Abstract
Background:
Magnetic resonance imaging (MRI) signal intensity (SI) measurements are being used increasingly in both clinical and research studies to assess the maturity of anterior cruciate ligament (ACL) grafts in humans. However, SI in conventional MRI with weighted images is a nonquantitative measure dependent on hardware and software.
Purpose:
To conduct a systematic review of studies that have used MRI SI as a proxy for ACL graft maturity and to identify potential confounding factors in assessing the ACL graft in conventional MRI studies.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
A systematic review was conducted by searching the MEDLINE/PubMed, Scopus, and Cochrane Library electronic databases according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines to identify studies that examined the healing of the intra-articular portion of the ACL graft by assessing SI on MRIs.
Results:
A total of 34 studies were selected for inclusion in this systematic review. The MRI acquisition techniques and methods to evaluate the ACL graft SI differed greatly across the studies. No agreement was found regarding the time frames of SI changes in MRI reflecting normal healing of the ACL tendon graft, and the graft SI and clinical outcomes after ACL reconstruction were found to be poorly correlated.
Conclusion:
The MRI acquisition and evaluation methods used to assess ACL grafts are very heterogeneous, impeding comparisons of SI between successive scans and between independent studies. Therefore, quantitative MRI-based biomarkers of ACL graft healing are greatly needed to guide the appropriate time of returning to sports after ACL reconstruction.
Keywords: anterior cruciate ligament, anterior cruciate ligament reconstruction, graft healing, signal intensity, magnetic resonance imaging
The biological process that occurs during normal graft healing after anterior cruciate ligament (ACL) reconstruction in the human knee remains incompletely understood.10,13,16,27,46 In general, 3 stages of graft maturity are described: an early inflammatory stage, a revascularization stage, and a late remodeling stage.10 The minimum time required postoperatively for the ACL graft to reach full maturity in humans is unknown.10 To date, objective guidelines permitting safe return to full sports activity following ACL reconstruction are lacking.7,22
Previous animal research has found magnetic resonance imaging (MRI) to be useful in predicting the biomechanical and histologic properties of an ACL graft after ACL reconstruction.6,18,57 MRI signal intensity (SI) measurements are also being used increasingly in clinical studies to assess maturity of the ACL graft in humans.21 In the 1990s, Howell et al26 were among the first to propose an MRI grading system that categorized the signal measured in the ACL graft as low, intermediate, or high, where the lower the SI, the more “mature” the reconstructed graft was assumed to be. However, SI in conventional “weighted” MRIs is expressed in relative units that are dependent on acquisition parameters and scanner characteristics. Therefore, SI does not enable absolute quantification of biophysical tissue properties.3,11 In an attempt to normalize the graft SI grayscale value, many studies have used the signal-to-noise quotient (SNQ) to assess graft maturity via MRI,¶ a method first proposed by Stockle et al.53 Unfortunately, routine methods commonly used to measure SI with single-coil imaging setups are no longer valid when multichannel coils and parallel imaging methods are used.12
The orientational variation in MRI signals, which is referred to as the magic angle effect (MAE), should also be considered when evaluating the ACL graft.14,19 Generally, the variety of situations in which a significant MAE can be seen is underestimated.47 Taken together, the technical dependencies of the MRI-based SI need to be taken into account for accurate image interpretation and comparison across studies. The purpose of this study was to conduct a systematic review of the studies that have used MRI SI as a proxy for ACL graft maturity and identify potential confounding factors in assessing the ACL graft in conventional MRI studies.
Methods
Search Criteria
A systematic review of the published literature was conducted in adherence with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. A literature search was independently performed by 2 authors (P.V.D. and K.Z.) across 3 electronic databases (MEDLINE/PubMed, Scopus, and Cochrane Library) for articles published from database inception until August 8, 2018, using combinations of the following search terms: ((((“Anterior Cruciate Ligament”[Mesh]) OR (“anterior cruciate” OR ACL))) AND ((“Magnetic Resonance Imaging”[Mesh]) OR (“magnetic resonance imaging” OR MRI OR MR))) AND (graft* OR autograft* OR allograft*). After the duplicates were removed, all identified articles were screened by title and abstract. Inclusion criteria were patients undergoing primary single-bundle ACL reconstruction, clinical follow-up, and MRI follow-up. Studies were included only if they examined normal healing of the intra-articular ACL graft by SI in conventional MRI. Exclusion criteria were case reports, review articles, congress abstracts and proceedings, biomechanical studies, nonhuman or cadaveric studies, and non-English publications. Studies were also excluded if they included patients undergoing double- or selective-bundle ACL reconstruction; examined patients by use of advanced MRI techniques (eg, magnetic resonance angiography, ultrashort echo-time imaging, diffusion MRI); or focused on graft failure, bone tunnel healing, or bony morphometry of the knee (eg, posterior tibial slope and dimensions of the femoral notch). Studies were also excluded if more than 20% of the study population had an unstable knee at clinical follow-up. Based on the above-mentioned inclusion and exclusion criteria, a population, intervention, (comparison), outcome (PI(C)O) model was designed to facilitate the relevant literature search. The authors reviewed the full texts of the articles that remained after screening by title and abstract for final inclusion using the PI(C)O model. Discrepancies were discussed and overcome by consensus. Additionally, reference lists from the included studies were reviewed and reconciled to verify that all eligible articles were considered.
Data Extraction
The 2 independent reviewers subsequently completed data extraction. Data extracted from each full-text article included the first author’s name, journal title, year of publication, study level of evidence, number of study participants undergoing follow-up MRI scan, type of graft used, MRI follow-up time points, MRI scan and sequence parameters, method of SI measurement, and reported correlations between clinical/patient-reported outcome measures and MRI findings (if available). Level of evidence was assigned through use of the criteria published by Marx et al37 in 2015, adapted from the 2011 Oxford Centre for Evidence-Based Medicine Working Group. The risk of bias of all the included studies was graded according to the Cochrane risk-of-bias tool for randomized controlled trials and the Newcastle-Ottawa quality assessment scale for cohort and case-control studies.
Results
Literature Search
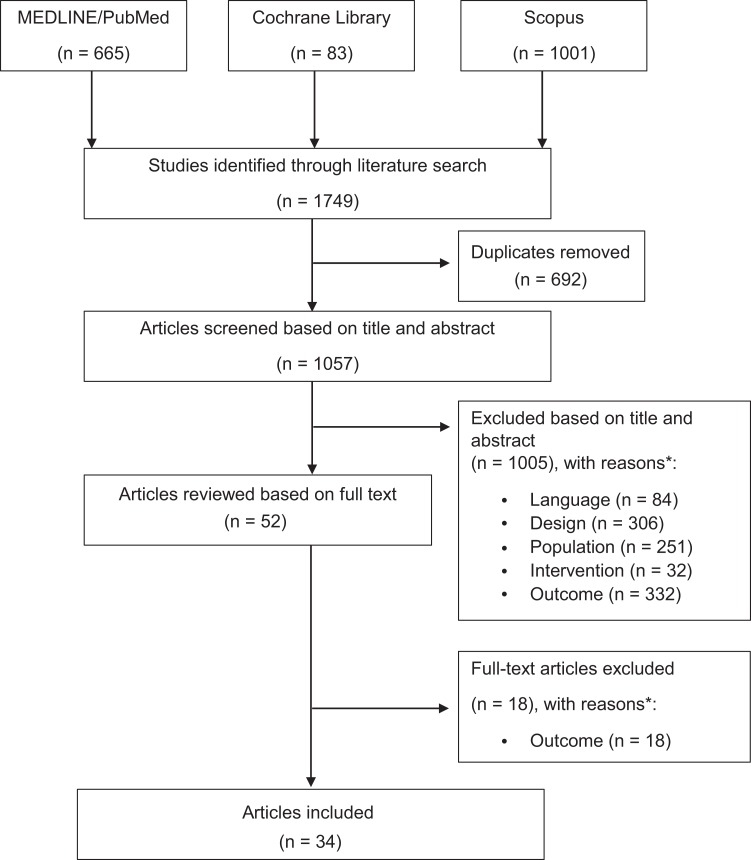
The initial search identified a total of 1749 articles. After duplicates were removed, titles and abstracts were reviewed for relevance, and inclusion and exclusion criteria were applied, a total of 34 articles were considered eligible for this systematic review (Figure 1).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Meta-Analyses) flowchart article selection. *Based on the inclusion criteria [population, intervention, (comparison), outcome model; PI(C)O model].
The publication dates ranged from 1991 to 2018. The study quality was rated as good in 13 studies,# fair in 4 studies,1,5,17,54 and poor in 3 studies.28,40,49 The main characteristics of the included studies are summarized in Table 1, and the MRI parameters are summarized in Table 2.
TABLE 1.
Characteristics of the Included Studiesa
| Lead Author (Year) | LOE | Patients, nb | Graft Type | MRI Follow-up |
|---|---|---|---|---|
| Hofbauer (2019)23 | 2 | 62 | HT autograft | 6 mo |
| Chen (2018)9 | 3 | 48 | HT autograft (n = 28) + allograft (n = 20) | 3/6/12 mo |
| Liu (2018)35 | 2 | 37 | HT autograft | 3/6/12/24 mo |
| Cavaignac (2018)8 | 2 | 62 | HT autograft | 12 mo |
| Rose (2017)48 | 2 | 32 | HT allograft (n = 16) + TA allograft (n = 16) | 6 mo |
| Sim (2018)52 | 3 | 64 | HT autograft | 24 mo |
| Lee (2017)30 | 2 | 73 | NA | 12 mo |
| Ahn (2017)1 | 3 | 81 | HT autograft | 6 mo |
| Li (2017)31 | 3 | 38 | HT autograft (n = 21) + TA allograft (n = 17) | 3/6/12 mo |
| Ruffilli (2016)49 | 2 | 40 | HT autograft | 6 mo |
| Li (2014)32 | 4 | 104 | HT autograft (n = 42) + TA allograft (n = 62) | 30 mo (range, 12-114 mo) |
| Biercevicz (2015)5 | 3 | 16 | BPTB autograft (n = 11) + HT (n = 5) | 36/60 mo |
| Valenti Azcarate (2014)54 | 2 | 150 | BPTB allograft | 6 mo |
| Ntoulia (2013)45 | 4 | 50 | BPTB autograft | 3 d/6 mo/12 mo/24 mo |
| Mutsuzaki (2012)41 | 1 | 57 | HT autograft | 12 mo |
| Li (2012)33 | 3 | 52 | TA allograft (n = 30) + HT autograft (n = 22) | 30 mo |
| Ntoulia (2011)44 | 4 | 32 | BPTB autograft | 3 d/6 mo/12 mo |
| Figueroa (2010)17 | 3 | 50 | HT autograft | 6 mo |
| Nin (2009)43 | 1 | 100 | BPTB allograft | 6 mo |
| Endele (2009)15 | 1 | 40 | BPTB autograft | 24 mo |
| Saupe (2008)50 | 4 | 47 | BPTB autograft | 80 mo (range, 52-144 mo) |
| Muramatsu (2008)40 | 3 | 44 | BPTB allograft (n = 24) + autograft (n = 20) | 1/4/6/12 mo |
| Lee (2007)29 | 4 | 59 | QT autograft | 12 mo (range, 3-31 mo) |
| Gohil (2007)20 | 2 | 46 | HT autograft | 2/6/12 mo |
| Hong (2005)24 | 4 | 29 | BPTB autograft (n = 15) + HT autograft (n = 13) + allograft BPTB (n = 1) | 12 mo (range, 6-13 mo) |
| Min (2001)38 | 4 | 23 | BPTB autograft | 2/3/6/12 mo |
| Vogl (2001)56 | 4 | 68 | BPTB autograft | 2 wk/3 mo/12 mo/18 mo/24 mo |
| Jansson (2001)28 | 2 | 20 | BPTB autograft (n = 10) + HT autograft (n = 10) | 24 mo |
| Nakayama (2001)42 | 4 | 54 | HT autograft | 5/12/24 mo |
| Stockle (1998)53 | 4 | 20 | BPTB autograft | 2 wk/3 mo/6 mo/12 mo/24 mo |
| Murakami (1998)39 | 4 | 44 | HT autograft | 12 mo (range, 1-54 mo) |
| Howell (1995)26 | 4 | 45 | HT autograft | 6 mo (range, 1-12 mo) |
| Yamato (1992)59 | 4 | 15 | BPTB autograft | 9 mo (range, 3-36 mo) |
| Autz (1991)2 | 4 | 20 | BPTB autograft | 10 mo (range, 1-18 mo) |
aBPTB, bone–patellar tendon–bone; HT, hamstring tendon; LOE, level of evidence; MRI, magnetic resonance imaging; NA, not available; QT, quadriceps tendon; TA, tibialis anterior.
bNumber refers to patients who underwent MRI scan.
TABLE 2.
MRI Parameters of the Included Studiesa
| Lead Author (Year) | Magnet Strength, T | Coil | Sequence/Weighting | TR | TE | Method |
|---|---|---|---|---|---|---|
| Hofbauer (2019)23 | 1.5 | NA | T2 FS | 5000 | 102 | SNQ = SI (graft-PCL)/bg |
| Chen (2018)9 | 3.0 | NA | PD FS | NA | NA | SNQ = SI (graft-PCL)/bg |
| Liu (2018)35 | 3.0 | NA | PD FS | 3000 | 28 | SNQ = SI (graft-QT)/bg |
| Cavaignac (2018)8 | 3.0 | Knee coil, 15-ch | 3D TSE PD | NA | NA | SNQ = SI (graft-PCL)/bg |
| Rose (2017)48 | 1.5 | NA | T2 FS | 3000 | 40 | SNQ = SI (graft-QT)/bg |
| Sim (2018)52 | 1.5 | NA | T2 | NA | NA | SI (graft/PCL) |
| Lee (2017)30 | 3.0 | NA | T2 | NA | NA | Subjective |
| Ahn (2017)1 | 3.0 | NA | NA | NA | NA | SNQ = SI (graft-QT)/bg |
| Li (2017)31 | 3.0 | NA | PD FS | 3000 | 28 | SNQ = SI (graft-QT)/bg |
| Ruffilli (2016)49 | 1.5 | Knee coil, 8-ch | TSE PD FS | 3500 | 43 | Subjective |
| Li (2014)32 | 3.0 | NA | PD FS | 3000 | 28 | SNQ = SI (graft-QT)/bg |
| Biercevicz (2015)5 | 3.0 | Knee coil | 3D FLASH T1 | 20 | 7.6 | SI graft/bone |
| Valenti Azcarate (2014)54 | NA | NA | PD and T2 | NA | NA | SI graft |
| Ntoulia (2013)45 | 1.5 | NA | TSE T1 Gd-DTPA | 500 | 17 | SNR = SI graft/SD bg |
| Mutsuzaki (2012)41 | 1.5 | NA | PD | NA | NA | Subjective |
| Li (2012)33 | 3.0 | NA | TSE PD FS | 3000 | 28 | SNQ = SI (graft-QT)/bg |
| Ntoulia (2011)44 | 1.5 | NA | 3D SPGR FS Gd-DTPA | 32 | 5.1 | SNR = SI graft/SD bg |
| Figueroa (2010)17 | 1.5 | NA | T2 | NA | NA | Subjective |
| Nin (2009)43 | NA | NA | PD and T2 | NA | NA | SI graft |
| Endele (2009)15 | 0.2 | NA | TSE T2 | NA | NA | Subjective |
| Saupe (2008)50 | 1.5 | Knee coil, 8-ch | TSE PD and T2 FS | 2000/4100 | 15/75 | Subjective |
| Muramatsu (2008)40 | 1.0 | NA | T1 Gd-DTPA | 517 | 17 | SNQ = SI (graft-QT)/bg |
| Lee (2007)29 | 1.5 | Knee coil | TSE PD FS and T2 | NA | NA | SI graft |
| Gohil (2007)20 | 1.5 | Knee coil | TSE PD | 3000 | 30 | SNR = SI graft/bg |
| Hong (2005)24 | 1.5 | NA | TSE PD and T2 | 2000 | 20/80 | Subjective |
| Min (2001)38 | 1.5 | NA | TSE PD and T2 | 20 | 70 | SI graft |
| Vogl (2001)56 | 1.5 | NA | SE T1 Gd-DTPA | 800 | 15 | SNQ = SI (graft-QT)/bg |
| Jansson (2001)28 | 1.5 | NA | SE T1 Gd-DTPA | 500 | 12 | Subjective |
| Nakayama (2001)42 | 0.5 | NA | 3D FFE | 51 | 14 | Subjective |
| Stockle (1998)53 | 1.5 | Knee coil | SE T2 and T1 Gd-DTPA | NA | NA | SNQ = SI (graft-PT)/bg |
| Murakami (1998)39 | 0.2 | Knee coil | SE PD and T2 | 2000 | 38/100 | Subjective |
| Howell (1995)26 | 1.5 | Knee coil | SE T1 Gd-DTPA | 618 | 22 | Subjective |
| Yamato (1992)59 | 1.5 | Knee coil | SE T1 | 800 | 15 | Subjective |
| Autz (1991)2 | 1.5 | Knee coil | SE PD and T2 | 2000 | 30/80 | Subjective |
abg, background; ch, channel; FFE, fast field echo; FLASH, fast low angle shot; FS, fat-suppressed; Gd-DTPA, gadolinium-diethylenetriamine penta-acetic acid; MRI, magnetic resonance imaging; NA, not available; PCL, posterior cruciate ligament; PD, proton density; PT, patellar tendon; QT, quadriceps tendon; SE, spin echo; SI, signal intensity; SNQ, signal-to-noise quotient; SNR, signal-to-noise ratio; SPGR, spoiled gradient recalled; T, tesla; TE, time-to-echo; TR, time-to-repetition; (T)SE, (turbo) spin echo; 3D, 3-dimensional.
Graft Type
Regarding graft type, 24 studies used autograft tendon for ACL reconstruction, 3 studies43,48,54 used allograft, and 6 studies9,24,31,32,33,40 used both auto- and allografts. One study did not specify the ACL graft type.30
Time Interval Between Surgery and MRI
The time interval between surgery and MRI ranged from 3 days to 144 months. MRI assessment of the ACL graft was performed within 24 months postoperatively in 27 studies. Of these, 11 studies collected longitudinal MRI follow-up data.**
MRI Technique
Magnetic field strength (in teslas) ranged from 0.2 to 3.0 T (0.2 T, n = 2; 0.5 T, n = 1; 1.0 T, n = 1; 1.5 T, n = 19; 3.0 T, n = 9). The magnetic field strength was not specified in 2 studies.43,54 A dedicated knee coil was used in 11 studies, only 3 of which mentioned the number of channels used (8 or 15 channels).8,49,50 The type of coil used was not specified in 23 studies. Of the 34 included studies, 20 studies mentioned the type of MRI sequence used (turbo spin-echo [TSE], n = 10,†† conventional spin-echo [SE], n = 7,‡‡ and gradient-echo [GRE], n = 35,42,44), whereas 13 studies§§ mentioned only the type of contrast-weighting. One study1 mentioned neither the MRI sequence nor the type of contrast used. Most of the included studies (24/34) used proton-density (PD) and/or T2-weighted contrast. However, 4 of the included studies used 3-dimensional (3D) MRI data acquisition (GRE T1-weighted, n = 2,5,44 GRE T2*-weighted, n = 1,42 and TSE PD-weighted, n = 18). Further, 7 of the included studies26,28,40,44,45,53,56 used T1-weighted gadolinium-diethylenetriamine penta-acetic acid [DTPA]-enhanced MRI to evaluate revascularization of the ACL graft.
Methods to Evaluate the Graft SI on MRI
We found that 13 studies evaluated the MRI signal of the ACL graft subjectively using adjacent tissue (gastrocnemius tendon,30 semimembranosus tendon,17,49 posterior cruciate ligament,28 fat tissue,26 not specified2,15,24,39,41,42,50,59) as a reference. Further, 21 studies used the mean intensity of a region of interest (ROI) to estimate the graft SI; this was done on a single image in all cases. The following measures were used to evaluate the SI:
-
1. SNQ = SIgraft – SIquadriceps tendon/SIbackground (n = 81,31–33,35,40,48,56)
= SIgraft – SIposterior cruciate ligament/SIbackground (n = 38,9,23)
= SIgraft – SIpatellar tendon/SIbackground (n = 153)
2. Signal-to-noise ratio (SNR) = SIgraft/SDbackground (n = 244,45)
3. SNR = SIgraft/SIbackground (n = 120)
4. SIgraft/SIposterior cruciate ligament (n = 152)
5. SIgraft/SIbone (n = 15)
Graft Healing
Longitudinal MRI assessment of ACL graft healing within 24 months postoperatively was reported in 11 studies, with all but 1 study42 using ROI analysis to determine the graft SI. Of these studies, 8 (6 of which used SNQ analysis9,31,35,40,53,56) found an initial increase in the graft SI, peaking at 6 months postoperatively, followed by a decrease in SI.9,20,31,35,40,45,53,56 In contrast, 3 studies38,42,44 (none of them using SNQ analysis) found a tendency of the graft signal to increase beyond 12 months postoperatively. A further 6 studies2,24,26,39,50,59 using subjective analysis and 2 studies29,32 using ROI analysis compared MRI SI of grafts with different maturities. Of these, 4 studies24,32,39,59 found graft SI to decrease with postoperative time, and 4 studies2,26,29,50 found no relation between graft SI and postoperative time.
Gadolinium-DTPA-Enhanced MRI
Gadolinium-DTPA-enhanced MRI was used in 7 studies to determine the progress of ACL graft revascularization changes over time. Of the 7 studies, 5 studies (3 of them using SNQ40,53,56 and 2 using SNR44,45 measurements) reported vascularity to be present in and around the ACL graft. In contrast, 2 studies26,28 (both using subjective analysis) found the unimpinged human ACL graft to remain hypovascular during the first 2 years of implantation.
Autograft vs Allograft Healing
In 5 studies,9,31–33,40 healing was compared between ACL autografts and allografts by use of SNQ analysis. Generally, these studies found higher SI in allograft tendons between 12 and 30 months postoperatively, suggesting inferior graft maturity. Of these studies, 2 studies9,31 (both using PD-weighted MRI sequences) found an increase in the graft SI until 6 months postoperatively, followed by a signal decrease for both auto- and allograft tendons at longitudinal MRI follow-up. In contrast, Muramatsu et al40 (using gadolinium-DTPA enhanced T1-weighted MRI sequences) found persistent increases in allograft SI between 6 and 12 months postoperatively.
Correlation Between MRI and Graft Position
Several studies reported on the association between the graft SI and its orientation in the knee joint. Chen et al9 (using PD-weighted MRI sequences and SNQ analysis) found that the graft bending angle was correlated with a high SI of the proximal graft in the early postoperative period and thus might affect proximal graft healing after ACL reconstruction. In contrast, Sim et al52 (using T2-weighted MRI sequences and SI analysis) did not find such an association. Lee et al30 found that positioning the femoral tunnel near the anteromedial bundle and center led to subjectively “better” graft SI on follow-up MRI in anatomic single-bundle ACL reconstruction than did positioning the femoral tunnel near the posterolateral bundle. Ahn et al1 compared the retrograde outside-in and transtibial femoral tunnel drilling techniques. Although these authors did not report the MRI sequence parameters, they noticed in the follow-up MRI a significantly higher graft SNQ value with the outside-in than the transtibial technique. In addition, these authors found that the mean sagittal and coronal ACL angles were more vertical in the transtibial than in the outside-in group. Rose and Crawford48 found that the allograft SNQ value at 6 months postoperatively was dependent on the position of the tibial tunnel in the sagittal plane as well as the sagittal graft orientation. Finally, Li et al32 found that the graft SNQ value correlated negatively with the ACL–Blumensaat line angle.
Correlation Between MRI and Clinical Outcomes
The correlation between graft SI and clinical outcomes was examined in 7 studies.5,23,29,31,32,48,50 Of these, 3 studies,23,31,48 all using SNQ analysis, found no correlation between the MRI graft maturity measurements and KT-1000 knee ligament arthrometer findings and patient-reported outcome measures during the first postoperative year. Saupe et al,50 using subjective analysis, and Lee et al,29 using SI analysis, did not find a significant correlation between graft signal on MRI and functional outcomes at long-term follow-up. Biercevicz et al5 found that 3D MRI–derived graft volume measurements combined with median graft SI could predict traditional outcome measures in patients at 3- and 5-year follow-up. Li et al32 found a positive correlation between graft SNQ and the Tegner score, suggesting that more active patients are at greater risk of graft failure.
Discussion
The main finding of this systematic review is that the MRI protocols (including sequence type, acquisition parameters, coil design, and field strength) and methods used for evaluating the ACL graft SI differed widely across the studies. This wide variety of scan protocols and image assessment techniques impedes comparison of SI between successive scans and between independent studies.
Clinical and functional outcomes and patient-reported outcome measures are most commonly used to evaluate overall patient knee outcome after ACL surgery.7,22 However, these assessments may lack the objectivity to measure subtle changes in tendon status, which is needed to evaluate and monitor progression of graft healing.7,22
MRI allows for a noninvasive graft-specific assessment and may have advantages over traditional outcomes.21,25 Qualitative measurements of graft SI are widely used in clinical studies to gain insight into the biological process of tendon graft healing following ACL reconstruction.21,25 These methods have focused mainly on the SI of PD/T2-weighted MRIs, an indirect measure of water content, which has been linked to graft vascularity.25,26 However, the MRI signal is dependent on hardware-specific factors, PD signal scaling factors, voxel volume, pulse sequence weighting, and patient positioning in the scanner.3,11 These factors complicate comparison between successive scans and between patients. In an attempt to normalize the graft SI values within each image and minimize concerns of variability between scan sessions, previous studies have used SNQ (or contrast-to-noise ratio [CNR]) measurements.∥∥ However, 2 important issues need to be considered when these measurements are applied in clinical settings.
First, the conventionally determined SNR based on separate signal and noise regions in a single image (the “2-region” approach) will in general not agree with the true SNR in most situations (that is, in acquisitions using multichannel phased-array surface coil systems and parallel imaging).12 In these cases, the noise distribution is described by the spatially varying geometry factor (g-factor) and depends on parameters such as the coil geometry and acceleration factor. Therefore, the SNR will appear lower or higher depending on the positioning of the background ROI, which in turn will affect the statistical significance of follow-up SNR and CNR measurements.3,11,12 Several alternative SNR measurement methods that are compatible with multichannel coil and parallel imaging applications have been described.12 In general, only those SNR measurement methods that determine the noise at the same spatial position as the signal remain valid. The “difference method,” which is based on analyzing a difference image of 2 repeated (identical) acquisitions, is most often applied in the clinical setting to reduce SNR bias.12,51 Nevertheless, in many of the studies included in this review, the SNR calculations were performed using the 2-region approach despite the questionable validity of this method in the case of inhomogeneous noise distribution. Additionally, most of these studies used the mean value of the background intensity for normalizing the graft signal, whereas using the standard deviation of the background intensity is generally recommended, given that its standard deviation is less variable than its mean value.3,11,12
Second, the SI changes of the ACL graft related to changes in its alignment are likely a result of the MAE.14 This MRI-specific artifact, which typically occurs in structures containing highly ordered collagen such as tendons and ligaments, results in increased T2-weighted signal when these structures are oriented at 55° relative to the magnet’s bore.19 Similarly, the SI decreases when these structures are aligned parallel with the main magnetic field. In general, the angular range over which one can expect to see significant MAEs is underestimated and may be as large as 39° for pulse sequences with short echo times (TE) (7 ms).47 Although less severe at longer TE, the MAE tends to occur below critical TE values as high as 40 and 70 ms for SE and TSE imaging sequences, respectively.34,47 These findings could explain, at least in part, the lower graft SI found in patients treated by the transtibial technique1 and in patients with a higher ACL–Blumensaat line angle,32 as both result in a more vertical ACL angle and a more parallel alignment of the ACL graft with the main magnetic field. Also, an MAE can be expected at the angle where the graft is acutely bent on the edge of the femoral tunnel aperture (high graft bending angle). Chen et al,9 using PD-weighted MRI sequences for evaluating the ACL graft, found an association between the graft bending angle and the SI of the proximal graft, possibly contributing to poor healing. In contrast, Sim et al,52 using T2-weighted MRI sequences, did not find such an association. Although these studies have evaluated the ACL graft with MRI using different ROI methods at different time points, we believe the different study results can be explained at least in part by the occurrence of MAE at the graft bending angle.
Conflicting results have been reported between studies regarding the sequential change in graft SI that reflects normal healing. In general, studies evaluating longitudinal ACL graft healing with PD-weighted or gadolinium-enhanced T1-weighted TSE sequences found an initial increase in the graft SI peaking at 6 months postoperatively, followed by a decrease in SI. In contrast, studies using GRE-based sequences found a tendency of the graft signal to either increase during the first 12 months postoperatively44 or remain low and not change over time.42 Min et al,38 using quite different parameter settings (time-to-repetition/TE 20/70), also reported the graft SI to increase on sequential MRI using PD- and T2-weighted images. Furthermore, studies comparing autograft and allograft tendon healing on MRI have found different results. Li et al,32,33 using PD-weighted TSE sequences and SNQ analysis in 2 separate studies, found an initial increase in the auto- and allograft SI, followed by a signal decrease within the first postoperative year. In contrast, Muramatsu et al40 found a persistent increase in allograft SI within the first postoperative year using gadolinium-enhanced T1-weighted TSE sequences and SNQ analysis. Our study results clearly demonstrate that even studies using similar SI measures may yield different results. In our opinion, these differences are explained mainly by variations in the MRI acquisition techniques between studies rather than variations in the tissues themselves.
The role of MRI in monitoring and predicting outcomes after ACL surgery remains uncertain. Only 7 studies included in this review examined the correlation between MRI findings and clinical outcomes after ACL surgery. Overall, the T2-weighted graft signal was not reliable and did not predict clinical or functional outcomes after ACL reconstruction at both early23,31,48 and long-term29,50 follow-up. Only Biercevicz et al5 found that the combined parameters of graft volume and median graft SI derived from T1-weighted 3D GRE MRI had the ability to predict clinical or in vivo outcomes in patients at 3- and 5-year follow-up after ACL reconstruction. It is clear from these findings that the use of SI measurements to evaluate graft healing is challenging because of the technical dependencies of conventional SI data, even if quantified by the SNQ.
Quantitative MRI methods allow absolute quantification of tissue relaxation times, which are much less sensitive to image acquisition parameters compared with conventional SI data. Several magnetic resonance approaches to quantifying the structural and functional integrity of the ACL graft have been proposed. T2* relaxation time is a magnetic resonance parameter that has been shown to correlate with the level of tissue organization.4,6,18 T2* captures fast-T2 relaxations (T2* <10 ms) that reflect spin-spin interactions of protons bound to collagen and the degree of collagen fibril alignment.58 Therefore, this technique is well suited for imaging highly organized collagenous structures. Diffusion tensor imaging (DTI) is another potential candidate to assess ACL graft maturation.55 DTI allows for noninvasive in vivo quantification of the diffusion of water in biological tissues and assessment of its directional anisotropy, thereby providing a proxy measure of tissue microstructure and microvasculature. Recently, Van Dyck et al55 showed that DTI is feasible and reliable for quantitative evaluation of the ACL graft using a clinical 3T system.55 However, DTI suffers from long scan times (typically >10 minutes), accompanied by a high risk of patient motion, and it does not provide morphologic images, thereby hobbling its use in clinical practice. The new technique of magnetic resonance fingerprinting (MRF) is promising to overcome these drawbacks.36 MRF permits the noninvasive, time-efficient quantification of multiple important properties simultaneously (eg, T1, T2, PD, diffusion) for different tissues through a new approach to data acquisition, postprocessing, and visualization. The tissue-specific features can then synergistically provide information that cannot be obtained with conventional quantitative techniques in the same time frame.
Several studies included in this review compared MRI and clinical outcomes of different ACL reconstruction techniques (eg, comparisons of graft type,9,33 graft position,1,30 insertion-preserved vs detached hamstring graft,35,49 navigated vs manual surgery,15 and minimal debridement vs conventional clearance of the intercondylar notch20), and many of them found differences in graft SI but similar clinical outcomes. Although the SI variations result from both technical and biological factors, these findings illustrate the lack of sensitivity of clinical outcome measures following ACL reconstruction.
There were limitations to this systematic review. First, the included studies had variable MRI protocols and none of these compared protocols to assess the ACL graft. Therefore, a meta-analysis was not performed and definitive conclusions cannot be drawn. Second, only English-language literature was searched, and we did not contact the study authors for missing data. Third, the lack of MRI correlation with clinical outcome measures suggests that conventional MRI may not be the best way to assess graft maturation and return to play. To date, no previous human study has established the relationship between the graft SI and its biomechanical and histologic properties in vivo. Further research in quantitative MRI methods is needed, as these methods are not dependent on the scanner and acquisition parameters and thus may provide a better indicator of the ACL graft maturation status.
Conclusion
Multiple studies have attempted to use MRI to assess graft maturity after ACL reconstruction as an adjunct to traditional clinical outcome measures. However, MRI results differed greatly across the studies due to the wide heterogeneity of MRI acquisition and interpretation methods used to assess the ACL graft. This impedes comparison of SI between successive scans and between independent studies. Therefore, objective quantitative MRI biomarkers of graft healing would be desirable to help guide the appropriate time of returning to sports after ACL reconstruction.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: P.V.D. and C.H.W.H. are supported by the Research Foundation Flanders (FWO-Vlaanderen), Belgium (grants 1831217N and T001017N, respectively). AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Ahn JH, Lee YS, Jeong HJ, et al. Comparison of transtibial and retrograde outside-in techniques of anterior cruciate ligament reconstruction in terms of graft nature and clinical outcomes: a case control study using 3T MRI. Arch Orthop Trauma Surg. 2017;137(3):357–365. [DOI] [PubMed] [Google Scholar]
- 2. Autz G, Goodwin C, Singson RD. Magnetic resonance evaluation of anterior cruciate ligament repair using the patellar tendon double bone block technique. Skeletal Radiol. 1991;20(8):585–588. [DOI] [PubMed] [Google Scholar]
- 3. Berns GS, Howzell SM, Farley TE. The accuracy of signal intensity measurements in magnetic resonance imaging as evaluated within the knee. Magn Reson Imaging. 1992;10(4):573–578. [DOI] [PubMed] [Google Scholar]
- 4. Beveridge JE, Machan JT, Walsh EG, et al. Magnetic resonance measurements of tissue quantity and quality using T2* relaxometry predict temporal changes in the biomechanical properties of the healing ACL. J Orthop Res. 2018;36(6):1701–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biercevicz AM, Akelman MR, Fadale PD, et al. MRI volume and signal intensity of ACL graft predict clinical, functional, and patient-oriented outcome measures after ACL reconstruction. Am J Sports Med. 2015;43(3):693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Biercevicz AM, Murray MM, Walsh EG, Miranda DL, Machan JT, Fleming BC. T2* MR relaxometry and ligament volume are associated with the structural properties of the healing ACL. J Orthop Res. 2014;32(4):492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bourke HE, Salmon LJ, Waller A, Patterson V, Pinczewski LA. Survival of the anterior cruciate ligament graft and the contralateral ACL at a minimum of 15 years. Am J Sports Med. 2012;40(9):1985–1992. [DOI] [PubMed] [Google Scholar]
- 8. Cavaignac E, Marot V, Faruch M, et al. Hamstring graft incorporation according to the length of the graft inside tunnels. Am J Sports Med. 2018;46(2):348–356. [DOI] [PubMed] [Google Scholar]
- 9. Chen L, Wu Y, Lin G, et al. Graft bending angle affects allograft tendon maturity early after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26(10):3048–3054. [DOI] [PubMed] [Google Scholar]
- 10. Claes S, Verdonk P, Forsyth R, Bellemans J. The “ligamentization” process in anterior cruciate ligament reconstruction: what happens to the human graft? A systematic review of the literature. Am J Sports Med. 2011;39(11):2476–2483. [DOI] [PubMed] [Google Scholar]
- 11. den Dekker AJ, Sijbers J. Data distributions in magnetic resonance images: a review. Phys Med. 2014;30(7):725–741. [DOI] [PubMed] [Google Scholar]
- 12. Dietrich O, Raya JG, Reeder SB, Reiser MF, Schoenberg SO. Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging. 2007;26(2):375–385. [DOI] [PubMed] [Google Scholar]
- 13. Dong S, Xie G, Zhang Y, Shen P, Huangfu X, Zhao J. Ligamentization of autogenous hamstring grafts after anterior cruciate ligament reconstruction: midterm versus long-term results. Am J Sports Med. 2015;43(8):1908–1917. [DOI] [PubMed] [Google Scholar]
- 14. Echigo J, Yoshioka H, Takahashi H, Niitsu M, Fukubayashi T, Itai Y. Signal intensity changes in anterior cruciate ligament autografts: relation to magnetic field orientation. Acad Radiol. 1999;6(4):206–210. [DOI] [PubMed] [Google Scholar]
- 15. Endele D, Jung C, Becker U, Bauer G, Mauch F. Anterior cruciate ligament reconstruction with and without computer navigation: a clinical and magnetic resonance imaging evaluation 2 years after surgery. Arthroscopy. 2009;25(10):1067–1074. [DOI] [PubMed] [Google Scholar]
- 16. Falconiero RP, DiStefano VJ, Cook TM. Revascularization and ligamentization of autogenous anterior cruciate ligament grafts in humans. Arthroscopy. 1998;14(2):197–205. [DOI] [PubMed] [Google Scholar]
- 17. Figueroa D, Melean P, Calvo R, et al. Magnetic resonance imaging evaluation of the integration and maturation of semitendinosus-gracilis graft in anterior cruciate ligament reconstruction using autologous platelet concentrate. Arthroscopy. 2010;26(10):1318–1325. [DOI] [PubMed] [Google Scholar]
- 18. Fleming BC, Vajapeyam S, Connolly SA, Magarian EM, Murray MM. The use of magnetic resonance imaging to predict ACL graft structural properties. J Biomech. 2011;44(16):2843–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fullerton GD, Rahal A. Collagen structure: the molecular source of the tendon magic angle effect. J Magn Reson Imaging. 2007;25(2):345–361. [DOI] [PubMed] [Google Scholar]
- 20. Gohil S, Annear PO, Breidahl W. Anterior cruciate ligament reconstruction using autologous double hamstrings: a comparison of standard versus minimal debridement techniques using MRI to assess revascularisation. A randomised prospective study with a one-year follow-up. J Bone Joint Surg Br. 2007;89(9):1165–1171. [DOI] [PubMed] [Google Scholar]
- 21. Grassi A, Bailey JR, Signorelli C, et al. Magnetic resonance imaging after anterior cruciate ligament reconstruction: a practical guide. World J Orthop. 2016;7(10):638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harris JD, Abrams GD, Bach BR, et al. Return to sport after ACL reconstruction. Orthopedics. 2014;37(2):e103–e108. [DOI] [PubMed] [Google Scholar]
- 23. Hofbauer M, Soldati F, Szomolanyi P, et al. Hamstring tendon autografts do not show complete graft maturity 6 months postoperatively after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27(1):130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hong SJ, Ahn JM, Ahn JH, Park SW. Postoperative MR findings of the healthy ACL grafts: correlation with second look arthroscopy. Clin Imaging. 2005;29(1):55–59. [PubMed] [Google Scholar]
- 25. Howell SM, Berns GS, Farley TE. Unimpinged and impinged anterior cruciate ligament grafts: MR signal intensity measurements. Radiology. 1991;179(3):639–643. [DOI] [PubMed] [Google Scholar]
- 26. Howell SM, Knox KE, Farley TE, Taylor MA. Revascularization of a human anterior cruciate ligament graft during the first two years of implantation. Am J Sports Med. 1995;23(1):42–49. [DOI] [PubMed] [Google Scholar]
- 27. Janssen RP, Scheffler SU. Intra-articular remodelling of hamstring tendon grafts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2102–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jansson KA, Karjalainen PT, Harilainen A, et al. MRI of anterior cruciate ligament repair with patellar and hamstring tendon autografts. Skeletal Radiol. 2001;30(1):8–14. [DOI] [PubMed] [Google Scholar]
- 29. Lee S, Seong SC, Jo CH, Han HS, An JH, Lee MC. Anterior cruciate ligament reconstruction with use of autologous quadriceps tendon graft. J Bone Joint Surg Am. 2007;89(suppl 3):116–126. [DOI] [PubMed] [Google Scholar]
- 30. Lee SM, Yoon KH, Lee SH, Hur D. The relationship between ACL femoral tunnel position and postoperative MRI signal intensity. J Bone Joint Surg Am. 2017;99(5):379–387. [DOI] [PubMed] [Google Scholar]
- 31. Li H, Chen J, Li H, Wu Z, Chen S. MRI-based ACL graft maturity does not predict clinical and functional outcomes during the first year after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25(10):3171–3178. [DOI] [PubMed] [Google Scholar]
- 32. Li H, Chen S, Tao H. Correlation analysis of potential factors influencing graft maturity after anterior cruciate ligament reconstruction. Orthop J Sports Med. 2014;2(10):2325967114553552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li H, Tao H, Cho S, Chen S, Yao Z. Difference in graft maturity of the reconstructed anterior cruciate ligament 2 years postoperatively: a comparison between autografts and allografts in young men using clinical and 3.0-T magnetic resonance imaging evaluation. Am J Sports Med. 2012;40(7):1519–1526. [DOI] [PubMed] [Google Scholar]
- 34. Li J, Pilla KB, Li Q, et al. Magic angle spinning NMR structure determination of proteins from pseudocontact shifts. J Am Chem Soc. 2013;135(22):8294–8303. [DOI] [PubMed] [Google Scholar]
- 35. Liu S, Li H, Tao H, Sun Y, Chen S, Chen J. A randomized clinical trial to evaluate attached hamstring anterior cruciate ligament graft maturity with magnetic resonance imaging. Am J Sports Med. 2018;46(5):1143–1149. [DOI] [PubMed] [Google Scholar]
- 36. Ma D, Gulani V, Seiberlich N, et al. Magnetic resonance fingerprinting. Nature. 2013;495(7440):187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marx RG, Wilson SM, Swiontkowski MF. Updating the assignment of levels of evidence. J Bone Joint Surg Am. 2015;97(1):1–2. [DOI] [PubMed] [Google Scholar]
- 38. Min BH, Chung WY, Cho JH. Magnetic resonance imaging of reconstructed anterior cruciate ligament. Clin Orthop Relat Res. 2001;393:237–243. [DOI] [PubMed] [Google Scholar]
- 39. Murakami Y, Sumen Y, Ochi M, Fujimoto E, Adachi N, Ikuta Y. MR evaluation of human anterior cruciate ligament autograft on oblique axial imaging. J Comput Assist Tomogr. 1998;22(2):270–275. [DOI] [PubMed] [Google Scholar]
- 40. Muramatsu K, Hachiya Y, Izawa H. Serial evaluation of human anterior cruciate ligament grafts by contrast-enhanced magnetic resonance imaging: comparison of allografts and autografts. Arthroscopy. 2008;24(9):1038–1044. [DOI] [PubMed] [Google Scholar]
- 41. Mutsuzaki H, Kanamori A, Ikeda K, Hioki S, Kinugasa T, Sakane M. Effect of calcium phosphate-hybridized tendon graft in anterior cruciate ligament reconstruction: a randomized controlled trial. Am J Sports Med. 2012;40(8):1772–1780. [DOI] [PubMed] [Google Scholar]
- 42. Nakayama Y, Shirai Y, Narita T, Mori A, Kobayashi K. The accuracy of MRI in assessing graft integrity after anterior cruciate ligament reconstruction. J Nippon Med Sch. 2001;68(1):45–49. [DOI] [PubMed] [Google Scholar]
- 43. Nin JR, Gasque GM, Azcarate AV, Beola JD, Gonzalez MH. Has platelet-rich plasma any role in anterior cruciate ligament allograft healing? Arthroscopy. 2009;25(11):1206–1213. [DOI] [PubMed] [Google Scholar]
- 44. Ntoulia A, Papadopoulou F, Ristanis S, Argyropoulou M, Georgoulis AD. Revascularization process of the bone–patellar tendon–bone autograft evaluated by contrast-enhanced magnetic resonance imaging 6 and 12 months after anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(7):1478–1486. [DOI] [PubMed] [Google Scholar]
- 45. Ntoulia A, Papadopoulou F, Zampeli F, Ristanis S, Argyropoulou M, Georgoulis A. Evaluation with contrast-enhanced magnetic resonance imaging of the anterior cruciate ligament graft during its healing process: a two-year prospective study. Skeletal Radiol. 2013;42(4):541–552. [DOI] [PubMed] [Google Scholar]
- 46. Pauzenberger L, Syre S, Schurz M. “Ligamentization” in hamstring tendon grafts after anterior cruciate ligament reconstruction: a systematic review of the literature and a glimpse into the future. Arthroscopy. 2013;29(10):1712–1721. [DOI] [PubMed] [Google Scholar]
- 47. Richardson ML, Amini B, Richards TL. Some new angles on the magic angle: what MSK radiologists know and don’t know about this phenomenon. Skeletal Radiol. 2018;47(12):1673–1681. [DOI] [PubMed] [Google Scholar]
- 48. Rose M, Crawford D. Allograft maturation after reconstruction of the anterior cruciate ligament is dependent on graft parameters in the sagittal plane. Orthop J Sports Med. 2017;5(8):2325967117719695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ruffilli A, Pagliazzi G, Ferranti E, Busacca M, Capannelli D, Buda R. Hamstring graft tibial insertion preservation versus detachment in anterior cruciate ligament reconstruction: a prospective randomized comparative study. Eur J Orthop Surg Traumatol. 2016;26(6):657–664. [DOI] [PubMed] [Google Scholar]
- 50. Saupe N, White LM, Chiavaras MM, et al. Anterior cruciate ligament reconstruction grafts: MR imaging features at long-term follow-up—correlation with functional and clinical evaluation. Radiology. 2008;249(2):581–590. [DOI] [PubMed] [Google Scholar]
- 51. Sijbers J, Scheunders P, Bonnet N, Van Dyck D, Raman E. Quantification and improvement of the signal-to-noise ratio in a magnetic resonance image acquisition procedure. Magn Reson Imaging. 1996;14(10):1157–1163. [DOI] [PubMed] [Google Scholar]
- 52. Sim JA, Kim JM, Lee S, Song EK, Seon JK. No difference in graft healing or clinical outcome between trans-portal and outside-in techniques after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26(8):2338–2344. [DOI] [PubMed] [Google Scholar]
- 53. Stockle U, Hoffmann R, Schwedke J, et al. Anterior cruciate ligament reconstruction: the diagnostic value of MRI. Int Orthop. 1998;22(5):288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Valenti Azcarate A, Lamo-Espinosa J, Aquerreta Beola JD, Hernandez Gonzalez M, Mora Gasque G, Valenti Nin JR. Comparison between two different platelet-rich plasma preparations and control applied during anterior cruciate ligament reconstruction: is there any evidence to support their use? Injury. 2014;45(suppl 4):S36–S41. [DOI] [PubMed] [Google Scholar]
- 55. Van Dyck P, Froeling M, De Smet E, et al. Diffusion tensor imaging of the anterior cruciate ligament graft. J Magn Reson Imaging. 2017;46(5):1423–1432. [DOI] [PubMed] [Google Scholar]
- 56. Vogl TJ, Schmitt J, Lubrich J, et al. Reconstructed anterior cruciate ligaments using patellar tendon ligament grafts: diagnostic value of contrast-enhanced MRI in a 2-year follow-up regimen. Eur Radiol. 2001;11(8):1450–1456. [DOI] [PubMed] [Google Scholar]
- 57. Weiler A, Peters G, Maurer J, Unterhauser FN, Sudkamp NP. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging: a two-year study in sheep. Am J Sports Med. 2001;29(6):751–761. [DOI] [PubMed] [Google Scholar]
- 58. Williams A, Qian Y, Golla S, Chu CR. UTE-T2* mapping detects sub-clinical meniscus injury after anterior cruciate ligament tear. Osteoarthritis Cartilage. 2012;20(6):486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yamato M, Yamagishi T. MRI of patellar tendon anterior cruciate ligament autografts. J Comput Assist Tomogr. 1992;16(4):604–607. [DOI] [PubMed] [Google Scholar]