ± 18.5%, p = 0.050). There were no significant differences in smoking status, chest HRCT findings, initial doses or treatment durations between the two groups. When relapses occurred, patients were treated again with prednisone with average dose of 35.0 ± 27.8 mg/d (range 10–100 mg/day), which was lower than the initial dose (83.5 ± 38.8 mg/day; range: 30–200 mg/day; p < 0.001). All re-treated patients showed rapid clinical and imaging improvement within 1 month.
Table 1.
Comparison of clinical features between relapse and nonrelapse groups in COP patients treated with corticosteroids.
| Variables | Relapse group (n = 23) | Nonrelapse group (n = 50) | p Value |
|---|---|---|---|
| Age (years) | 58.9 ± 8.9 | 55.3 ± 11.6 | 0.193 |
| Male, n (%) | 11 (47.8) | 23 (46.0) | 0.884 |
| Smoker, n (%) | 6 (26.1) | 16 (32.0) | 0.609 |
| Duration from onset of symptoms to diagnosis (months) | 3.1 ± 5.2 | 3.2 ± 9.0 | 0.490 |
| Symptoms and signs, n (%) | |||
| Fever | 15 (65.2) | 15 (30.0) | 0.004 |
| Crackles | 12 (52.2) | 27 (54.0) | 0.884 |
| Lab data | |||
| Serum CRP (mg/L) | 31.5 ± 39.4 | 17.5 ± 32.2 | 0.038 |
| Presence of autoantibodies, n
(%) |
3 (13.1) | 6 (12.0) | >0.999 |
| Pulmonary function test | |||
| FVC (L) | 2.1 ± 0.6a | 2.3 ± 0.6b | 0.383 |
| FVC (% predicted) | 66.3 ± 14.9* | 67.8 ± 17.6b
|
0.767 |
| DLCO (mmol/min/kPa) | 4.2 ± 1.5a | 5.40 ± 2.2b | 0.081 |
| DLCO (% predicted) | 45.9 ± 14.2a | 57.6 ± 18.5b | 0.050 |
| HRCT features, n (%) | |||
| Consolidation | 10 (43.5) | 33 (66.0) | 0.069 |
| GGO | 12 (52.2) | 23 (46.0) | 0.624 |
| Nodule | 1 (4.3) | 3 (6.0) | >0.999 |
| Reticulation | 4 (17.4) | 6 (12.0) | 0.798 |
| Honeycombing | 0 (0) | 0 (0) | >0.999 |
| Bronchovascular bundles | 6 (26.1) | 18 (36.0) | 0.402 |
| Subpleural areas | 7 (30.4) | 15 (30.0) | 0.970 |
| Upper lung zone | 1 (4.3) | 3 (6.0) | >0.999 |
| Lower lung zone | 21 (91.3) | 42 (84.0) | 0.634 |
| Initial dose of prednisone (mg/day) | 83.5 ± 38.8 | 89.8 ± 44.7 | 0.642 |
| Treatment duration (months) | 17.6 ± 15.5 | 16.8 ± 16.0 | 0.783 |
COP: cryptogenic organizing pneumonia; CRP: C-reactive protein, FVC: forced vital capacity; DLCO: diffusion capacity for carbon monoxide; HRCT: high-resolution computerized tomography; GGO: ground-glass opacity.
aData were available for 14 patients.
bData were available for 34 patients.
Steroids sparing treatment
There were 11 patients who received macrolide treatment at diagnosis. Eight patients were treated with clarithromycin and three with azithromycin. The mean treatment duration was 4.2 ± 2.7 months (range 3.0–12.0 months). The average follow-up duration was 51.7 ± 22.7 months (range 15.5–73.8 months). Seven patients achieved remission without relapse. Three patients received corticosteroid treatment due to nonresolution in chest HRCT abnormalities after being treated with macrolides for at least 3 months. One patient developed massive hemoptysis after the treatment of clarithromycin for 4 months and received surgical resection. The histology confirmed OP.
In this cohort, three patients who were asymptomatic and presented with solitary focal OP on HRCT received no treatment at diagnosis. Their follow-up durations were 57.5 months, 27.3 months, and 47.5 months, respectively. Two patients achieved spontaneous remission 6 months later. One patient complained of deterioration of cough and dyspnea and had corticosteroids initiated after 1 month from diagnosis. He stopped corticosteroid treatment 13 months later, and no relapse was observed.
Detailed description of patients diagnosed with OP secondary to autoimmune diseases in follow-up
As presented in Table 2, four patients initially classified as COP were later identified as OP secondary to autoimmune diseases. Extrapulmonary manifestations were absent in all patients and only one patient (patient 4) presented positive autoantibody (anti-Ro52) at admission without fulfilling the diagnostic criteria of specific CTD. Patient 1 achieved remission on chest imaging but developed muscle weakness with electromyogram (EMG) showing myogenic abnormality 1 year later. She was subsequently diagnosed with polymyositis (PM). Patient 2 experienced one relapse after initial episode and complained of hematuria with positive peri-antineutrophil cytoplasmic antibody (p-ANCA) after 2 years from initial diagnosis. He was diagnosed with ANCA-associated vasculitis (AAV). Patient 3 revisited our center 5 months later because of worsening pulmonary symptoms and was diagnosed with interstitial pneumonia with autoimmune features (IPAF) due to positive antinuclear antibody (ANA) and Raynaud’s phenomenon. Patient 4 showed muscle weakness and positivity of anti-EJ antibody 15 months later and was diagnosed with PM.
Table 2.
Details for patients diagnosed with OP secondary to systemic diseases at follow-up.
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Sex | F | M | F | F |
| Age (years) | 42 | 77 | 70 | 52 |
| Smoking history | N | N | N | N |
| Duration from onset of symptoms to diagnosis (months) | 2 | 1 | 2 | 3 |
| Initial hospitalization | ||||
| Extrapulmonary symptom | N | N | N | N |
| Presence of autoantibody | N | N | N | Anti-Ro52(+) |
| HRCT features | Consolidation along bronchovascular bundles | Reticulation and GGO along bronchovascular bundles | GGO with subpleural areas predominance | Consolidation along bronchovascular bundles and subpleural areas predominance |
| Follow-up period | ||||
| Extrapulmonary symptom | Muscle weakness | Hematuria | Raynaud’s phenomenon | Muscle weakness |
| Presence of autoantibody | N | p-ANCA (+); Anti-SSA (±) | ANA 1:320 titer | Anti-EJ (++); Anti-Ro52 (+) |
| Other examinations | EMG shown myogenic abnormality | Urine protein (++) Urine occult blood (+++) |
N | N |
| Duration from initial diagnosis to final diagnosis (months) | 14 | 24 | 6 | 15 |
| Final diagnosis | PM | AAV | IPAF | PM |
M: male; F: female; N: no; EMG: electromyogram; p-ANCA: peri-antineutrophil cytoplasmic antibody; ANA: antinuclear antibody; PM: polymyositis; AAV: ANCA-associated vasculitis; IPAF: Interstitial pneumonia with autoimmune features.
Discussion
This was a retrospective study to describe clinical characteristics and long-term outcomes of patients with biopsy-proven COP in a Chinese interstitial lung disease center. We showed that 31.5% (23 of 73) patients treated with corticosteroids developed relapses following treatment reduction or cessation. Fever, elevated CRP, and a reduced DLCO were associated with relapse. A subgroup of patients who presented with COP as the first manifestation were subsequently diagnosed with OP associated with autoimmune disease in follow-up.
Relapse is not uncommon in COP patients treated with corticosteroids; relapse rates vary from 9% to 58%.12,14,15 In 20% of patients, there have been two relapses.12 In the present study, most relapses occurred within 6 months following corticosteroid withdrawal (47.8%) or when the dose of corticosteroids was reduced below 10 mg daily (39.1%). Although the initial dose and treatment duration differed, similar results have been reported; relapses occur when the doses of prednisone range from 5 mg/day to 10 mg/day or after discontinuation of treatment.2,15,16 In accordance with previous studies,8,12 most cases responded favorably with re-instigation of corticosteroid therapy. In this cohort, no patients died from relapse in a 7-year period and the disease showed a benign clinical course.
We compared the clinical features between relapse and nonrelapse groups in COP treated with corticosteroids and assessed the factors that might increase the likelihood of relapse. We found that fever was more frequent in patients who relapsed than those who did not. Additionally, the level of CRP and DLCO% predicted had significant differences between the two groups. Lazor and his co-authors reported delayed treatment and mild cholestasis favored multiple relapses.12 A study from Wanatabe and his colleagues identified PaO2 a predictor of relapse.15 COP was also deemed as an inflammatory rather than a fibrotic disease.16 We hypothesized that fever and the level of CRP represented an inflammatory process and the decreased PaO2 and DLCO reflected the disease severity. Our study provides some information that future clinical trials should take into account to stratify the disease and personalize treatment for an individual patient.
Effective macrolide treatment of COP is not a novel notion.17–19 In the present study, 11 patients received macrolides and 7 of them achieved remission. The effect of macrolides in treatment of COP may be related to the anti-inflammatory mechanism. In an in vitro study, alveolar macrophages collected from bronchoalveolar lavage of patients with COP produced significant number of pro-inflammatory cytokines, which could be inhibited by macrolides.20 Clinicians can introduce macrolides in patients with COP who refuse or cannot tolerate the side effects of corticosteroids. Nevertheless, the appropriate dose and duration of macrolide treatment remain to be ascertained.
In this cohort, four patients initially diagnosed with COP were eventually confirmed as OP secondary to systemic diseases. Of note, there was no clinical or pathological evidence of CTD at the initial hospital admission; OP was the initial manifestation of CTD. Similar presentation has been observed in lung dominant anti-synthetase syndrome and AAV.21,22 More recently, an observational study from Li HP and her colleagues demonstrated that 65% of patients who showed pulmonary symptoms at initial admission were ultimately diagnosed with CTD-ILD on subsequent follow-up examinations.23 Thus, it should be acknowledged that OP may not always be cryptogenic and regular prospective monitoring of extrapulmonary manifestation and routine assessment of serum antibodies may be helpful in identifying underlying etiology.
In summary, in this long-term retrospective study of biopsy-proven COP, we have shown a relapse rate of 31.5% in patients treated with corticosteroids and absence of relapse in those treated with macrolides. Three parameters, fever, elevated CRP, and a reduced DLCO were predictors of relapse in patients treated with corticosteroids.OP may not always be cryptogenic and should be followed up to identify the systemic diseases that may be driving the underlying process.
Acknowledgements
The authors thank all members of the department of Pulmonary and Critical Care Medicine, Nanjing Drum Tower Hospital, Clinical College of Nanjing Medical School. The work should be attributed to Nanjing Drum Tower Hospital, Clinical College of Nanjing Medical School, No. 321 Zhongshan Road, Nanjing, Jiangsu, China.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the National Natural Science Foundation of China (81570058), Jiangsu Provincial Medical Talent (ZDRCA2016058) and Jiangsu Social Development Project (BE2017604).
ORCID iD: Jinghong Dai  https://orcid.org/0000-0003-3165-0623
https://orcid.org/0000-0003-3165-0623
References
- 1. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002; 165: 277–304. [DOI] [PubMed] [Google Scholar]
- 2. Epler GR, Colby TV, McLoud TC, et al. Bronchiolitis obliterans organizing pneumonia. N Engl J Med 1985; 312: 152–158. [DOI] [PubMed] [Google Scholar]
- 3. King TE, Jr, Mortenson RL. Cryptogenic organizing pneumonitis. The North American experience. Chest 1992; 102: 8s–13s. [PubMed] [Google Scholar]
- 4. Drakopanagiotakis F, Paschalaki K, Abu-Hijleh M, et al. Cryptogenic and secondary organizing pneumonia: clinical presentation, radiographic findings, treatment response, and prognosis. Chest 2011; 139: 893–900. [DOI] [PubMed] [Google Scholar]
- 5. Travis WD, Hunninghake G, King TE, Jr, et al. Idiopathic nonspecific interstitial pneumonia: report of an American Thoracic Society project. Am J Respir Crit Care Med 2008; 177: 1338–1347. [DOI] [PubMed] [Google Scholar]
- 6. Pathak V, Kuhn JM, Durham C, et al. Macrolide use leads to clinical and radiological improvement in patients with cryptogenic organizing pneumonia. Ann Am Thorac Soc 2014; 11: 87–91. [DOI] [PubMed] [Google Scholar]
- 7. Friedlander AL, Albert RK. Chronic macrolide therapy in inflammatory airways diseases. Chest 2010; 138: 1202–1212. [DOI] [PubMed] [Google Scholar]
- 8. Cottin V, Cordier JF. Cryptogenic organizing pneumonia. Semin Respir Crit Care Med 2012; 33: 462–475. [DOI] [PubMed] [Google Scholar]
- 9. Travis WD, Costabel U, Hansell DM, et al. An official American thoracic society/European respiratory society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cordier JF. Cryptogenic organizing pneumonia. Clin Chest Med 2004; 25: 727–738, vi–vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Epler GR. Bronchiolitis obliterans organizing pneumonia. Arch Intern Med 2001; 161: 158–164. [DOI] [PubMed] [Google Scholar]
- 12. Lazor R, Vandevenne A, Pelletier A, et al. Cryptogenic organizing pneumonia. Characteristics of relapses in a series of 48 patients. The Groupe d’Etudes et de Recherche sur les Maladles “Orphelines” Pulmonaires (GERM”O”P). Am J Respir Crit Care Med 2000; 162: 571–577. [DOI] [PubMed] [Google Scholar]
- 13. Barroso E, Hernandez L, Gil J, et al. Idiopathic organizing pneumonia: a relapsing disease. 19 years of experience in a hospital setting. Respiration 2007; 74: 624–631. [DOI] [PubMed] [Google Scholar]
- 14. Yamamoto M, Ina Y, Kitaichi M, et al. Clinical features of BOOP in Japan. Chest 1992; 102: 21s–25s. [DOI] [PubMed] [Google Scholar]
- 15. Watanabe K, Senju S, Wen FQ, et al. Factors related to the relapse of bronchiolitis obliterans organizing pneumonia. Chest 1998; 114: 1599–1606. [DOI] [PubMed] [Google Scholar]
- 16. Cordier JF. Cryptogenic organising pneumonia. Eur Respir J 2006; 28: 422–446. [DOI] [PubMed] [Google Scholar]
- 17. Stover DE, Mangino D. Macrolides: a treatment alternative for bronchiolitis obliterans organizing pneumonia? Chest 2005; 128: 3611–3617. [DOI] [PubMed] [Google Scholar]
- 18. Chang WJ, Lee EJ, Lee SY, et al. Successful salvage treatment of steroid-refractory bronchiolar COP with low-dose macrolides. Pathol Int 2012; 62: 144–148. [DOI] [PubMed] [Google Scholar]
- 19. Lee J, Cha SI, Park TI, et al. Adjunctive effects of cyclosporine and macrolide in rapidly progressive cryptogenic organizing pneumonia with no prompt response to steroid. Intern Med 2011; 50: 475–479. [DOI] [PubMed] [Google Scholar]
- 20. Cai M, Bonella F, Dai H, et al. Macrolides inhibit cytokine production by alveolar macrophages in bronchiolitis obliterans organizing pneumonia. Immunobiology 2013; 218: 930–937. [DOI] [PubMed] [Google Scholar]
- 21. Priyangika SM, Karunarathna WG, Liyanage I, et al. Organizing pneumonia as the first manifestation of anti-synthetase syndrome. BMC Res Notes 2016; 9: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Omar M, Goli S, Ramnarine I, et al. Organizing Pneumonia: contemplate beyond cryptogenic. Am J Med 2018; 131: e81–e85. [DOI] [PubMed] [Google Scholar]
- 23. Hu Y, Wang LS, Wei YR, et al. Clinical characteristics of connective tissue disease-associated interstitial lung disease in 1,044 Chinese patients. Chest 2016; 149: 201–208. [DOI] [PubMed] [Google Scholar]