Abstract
Sex-determining region Y-related high-mobility-group box transcription factor 11 (SOX11) is an essential member of the SOX transcription factors and has been highlighted as an important regulator in embryogenesis. SOX11 studies have only recently shifted focus from its role in embryogenesis and development to its function in disease. In particular, the role of SOX11 in carcinogenesis has become of major interest in the field. SOX11 expression is elevated in a wide variety of tumors. In many cancers, dysfunctional expression of SOX11 has been correlated with increased cancer cell survival, inhibited cell differentiation, and tumor progression through the induction of metastasis and angiogenesis. Nevertheless, in a limited number of malignancies, SOX11 has also been identified to function as a tumor suppressor. Herein, we review the correlation between the expression of SOX11 and tumor behaviors. We also summarize the mechanisms underlying the regulation of SOX11 expression and activity in pathological conditions. In particular, we focus on the pathological processes of cancer targeted by SOX11 and discuss whether SOX11 is protective or detrimental during tumor progression. Moreover, SOX11 is highlighted as a clinical biomarker for the diagnosis and prognosis of various human cancer. The information reviewed here should assist in future experimental designs and emphasize the potential of SOX11 as a therapeutic target for cancer.
Keywords: biomarker, cancer, cell differentiation, cell proliferation, metastasis, prognosis, SOX11
Introduction
Since its first description in 1990, the family of sex-determining region Y (SRY)-related high-mobility-group (HMG) box (SOX) transcription factors have gained attention for their potential physiological and pathological role in cell biology. The SOX family are characterized by their high degree of sequence homology with the SRY HMG DNA-binding domain.1,2 To date, approximately 20 SOX genes have been identified in both vertebrates and invertebrates, which can be further categorized into eight subgroups (SOXA to SOXH) based on sequence homology inside and outside the HMG domain.3 SOX transcription factors are essential in regulating stem cell maintenance and terminal differentiation of different cell types.4 Given that SOX proteins control multiple essential developmental and homeostatic processes, it is not surprising that dysfunction and mutation of SOX genes can lead to a variety of hereditary human diseases. Growing evidence has shown that several SOX members are involved in different types of cancer, such as SOX2 in prostate cancer (PCa),5 SOX4 in leukemia,6 and SOX9 in breast cancer.7
SOX11 belongs to the highly conserved SOXC group, also including SOX4 and SOX12.8 The past two decades witnessed an evolution in our understanding of the biological function of SOX11. In 1993, SOX11 was accepted as a transcriptional activator in the development of the nervous system.8 Subsequently, defects in SOX11 expression were demonstrated to be responsible for malformations of a variety of human organs, such as heart, lung, stomach, and the skeletal system.9 Recent studies have demonstrated SOX11 mRNA as one of the most frequently increased transcripts in various human cancers, including mantle cell lymphoma (MCL),10 epithelial ovarian cancer (EOC),11 breast cancer,12 gastrointestinal tumors,13 and nervous system neoplasms. Various regulatory mechanisms of SOX11, including promoter methylation, histone modifications and microRNA (miRNA) interference, underlie the regulation of its expression and activity.10,14 Owing to its pro-proliferative and anti-apoptotic effects, SOX11 has been considered as an oncogene.15,16 Nevertheless, recent investigations have described paradoxical roles of SOX11 in tumor suppression and in the prognosis of different types of cancer.17,18 Therefore, it is imperative to define the potential role of SOX11 in tumorigenesis and elucidate how alteration of SOX11 is linked to the development of human cancers.
The focus of this review is to summarize the latest progress regarding the associations between SOX11 and cancers. First, we introduce the structure and function of the SOX11 gene. Then, we direct attention to the regulatory mechanisms of SOX11 in cancers. Next, we highlight the dual roles of SOX11 in pathological processes and molecular pathways underlying its oncogenic or antitumor effects. Moreover, we discuss the clinical relevance of SOX11 in cancer treatment and prognosis. Finally, we set out several novel potential directions of SOX11 studies. Collectively, the information compiled here will serve as a comprehensive reference for the actions of SOX11 in cancer identified to date and hopefully aid in the design of further experimental research and increase the potential of SOX11 as a therapeutic target for cancers in the future.
Molecular features of SOX11
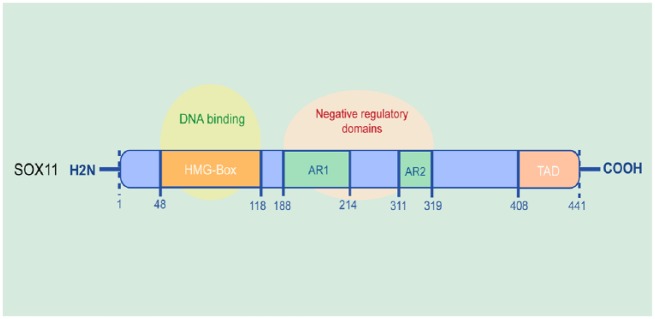
SOX11 was cloned and characterized by the partial cloning of both human and mouse SOX11 genes and was mapped to chromosome 2p25.3.8 SOX11 is a single-exon gene, the full-length cDNA of which was 8743 bp, with a long 3’ untranslated region.8,19 The SOX11 protein is composed of 441 amino acids. SOX11 features two functional domains: the N-terminal HMG domain and the conserved C-terminal transactivation domain (TAD).20,21 (Figure 1) These two domains are conserved in all three SOXC members. The homology between SOXC members is relatively low outside these two conserved domains. Interestingly, these two domains are more highly conserved between SOX4 and SOX11 orthologs than between SOX12 and SOX11 orthologs.20
Figure 1.
The molecular structure of SOX11.
The SOX11 protein is composed of 441 amino acids. SOX11 contains two functional domains, the N-terminal HMG domain and the conserved TAD.
SOX11, sex-determining region Y-related high-mobility-group box transcription factor 11; HMG, high mobility group; TAD, C-terminal transactivation domain.
The N-terminal HMG domain of SOX11 can bind to the consensus sequence 5’-(A/T)(A/T)CAA(A/T)G-3’ in the minor groove of DNA.22 Binding of the HMG domain to the minor groove in the DNA helix causes a sharp bend, leading to alteration of local chromatin architecture and the formation of functional transcriptional enhancer complexes.22,23 All SOXC proteins have been demonstrated to share 67% identity and 94% similarity in the TAD, which comprises the 33 C-terminal residues. The TAD of SOX11 is predicted to form a stable uninterrupted helical structure, which is shorter and interrupted by 3 and 7 residues in SOX4 and SOX12, respectively.20 Protein secondary structure models indicate that 20 residues in the SOX11 TAD domain are responsible for this essential helical structure.20 In accordance with the belief that this configuration plays a critical role in regulating TAD activity, SOX11 is most effective in transactivating transfected reporter genes among the three SOXC proteins.20,24,25 However, the mechanism through which the TAD controls transcriptional activation remains to be investigated. Moreover, prosite assay revealed three supposed phosphorylation sites in the TAD of SOX11: a casein kinase-2 phosphorylation site at Ser363, a glycogen synthase kinase-3 and a polo-like kinase site at Thr371, and a polo-like kinase site at Thr376.20 Recently, Balta et al.26 carried out mass-spectrometry-based identification of putative phosphorylation sites followed by mutational analysis. They identified one serine residue, S30, which strongly modulated SOX11’s subcellular localization, and nine other serine residues with the potential for phosphorylation. It will be interesting to explore how SOX11 is targeted by different kinases and pathways, which may result in combinatorial phosphorylation code that modulates SOX11’s activity and target specificity.
In addition, two acidic regions in SOX11, AR1 (amino acids 188-214) and AR2 (amino acids 319-311), have been demonstrated to function as negative regulatory domains to suppress DNA binding.20 An approximately twofold increase in the capability of SOX11 to bind DNA in electrophoresis mobility shift assays can be observed after deletion of these two inhibitory domains in SOX11.24 These two autoinhibitory regions sharply decrease DNA binding, probably by providing a hinge that masks the HMG domain and the TAD. Thus, the acidic regions, the HMG domain and the TAD in SOX11 may cooperate to precisely regulate the recruitment of SOX11 to activate specific genes and prevent the promiscuous binding of SOX11 to DNA. Nevertheless, the interactions of this assumed autoregulatory mechanism still need to be elucidated.
Expression and biological functions of SOX11
To further understand the function of SOX11 in malignancies, it is significant to highlight its expression and critical roles in vertebrate development and normal physiological conditions.
SOX11 is widely expressed during organogenesis in the mouse embryo, and is highly expressed in human and mouse fetus in the peripheral and central nervous system, gastrointestinal tract, lung, spleen, pancreas, kidneys, and gonads.27–29 High levels of SOX11 genes are also expressed in mesenchymal and neural progenitor cells.30 Interestingly, during embryonic development, Kuhlbrodt and colleagues31 found that the abundance of SOX11 transcripts is reduced early with ongoing development, indicating that SOX11 may only play an essential role in early determination and differentiation processes. Further, expression of SOX11 is lack or even absent in adult neurons and other tissues. SOX11 expression cannot be detected in normal lymphoid progenitors or mature B cells.32,33
As clearly indicated by its protein properties and expression pattern, SOX11 plays a crucial role in human developmental and differentiation processes. SOX11-deficient newborn mice suffer from a variety of developmental defects and immediately die of heart arterial outflow tract malformations after birth.28 In addition, these SOX11-deficient mice present widespread malformations, including cleft lip and palate, lung hypoplasia, asplenia, omphalocele, split lumbar vertebrae, undermineralized skull, open eyelids, and microphthalmia with anterior segment dysgenesis.9,28 Developing SOX11 knock-out mice also suffer from hypoplasia in the renal and nervous systems. SOX11-deficient retinas show impeded differentiation of retinal ganglion and cone cells during the embryonic stages.34,35 Targeted ablation of both SOX11 and SOX4 in the retinal ganglion cells can lead to a complete absence of retinal ganglion cells during embryonic development.36 In addition, deletion of SOX11 significantly impairs the proliferation of sympathetic ganglia in the early embryonic stages and influences their survival during later developmental stages.27 Genetic studies of SOX11, regarding both embryonic and adult neurogenesis, indicate that SOX11 plays essential roles in the differentiation of neural stem cells, maintenance of the immature state of oligodendrocyte lineages and the control of sensory neuron survival.37–39
The expression of SOX11 may vary in different cancer types (Table 1). For example, SOX11 mRNA can be detected at a high level in a wide variety of cancers, including breast cancer,12 gastric cancer (GC),18 lymphoma,40 and hematopoietic tumors.41 In contrast, low expression of SOX11 can also be observed in other cancers, such as nasopharyngeal carcinoma (NPC),17 diffuse large B-cell lymphoma (DLBCL),41 and bladder cancer.42
Table 1.
Overview of SOX11 function reported in diverse types of cancer.
| Cancer categories | Expression of SOX11 | Correlation between the SOX11 expression level with clinicopathological features of tumors | Effect of SOX11 | Reference |
|---|---|---|---|---|
| Glioma | mRNA is higher | SOX11 expression was not correlated with tumor histological grade or any other clinicopathological features. | Overexpression of SOX11 reduces cell proliferation and colony formation and induces neuronal differentiation of GICs. | Weigle et al.43
Hide et al.44 |
| Meningioma | mRNA is higher | Overexpression of SOX11 is related to high grade of meningioma. | — | Stuart et al.45 |
| Medulloblastoma | mRNA and protein are higher | There is no significant correlation between SOX11 expression and histological type. | — | Lee et al.46 |
| MCL | mRNA and protein are higher | Nuclear expression of SOX11 is significant for diagnosis MCL from other lymphoma types. | On one hand, SOX11 can promote cell survival and tumor angiogenesis and inhibit B-cell differentiation; on the other side, SOX11 reduces the cell proliferation. | Nygren et al.47
Palomero et al.48 Palomero et al.49 |
| CLL | mRNA expression positively correlates with the mutational status of IGHV genes | — | — | Roisman et al.50 |
| DLBCL | mRNA is minimal to absence with DNA hypermethylation | — | — | Dictor et al.51
Zhang et al.52 |
| Burkitt’s lymphoma | mRNA and protein are higher in childhood |
— | — | Dictor et al.51 |
| Breast cancer | mRNA and protein are higher | Elevated SOX11 expression is significantly associated with smaller tumor size and earlier tumor grade, and inversely correlated with lymph node metastasis. | SOX11 is required for growth of ER-negative breast cancer cell lines, and SOX11 overexpression promotes the migratory ability of breast cancer cells. | Liu et al.53 |
| BLBC | mRNA and protein are higher | Upregulation of SOX11 is related to worse clinicopathological features in BLBC. | SXO11 increases cell proliferation, and promotes migration. | Shepherd et al.12 |
| EOC | mRNA and protein are higher | SOX11 status shows no correlation with distribution of high- or low-grade tumors in individual histological subtypes. | Overexpression of SOX11 induces growth arrest in EOC cell lines. | Brennan et al.54
Sernbo et al.11 |
| Prostate cancer | mRNA is lower | Low expression levels of SOX11 is related to the severity of the tumor malignancy. | SOX11 represses cell growth, tumor invasion and metastasis. | Yao et al.55 |
| Hepatocellular carcinoma | mRNA and protein are higher | SOX11 methylation and SOX11 expression are positively related with tumor size, microvascular invasion, and TNM stage. | — | Teng et al.56 |
| Gastric cancer | mRNA and protein are lower | Expression of SOX11 negatively correlates with Lauren’s classification, differentiation status, TNM stage, invasive depth and lymph node. | SOX11 can suppress GC cell migration and invasion. | Qu et al.18
Xu et al.13 |
| Pancreatic cancer | mRNA and protein are higher | — | SOX11 promotes cell proliferation but inhibits invasion or metastasis. | Li et al.57 |
| Nasopharyngeal carcinoma | mRNA level is associated with DNA methylation status | SOX11 overexpression is negatively related to clinical stage and lymph node metastasis. | SOX11 overexpression decreases the capacity of tumor growth and invasion. | Zhang et al.17 |
| Lung cancer | mRNA is higher | Increased SOX11 expression is positively correlated with tumor grade, TNM stage, and lymph node metastasis. | SOX11 may increase migration and invasion. | Walter et al.58 |
| Melanoma | mRNA and protein are higher | SOX11 upregulation is associated with tumor type, tumor location, and increased lymph node metastasis. | Overexpressed SOX11 promotes proliferation, colony formation, migration, and invasion of melanoma cell. | Jian et al.59 |
| Fibromatosis | mRNA and protein are lower | Low expression of SOX11 correlates with worse clinical outcome and earlier recurrence. | — | Misemer et al.60 |
| Bladder cancer | mRNA is lower | — | — | Chung et al.42 |
BLBC, basal-like breast cancer; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; EOC, epithelial ovarian cancer; GIC, glioma-initiating cell; IGHV, immunoglobulin heavy chain variable gene; MCL, mantle cell lymphoma; SOX11, Sex-determining region Y-related high-mobility-group box transcription factor 11; TNM, tumor node metastasis classification; —, unavailable in the original article.
Nevertheless, the re-expression of SOX11 in different cancer types has a prominent impact on tumor formation and initiation. Clonal expansion of cells with tumorigenic properties is the pivotal process towards tumor initiation.61 SOX11 may exert both stimulative and suppressive effect on these tumorigenic cells, which can evade normal cell cycle checkpoints and invade tissues.12,41 In addition to its diverse role in controlling cell fate, evidence has showed the important function of SOX11 in regulating the expansion and differentiation of cancer-initiating cells (CICs).62 The orchestration of tumor formation and initiation has been identified in several cancers to be driven by CICs, also termed cancer stem-like cells.63 SOX11 has the ability to repress expansion and maintenance of highly aggressive CICs in the development of glioma.44 Therefore, SOX11 participates in tumorigenesis partly through controlling CIC maintenance, and may act as a stem cell marker in some cases.
Regulatory mechanisms of SOX11 expression
The differential expression patterns in development and tumor-specific effects of SOX11 originate from the underlying mechanisms that control the expression and activity of its mRNA and protein, including epigenetic alterations and miRNA interference. In addition, several upstream regulators, such as Wnt and Notch signaling pathways, have also been implicated in regulating SOX11 expression and activity.
Epigenetic alterations
Epigenetic alterations such as DNA methylation and histone modifications, which regulate gene expression without changing the DNA sequence, play a vital role in development and tumorigenesis.64,65 The silence of SOX11 in normal adult tissues may be attributed to DNA methylation in its promoter region. More sites of SOX11 promoter have been found to be methylated in tissues of adult stages, including brain, kidney, and testis, in comparison with fetal as well as neonatal counterparts.29 Of major interest, the methylation rate of the SOX11 promoter region is significantly higher in a wide variety of carcinoma tissues, including DLBCL,41 NPC,17 EOC,11 estrogen receptor (ER) positive breast cancer,66 and bladder cancer,42 compared with adjacent normal tissues, which may downregulate SOX11. Interestingly, hypomethylation of SOX11 promoter region can also be observed in both SOX11-positive and SOX11-negative MCL cell lines, whereas DNA methyltransferase inhibitor, 5-azacytidine, can downregulate SOX11 expression in SOX11-positive MCL cell lines. The effect of 5-azacytidine on SOX11 levels might be indirect owing to the fact that the SOX11 promoter region is already hypomethylated in MCL. In fact, 5-azacytidine inhibits the enzymes that instigate those repressive marks, leading to their dilution in the treated cell population.10,41
Hypermethylation was not detected in either adult stem cells or normal hematopoietic cells that have low levels of SOX11, whereas strong enrichment of repressive histone markers H3K9me2 and H3K27me3 was responsible for the repression of SOX11 expression in these cells.14 Furthermore, analysis of H3K27me3 enrichment in neoplastic cells revealed that acquired methylation of the SOX11 promoter might be associated with the loss of these repressive histone markers.66 In addition, in embryonic stem cells and SOX11-positive MCL cell lines, high SOX11 expression is correlated with the activation of histone markers, including H3K9/14Ac and H3K4me3, instead of to DNA methylation.14 In addition to DNA and histone methylation, histone acetylation is also predominant in the regulation of SOX11. Nordstrom and colleagues found that histone deacetylase inhibitors can induce SOX11 expression in cancer cells with low levels of SOX11 methylation, but not in methylated cancer cell lines, suggesting that histone acetylation may induce SOX11 expression and that promoter methylation can prevent chromatin acetylation.66 Taken together, these results demonstrate that repressed SOX11 expression in development or tumorigenesis is mainly caused by epigenetic alterations such as elevated methylation or histone deacetylase activity. However, integrative studies are required to elucidate which DNA methyltransferases and histone modifying enzymes are involved in this tight control of SOX11.
Apart from DNA methylation and histone modifications, the 3D reconfiguration of the SOX11 enhancer is also associated with aberrant SOX11 expression. Recently, Queirós et al.67 have identified that a distal enhancer associated with aberrant SOX11 expression only showed high contact frequencies with the SOX11 gene in 3D space in the SOX11-positive MCL samples. Moreover, the authors have also showed the presence of an enhancer-related histone mark (H3K4me1) and the epigenetic mark (H3K27ac) related to genomic activation at the putative SOX11 enhancer (a cluster of hypomethylated DMRs located 650 kb downstream of SOX11) only in the SOX11-positive samples, strongly suggesting that this regulatory element plays a role in SOX11 expression in MCL.68
miRNA interference
miRNAs are a series of 18- to 25-nucleotide non-coding RNAs that suppress protein translation through sequence-specific pairing with 3’-untranslated regions (3’-UTRs) of target mRNAs.69 In neonatal rat neurons, miR-212-3p and miR-132-3p showed the most prominent interactions with the 3’-UTR sequence of SOX11, thus suppressing SOX11 expression, which indicates that SOX11 expression can be regulated by miRNAs in neurogenesis.70 In melanoma, a reporter assay with the 3’-UTR of SOX11 cloned downstream of the luciferase gene exhibited decreased luciferase activity in the presence of miR-211, indicating that miR-211 is a direct repressor of SOX11 expression.71 In addition, Navarro and colleagues72 used supervised analyses to identify that a total of 22 miRNAs are visibly overexpressed and lead to the silencing of SOX11 in MCL, in particular the most important miRNAs were miR-455-5p and miR-455-3p. Even with these studies, the potential role of miRNAs in the regulation of SOX11 expression in tumorigenesis is largely unknown. Thus, further studies are required to illustrate the correlation between SOX11 and miRNAs.
Upstream regulatory pathways
Differential expression of SOX11 could also be correlated with the activity of a variety of signaling pathways. The canonical Wnt signaling pathway, which signals through β-catenin and T-cell factor (TCF) transcription factors, is indispensable for embryonic development and the maintenance of adult stem cells.73 Previous studies have reported that SOX members, including SOX2 and SOX4, are upregulated by Wnt signaling in both benign and malignant intestinal tissues.74 In addition, the Notch pathway has been implicated in suppressing SOX4 and SOX11 expression in the early developing retina.34
Furthermore, although recent investigations have identified genetic alterations in SOX members such as SOX2 and SOX9 in several cancer types, mutational analyses of the entire SOX11 coding sequence in MCL have not revealed tumor-associated DNA sequence alterations.75–77 Nevertheless, further studies should also focus on whether there exist accumulated genetic alterations, including gene deletion and gene amplification, of SOX11 in other cancer types.
Oncogenic function of SOX11 in tumors
There is compelling evidence that SOX11 functions as a crucial transcription factor targeting downstream genes to exert tumor-stimulative effects in a wide variety of tumors. To further understand the pleiotropic functions of SOX11 in tumors and to provide insight into its mechanisms of action and potential applications, we have focused on specific molecular pathways of SOX11 during distinct tumor processes (Figure 2).
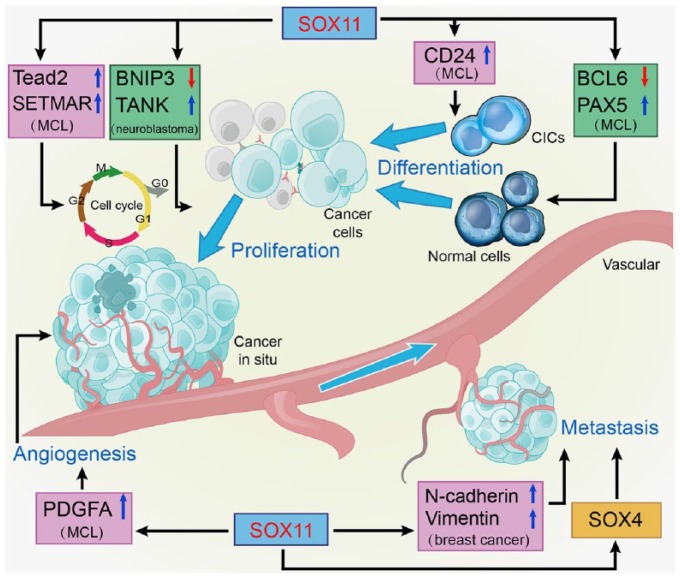
Figure 2.
Overall carcinogenic actions of SOX11 on the hallmarks of tumor biology.
SOX11 exerts tumor-stimulative effects through increasing cell proliferation, repressing cell differentiation, inducing angiogenesis, and promoting metastasis.
BCL6, B-cell lymphoma 6; BNIP3, B-cell lymphoma 2 (BCL2)/adenovirus E1B 19 kDa protein-interacting protein 3; CIC, cancer-initiating cell; PAX5, Paired box protein 5; PDGFA, platelet-derived growth factor A; SETMAR, SET domain and mariner transposase fusion gene; SOX11, Sex-determining region Y-related high-mobility-group box transcription factor 11; TANK, TRAF family member-associated NF-κB activator.
Stimulating cell survival in tumorigenesis
The regulation of apoptosis-related pathways appears to serve as a critical juncture for the control of unbridled cell proliferation and tumor growth.78,79 The fact that abnormal expression of SOX11 has the capacity to evoke tumorigenesis primarily through promoting cell survival and suppressing apoptosis has been highlighted.15,16
The pro-survival role of SOX11 could be associated with its direct transcriptional upregulation of Tead2, which is an important transcription factor in the HIPPO signaling pathway and confers SOX11-mediated survival signals during organogenesis.15 Notably, the pro-survival effect of the HIPPO pathway has also been implicated in the development and metastasis of a large variety of tumors.15,80 In addition, SOX11 silencing was reported to decrease the expression of SET domain and mariner transposase fusion gene (SETMAR).62,81 SETMAR is a methyltransferase that can cooperate with topoisomerase II alpha to promote mitosis.82 Of major interest, recent studies show that SETMAR is involved in chromosomal rearrangements in B-cell malignancies.83 These results demonstrate that SOX11 may exert its oncogenic effect by maintaining cell proliferation through its direct regulation of SETMAR.
Moreover, SOX11 can promote cell survival via transcriptional regulation of the pro-apoptotic gene B-cell lymphoma 2 (BCL2)/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3) as well as the anti-apoptotic gene TRAF family member-associated NF-κB activator (TANK).16 BNIP3 is a mitochondrial protein, the increased expression of which can induce the acute onset of apoptosis in neurons.84 TANK works with TNF receptor-associated factor 2 (TRAF2) to induce genes supporting neuron survival.85,86 In Neuro2a neuroblastoma cells, siRNA-mediated SOX11 knock-down led to enhanced apoptosis by increasing BNIP3 and decreasing TANK.16 These results suggest that regulation of anti-apoptotic pathways by SOX11 serves as an essential mechanism of its oncogenic effects.
Promoting cell proliferation in tumorigenesis
SOX11 is also critical for growth and proliferation of multiple cancer types. In vitro, SOX11 knock-down in ER-negative breast cancer cell lines, MCL-derived cell lines, and glioma-derived cell lines reduces cell proliferation, suggesting that SOX11 contributes to cancer cell proliferation in tumorigenesis.12,32,43 Concordant with the oncogenic role of SOX11 in vitro, Vegliante and colleagues87 created an MCL-xenotransplant model by transplanting Z138shSOX11 cell lines into CB17-SCID mice and found that SOX11 silencing reduced tumor growth compared with SOX11-positive control tumors in vivo.
Kuo et al.88 have recently developed a transgenic C57BL/6 mouse model (Eμ-SOX11-EGFP) expressing murine SOX11 and EGFP under the control of a B-cell-specific IgH-Eμ enhancer. They demonstrated that B-cell-specific overexpression of SOX11 promoted the oncogenic proliferation of B1a B cells and drove an MCL-like phenotype. In addition, the increased signaling through the B-cell receptor (BCR) pathway associated with SOX11 overexpression can be reversed by pharmacological BTK inhibition, indicating that the oncogenic power of SOX11 promoting the transformation and aberrant expansion of the B1a B cells, in part through the activation of the BCR.88
Inhibiting cancer cell differentiation
Cell differentiation blockade is generally observed in tumorigenesis and tumor progression. Recent studies have provided evidence that re-expression of SOX11, which is normally decreased after brain maturation, in malignant glioma may reflect a dedifferentiation process during tumorigenesis.43,89 In addition, blocking of plasma cell differentiation mediated by SOX11 has been regarded as a relevant oncogenic mechanism in lymphoid neoplasia. B-cell lymphoma 6 (BCL6) is a transcription factor important for germinal center formation, and is indispensable for the differentiation of B lymphocytes into plasma cells or memory B cells.90 SOX11 binding at the promoter of BCL6 in MCL cells causes the powerful transcriptional inhibition of BCL6.49 The powerful inhibitory effect of SOX11 on BCL6 transcription may interfere with the B-cell differentiation program in lymphomagenesis.
Paired box protein 5 (PAX5) is a critical transcription factor regulating B-cell identity in B-cell developmental progression.91 PAX5 has the capacity to inhibit plasma cell development via repressing B-lymphocyte-induced maturation protein 1 (BLIMP1), an essential transcription factor that promotes plasma cell differentiation.92 Thus, alterations in the expression of PAX5 are thought to promote neoplastic transformation in lymphocytes.93 Of note, SOX11 silencing downregulates PAX5 and upregulates BLIMP1, thus promoting a shift from a mature B cell into the initial plasmacytic differentiation phenotype in primary MCL cell lines.87 Therefore, SOX11 may participate in the pathogenesis of MCL through preventing terminal B-cell differentiation by regulating PAX5.
Furthermore, CD24 is known to be expressed in different carcinomas and has been identified as an essential CIC surface marker.94 CD24 is significantly reduced or even abolished in MCL cells where SOX11 is stably or transiently knocked down.62 The visible downregulation of CD24 upon silencing of SOX11 suggests that SOX11 can maintain the important properties of CICs that facilitate expansion and renewal in tumorigenesis.
Initiating invasion and metastasis in cancer
One major characteristic of cancer is its capacity for metastasis to distant organs. Activation of epithelial–mesenchymal transition (EMT), an essential metastatic mechanism, increases the potential for motility and metastasis of polarized epithelial cells.79,95 Venkov and colleagues96 found that RNAi-mediated downregulation of SOX11 in fibroblasts attenuates the expression of EMT markers such as N-cadherin and vimentin, which are normally upregulated in EMT,95 and thus slows down the initiation and propagation of EMT. In breast cancer, overexpressed SOX11 has been identified as a critical regulator responsible for the enhanced EMT-like characteristics, including motility and migratory ability, of aggressive MDA-MB-231 and MCF7 cells.12 Interestingly, SOX11 depletion in ER-negative cells leads to the decreased expression of SOX4.12 As SOX4 functions cooperatively with SOX11 during development and is also a regulator of EMT in breast cancer, it is necessary to elucidate whether SOX11 cooperates with SOX4 to promote EMT-like characteristics in aggressive basal-like breast cancers.12,27,97 In addition, elevated expression of SOX11 is significantly associated with lymph node invasion and distant metastasis in small cell lung cancer.58 In general, further studies should determine whether SOX11 contributes to the activation or mediation of EMT signaling or other migration-associated pathways in multiple types of cancer.
Balsas et al.98 reported that specific FAK and CXCR4 inhibitors impaired SOX11-enhanced MCL engraftment in intravenous xenograft mouse models with a significant decrease of SOX11-positive MCL cells in bone marrow and lymph nodes and a simultaneous increase in peripheral blood. These data indicated that SOX11 directly regulated the expression of CXCR4 and FAK and the activation of FAK/PI3K/AKT pathway, which further contributed to a more aggressive phenotype, featured by promoted MCL homing and invasion and increased cell proliferation, survival, and drug resistance.68,99 Hence, inhibition of this pathway may represent an efficient strategy to overcome stromal-mediated chemotherapy refractoriness in aggressive MCL.
Inducing tumor angiogenesis
Angiogenesis is one of the most important mechanisms used by cancer cells and is particularly widely studied in solid malignancies where it has critically been correlated with neoplastic growth and metastasis.61,100 Palomero and colleagues48 found more enrichment of angiogenic genes and much larger microvessel density areas in SOX11-positive MCL xenografts than in SOX11-negative xenografts. In particular, platelet-derived growth factor A (PDGFA) is the only SOX11-targeted gene found in chromatin immunoprecipitation investigations.48 PDGFA is involved in the promotion of neoplastic angiogenesis by influencing the behaviors of endothelial cells and recruiting mesenchymal stromal cells.101,102 Luciferase reporter assays revealed that SOX11 is capable of increasing transcription of PDGFA in a paracrine manner. Functional evidence of PDGFA secretion was exhibited in the conditioned media of SOX11-positive MCL cell lines, which induced proliferation and migration of vascular endothelial cells and vessel formation to support angiogenesis.48 Therefore, promotion of angiogenesis via the SOX11–PDGFA axis supports the oncogenic function of SOX11 in the aggressive behavior of cancer.
Tumor-suppressive role of SOX11 in carcinoma
In contrast to the roles of SOX11 in anti-apoptosis, promotion of metastasis and tumor angiogenesis, a number of recent reports have shown that augmented SOX11 expression might be associated with slower cancer progression in a variety of human cancers, including glioma,44 MCL,103 NPC,17 GC,18 and PCa.55 These indicate that SOX11 has the potential to act as a tumor suppressor in many malignancies. The antitumor effect of SOX11 has been attributed to its capacity to halt cell proliferation, inhibit CICs maintenance, and repress cancer invasion and metastasis (Figure 3).
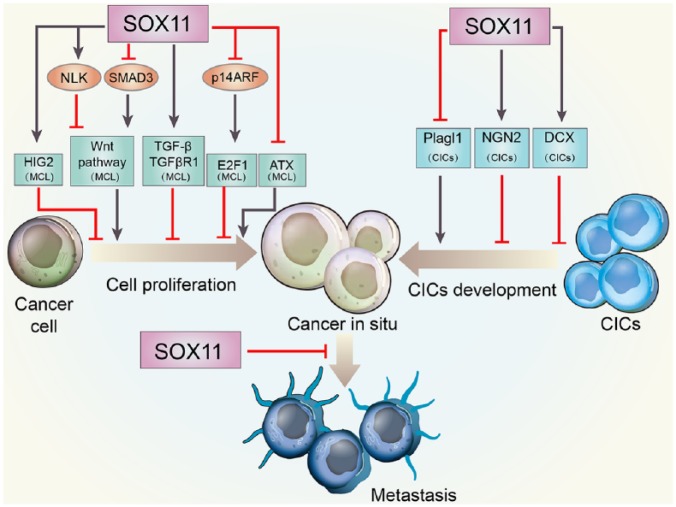
Figure 3.
Schematic representation of tumor-suppressive roles of SOX11 in cancer development.
SOX11 overexpression might be correlated with slower cancer progression. The tumor-suppressive effect of SOX11 has been attributed to its ability to decrease cell proliferation, repress CICs development, and reduce cancer invasion and metastasis.
ATX, autotaxin; CIC, cancer-initiating cell; DCX, doublecortin; HIG-2, hypoxia-inducible protein 2; NGN2, neurogenin 2; NLK, Nemo-like kinase; SOX11, sex-determining region Y-related high-mobility-group box transcription factor 11; TGF-β, transforming growth factor-β.
Inhibiting cell proliferation in carcinogenesis
The Wnt signaling pathway mediates cell proliferation and differentiation through activation of its downstream targets, such as TCF/lymphoid enhancer factor-1 (LEF) and β-catenin, in many physiological and pathophysiological conditions.104,105 The enhanced nuclear localization of β-catenin and activation of TCF/LEF appear to be essential processes for carcinogenesis in many tissues.106,107 Previous studies have identified activation of the Wnt pathway as essential for prompting cell proliferation in MCL cells.108,109 Nemo-like kinase (NLK) functions as a negative mediator of the Wnt pathway by downregulating the transcriptional activity of β-catenin and TCF/LEF.110 SMAD3 is a key mediator in the stabilization and nuclear translocation of β-catenin.111,112 SOX11 has been identified to significantly inhibit the Wnt signaling pathway by directly increasing NLK and reducing SMAD3, thus preventing the formation of the β-catenin/TCF4 complex in MCL.113 In addition, pathway analysis of ChIP-sequencing data revealed other SOX11 binding targets in the Wnt pathway, including chromodomain helicase DNA binding protein 8 (CHD8), glycogen synthase kinase-3 beta (GSK3β), cullin-1 (CUL1), and calcyclin binding protein (CACYBP), all of which are negative regulators of Wnt signaling and become attenuated after SOX11 depletion in MCL.113 Taken together, inhibition of Wnt signaling exhibits one crucial pathway through which SOX11 can exert its anti-proliferative effect in malignancies, and the interactions between SOX11 and the components of Wnt pathway still need further investigation.
Hypoxia-inducible protein 2 (HIG-2) is a lipid droplet protein that boosts neutral lipid deposition and has been identified to play roles in pathological lipid accumulation.114 Alterations in HIG-2 gene expression are commonly observed in lymphomas and leukemia.115 It has been reported that HIG-2 acts as a direct target of SOX11, and they reciprocally co-regulate each other.62,81 Silencing of SOX11 by siRNA causes downregulation of HIG-2, whereas knock-down of HIG-2 conversely reduces the expression of SOX11. Of major interest, SOX11 knock-down in MCL cell lines can significantly downregulate HIG-2 and subsequently increase cell proliferation. However, both HIG-2 and SOX11 knock-down did not lead to any additive increase in proliferation. Thus, we cannot exclude the possibility that SOX11 plays its antitumor role in MCL by preventing excessive proliferation by sustaining the expression of HIG-2.
In addition, gene ChIP analysis revealed that the Rb-E2F growth regulatory pathway is involved in SOX11-induced growth repression.41 Cyclin-dependent kinase inhibitor 2A (CDKN2A) and E2F1 are important components of the Rb-E2F pathway.116 The CDKN2A locus consists of two proteins, including p16INK4A and p14ARF, both of which act as tumor suppressors by controlling the cell cycle.117 Overexpression of SOX11 in GRANTA-519 and JEKO-1 lymphoma cells can augment the expression of the CDKN2A locus.41 E2F1, one of the downstream targets of p14ARF, can promote cell proliferation by regulating the expression of genes correlated with the cell cycle transition and the progression of the cell cycle from G1 into S phase.117–119 Downregulation of E2F1 along with the reduction in cell proliferation can be observed in SOX11-overexpressed lymphoma cells.41 Collectively, SOX11 may inhibit cell proliferation to exert anti-tumor effects by increasing CDKN2A and decreasing E2F1.
Transforming growth factor-β (TGF-β) is widely known as an essential tumor suppressor. Upregulation of TGF-β is also observed 24 h after SOX11 overexpression in MCL, followed by the increased expression of TGF-β receptor type-1 (TGFβR1), a direct binding target of SOX11 via high-resolution ChIP sequencing.41,113 Of note, increased TGFβR1 has been reported to be needed for TGF-β-mediated growth inhibition in MCL,120 which highlights the antiproliferative effect of overexpressed SOX11. Thus, the TGF-β pathway may also contribute to the antiproliferative effect of SOX11 in tumorigenesis.
The antiproliferative effect of SOX11 is also related to its negative regulation of autotaxin (ATX). The primary product of ATX is lysophosphatidic acid 1-acyl 2-hydroxyl glycerol 3-phosphate (LPA), which can promote growth, survival and motility of cells involved in tumorigenesis.121 Knock-down of SOX11 by shRNA in Z138 MCL cell lines caused the increase in both the expression of ATX and the proliferation of cancer cells.103 Accordingly, Conrotto and colleagues103 also found that SOX11 exerted its anti-tumor function by repressing ATX in tumorigenic cells and tumor growth rate in nonobese diabetic–severe combined immunodeficient (NOD-SCID) mice.
Repressing the development of CICs
In addition to its inhibitory role at the initial stage of tumor development, SOX11 can suppress tumor progression by inhibiting expansion and differentiation of highly aggressive CICs. CICs are able to infinitely self-renewal and generate additional cancer cells.122 Hide and colleagues44 found that glioma-initiating cell (GIC)-like cells NSCL61s lose the expression of SOX11. In contrast, overexpression of SOX11 in these cells exhibits repression of tumorigenesis by not only inducing their neuronal differentiation but also attenuating Plagl1 levels.44 Plagl1 regulates both cell cycle arrest and apoptosis and can be detected in many brain areas with high cell proliferation activity.123,124 However, knock-down of Plagl1 has been reported to significantly suppress the tumorigenicity of GICs.44,125 In consequence, inhibited expression of oncogenic Plagl1 may contribute to the antitumor effect of SOX11 by effectively decreasing CICs in cancer. In addition, SOX11 can synergize with neurogenin 2 (NGN2) to efficiently convert human GIC-like cells to postmitotic neuron-like cells in vitro and in vivo.126 NGN2 is a transcription factor that controls the commitment of neural progenitors to a neuronal fate during development.127 Aberrant expression of NGN2 can induce the differentiation of postnatal glial cells into neurons and promote cell cycle arrest and apoptosis of glioblastoma stem cells.128 Forced expression of NGN2/SOX11 results in rapid cell cycle exit and repression of cell proliferation in GIC-like cells.126 Moreover, doublecortin (DCX), a microtubule-associated protein highly expressed in neuroblasts and immature neurons, is shown to inhibit GIC self-renewal and migration.129 The expression of DCX can be induced by NGN2/SOX11 in human GICs and may contribute to their renewal arrest.126 In conclusion, not only is SOX11 a critical inhibitor of CIC maintenance, but its suppression is also mandatory for CIC differentiation. Thus, control of the self-renewal of CICs and their conversion into terminally differentiated cell types by SOX11 represents a novel therapeutic strategy to prevent tumorigenesis.
Suppressing tumor invasion and metastasis
The tumor-suppressive role of SOX11 may also stem from its capacity to inhibit cancer cell invasion and metastasis.130 A recent study investigating the role of SOX11 in NPC CNE2 cell lines revealed that re-expression of SOX11 mRNA and protein after treatment with 5-aza-2’-deoxycytidine, a DNA methyltransferase inhibitor that can demethylate SOX11, may significantly attenuate the growth and invasion ability of CNE2 cells.17 Notably, a higher methylation rate of SOX11 can be observed in NPC tissues from patients with lymph node metastasis than in those without lymph node metastasis.17 These indicate that the expression of SOX11 may be one of the factors that inhibit the invasion and metastasis of NPC, whereas this suppressive effect can be abolished by DNA methylation in cancer development. Transwell assays were used to investigate the migration and invasion capabilities of PCa PC-3 and DU145 cell lines in vitro, revealing that overexpression of SOX11 observably decreases migration of these PCa cells.55 In addition, overexpression of SOX11 in GC SGC-7901 cell lines exhibited suppressed migration and invasion compared to vector-SGC-7901 cells.18 However, the mechanisms underlying the inhibition of invasion and metastasis by SOX11 overexpression are still unknown. Further investigations are required to determine which target or signaling pathway SOX11 uses to inhibit migration and invasion in various human cancers.
Clinical relevance of SOX11 in carcinoma
The clinical relevance of the SOX11 gene has risen to a high level in the past 10 years as plenty of studies have indicated that SOX11 may contribute to diagnosis, prognosis, and different drug options in various human cancers.
Diagnostic value of SOX11
The diagnosis of MCL is achieved by identification of overexpressed cyclin D1 (CCND1) protein by immunohistochemistry (IHC) or by evidence of CCND1/immunoglobulin heavy chain (IGH) fusion by fluorescence in situ hybridization (FISH).131 However, approximately 10% of MCL lack this specific expression of CCND1. Notably, SOX11 is specifically expressed in almost all MCL cases regardless of the presence or absence of CCND1.32,68,132 Mozos and colleagues132 analyzed 50 conventional MCL cases and 12 CCND1-negative MCL cases for SOX11 expression, and found that nuclear SOX11 protein expression is a highly specific marker for both CCND1 positive and negative MCL. Consistently, Ek and colleagues32 identified the significant diagnostic value of SOX11 for MCL by detecting the nuclear expression of SOX11 in 18 whole tissue sections and 10 tissue microarray sections of MCL. Furthermore, Hsiao and colleagues133 retrospectively stained a separate cohort of 98 DLBCL cases and a total of 22 MCL cases for SOX11 and CCND1, and demonstrated that immunostaining of SOX11 is helpful in the differential diagnosis of CCND1-positive DLBCL from MCL.
In general, the distinction between MCL and CLL is based on well-characterized morphologic, phenotypic, and cytogenetic differences, including cyclin D1 expression in most MCLs and the absence of cyclin D1 in most CLLs. It is sometimes tricky to distinguish between them because some MCLs may mimic CLL clinically, histologically, or phenotypically and vice versa. Nevertheless, it is crucial to distinguish CLL from MCL, because CLL is usually considered a low-grade neoplasm even when the disease is nodal, whereas cases of MCL are clinically more aggressive.134 Mozos et al. reported that SOX11 is expressed in 93–95% of cyclin D1 positive MCL cases but in none of the large series of CLL.132 In addition, it has been demonstrated that SOX11 expression can be reliably analyzed by multiparameter flow cytometry and can, thus, be useful in distinguishing between MCL and CLL.135 Detection of SOX11 by IHC is therefore a diagnostic marker for MCL, especially cyclin D1 negative ones.132,47
Although SOX11 has no known lymphopoietic function and is not usually expressed in B cells, SOX11 has been shown to be expressed in specific subtypes of B-cell lymphoid malignancies, indicating that the dysfunction of this gene is not completely restricted to MCL.132 In addition, Lord et al. found that SOX11 protein expressing CD20+ cells might infrequently be detected during immune responses in reactive lymph nodes. Therefore, it is important to score SOX11 by IHC only in the tumor areas of MCL.136 We should also be aware that SOX11 negativity has been associated with indolent MCL that might look and behave like CLL.137 These are the limitations that restrict the diagnostic value of SOX in distinguishing MCL from CLL.
In medulloblastoma (MB) cases, many studies have confirmed obvious SOX11 overexpression and its availability as a biomarker in the diagnosis of MB.46,138,139 In addition, Teng and colleagues56 analyzed 111 hepatocellular carcinoma (HCC) patients with 66 chronic hepatitis B (CHB) patients, and revealed that the methylation frequency of serum SOX11 promoter serves as a useful and noninvasive biomarker for the discrimination of hepatitis B virus associated HCC from CHB.56 The frequency of SOX11 methylation is also utilized in a five-gene biomarker panel to detect bladder cancer at an early stage.42
Prognostic significance of SOX11
Of major interest, SOX11 may play a paradoxical role in tumor prognosis, as summarized in Table 2. Recent studies indicated that positive SOX11 expression is significantly correlated with increased recurrence-free survival (RFS) and a less-aggressive phenotype in EOC,54 GC,18 gliomas,44 and glioblastomas.89 Notably, hypermethylation of the SOX11 promoter has been found to be tightly associated with a poor prognosis in 89 GC patients via multivariate Cox regression analysis.13 By analyzing the relevance of gene expression with clinical evolution in 86 cases of CLL, Roisman and colleagues50 found shorter RFS and overall survival (OS) in SOX11-positive patients compared with SOX11 negative cases. It is worthwhile to note that high nuclear expression of SOX11 was correlated with a more prolonged OS and less-advanced clinicopathological features than those with low expression in 116 breast cancer patient samples.53 However, Shepherd and colleagues12 found that positive SOX11 expression indicated poor survival in 995 women with basal-like breast cancer. In the study that investigated SOX11 gene expression in 50 de novo adult acute myeloid leukemia (AML) patients, SOX11 overexpression influenced disease-free survival (DFS) and OS time through shortening it, indicating that SOX11high status might be considered as a prognostic marker for AML patients.140
Table 2.
Different prognostic significance of SOX11 in tumor cases.
| Tumor type | Research case | Method | Antibody specificity (company) |
Correlation between the SOX11 expression level with tumor prognosis | Reference |
|---|---|---|---|---|---|
| CLL | 86 | qRT-PCR | — | Elevated SOX11 expression is inversely associated with RFS and OS in patients. | Roisman et al.50 |
| EOC | 54 | Tissue microarray IHC |
— | Increased SOX11 is associated with an improved RFS among patients. | Brennan et al.54 |
| GC | 151 | IHC | Mouse monoclonal (Cell Marque) |
SOX11-positive patients showed longer medium survival and improved metastasis-free survival. | Qu et al.18 |
| Glioma | NR | NR | Rabbit polyclonal (Sigma) |
Upregulation of SOX11 in patients is related to a prolonged survival. | Hide et al.44 |
| Glioblastoma | 132 | IHC | Rabbit polyclonal (Spring) |
SOX11 overexpression is correlated with favorable prognosis and improved OS. | Korkolopoulou et al.89 |
| Melanoma | 40 | IHC | Rabbit polyclonal (Abcam) |
Positive SOX11 expression leads to a lower survival rate in patients. | Jian et al.59 |
| Lung cancer | 60 | nCounter analysis | — | Patients with absent expression of SOX11 have prolonged survival. | Walter et al.58 |
| BLBC | 995 | — | — | High expression of SOX11 is with poor RFS, OS, and metastasis-free survival. | Shepherd et al.12 |
| MCL | 53 | IHC | Rabbit polyclonal (Atlas Antibodies AB) |
SOX11-negative patients show worse prognosis and shorter OS. | Wang et al.141 |
| MCL | 186 | IHC | Rabbit polyclonal (Atlas Antibodies AB) |
Loss of SOX11 is associated with shorter OS, but is not associated with an indolent clinical course. | Nygren et al.47 |
| MCL | 112 | IHC | Rabbit polyclonal (Atlas Antibodies AB) |
SOX11-negative tumors tends to be nonnodal with better survival. | Fernandez et al.137 |
| MCL | 207 | IHC | Rabbit polyclonal (Sigma) |
SOX11-negative patients exhibit slow or absent clinical progression. | Ondrejka et al.142 |
| AML | 50 | qRT-PCR | — | SOX11 overexpression shortened DFS and OS time. | Tosic et al.140 |
BLBC, basal-like breast cancer; CLL, chronic lymphocytic leukemia; EOC, epithelial ovarian cancer; GC, gastric cancer; IHC, immunohistochemistry; MCL, mantle cell lymphoma; NR, not reported; OS, overall survival; qRT-PCR, quantitative real-time polymerase chain reaction; RFS, recurrence-free survival; SOX11, sex-determining region Y-related high-mobility-group box transcription factor 11.
Controversies about the clinical value of SOX11
The value of SOX11 IHC as a prognostic marker in MCL is, however, partly conflicting because of the lack of defined cut-off levels of SOX11 expression, which is reflected by the different immunohistochemical cut-offs used in recent studies. Wang and colleagues141 reported that loss of nuclear SOX11 is related to shorter OS in MCL with lymph node presentation (nodal MCL) by analyzing 53 patient samples. In addition, Nygren and colleagues47 reported that lack of SOX11 expression is associated with shorter OS and that SOX11-negative MCL patients develop more aggressive features than SOX11-positive patients. Nordström et al.143 have also reported that the absence of nuclear SOX11 in MCL is associated with shorter OS. Aukema et al.144 have recently conducted the currently largest (n = 365) multivariate analyses on the prognostic importance of SOX11 in MCL by taking MCL international prognostic index (MIPI) and Ki67 into account, and showed that SOX11 expression was not associated with time to treatment failure but patients with low SOX11 expression had shorter OS. In contrast, Dreyling and colleagues145 determined that positive SOX11 expression was correlated with poor outcome based on 112 total MCL patient samples. The analysis of clinical evolution by Roisman et al.50 also showed shorter TFS and OS in SOX11-positive MCL patients compared with SOX11-negative MCL patients.
Importantly, a multivariate analysis of 112 cases conducted by Fernandez and colleagues defined nonnodal presentation, predominantly hypermutated IGVH, lack of genomic complexity, and absence of SOX11 expression as qualities of a specific subtype of indolent MCL with excellent outcomes that might be managed more conservatively than conventional MCL.137 Navarro et al.146 conducted an integrative and multidisciplinary analysis of 177 MCL and further confirmed that MCL with these characteristics correspond to a subtype of the disease with more indolent behavior. Moreover, Ondrejka and colleagues142 reported that loss of SOX11 in indolent MCL resulted in slow or absent clinical progression.
Conventional SOX11-positive MCL usually had generalized lymphadenopathy, unmutated IGHV, complex karyotypes, required treatment at diagnosis, and had an aggressive evolution. In contrast, SOX11-negative MCL were characterized by leukemic presentation, frequently associated with splenomegaly, and with no or minimal lymphadenopathy. The tumors carried mutated IGHV and very few chromosomal alterations in addition to the t(11;14). The disease tended to be stable and asymptomatic for long periods, although some tumors progressed to more aggressive forms, which were usually associated with the acquisition of TP53 mutations and more complex karyotypes. These differences have led to the recognition of leukemic nonnodal MCL (nn-MCL) as a new subtype in the updated World Health Organization (WHO) classification.147 These results suggest that lack of SOX11 expression is a feature of nn-MCL subtype that may present with an indolent clinical behavior, though it may eventually progress to an aggressive form.
Notably, the apparent worse prognosis of SOX11-negative MCL in those studies 131-134 seems to be related to lymph node presentation and TP53 alterations, suggesting that these cases correspond to a selected subset of progressed tumors. This idea is consistent with the observed poor outcome conferred by TP53 mutations in both SOX11-positive and negative MCL,148,149 and hints that both conclusions may not be as contradictory as suggested.
Nevertheless, it has also controversial to use SOX11 as the marker to distinguish between indolent MCL and conventional MCL owing to the identification of cases with borderline SOX11 expression levels, technical difficulties, and concomitant confounding factors, such as the presence of TP53 alterations that impair the outcome of both indolent MCL and conventional MCL. Thus, Clot et al.150 developed a novel molecular assay using blood samples and confirmed that indolent MCL had a better OS than conventional MCL from the time of diagnosis and longer time to treatment. More importantly, they revealed that genomic complexity and TP53/CDKN2A aberrations predicted for shorter OS in the whole series and conventional MCL, whereas only genomic complexity was associated with shorter time to treatment and OS in indolent MCL.
Clinical applications of SOX11 antibodies
As SOX11 provides promising diagnostic and prognostic value in cancers, the key issue is how to improve the specificity and sensitivity in detecting SOX11 levels. Until 2012, the only available polyclonal antibody targeting SOX11 exhibited significant inter-batch variability and could not be incorporated into routine IHC because of nonspecific staining.151 However, a specific monoclonal mouse antibody, SOX11-C1, was developed against a C-terminal peptide of SOX11 for use in MCL by Nordstrom and colleagues in 2012.152 Compared with the polyclonal antibodies, this monoclonal antibody shows enhanced sensitivity, single epitope specificity, and no inter-batch variation, which enables the detection of tumor cells when they are present in <1% of total cells in blood specimens by IHC and flow cytometry.41,152 Walter and colleagues58 have suggested that SOX11-C1 may allow for the early detection of small cell lung cancer (SCLC) in blood samples. Above all, the SOX11-C1 antibody exhibits robust performance superior to polyclonal antibodies in diagnosing and predicting the clinic outcome of MCL. However, the application of the monoclonal antibody SOX11-C1 in other tumor types still requires further investigation.
Recently, Soldini et al.153 screened several different anti-SOX11 antibodies in IHC and WB applications. Among these tested antibodies, MRQ-58 showed to be the most sensitive and meanwhile did not cross-react with the SOX4 protein, which has high similarity in amino acid sequence to SOX11. The application of MRQ-58 in flow cytometry analysis was later investigated by Wasik et al.135 They revealed that the endogenous levels of SOX11 in MCL cell lines detected by WB correspond to the SOX11 levels detected by flow cytometry. Furthermore, they validated by confocal microscopy that MRQ-58 antibody recognized protein expressed in nuclei, consistent with IHC data presented by Soldini et al. In addition, several MCL cases that were negative with the polyclonal SOX11 antibody HPA000536 became positive when using the MRQ-58 antibody, and the results of IHC by MRQ-58 are more consistent with the mRNA expression.136 Therefore, this antibody greatly expands the value of SOX11 IHC as a prognostic marker in MCL owing to its high specificity.
SOX11 and therapy options
As SOX11 has proven its functional role in multiple aspects of tumor biology, using SOX11 for cancer therapy may present new therapeutic opportunities. Hide and colleagues44 observed an increased sensitivity of GICs to anticancer drugs, including etoposide and taxol, that was induced by SOX11 overexpression, suggesting that high expression of SOX11 may provide favorable outcomes in clinical chemotherapy. Moreover, Kuo and colleagues113 analyzed the association between SOX11 and the effect of R-HyperCVAD in 131 patient MCL samples, and found that R-HyperCVAD treatment significantly prolonged OS in MCL patients with high expression of SOX11 compared with in those with low SOX11 expression. Although intensive R-HyperCVAD therapy improves the clinical outcome of patients with MCL, the utilization of intensive R-HyperCVAD is limited in the clinic in part for its considerable side effects, including myelosuppression, infection, and other chemotherapy toxicities.154 However, bio-assay of SOX11 expression may represent a unique approach for preselecting patients who are more likely to achieve better survival with this intensive chemotherapy regimen not only in MCL, but also in other SOX11 expressing solid tumors, such as gliomas, medulloblastomas, and ovarian tumors. In addition, Palomero and colleagues48 discussed the effect of imatinib treatment in SOX11-positive MCL in vivo. They observed reduced lymphoma growth and inhibited tumor angiogenesis in SOX11-positive xenografts than in SOX11-negative MCL xenografts after imatinib treatment. Imatinib acts as a tyrosine kinase inhibitor of platelet-derived growth factor receptor (PDGFR), and therefore, modulation of angiogenic SOX11-related pathways by imatinib represents an attractive therapeutic strategy for treatment of aggressive cancers.48,155
Epigenetic alterations play a key role in tumorigenesis. Given its tumor-suppressive role, repressing DNA methylation or histone modification with pharmaceutical interventions to re-express SOX11 may have a conducive effect in cancer treatment. Trichostatin A (TSA) and vorinostat (SAHA) are commonly used as histone deacetylase inhibitors, and increased expression of SOX11 can be observed in breast cancer, neuroblastoma, Burkitt lymphoma, and MCL by TSA and SAHA treatment via the inhibition of histone deacetylation.14,66 Furthermore, the treatment of 5-aza-2’-deoxycytidine, a DNA methyltransferase inhibitor, can upregulate expression of SOX11 in the NPC cell line CNE2 and consequently inhibit growth and invasion of NPC.17 Although the epigenetic regulation of SOX11 in cancer is complicated, the application of epigenetic agents targeting SOX11 may provide new options for cancer therapy, which urgently need further investigation. In summary, detecting SOX11 expression can be used to indicate diagnosis, predict prognosis and provide new insights for targeted therapy. Therefore, the accurate assessment of SOX11 expression levels in primary tumors is important for patient management. As studies are in progress to identify conditions in which the expression levels of SOX11 are increased, we suggest that the following areas need further elucidation: (i) whether increased SOX11 levels can identify a population at high risk for tumorigenesis; (ii) how to determine standards for evaluating SOX11 expression levels to predict tumor prognosis and status; (iii) how to detect SOX11 via more specific and efficient approaches in the clinic; and (iv) how to prevent SOX11 levels from increasing with tumor progression
Conclusion and prospects
Over the past 20 years, the roles of transcription factors in tumors have become a popular research topic.156–158 SOX11, a newcomer to the SOX family of transcription factors, has been demonstrated to affect physiological and pathological processes related to organogenesis and tumorigenesis. Considering the paradoxical effects of SOX11 in controlling tumorigenesis, the traditional terms ‘oncogene’ and ‘tumor-suppressor gene’ are far from sufficient to describe the role of SOX11 in tumorigenesis and progression. SOX11 is involved in tumorigenesis by promoting proliferation, tumor metastasis, and angiogenesis and by inhibiting cell differentiation. From another point of view, SOX11 exerts antitumor effects by inducing apoptosis, suppressing CIC maintenance, and reducing cancer invasion. In particular, SOX11 plays a diverse role in the pathogenesis of conventional MCL through regulating a complex transcriptional program that may contribute to both aggressive behavior and tumor prevention. Notably, no mutations, genetic aberrations, or aberrant DNA methylations at the promoter region related to its expression have been found.10,159 Only the presence of active histone marks at the SOX11 promoter has been observed to be associated with its RNA expression.14 Thus, an essential question remains what the initial event is that causes SOX11 activation in MCL. Another challenge regarding the paradoxical results of SOX11 in MCL may derive from the extensive spectrum of growth patterns of MCL and the discrepancy between MCL subtypes.160 Studies regarding SOX11 in iMCL only revealed its prognostic value.137 Large-scale investigation is still needed to explore detailed mechanisms underlying SOX11 in lymphoma development, which will be helpful for understanding the different ideas of SOX11 in initiation and prognosis of MCL. Furthermore, both in vivo and in vitro studies have suggested that SOX11 can also exert both oncogenic and antitumor functions in the development and progression of other types of cancer. These paradoxical results might be explained by cellular context-dependent or cell-type-dependent effects of SOX11 even though the cells derive from the same type of tissue. Moreover, the divergent study designs and experimental materials, for example, the different choices and titers of antibodies in IHC or immunoblotting, might also be partly responsible for the inconsistent results. In addition, improved mouse models of cancer that can recapitulate most aspects of cancer initiation and progression could be helpful in unravelling more mechanisms underlying the paradoxical functions of SOX11. Undoubtedly, corroborating mouse results with human cancer samples is also crucial to elucidate the roles of SOX11. Furthermore, to better understand the oncogenic or antitumor roles of SOX11, further studies should focus on (i) whether upregulation of SOX11 is a cause or a consequence of the progression from normal tissue to carcinoma, (ii) which type(s) of post-transcriptional or post-translational regulation contribute(s) to its upregulation or activation in tumorigenesis, and (iii) the identification of more downstream targets or pathways activated by SOX11 and their connections to tumorigenesis.
The activity of various transcription factors is modulated by post-translational modifications, whereas there is no evidence reported that this is also the case for the SOX11 protein. As described previously, SOX11 can significantly regulate Wnt signaling to control tumor cell fate in tumorigenesis. In reverse, it requires more investigations whether Wnt pathway can provoke SOX11 activity in a way similar to the regulatory mechanism of SOX4 and further facilitates its tumor-suppressive function. In addition, recent studies have reported that the crosstalk of SOX4 with the Wnt, Notch, and PI3K pathways can contribute to PCa progression.161 SOX4 has been demonstrated to both activate and inversely be activated by Wnt, Notch, and PI3K pathways. Interestingly, another possibility of SOX11 activation may come from the presence of binding sides for SOX members in its enhancer, suggesting a positive feedback loop in which SOX11 enhances its own expression. Currently, the progress in SOX11 is far from sufficient to meet the need for therapeutic research. Targeting SOX11 may have many unwanted complications. The detailed regulatory mechanisms of SOX11 have to be elucidated before SOX11 can finally serve as a therapeutic target. Therefore, it is advantageous to put future investigations into the regulatory mechanisms underlying activating SOX11, such as Wnt, Notch, and PI3K signaling, and its downstream targets in the context of carcinogenesis and cancer progression. This might also aid in the design of pharmacological compounds that control the activity of this transcription factor.
On the other hand, despite the prospective therapeutic benefits of pharmacological compounds that inhibit SOX11 activity, it is indispensable to carefully evaluate the cellular context in which it would be used and the function of SOX11 in those environments to prevent any adverse effects brought on by repression of its antitumor effect. Owing to its critical functions in inhibiting proliferation, CIC maintenance, and cancer metastasis, SOX11 has been considered as tumor suppressors in some cases. Although the tumor-suppressive function of SOX11 is supported by ample experimental evidence, this has not yet been confirmed by cancer genetics. So far only rare alterations involving SOX11 genes have been identified in tumors compared with classical tumor suppressors, especially the same subgroup of SOX family. Given the dual nature of SOX11 and its remarkable tissue context dependence, the challenge of further trails must pay more attention to maximizing the effect on the tumor-suppressive arms of SOX11 and, at the same time, minimizing the tumor-promoting aspect of SOX11. Ultimately, from a therapeutic perspective, these options open new avenues for investigation
In addition, SOX11 expression levels can provide both diagnostic and prognostic value in the clinic. As discussed previously, those opposing outcomes in the clinic further underscore the different roles of SOX11 in various cancer types. However, the following issues need to be addressed: (i) the discrepancy of prognosis in different types of cancer may result from tumor heterogeneity; (ii) the meta-analyses may have some potential limitations, such as deficient experimental design and insufficient clinical cases; and (iii) there is statistical heterogeneity in clinical investigations which may be caused by the different criteria used to assess SOX11 expression, the patient population, the tumor types, and the disease stages. Although the current knowledge of SOX11 continues to expand at an almost exponential rate, several types of integrative studies are especially useful for the development of therapeutic strategies. Therefore, future clinical investigations may benefit from concentrating on (i) defining the standards for assessing SOX11 concentration to predict clinical outcomes, (ii) determining how to modulate SOX11 levels to appropriately increase therapeutic response and prevent cancer relapse, and (iii) determining whether the clinical application of therapeutics targeting SOX11 has other unwanted side effects. Together, the development of transcriptomics, proteomics, and drug screening technologies and further investigations regarding SOX11 and its oncogenic effects will facilitate improved applications of SOX11 in cancer treatment.
Footnotes
Authors’ note: Zhi Yang is also affiliated to Department of Cardiovascular Surgery, General Hospital of Guangzhou Military Command of PLA, Guang Zhou, China.
Funding: This work was supported by the National Natural Science Foundation of China (grant numbers 81871607, 81570231, 81700236, 81600306, and 81500263), Natural Science Foundation of Shaanxi Province (grant number 2018JM3042), Major project of Military Logistics (grant number ALJ17J001), Achievement Training Program of Air Force Military Medical University (grant number 2016CGPY0301), Natural Science Foundation of Xi’an City (grant number 2016049SF/YX05(2)), and High-tech Project of Xijing Hospital (grant number XJGX15Y32).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Yang Yang  https://orcid.org/0000-0002-1163-2359
https://orcid.org/0000-0002-1163-2359
Contributor Information
Zhi Yang, Department of Cardiovascular Surgery, Xijing Hospital, The Fourth Military Medical University, Xi’an, China.
Shuai Jiang, Department of Aerospace Medicine, The Fourth Military Medical University, Xi’an, China.
Chenxi Lu, Key Laboratory of Resource Biology and Biotechnology in Western China, Ministry of Education, Faculty of Life Sciences, Northwest University, Xi’an, China.
Ting Ji, Key Laboratory of Resource Biology and Biotechnology in Western China, Ministry of Education, Faculty of Life Sciences, Northwest University, Xi’an, China.
Wenwen Yang, Key Laboratory of Resource Biology and Biotechnology in Western China, Ministry of Education, Faculty of Life Sciences, Northwest University, Xi’an, China.
Tian Li, Department of Biomedical Engineering, The Fourth Military Medical University, Xi’an, China.
Jianjun Lv, Department of Biomedical Engineering, The Fourth Military Medical University, Xi’an, China.
Wei Hu, Department of Immunology, The Fourth Military Medical University, Xi’an, China.
Yang Yang, Key Laboratory of Resource Biology and Biotechnology in Western China, Ministry of Education, Faculty of Life Sciences, Northwest University, Xi’an, China.
Zhenxiao Jin, Department of Cardiovascular Surgery, Xijing Hospital, The Fourth Military Medical University, 127 Changle West Road, Xi’an 710032, China.
References
- 1. Sinclair AH, Berta P, Palmer MS, et al. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 1990; 346: 240–244. [DOI] [PubMed] [Google Scholar]
- 2. Gubbay J, Collignon J, Koopman P, et al. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 1990; 346: 245–250. [DOI] [PubMed] [Google Scholar]
- 3. Bowles J, Schepers G, Koopman P. Phylogeny of the sox family of developmental transcription factors based on sequence and structural indicators. Dev Biol 2000; 227: 239–255. [DOI] [PubMed] [Google Scholar]
- 4. Sarkar A, Hochedlinger K. The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell 2013; 12: 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mu P, Zhang Z, Benelli M, et al. Sox2 promotes lineage plasticity and antiandrogen resistance in Tp53- and Rb1-deficient prostate cancer. Science 2017; 355: 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang H, Alberich-Jorda M, Amabile G, et al. Sox4 is a key oncogenic target in C/Ebpalpha mutant acute myeloid leukemia. Cancer Cell 2013; 24: 575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang H, He L, Ma F, et al. Sox9 regulates low density lipoprotein receptor-related protein 6 (Lrp6) and T-cell factor 4 (Tcf4) expression and Wnt/Beta-catenin activation in breast cancer. J Biol Chem 2013; 288: 6478–6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jay P, Goze C, Marsollier C, et al. The human Sox11 gene: cloning, chromosomal assignment and tissue expression. Genomics 1995; 29: 541–545. [DOI] [PubMed] [Google Scholar]
- 9. Wurm A, Sock E, Fuchshofer R, et al. Anterior segment dysgenesis in the eyes of mice deficient for the high-mobility-group transcription factor Sox11. Exp Eye Res 2008; 86: 895–907. [DOI] [PubMed] [Google Scholar]
- 10. Wasik AM, Lord M, Wang X, et al. Soxc transcription factors in mantle cell lymphoma: the role of promoter methylation in Sox11 expression. Sci Rep 2013; 3: 1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sernbo S, Gustavsson E, Brennan DJ, et al. The tumour suppressor Sox11 is associated with improved survival among high grade epithelial ovarian cancers and is regulated by reversible promoter methylation. BMC Cancer 2011; 11: 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shepherd JH, Uray IP, Mazumdar A, et al. The Sox11 transcription factor is a critical regulator of basal-like breast cancer growth, invasion, and basal-like gene expression. Oncotarget 2016; 7: 13106–13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu X, Chang X, Li Z, et al. Aberrant Sox11 promoter methylation is associated with poor prognosis in gastric cancer. Cell Oncol (Dordr) 2015; 38: 183–194. [DOI] [PubMed] [Google Scholar]
- 14. Vegliante MC, Royo C, Palomero J, et al. Epigenetic activation of Sox11 in lymphoid neoplasms by histone modifications. PLoS One 2011; 6: e21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gadi J, Jung SH, Lee MJ, et al. The transcription factor protein Sox11 enhances early osteoblast differentiation by facilitating proliferation and the survival of mesenchymal and osteoblast progenitors. J Biol Chem 2013; 288: 25400–25413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jankowski MP, Cornuet PK, Mcilwrath S, et al. SRY-box containing gene 11 (Sox11) transcription factor is required for neuron survival and neurite growth. Neuroscience 2006; 143: 501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang S, Li S, Gao JL. Promoter methylation status of the tumor suppressor gene Sox11 Is associated with cell growth and invasion in nasopharyngeal carcinoma. Cancer Cell Int 2013; 13: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qu Y, Zhou C, Zhang J, et al. The metastasis suppressor Sox11 is an independent prognostic factor for improved survival in gastric cancer. Int J Oncol 2014; 44: 1512–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Azuma T, Ao S, Saito Y, et al. Human Sox11, an upregulated gene during the neural differentiation, has a Long 3’ untranslated region. DNA Res 1999; 6: 357–360. [DOI] [PubMed] [Google Scholar]
- 20. Dy P, Penzo-Mendez A, Wang H, et al. The three Soxc proteins—Sox4, Sox11 and Sox12—exhibit overlapping expression patterns and molecular properties. Nucleic Acids Res 2008; 36: 3101–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weiss MA. Floppy Sox: mutual induced fit in hmg (High-Mobility Group) box-DNA recognition. Mol Endocrinol 2001; 15: 353–362. [DOI] [PubMed] [Google Scholar]
- 22. Van Beest M, Dooijes D, Van De Wetering M, et al. Sequence-specific high mobility group box factors recognize 10–12-base pair minor groove motifs. J Biol Chem 2000; 275: 27266–27273. [DOI] [PubMed] [Google Scholar]
- 23. Pontiggia A, Rimini R, Harley VR, et al. Sex-reversing mutations affect the architecture of SRY-DNA complexes. EMBO J 1994; 13: 6115–6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wiebe MS, Nowling TK, Rizzino A. Identification of novel domains within Sox-2 and Sox-11 involved in autoinhibition of DNA binding and partnership specificity. J Biol Chem 2003; 278: 17901–17911. [DOI] [PubMed] [Google Scholar]
- 25. Hoser M, Potzner MR, Koch JM, et al. Sox12 deletion in the mouse reveals nonreciprocal redundancy with the related Sox4 and Sox11 transcription factors. Mol Cell Biol 2008; 28: 4675–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balta EA, Wittmann MT, Jung M, et al. Phosphorylation modulates the subcellular localization of Sox11. Front Mol Neurosci 2018; 11: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Potzner MR, Tsarovina K, Binder E, et al. Sequential requirement of Sox4 and Sox11 during development of the sympathetic nervous system. Development 2010; 137: 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sock E, Rettig SD, Enderich J, et al. Gene targeting reveals a widespread role for the high-mobility-group transcription factor Sox11 in tissue remodeling. Mol Cell Biol 2004; 24: 6635–6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pamnani M, Sinha P, Nara S, et al. Study of promoter DNA methylation of Sox11 and its correlation with tissue-specific expression in the laboratory mouse. Gene 2014; 552: 133–139. [DOI] [PubMed] [Google Scholar]
- 30. Hargrave M, Wright E, Kun J, et al. Expression of the Sox11 gene in mouse embryos suggests roles in neuronal maturation and epithelio-mesenchymal induction. Dev Dyn 1997; 210: 79–86. [DOI] [PubMed] [Google Scholar]
- 31. Kuhlbrodt K, Herbarth B, Sock E, et al. Cooperative function of POU proteins and Sox proteins in glial cells. J Biol Chem 1998; 273: 16050–16057. [DOI] [PubMed] [Google Scholar]
- 32. Ek S, Dictor M, Jerkeman M, et al. Nuclear expression of the non B-cell lineage Sox11 transcription factor identifies mantle cell lymphoma. Blood 2008; 111: 800–805. [DOI] [PubMed] [Google Scholar]
- 33. Bergsland M, Werme M, Malewicz M, et al. The establishment of neuronal properties is controlled by Sox4 and Sox11. Genes Dev 2006; 20: 3475–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Usui A, Iwagawa T, Mochizuki Y, et al. Expression of Sox4 and Sox11 is regulated by multiple mechanisms during retinal development. FEBS Lett 2013; 587: 358–363. [DOI] [PubMed] [Google Scholar]
- 35. Usui A, Mochizuki Y, Iida A, et al. The early retinal progenitor-expressed gene Sox11 regulates the timing of the differentiation of retinal cells. Development 2013; 140: 740–750. [DOI] [PubMed] [Google Scholar]
- 36. Jiang Y, Ding Q, Xie X, et al. Transcription factors Sox4 and Sox11 function redundantly to regulate the development of mouse retinal ganglion cells. J Biol Chem 2013; 288: 18429–18438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haslinger A, Schwarz TJ, Covic M, et al. Expression of Sox11 in adult neurogenic niches suggests a stage-specific role in adult neurogenesis. Eur J Neurosci 2009; 29: 2103–2114. [DOI] [PubMed] [Google Scholar]
- 38. Lin L, Lee VM, Wang Y, et al. Sox11 regulates survival and axonal growth of embryonic sensory neurons. Dev Dyn 2011; 240: 52–64. [DOI] [PubMed] [Google Scholar]
- 39. Wang Y, Lin L, Lai H, et al. Transcription factor Sox11 is essential for both embryonic and adult neurogenesis. Dev Dyn 2013; 242: 638–653. [DOI] [PubMed] [Google Scholar]
- 40. Cao X, Fan L, Fang C, et al. The expression of Sox11, Cyclin D1, Cyclin D2, and Cyclin D3 in B-cell lymphocytic proliferative diseases. Med Oncol 2012; 29: 1190–1196. [DOI] [PubMed] [Google Scholar]
- 41. Gustavsson E, Sernbo S, Andersson E, et al. Sox11 expression correlates to promoter methylation and regulates tumor growth in hematopoietic malignancies. Mol Cancer 2010; 9: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chung W, Bondaruk J, Jelinek J, et al. Detection of bladder cancer using novel DNA methylation biomarkers in urine sediments. Cancer Epidemiol Biomarkers Prev 2011; 20: 1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weigle B, Ebner R, Temme A, et al. Highly specific overexpression of the transcription factor SOX11 in human malignant gliomas. Oncol Rep 2005; 13: 139–144. [PubMed] [Google Scholar]
- 44. Hide T, Takezaki T, Nakatani Y, et al. Sox11 prevents tumorigenesis of glioma-initiating cells by inducing neuronal differentiation. Cancer Res 2009; 69: 7953–7959. [DOI] [PubMed] [Google Scholar]
- 45. Stuart JE, Lusis EA, Scheck AC, et al. Identification of gene markers associated with aggressive meningioma by filtering across multiple sets of gene expression arrays. J Neuropathol Exp Neurol 2011; 70: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee CJ, Appleby VJ, Orme AT, et al. Differential expression of SOX4 and SOX11 in medulloblastoma. J Neurooncol 2002; 57: 201–214. [DOI] [PubMed] [Google Scholar]
- 47. Nygren L, Baumgartner WS, Klimkowska M, et al. Prognostic role of SOX11 in a population-based cohort of mantle cell lymphoma. Blood 2012; 119: 4215–4223. [DOI] [PubMed] [Google Scholar]
- 48. Palomero J, Vegliante MC, Rodriguez ML, et al. Sox11 promotes tumor angiogenesis through transcriptional regulation of PDGFA in mantle cell lymphoma. Blood 2014; 124: 2235–2247. [DOI] [PubMed] [Google Scholar]
- 49. Palomero J, Vegliante MC, Eguileor A, et al. SOX11 defines two different subtypes of mantle cell lymphoma through transcriptional regulation of BCL6. Leukemia 2016; 30: 1596–1599. [DOI] [PubMed] [Google Scholar]
- 50. Roisman A, Stanganelli C, Nagore VP, et al. SOX11 expression in chronic lymphocytic leukemia correlates with adverse prognostic markers. Tumour Biol 2015; 36: 4433–4440. [DOI] [PubMed] [Google Scholar]
- 51. Dictor M, Ek S, Sundberg M, et al. Strong lymphoid nuclear expression of SOX11 transcription factor defines lymphoblastic neoplasms, mantle cell lymphoma and Burkitt’s lymphoma. Haematologica 2009; 94: 1563–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang WM, Wang C, Li BZ. Expression of Sox11 transcription factor in different types of B-cell lymphomas. Zhonghua Bing Li Xue Za Zhi 2011; 40: 698–699. [PubMed] [Google Scholar]
- 53. Liu DT, Peng Z, Han JY, et al. Clinical and prognostic significance of SOX11 in breast cancer. Asian Pac J Cancer Prev 2014; 15: 5483–5486. [DOI] [PubMed] [Google Scholar]
- 54. Brennan DJ, Ek S, Doyle E, et al. The transcription factor Sox11 is a prognostic factor for improved recurrence-free survival in epithelial ovarian cancer. Eur J Cancer 2009; 45: 1510–1517. [DOI] [PubMed] [Google Scholar]
- 55. Yao Z, Sun B, Hong Q, et al. The role of tumor suppressor gene SOX11 in prostate cancer. Tumour Biol 2015; 36: 6133–6138. [DOI] [PubMed] [Google Scholar]
- 56. Teng Y, Fan YC, Mu NN, et al. Serum SOX11 promoter methylation is a novel biomarker for the diagnosis of hepatitis B virus-related hepatocellular carcinoma. Neoplasma 2016; 63: 419–426. [DOI] [PubMed] [Google Scholar]
- 57. Li P, Hu Y, Yi J, et al. Identification of potential biomarkers to differentially diagnose solid pseudopapillary tumors and pancreatic malignancies via a gene regulatory network. J Transl Med 2015; 13: 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Walter RF, Mairinger FD, Werner R, et al. SOX4, SOX11 and PAX6 mRNA expression was identified as a (prognostic) marker for the aggressiveness of neuroendocrine tumors of the lung by using next-generation expression analysis (NanoString). Future Oncol 2015; 11: 1027–1036. [DOI] [PubMed] [Google Scholar]
- 59. Jian J, Guoying W, Jing Z. Increased expression of sex determining region Y-box 11 (SOX11) in cutaneous malignant melanoma. J Int Med Res 2013; 41: 1221–1227. [DOI] [PubMed] [Google Scholar]
- 60. Misemer BS, Skubitz AP, Carlos Manivel J, et al. Expression of FAP, ADAM12, WISP1, and SOX11 is heterogeneous in aggressive fibromatosis and spatially relates to the histologic features of tumor activity. Cancer Med 2014; 3: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 62. Kuci V, Nordstrom L, Conrotto P, et al. Sox11 and Hig-2 are cross-regulated and affect growth in mantle cell lymphoma. Leuk Lymphoma 2016; 57: 1883–1892. [DOI] [PubMed] [Google Scholar]
- 63. Bjerkvig R, Tysnes BB, Aboody KS, et al. Opinion: the origin of the cancer stem cell: current controversies and new insights. Nat Rev Cancer 2005; 5: 899–904. [DOI] [PubMed] [Google Scholar]
- 64. Esteller M. Epigenetics in cancer. N Engl J Med 2008; 358: 1148–1159. [DOI] [PubMed] [Google Scholar]
- 65. Xin Z, Ma Z, Hu W, et al. Foxo1/3: potential suppressors of fibrosis. Ageing Res Rev 2018; 41: 42–52. [DOI] [PubMed] [Google Scholar]
- 66. Nordstrom L, Andersson E, Kuci V, et al. DNA methylation and histone modifications regulate Sox11 expression in lymphoid and solid cancer cells. BMC Cancer 2015; 15: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Queiros AC, Beekman R, Vilarrasa-Blasi R, et al. Decoding the DNA methylome of mantle cell lymphoma in the light of the entire B cell lineage. Cancer Cell 2016; 30: 806–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Beekman R, Amador V, Campo E. Sox11, a key oncogenic factor in mantle cell lymphoma. Curr Opin Hematol 2018; 25: 299–306. [DOI] [PubMed] [Google Scholar]
- 69. Bartel DP. Micrornas: genomics, biogenesis, mechanism, and function. Cell 2004; 116: 281–297. [DOI] [PubMed] [Google Scholar]
- 70. Haenisch S, Zhao Y, Chhibber A, et al. Sox11 identified by target gene evaluation of miRNAs differentially expressed in focal and non-focal brain tissue of therapy-resistant epilepsy patients. Neurobiol Dis 2015; 77: 127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Margue C, Philippidou D, Reinsbach SE, et al. New target genes of MITF-induced microRNA-211 contribute to melanoma cell invasion. PLoS One 2013; 8: e73473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Navarro A, Clot G, Prieto M, et al. MicroRNA expression profiles identify subtypes of mantle cell lymphoma with different clinicobiological characteristics. Clin Cancer Res 2013; 19: 3121–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fevr T, Robine S, Louvard D, et al. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol 2007; 27: 7551–7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Reichling T, Goss KH, Carson DJ, et al. Transcriptional profiles of intestinal tumors in Apc(Min) mice are unique from those of embryonic intestine and identify novel gene targets dysregulated in human colorectal tumors. Cancer Res 2005; 65: 166–176. [PubMed] [Google Scholar]
- 75. Hussenet T, Dali S, Exinger J, et al. Sox2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS One 2010; 5: e8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hartmann EM, Campo E, Wright G, et al. Pathway discovery in mantle cell lymphoma by integrated analysis of high-resolution gene expression and copy number profiling. Blood 2010; 116: 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hall MD, Murray CA, Valdez MJ, et al. Mesoderm-specific Stat3 deletion affects expression of Sox9 yielding Sox9-dependent phenotypes. PLoS Genet 2017; 13: e1006610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jiang S, Li T, Yang Z, et al. Deciphering the roles of foxo1 in human neoplasms. Int J Cancer 2018: [DOI] [PubMed] [Google Scholar]
- 79. Yang Z, Jiang S, Cheng Y, et al. Foxc1 in cancer development and therapy: deciphering its emerging and divergent roles. Ther Adv Med Oncol 2017; 9: 797–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pan D. The hippo signaling pathway in development and cancer. Dev Cell 2010; 19: 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang X, Bjorklund S, Wasik AM, et al. Gene expression profiling and chromatin immunoprecipitation identify DBN1, SETMAR and HIG2 as direct targets of SOX11 in mantle cell lymphoma. PLoS One 2010; 5: e14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wray J, Williamson EA, Sheema S, et al. Metnase mediates chromosome decatenation in acute leukemia cells. Blood 2009; 114: 1852–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jeyaratnam DC, Baduin BS, Hansen MC, et al. Delineation of known and new transcript variants of the SETMAR (Metnase) gene and the expression profile in hematologic neoplasms. Exp Hematol 2014; 42: 448–456 e444. [DOI] [PubMed] [Google Scholar]
- 84. Althaus J, Bernaudin M, Petit E, et al. Expression of the gene encoding the pro-apoptotic BNIP3 protein and stimulation of hypoxia-inducible factor-1alpha (HIF-1alpha) protein following focal cerebral ischemia in rats. Neurochem Int 2006; 48: 687–695. [DOI] [PubMed] [Google Scholar]
- 85. Mattson MP, Meffert MK. Roles for NF-KappaB in nerve cell survival, plasticity, and disease. Cell Death Differ 2006; 13: 852–860. [DOI] [PubMed] [Google Scholar]
- 86. Lv J, Jiang S, Yang Z, et al. PGC-1α sparks the fire of neuroprotection against neurodegenerative disorders. Ageing Res Rev 2018; 44: 8–21. [DOI] [PubMed] [Google Scholar]
- 87. Vegliante MC, Palomero J, Perez-Galan P, et al. SOX11 regulates PAX5 Expression and blocks terminal B-cell differentiation in aggressive mantle cell lymphoma. Blood 2013; 121: 2175–2185. [DOI] [PubMed] [Google Scholar]
- 88. Kuo PY, Jatiani SS, Rahman AH, et al. SOX11 augments BCR signaling to drive MCL-Like tumor development. Blood 2018; 131: 2247–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Korkolopoulou P, Levidou G, El-Habr EA, et al. Sox11 expression in astrocytic gliomas: correlation with nestin/C-Met/IDH1-R132H expression phenotypes, p-Stat-3 and survival. Br J Cancer 2013; 108: 2142–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Basso K, Dalla-Favera R. Roles of BCL6 in normal and transformed germinal center B cells. Immunol Rev 2012; 247: 172–183. [DOI] [PubMed] [Google Scholar]
- 91. Busslinger M, Klix N, Pfeffer P, et al. Deregulation of PAX-5 by Translocation of the Emu enhancer of the IgH locus adjacent to two alternative PAX-5 promoters in a diffuse large-cell lymphoma. Proc Natl Acad Sci U S A 1996; 93: 6129–6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Shaffer AL, Lin KI, Kuo TC, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 2002; 17: 51–62. [DOI] [PubMed] [Google Scholar]
- 93. Teo AE, Chen Z, Miranda RN, et al. Differential PAX5 levels promote malignant B-cell infiltration, progression and drug resistance, and predict a poor prognosis in MCL patients independent of CCND1. Leukemia 2016; 30: 580–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jaggupilli A, Elkord E. Significance of CD44 and CD24 as cancer stem cell markers: an enduring ambiguity. Clin Dev Immunol 2012; 2012: 708036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kalluri R, Weinberg RA. The basics of epithelial–mesenchymal transition. J Clin Invest 2009; 119: 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Venkov C, Plieth D, Ni T, et al. Transcriptional networks in epithelial–mesenchymal transition. PLoS One 2011; 6: e25354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tiwari N, Tiwari VK, Waldmeier L, et al. Sox4 is a master regulator of epithelial–mesenchymal transition by controlling EZH2 expression and epigenetic reprogramming. Cancer Cell 2013; 23: 768–783. [DOI] [PubMed] [Google Scholar]
- 98. Balsas P, Palomero J, Eguileor A, et al. SOX11 promotes tumor protective microenvironment interactions through CXCR4 and FAK regulation in mantle cell lymphoma. Blood 2017; 130: 501–513. [DOI] [PubMed] [Google Scholar]
- 99. Bea S, Amador V. Role of Sox11 and genetic events cooperating with cyclin D1 in mantle cell lymphoma. Curr Oncol Rep 2017; 19: 43. [DOI] [PubMed] [Google Scholar]
- 100. Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature 2000; 407: 242–248. [DOI] [PubMed] [Google Scholar]
- 101. Dong J, Grunstein J, Tejada M, et al. VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J 2004; 23: 2800–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ding W, Knox TR, Tschumper RC, et al. Platelet-derived growth factor (PDGF)-PDGF receptor interaction activates bone marrow-derived mesenchymal stromal cells derived from chronic lymphocytic leukemia: implications for an angiogenic switch. Blood 2010; 116: 2984–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Conrotto P, Andreasson U, Kuci V, et al. Knock-down of Sox11 induces autotaxin-dependent increase in proliferation in vitro and more aggressive tumors in vivo. Mol Oncol 2011; 5: 527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Berndt JD, Moon RT. Cell biology. Making a point with Wnt signals. Science 2013; 339: 1388–1389. [DOI] [PubMed] [Google Scholar]
- 105. Jiang S, Li T, Yang Z, et al. AMPK orchestrates an elaborate cascade protecting tissue from fibrosis and aging. Ageing Res Rev 2017; 38: 18–27. [DOI] [PubMed] [Google Scholar]
- 106. Taketo MM. Shutting down Wnt signal-activated cancer. Nat Genet 2004; 36: 320–322. [DOI] [PubMed] [Google Scholar]
- 107. Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 2003; 1653: 1–24. [DOI] [PubMed] [Google Scholar]
- 108. Gelebart P, Anand M, Armanious H, et al. Constitutive activation of the Wnt canonical pathway in mantle cell lymphoma. Blood 2008; 112: 5171–5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kimura Y, Arakawa F, Kiyasu J, et al. The Wnt signaling pathway and mitotic regulators in the initiation and evolution of mantle cell lymphoma: gene expression analysis. Int J Oncol 2013; 43: 457–468. [DOI] [PubMed] [Google Scholar]
- 110. Ishitani T, Ninomiya-Tsuji J, Matsumoto K. Regulation of lymphoid enhancer factor 1/T-cell factor by mitogen-activated protein kinase-related Nemo-like kinase-dependent phosphorylation in Wnt/beta-catenin signaling. Mol Cell Biol 2003; 23: 1379–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Labbe E, Letamendia A, Attisano L. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-beta and Wnt pathways. Proc Natl Acad Sci U S A 2000; 97: 8358–8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhang M, Wang M, Tan X, et al. Smad3 prevents beta-catenin degradation and facilitates beta-catenin nuclear translocation in chondrocytes. J Biol Chem 2010; 285: 8703–8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kuo PY, Leshchenko VV, Fazzari MJ, et al. High-resolution chromatin immunoprecipitation (ChIP) sequencing reveals novel binding targets and prognostic role for Sox11 in mantle cell lymphoma. Oncogene 2015; 34: 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gimm T, Wiese M, Teschemacher B, et al. Hypoxia-inducible protein 2 is a novel lipid droplet protein and a specific target gene of hypoxia-inducible factor-1. FASEB J 2010; 24: 4443–4458. [DOI] [PubMed] [Google Scholar]
- 115. Kenny PA, Enver T, Ashworth A. Receptor and secreted targets of Wnt-1/beta-catenin signalling in mouse mammary epithelial cells. BMC Cancer 2005; 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Leoncini L, Bellan C, De Falco G. Retinoblastoma gene family expression in lymphoid tissues. Oncogene 2006; 25: 5309–5314. [DOI] [PubMed] [Google Scholar]
- 117. Dominguez-Brauer C, Brauer PM, Chen YJ, et al. Tumor Suppression by ARF: gatekeeper and caretaker. Cell Cycle 2010; 9: 86–89. [DOI] [PubMed] [Google Scholar]
- 118. Schuldt A. Cell cycle: E2F1 ensures the endocycle. Nat Rev Mol Cell Biol 2011; 12: 768. [DOI] [PubMed] [Google Scholar]
- 119. Shats I, Deng M, Davidovich A, et al. Expression level is a key determinant of E2F1-mediated cell fate. Cell Death Differ 2017; 24: 626–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ek S, Bjorck E, Hogerkorp CM, et al. Mantle cell lymphomas acquire increased expression of CCL4, CCL5 and 4–1BB-L implicated in cell survival. Int J Cancer 2006; 118: 2092–2097. [DOI] [PubMed] [Google Scholar]
- 121. Liu S, Umezu-Goto M, Murph M, et al. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell 2009; 15: 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature 2001; 414: 105–111. [DOI] [PubMed] [Google Scholar]
- 123. Spengler D, Villalba M, Hoffmann A, et al. Regulation of apoptosis and cell cycle arrest by zac1, a novel zinc finger protein expressed in the pituitary gland and the brain. EMBO J 1997; 16: 2814–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Valente T, Auladell C. Expression pattern of Zac1 mouse gene, a new zinc-finger protein that regulates apoptosis and cellular cycle arrest, in both adult brain and along development. Mech Dev 2001; 108: 207–211. [DOI] [PubMed] [Google Scholar]
- 125. Kondo T. Mouse induced glioma-initiating cell models and therapeutic targets. Anticancer Agents Med Chem 2010; 10: 471–480. [DOI] [PubMed] [Google Scholar]
- 126. Su Z, Zang T, Liu ML, et al. Reprogramming the fate of human glioma cells to impede brain tumor development. Cell Death Dis 2014; 5: e1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Sun Y, Nadal-Vicens M, Misono S, et al. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell 2001; 104: 365–376. [DOI] [PubMed] [Google Scholar]
- 128. Guichet PO, Bieche I, Teigell M, et al. Cell death and neuronal differentiation of glioblastoma stem-like cells induced by neurogenic transcription factors. Glia 2013; 61: 225–239. [DOI] [PubMed] [Google Scholar]
- 129. Santra M, Santra S, Roberts C, et al. doublecortin induces mitotic microtubule catastrophe and inhibits glioma cell invasion. J Neurochem 2009; 108: 231–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Li T, Jiang S, Yang Y. Database selection and heterogeneity-more details, more credibility. JAMA Oncol 2018; 4: 1295. [DOI] [PubMed] [Google Scholar]
- 131. Islam TC, Asplund AC, Lindvall JM, et al. High level of cannabinoid receptor 1, absence of regulator of G protein signalling 13 and differential expression of Cyclin D1 in mantle cell lymphoma. Leukemia 2003; 17: 1880–1890. [DOI] [PubMed] [Google Scholar]
- 132. Mozos A, Royo C, Hartmann E, et al. Sox11 expression is highly specific for mantle cell lymphoma and identifies the Cyclin D1-negative subtype. Haematologica 2009; 94: 1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hsiao SC, Cortada IR, Colomo L, et al. SOX11 is useful in differentiating cyclin D1-positive diffuse large B-cell lymphoma from mantle cell lymphoma. Histopathology 2012; 61: 685–693. [DOI] [PubMed] [Google Scholar]
- 134. Gradowski JF, Sargent RL, Craig FE, et al. Chronic lymphocytic leukemia/small lymphocytic lymphoma with cyclin D1 positive proliferation centers do not have CCND1 translocations or gains and lack SOX11 expression. Am J Clin Pathol 2012; 138: 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wasik AM, Priebe V, Lord M, et al. Flow cytometric analysis of SOX11: a new diagnostic method for distinguishing B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma from mantle cell lymphoma. Leuk Lymphoma 2015; 56: 1425–1431. [DOI] [PubMed] [Google Scholar]
- 136. Lord M, Wasik AM, Christensson B, et al. The utility of mRNA analysis in defining SOX11 expression levels in mantle cell lymphoma and reactive lymph nodes. Haematologica 2015; 100: e369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Fernandez V, Salamero O, Espinet B, et al. Genomic and gene expression profiling defines indolent forms of mantle cell lymphoma. Cancer Res 2010; 70: 1408–1418. [DOI] [PubMed] [Google Scholar]
- 138. Czapiewski P, Gorczynski A, Radecka K, et al. Expression of SOX11, PAX5, TTF-1 and ISL-1 in medulloblastoma. Pathol Res Pract 2016; 212: 965–971. [DOI] [PubMed] [Google Scholar]
- 139. De Bont JM, Kros JM, Passier MM, et al. Differential expression and prognostic significance of sox genes in pediatric medulloblastoma and ependymoma identified by microarray analysis. Neuro Oncol 2008; 10: 648–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Tosic N, Petrovic I, Grujicic NK, et al. Prognostic significance of SOX2, SOX3, SOX11, SOX14 and SOX18 gene expression in adult de novo acute myeloid leukemia. Leuk Res 2018; 67: 32–38. [DOI] [PubMed] [Google Scholar]
- 141. Wang X, Asplund AC, Porwit A, et al. The subcellular Sox11 distribution pattern identifies subsets of mantle cell lymphoma: correlation to overall survival. Br J Haematol 2008; 143: 248–252. [DOI] [PubMed] [Google Scholar]
- 142. Ondrejka SL, Lai R, Smith SD, et al. Indolent mantle cell leukemia: a clinicopathological variant characterized by isolated lymphocytosis, interstitial bone marrow involvement, kappa light chain restriction, and good prognosis. Haematologica 2011; 96: 1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nordstrom L, Sernbo S, Eden P, et al. SOX11 and TP53 add prognostic information to MIPI in a homogenously treated cohort of mantle cell lymphoma—a Nordic Lymphoma group Study. Br J Haematol 2014; 166: 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Aukema SM, Hoster E, Rosenwald A, et al. Expression of TP53 is associated with the outcome of MCL independent of MIPI and Ki-67 in Trials of the European MCL Network. Blood 2018; 131: 417–420. [DOI] [PubMed] [Google Scholar]
- 145. Dreyling M, Kluin-Nelemans HC, Bea S, et al. Update on the molecular pathogenesis and clinical treatment of mantle cell lymphoma: report of the 10th annual conference of the european mantle cell lymphoma network. Leuk Lymphoma 2011; 52: 2226–2236. [DOI] [PubMed] [Google Scholar]
- 146. Navarro A, Clot G, Royo C, et al. Molecular subsets of mantle cell lymphoma defined by the IGHV mutational status and SOX11 expression have distinct biologic and clinical features. Cancer Res 2012; 72: 5307–5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016; 127: 2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Sander B, Quintanilla-Martinez L, Ott G, et al. Mantle cell lymphoma—a spectrum from indolent to aggressive disease. Virchows Arch 2016; 468: 245–257. [DOI] [PubMed] [Google Scholar]
- 149. Royo C, Navarro A, Clot G, et al. Non-nodal type of mantle cell lymphoma is a specific biological and clinical subgroup of the disease. Leukemia 2012; 26: 1895–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Clot G, Jares P, Gine E, et al. A gene signature that distinguishes conventional and leukemic nonnodal mantle cell lymphoma helps predict outcome. Blood 2018; 132: 413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhang LN, Cao X, Lu TX, et al. Polyclonal antibody targeting SOX11 cannot differentiate mantle cell lymphoma from B-cell non-Hodgkin lymphomas. Am J Clin Pathol 2013; 140: 795–800. [DOI] [PubMed] [Google Scholar]
- 152. Nordstrom L, Andreasson U, Jerkeman M, et al. Expanded clinical and experimental use of SOX11 - using a monoclonal antibody. BMC Cancer 2012; 12: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Soldini D, Valera A, Sole C, et al. Assessment of SOX11 expression in routine lymphoma tissue sections: characterization of new monoclonal antibodies for diagnosis of mantle cell lymphoma. Am J Surg Pathol 2014; 38: 86–93. [DOI] [PubMed] [Google Scholar]
- 154. Romaguera JE, Fayad LE, Feng L, et al. Ten-year follow-up after intense chemoimmunotherapy with Rituximab-HyperCVAD alternating with Rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br J Haematol 2010; 150: 200–208. [DOI] [PubMed] [Google Scholar]
- 155. Kartha RV, Sundram UN. Silent mutations in KIT and PDGFRA and coexpression of receptors with SCF and PDGFA in Merkel cell carcinoma: implications for tyrosine kinase-based tumorigenesis. Mod Pathol 2008; 21: 96–104. [DOI] [PubMed] [Google Scholar]
- 156. Darnell JE., Jr. Transcription factors as targets for cancer therapy. Nat Rev Cancer 2002; 2: 740–749. [DOI] [PubMed] [Google Scholar]
- 157. Ma Z, Xin Z, Hu W, et al. Forkhead box O proteins: crucial regulators of cancer EMT. Semin Cancer Biol 2018; 50: 21–31. [DOI] [PubMed] [Google Scholar]
- 158. Jiang S, Yang Z, Di S, et al. Novel role of forkhead box O 4 transcription factor in cancer: bringing out the good or the bad. Semin Cancer Biol 2018; 50: 1–12. [DOI] [PubMed] [Google Scholar]
- 159. Royo C, Salaverria I, Hartmann EM, et al. The complex landscape of genetic alterations in mantle cell lymphoma. Semin Cancer Biol 2011; 21: 322–334. [DOI] [PubMed] [Google Scholar]
- 160. Bea S, Valdes-Mas R, Navarro A, et al. Landscape of somatic mutations and clonal evolution in mantle cell lymphoma. Proc Natl Acad Sci U S A 2013; 110: 18250–18255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Moreno CS. The sex-determining region Y-Box 4 and homeobox C6 transcriptional networks in prostate cancer progression: crosstalk with the Wnt, Notch, and Pi3K Pathways. Am J Pathol 2010; 176: 518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]