Abstract
The current study focuses on the ability to improve cognitive function after stroke with interventions administered at delayed/chronic time points. In light of recent studies demonstrating delayed GABA antagonists improve motor function, we utilized electrophysiology, biochemistry and neurobehavioral methods to investigate the role of α5 GABAA receptors on hippocampal plasticity and functional recovery following ischemic stroke. Male C57Bl/6 mice were exposed to 45 min transient middle cerebral artery occlusion and analysis of synaptic and functional deficits performed 7 or 30 days after recovery. Our findings indicate that hippocampal long-term potentiation (LTP) is impaired 7 days after stroke and remain impaired for at least 30 days. We demonstrate that ex vivo administration of L655,708 reversed ischemia-induced plasticity deficits and importantly, in vivo administration at delayed time-points reversed stroke-induced memory deficits. Western blot analysis of hippocampal tissue reveals proteins responsible for GABA synthesis are upregulated (GAD65/67 and MAOB), increasing GABA in hippocampal interneurons 30 days after stroke. Thus, our data indicate that both synaptic plasticity and memory impairments observed after stroke are caused by excessive tonic GABA activity, making inhibition of specific GABA activity at delayed timepoints a potential therapeutic approach to improve functional recovery and reverse cognitive impairments after stroke.
Keywords: Cognition and memory, GABAA receptors, hippocampus, stroke, synaptic plasticity
Introduction
Recent clinical studies have shown great promise in acute stroke therapy, with the use of tissue plasminogen activator in combination with mechanical endovascular thrombectomy improving outcomes. However, the vast majority of stroke patients do not receive care within the limited window of opportunity (>4.5 h). This, combined with the failure of numerous acute neuroprotective treatments to translate to clinical utility, has motivated researchers to investigate treatments that improve functional status when administered at delayed/chronic time points. Emerging experimental data demonstrate that an imbalance between inhibitory and excitatory neurotransmission after ischemic stroke can be reversed, leading to recovery of motor deficits. Decades of research focused on the excessive hyperexcitability observed during the acute phase following ischemia, termed excitotoxicity. While great advances in our understanding of cell death signaling have been made, all therapeutic approaches to prevent excitotoxic injury have failed to translate in human studies. More recently, a delayed decrease in excitability has been reported, either via decreased excitatory transmission and/or increased inhibitory tone. Clarkson et al.1 and Clarkson2 showed that reducing GABAergic inhibitory transmission with the tonic GABA inhibitor L655,708 enhances sensorimotor recovery after experimental stroke.1,2 An important follow-up study was recently performed that showed that delayed administration of L655,708 remained effective in improving sensorimotor function. Together, these data suggest that hypoexcitable states contribute to motor deficits following stroke can be reversed.
Extensive research has focused on motor deficits and strategies to improve outcomes; however, there has been less emphasis on cognitive domains. It is becoming increasingly clear that many of those recovering from stroke endure continuing problems with memory and cognition. Several clinical studies have observed that cognitive impairment and memory loss are common after stroke, with estimates as high as 30–50% suffering dementia-like symptoms within the first year after stroke.3–8 Thus, it is critical that new strategies be developed that can be utilized in the vast majority of stroke victims who are not able to benefit from acute therapies, but suffer from significant cognitive deficits during the subacute or chronic stage.
Our study focuses on the hypothesis that stroke causes chronic impairments in hippocampal synaptic plasticity that underlies cognition and memory dysfunction. It is increasingly clear that in addition to neuronal injury following cerebral ischemia, impaired functional networks contribute to long-term cognitive deficits. The hippocampus plays a central role in memory acquisition, storage and retrieval, and hippocampal CA1 pyramidal neurons are extremely sensitive to ischemic injury.9,10 Therefore, extensive research has focused on interventions to reduce hippocampal cell death as a strategy to improve cognitive function after brain ischemia. Interestingly, middle cerebral artery occlusion (MCAO) does not appear to induce hippocampal CA1 neuronal death after stroke,11 suggesting that memory impairments may not be due to cell death, but rather synaptic deficits. To analyze synaptic deficits after experimental stroke, we measure activity-dependent enhancement of synaptic strength, termed long-term potentiation (LTP), as the cellular substrate of hippocampal memory. Importantly, the current study moves beyond neuroprotection and, rather, focuses on a neuro-restorative strategy to improve memory function at delayed timepoints following stroke. The strategy presented here relies on reversing the hypoexcitable state of hippocampal neurons that results in plasticity and memory impairment after stroke. While recent reports suggest that reducing GABAergic inhibitory transmission enhances sensorimotor recovery after experimental stroke,1,2 to this point, it is unclear whether similar tonic inhibitory mechanisms are at play in hippocampal deficits after stroke or if reversing GABAergic inhibitory tone can lead to similar recovery in memory potential as those seen in motor recovery. Thus, we tested the new hypothesis that inhibition of α5-mediated tonic GABAergic neurotransmission can rescue stroke-induced hippocampal synaptic plasticity deficits and improve memory function.
Methods
Experimental animals
All experimental protocols were approved by the University of Colorado School of Medicine Institutional Animal Care and Use Committee and conformed to the National Institutes of Health guidelines for the care and use of animals in research. A total of 84 adult (20–25 g) male C57Bl/6 (8–12 weeks) mice purchased from Charles River Laboratory (Fredrickson, NC) were used for this study. All mice were housed in standard 12-h light dark cycle with free access to food and water. All experiments in the study adhered to the ARRIVE guidelines for animal experiments. All experiments were performed randomized (software generated randomization code provided to surgeon) and surgeon blinded to treatment group (separate investigator performed drug treatments and analyses (electrophysiology/biochemistry)). Groups included MCAO versus Sham survived for 7 or 30 days for electrophysiology (n = 5–9 mice/group); MCAO versus Sham and Vehicle versus L655,708 groups (n = 5–7 mice/group) for behavior and biochemistry.
MCAO model
Transient focal cerebral ischemia (45 min procedure) was induced using reversible MCAO through the intraluminal filament techniques as previously described.12,13 Briefly, mice were anesthetized with isoflurane (induction 3.0% and maintenance 1.5–2.0%). Head and body temperature were maintained at 36.5 ± 1.0℃ throughout the MCAO surgery. A laser Doppler probe (Moor Instruments, Oxford, England, UK) was placed over the ipsilateral cortex to assure adequate occlusion. Probe placement was established in a similar location for all mice by making a small incision (probe hole) in the middle of a line drawn between the outer canthus (lateral corner of the eye) and ear canal. Only animals with a blood flow reduction to less than 25% of baseline were included.
Hippocampal slice preparation
Hippocampal slices were prepared at varying times after recovery from MCAO. Animals were anesthetized with 3% isoflurane in an O2 enriched chamber. Mice were decapitated and the brains quickly extracted and placed in ice-cold (2–5℃) oxygenated (95% O2/5% CO2) artificial cerebral spinal fluid (aCSF) composed of the following (in mmol/L): 125 NaCl, 2.5. KCl, 25 NaHCO3, 1.25 NaH2PO4, 2.0 CaCl2, 1.0 MgCl2, 12 glucose). Horizontal hippocampal slices (300 µm thick) were cut with a Vibratome 1000 (Leica) and transferred to a holding chamber containing ACSF for 1.5–2 h before recording.
Electrophysiology
This procedure was done as previously described.14 Briefly, for synaptically evoked potentials, hippocampal slices were placed on a temperature controlled (32 ± 1℃) interface chamber perfused with oxygenated aCSF at a rate of 1.5 ml/min. Extracellular field recordings were performed by stimulating the Schaffer collaterals and recording the field excitatory post synaptic potential (fEPSP). fEPSPs were adjusted to 50% of the maximum slope and test pulses were evoked at a rate of 0.05 Hz. A 20-min stable baseline period was established before delivering a theta burst stimulation (TBS) train (4 pulses delivered at 100 Hz in 30 ms bursts, repeated 10, times with 200 ms inter-burst-intervals). Following TBS, the fEPSP was recorded for 60 min. Analog fEPSPs were amplified (1000×) and filtered through a pre-amplifier (Grass Instruments, Model P511) at 0.03 Hz–1.0 kHz, digitized at 10 kHz and stored on computer for later off-line analyses. The derivative of the initial 2–3 ms onset of the fEPSP slope was measured (dV/dT), and the amount of potentiation calculated as the percent change from baseline (the averaged 10 min slope value from 50 to 60 min post-TBS divided by the averaged slope value 10 min prior to TBS). For the time course graphs, normalized fEPSP slope values were averaged and plotted as the percent change from baseline and referenced to 100%.
Drug treatment
L-655,708 was purchased from Tocris and dissolved in DMSO. For both sham and stroke electrophysiology recordings, hippocampal slices were superfused in aCSF + L655,708 (100 nM) during baseline recordings and 1 h after TBS. For behavioral testing, mice (MCAO and Sham) were subcutaneously implanted with mini-osmotic pumps (Alzet Osmotic Pumps) filled with either vehicle (DMSO/Saline) or 5 mM L655,708, delivering 200 mg/kg/day on days 26–30, with behavioral testing beginning on day 30. Drug dosage and time of delivery were as previously described.1
Contextual fear conditioning
The contextual fear conditioning paradigm was utilized as a hippocampal-dependent memory task.15 The apparatus consisted of two fear conditioning chambers with shock grid floors, consisting of 16 stainless steel rods connected to a shock generator (Colbourn Instruments, Model H13-15, Whitehall, PA, USA). Mice were transported in white buckets during the training and testing sessions. During training on day 7 or day 30 after MCAO, mice were allowed to habituate the conditioning chamber for two separate 2-min pre-exposure sessions followed by a foot shock (2 s/1.0 mA electric shock) immediately after the second exposure. Following shock, mice were returned to their home cages. Testing occurred 24 h later, mice were transported in white buckets and placed back into the fear conditioning chambers. Freezing behavior was measured in 10-s intervals across a 5-min test by a blinded observer, and was defined as the absence of movement except for heart beat/respiration.
Western blot analysis
Frozen hippocampi in 250 µl of sucrose buffer (10 mM Tris, pH 7.4, 320 mM sucrose, protease and phosphatase inhibitors-Thermo Scientific) were homogenized with a pestle and drill homogenizer. Homogenates were centrifuged for 10 min at 1000g and supernatant was subsequently transferred to a fresh tube and centrifuged for 15 min at 10,000 g. Supernatant (cytosolic fraction) was removed and pellet (membrane fraction) was kept and re-suspended in Neuronal Protein Extraction Reagent (Thermo Scientific) for cell lysis. All previous steps were performed at 4℃. Protein concentration was measured with Pierce BCA Protein Assay Kit (Thermo Scientific). Equal quantity of total protein was loaded per lane (20 µg) and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF). Membranes were blocked with 10% BSA in 1× Tris-buffered saline (1× TBS) for 1 h at RT and incubated overnight at 4℃ with antibodies targeting synaptic and extra-synaptic proteins. Primary antibodies used: GABA-α1, α3, δ (Millipore), α5 (Thermo Fisher), GAT-1, GAT-3 (Abcam), MAOB (Sigma Aldrich), GAD6567 (Millipore) and β-actin (Sigma Aldrich). Secondary antibodies used: goat anti-mouse or goat anti rabbit depending on primary antibody’s host. Immuno-reactive bands were detected with West Pico/West Femto maximum sensitivity substrate (Thermo Fisher). Integrated volume of protein bands was quantified with Bio-Rad ChemiDoc MP Imaging software and normalized to β-actin.
Immunohistochemistry
Staining of 50 µm sections consisted of phosphate-buffered saline washes (1× PBS, 3 × 5 min), quenching of endogenous peroxidase (0.3% H2O2 in 1× PBS, 30 min), 2-h incubation in blocking serum (10% normal donkey serum in 0.3% Triton X-100), 48-h incubation at 4℃ in primary antibody, PBS washes (3 × 5 min), 1-h incubation in secondary antibody, PBS washes (3 × 5 min), mounting and coverslip with anti-fade mounting medium (Vectashield, H-1000). Primary antibodies used were guinea pig anti-gamma-Aminobutyric acid (GABA, 1:2000, Protos Biotech, NT108) and mouse anti-glutamic acid decarboxylase (GAD67, 1:1000, Millipore, MAB5406), with Alexa Fluor 488 or 594-conjugated IgG (1:600; Jackson Immuno) secondary antibody. Photomicrographs were obtained from four sections/animal in the stratum oriens of the CA1 region on a confocal microscope (Olympus FV1000) using Olympus Fluoview imaging software (Center Valley, PA). Image analysis was performed by obtaining pixel intensity using ImageJ software. Each image underwent auto-thresholding with identical parameters for each image. Mean pixel intensity was measured for each positive cell. The mean pixel intensities for each cell were then averaged across each image, resulting in an average mean pixel intensity for every image.
Histology
Terminal deoxynucleotidyl transferase dUTP nick end labeling
Hippocampal cell death was measured using the In Situ Cell Death Detection Kit, TMR red. In short, tissue sections were incubated in 50 uL of the active TMR red solution, for 1 h at 37℃. Sections were then washed in 1× PBS three times before being mounted for confocal imaging.
Statistical analysis
All data were presented as mean ± SEM. Power analysis was performed using previous data generated in our laboratory. For LTP experiments to detect differences of 40% with a standard deviation of 20 a group size of 6 is required with alpha < 0.05 and 80% power. For biochemistry experiments, to detect 2-fold differences with a standard deviation of 0.5 a group size of 5 is required to generate data with alpha < 0.05 and 80% Power. Statistical analysis of all data was determined using the Student’s t-test for two group comparisons and one-way analysis of variance (ANOVA) and post hoc Dunnett’s test for comparison of multiple groups. Differences considered statistically significant with p < 0.05.
Results
Synaptic function is impaired in the CA1 of the hippocampus after MCAO
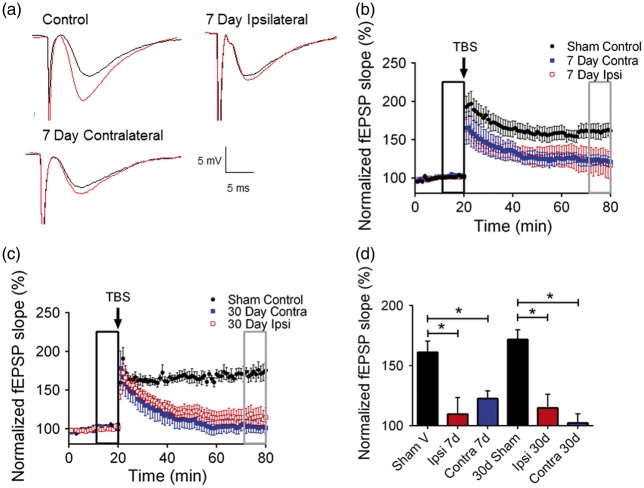
To determine the effect of experimental stroke on hippocampal function, we exposed mice to transient MCAO and performed extracellular field recordings of CA1 neurons to analyze synaptic function in the hippocampus. Hippocampal slices obtained from sham mice or mice previously exposed to 45 min MCAO were placed in an interface chamber and evoked LTP was recorded by placing a stimulating electrode in the stratum radiatum and a recording electrode in the CA1 dendritic area to record Schaffer collateral-CA1 field excitatory post-synaptic potentials (fEPSPs). In sham control slices, a brief TBS resulted in LTP, increasing the fEPSP slope to 161.2 ± 9.2% (n = 8, p < 0.05) of baseline (set at 100%) after 60 min. (Figure 1(a) and (b)). In contrast, recordings obtained during the subacute recovery phase following MCAO (seven days) showed the impairment of LTP [7-day ipsilateral: 109.7 ± 13.9% (n = 7) and 7-day contralateral: 122.6 ± 6.43% (n = 9), both p < 0.05 compared to sham controls] (Figure 1(b)). MCAO-induced impairment in LTP was observed to persist for up to 30 days; fEPSP 1 h after TBS in slices obtained 30 days after MCAO was 114.8 ± 11.49% (n = 7) in ipsilateral hippocampus and 102.4 ± 7.595% (n = 7) in contralateral hippocampus after MCAO (p < 0.05 compared to sham controls (Figure 1(c)). Figure 1(d) shows the summary of these experiments, demonstrating that MCAO causes reduced hippocampal synaptic plasticity (LTP) in both ipsilateral and contralateral hippocampi and the effect persisted for at least 30 days after the ischemic insult.
Figure 1.
Stroke impairs synaptic plasticity in the hippocampus. (a) Example fEPSPs from sham-operated control mice at seven days after MCAO before (black) and after (red) TBS in both ipsilateral (Ipsi) and contralateral (Contral) hippocampus. (b) Time-course of fEPSP slope (mean ± SEM) from sham control mice (black-filled circles) and from contralateral hippocampus (blue-filled squares) or ipsilateral hippocampus (red open squares) at seven days after MCAO. Arrow indicates timing of TBS (40 pulses). (c) Time-course of fEPSP slope (mean ± SEM) from sham mice (black filled circles) and from contralateral hippocampus (blue filled squares) or ipsilateral hippocampus (red open squares) 30 days after MCAO. Arrow indicates timing of TBS (40 pulses). (d) Quantification of change in fEPSP slope following TBS. Average fEPSP slope (mean + SEM) at 60 min after TBS (in gray box in B) normalized to baseline (black box in B). *p < 0.05 compared with sham controls.
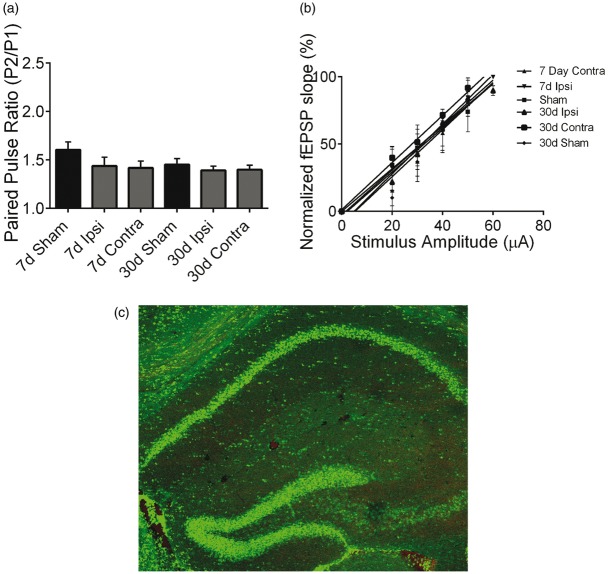
To determine whether ischemia alters pre-synaptic function, we examined the paired-pulse ratio (PPR) as a measure of pre-synaptic neurotransmitter release probability.14,16,17 PPR responses were recorded as described above using 50 ms interpulse interval. We did not observe differences in PPR in any groups (Figure 2(a)), suggesting that changes in pre-synaptic glutamate release does not account for MCAO-induced changes in LTP. We analyzed input-output (I/O) curves of fEPSP versus stimulus intensity to assess the effect of stroke on CA3 axonal excitability. Figure 2(b) shows that analysis of I/O in ipsilateral and contralateral hippocampus at each timepoint showed that there was no significant effect of stroke on the slope of the I/O curve. These data suggest that synaptic transmission and axonal excitability are not altered after MCAO, implicating changes in post-synaptic responses as the mediator of impaired LTP. Consistent with unaltered I/O curves after MCAO, we did not observe CA1 neuronal injury following MCAO. MCAO-induced cell death was analyzed by staining for TUNEL. We did not detect any TUNEL+ cells in the CA1 region of the hippocampus 24 h (Figure 2(c)) or seven days (data not shown) after MCAO.
Figure 2.
Stroke does not affect cell viability or basal synaptic transmission. (a) Quantification of paired-pulse ratio of sham control mice and at each timepoint after MCAO recorded from both the ipsilateral (Ipsi) and contralateral (Contra) hippocampi. (b) Normalized fEPSP slope plotted against stimulus intensity, showing no differences in any experimental condition. (c) Representative fluorescent photomicrographs of hippocampus illustrating intact pyramidal cell layer (green) and no TUNEL positive injured cells (red) 24 h after MCAO.
Altering GABAA α5 receptors improve synaptic plasticity in the hippocampus after MCAO
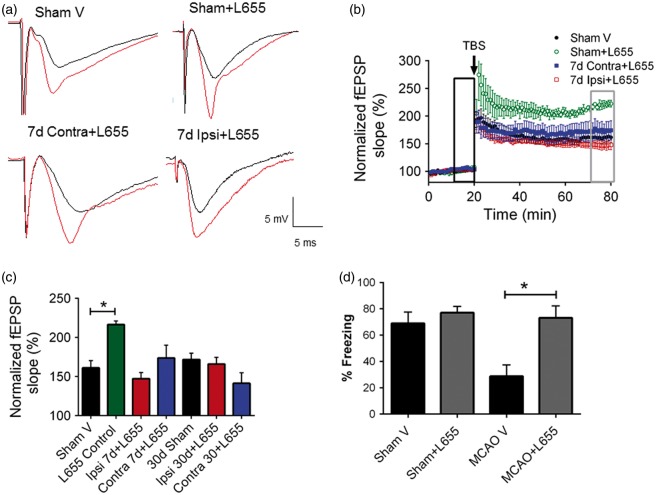
It was recently shown that a broad spectrum GABAA receptor antagonist (picrotoxin) improves MCAO-induced impairments in hippocampal LTP.11 To test the role of tonic GABAergic transmission mediated by GABAA α5 receptor subunits on impaired hippocampal plasticity after MCAO, hippocampal slices were superfused in aCSF + L655,708 (100 nM), an inverse agonist of GABAA-alpha 5 receptors.18 In acute hippocampal slices from sham controls, LTP was significantly increased in the presence of L655,708 with the fEPSP slope increasing to 216.4 ± 4.861 (n = 4, p < 0.05) compared to 161.2 ± 9.2 (n = 6) of baseline under control conditions (Figure 3(a) and (b)), consistent with previous reports indicating that reducing GABAA α5 tonic current function enhances synaptic plasticity. Importantly, recordings obtained in slices superfused with L655,708 reversed the MCAO-induced impairment in LTP observed at seven days [7-Day MCAO + L655,708 ipsilateral: 147.4 ± 7.856 (n = 7) and 7-Day MCAO + L655,708 contralateral: 173.7 ± 16.47% (n = 6), were significantly increased compared to 7-Day MCAO untreated (p < 0.05) and not significantly different from sham controls (161.2 ± 9.244, n = 6, p < 0.05; Figure 3(b))]. Furthermore, acute hippocampal slices treated with L655,708 rescued LTP impairment in both ipsilateral (165.9 ± 8.78% (n = 6)) and contralateral (141.13 ± 13.47% (n = 7)) when superfused 30 days after MCAO compared to controls (p < 0.05, Figure 3(c)).
Figure 3.
Altering GABAA α5 receptors rescue synaptic function after MCAO. (a) Example fEPSPs from sham-operated control mice at seven days after MCAO before (black) and after (red) TBS in both ipsilateral and contralateral hippocampus. (b) Time-course of fEPSP slope (mean ± SEM) from the hippocampus in sham mice (black filled circles), Sham + 100 nM L655,708 treated (green open circles) and L655,708 treated mice in contralateral (blue filled squares) and Ipsilateral (red open squares) seven days after MCAO. Arrow indicates timing of TBS (40 pulses). (c) Quantification of change in fEPSP slope following TBS. Average fEPSP slope (mean + SEM) at 60 min after TBS (in gray box in B) normalized to baseline (black box in B). *p < 0.05 compared with sham controls. (d) Memory impairment 30 days after MCAO. Adult mice displayed memory dysfunction after contextual fear conditioning. Mice implanted with mini-osmotic pumps delivered either vehicle or L655,708 4 days before behavioral testing. Quantification of freezing behavior 24 h after contextual fear conditioning in a novel environment (n = 5–8/group). *p < 0.05 compared to sham; behavior was scored. (n = 6–11/group. *p < 0.05 compared to sham controls).
GABAA α5 receptor activity contributes to memory deficits following MCAO
Experimental stroke has been shown to impair memory both acutely and chronically after injury. To determine if memory impairment after MCAO could be rescued, we administered L655,708 four days prior to behavioral testing. Mice were individually implanted with subcutaneous mini-osmotic pumps (vehicle or L655,708 at 5 mM, delivering 200 mg/kg/day) 26 days (days 26–30) after recovery from MCAO. Mice were subjected to contextual fear conditioning 30 days after MCAO to determine its effect on learning and memory. MCAO mice show a significant decrease in freezing behavior at 30 days after MCAO (percentage freezing: MCAO + vehicle 28.8 ± 8.6, n = 5; Sham + vehicle 69.0 ± 8.5, n = 7, p < 0.05, Figure 3(d)) when compared to sham, indicating impaired memory. In contrast, mice treated with L655,708 exhibited intact memory 30 days post injury (percentage freezing: MCAO+L655,708 73.20 ± 9.019, n = 5) when compared to vehicle-treated MCAO injured mice. Interestingly, L655,708 had no effect on memory function in sham mice (Figure 3(d)). Importantly, open field experiments showed no changes in sensorimotor function (total distance moved or velocity) or anxiety (time spent near the outer wall of chamber) in these mice (data not shown). These data correlate with the rescue of LTP observed in our electrophysiology experiments and indicate that delayed administration of inhibitors of tonic GABAergic neurotransmission is able to reverse ischemia-induced memory deficits.
Molecular analysis of GABAA α5 receptor activity
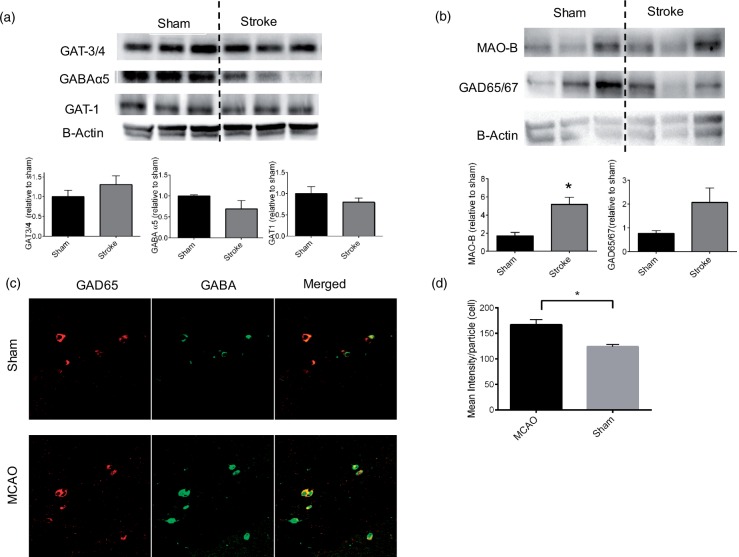
Tonic inhibition is controlled by the uptake of GABA through neuronal and astrocytic transporters (GATs) and a recent study demonstrated reduced expression of the astrocytic transporter GAT3/4 in the peri-infarct cortex after photothrombotic stroke.1 To test the hypothesis that a similar reduction in GABA transporter expression underlies chronic alterations in hippocampal excitability following stroke, we harvested both ipsilateral and contralateral hippocampi 30 days after MCAO and measured the expression levels of GABAA receptors and transporters. We did not observe changes in expression of the astrocytic GABA transporter GAT3/4 (n = 5, p > 0.05; Figure 4(a)). Similarly, the neuronal GABA transporter GAT1 was unaltered by ischemia (n = 5, p > 0.05; Figure 4(a)). In light of the ability of the GABAA α5 subunit specific inhibitor L655,708 to rescue synaptic impairment, we analyzed the effect of stroke on expression of GABAA α5 subunits. Surprisingly, we observed no change in GABAA α5 expression (Figure 4(a)). Similarly, we did not observe changes in any of the GABAA receptor subunits analyzed (δ, α1 and α3-Data not shown). In light of no changes in receptors or transporters being observed, we analyzed the expression of GABA synthetic enzymes, both neuronal GAD65/67 and MAOB. We observed that 30 days after MCAO, MAOB protein expression was significantly increased (n = 6, p < 0.05; Figure 4(b)) and GAD65/67 was also elevated (Figure 4(b)) in the hippocampus when compared to sham controls. Finally, to resolve the cellular source of increased GABA, we performed IHC with an anti-GABA antibody. We observed significantly increased GABA 30 days after MCAO (n = 4, p < 0.05 compared to sham), with intensity observed within GAD67 + interneurons (Figure 4(c) and (d)). These data indicate that stroke-induced increase in GABAA α5 subunit-mediated tonic inhibition is likely a result of increased synthesis and release of GABA from inhibitory interneurons.
Figure 4.
Biochemical evidence for increased GABA synthesis following MCAO. (a) Western blot analysis showed no significant changes in any of the GABAA receptor α5 subunits or GATA3/4, GAT1 GABA transporters. (b) Stroke caused a significant increase in MAOB, with a similar increase observed in GAD65/67 levels. (c) Representative photomicrographs of the CA1 region of the hippocampus in sham (top) and 30 days after MCAO (bottom) show increased GABA immunofluorescence in stroke animals. (d) Quantification of cellular GABA pixel intensity. n = 4/group. *p < 0.05 compared to sham controls.
Discussion
This study presents data showing that inhibition of α5 containing GABA-A receptors can reverse stroke-induced deficits in synaptic plasticity and improve cognitive outcomes. Further, our data indicate that stroke-induced memory deficits are sustained for at least 30 days but that delayed administration of the α5 inverse agonist L655,708 rapidly improves memory function. No change in expression of GABAA receptors (α5, δ, α1 or α3) or GABA transporters (GAT1 or GAT3/4) was observed after stroke. However, increased expression of the enzymes responsible for synthesis of GABA, MAOB and GAD65/67 appears to explain the increased tonic inhibition observed in the hippocampus after MCAO. These findings have important translational implications as we demonstrate the ability to reverse stroke-induced memory deficits when intervention is delayed well beyond the acute critical period.
The experimental stroke research community has demonstrated the ability to reduce brain injury when a variety of compounds are administered during the acute phase following ischemic stroke. However, clinical trials for the treatment of ischemic stroke have been unsuccessful. Thus, it is important to move beyond neuroprotection as a therapeutic strategy and begin to target processes that impact functional recovery during the subacute and chronic times after stroke. Currently, therapies aimed at promoting functional recovery are limited to improvements in the motor function domain using physical rehabilitation. In addition to motor recovery, it is critical that stroke therapies begin to address the cognitive deficits associated with stroke that play a major role in quality of life outcomes. Memory deficits are commonly reported following stroke, both acutely and chronically and lend themselves to experimental interrogation. The hippocampus plays a central role in memory acquisition and hippocampal synaptic plasticity is the leading model for cellular changes that underlie learning and memory. Here we report a new approach to improved functional recovery by targeting excessive inhibitory transmission in the hippocampus, which we demonstrate restores hippocampal synaptic plasticity (LTP) and memory function. Our observation of impaired hippocampal LTP is consistent with recent reports following experimental stroke11 and global cerebral ischemia.14,19,20 As noted here, a recent stroke study showed that inhibition of GABAergic transmission using picrotoxin in acute hippocampal slices improved LTP; however, this treatment was not amenable to behavioral testing or clinical application, as global inhibition of GABA produces significant behavioral effects, including seizures. In contrast, our use of the GABAA α5 receptor subtype inhibitor L655,708 allowed us to demonstrate in vivo efficacy of delayed inhibition of tonic GABAergic neurotransmission.
Experimental data have demonstrated an imbalance between inhibitory and excitatory neurotransmission in the cortex following experimental ischemic stroke and importantly that delayed administration of agents aimed at restoring excitation/inhibition balance enhances motor recovery in mice.1,2 Clarkson et al.1 showed that after stroke, tonic inhibition is increased in the peri-infarct cortex, that this increase was mediated by extrasynaptic GABAA receptor activation, and that delayed administration of L655,708 improved motor function.1 Similarly, we observed that administering L655,708 in vivo with an osmotic pump rescued memory impairment 30 days after stroke. Here, extracellular field recordings in the CA1 area reveal that acute application of L655,708 on slices at delayed time points after MCAO recovers synaptic plasticity in the hippocampus 7 and 30 days after MCAO. While we observed a similar restoration of function using L655,708 to assess memory dysfunction following stroke, there are differences in the cortical and hippocampal responses. Firstly, it is important to note that transient MCAO does not result in hippocampal neuronal cell death, as shown here and Li et al.11 Consistent with lack of ischemic injury, basal synaptic transmission in the hippocampus is not altered by stroke. In contrast to recent studies in the cortex,1 we did not observe a change in astrocytic GABA transporter (GAT3/4) expression following MCAO. Interestingly, we observed increased expression in enzymes responsible for synthesis of GABA (MAOB and GAD65/67) within the hippocampus following stroke. GAD65/67 is a well-established synthetic enzyme expressed in GABAergic interneurons, while the role of MAOB in GABA synthesis is less studied. Recent studies in Alzheimer’s (AD) has demonstrated that MAOB is expressed at low levels within the hippocampus under control conditions and that increased expression has been demonstrated to result in elevated production and release of GABA.21,22 Interestingly, astrocytic MAOB was implicated in the AD-induced increase in GABA release. However, a recent study showed an increase in MAOB in neurons in an AD model.23 In the current study, we observed increased GABA expression at chronic timepoints (30 days) after ischemic stroke in GABAergic interneurons, thus establishing a role for increased neuronal production and likely release of GABA in hippocampal function following stroke. Thus, while strong evidence for neuronal contribution to excessive inhibition following MCAO in the hippocampus was found in the current study, further investigation of the role of astrocytes in excessive inhibition remains warranted.
Summary
This study shows for the first time that pharmacological intervention administered during the subacute/chronic phase of stroke recovery reverses functional cognitive deficits, providing evidence that pharmacological interventions may be amenable to improving rehabilitation, unlike previous studies focused on prevention of brain injury and development of functional impairments. These data highlight emerging strategies that involve rescuing functional outcomes after ischemia, rather than focusing on neuroprotection. As neuronal and network repair strategies continue to mature, studies similar to ours will likely lead the way in identifying translatable targets to improve the lives of those who suffer from stroke.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Bugher Collaborative Research Center Project 14 BFSC17690001; NIH R01NS092645. Imaging experiments were performed in the University of Colorado Anschutz Medical Campus Advance Light Microscopy Core supported in part by NIH/NCATS Colorado CTSI Grant Number UL1 TR001082. Contents are the authors' sole responsibility and do not necessarily represent official NIH views.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
James E Orfila – Acquisition of data, analysis and interpretation of data, drafting of manuscript.
Himmat Grewal – Acquisition of data, analysis and interpretation of data.
Robert M Dietz – Acquisition of data, analysis and interpretation of data, critical revision.
Frank Strnad – Acquisition of data, drafting of manuscript.
Takeru Shimizu – Acquisition of data, drafting of manuscript.
Myriam Moreno-Garcia – Acquisition of data, drafting of manuscript.
Christian Schroeder – Acquisition of data, drafting of manuscript.
Joan Yonchek – Acquisition of data, drafting of manuscript.
Krista M Rodgers – Acquisition of data, drafting of manuscript.
Andra Dingman – Acquisition of data, drafting of manuscript.
Timothy J Bernard – Study conception and design, critical revision.
Nidia Quillinan – Study conception and design, critical revision.
Wendy B Macklin – Study conception and design, critical revision.
Richard J Traystman – Study conception and design, critical revision.
Paco S Herson – Study conception and design, analysis and interpretation of data, critical revision.
References
- 1.Clarkson AN, Huang BS, Macisaac SE, et al. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature 2010; 468: 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarkson AN. Perisynaptic GABA receptors the overzealous protector. Adv Pharmacol Sci 2012; 2012: 708428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snaphaan L, de Leeuw FE. Poststroke memory function in nondemented patients: a systematic review on frequency and neuroimaging correlates. Stroke 2007; 38: 198–203. [DOI] [PubMed] [Google Scholar]
- 4.Al-Qazzaz NK, Ali SH, Ahmad SA, et al. Cognitive impairment and memory dysfunction after a stroke diagnosis: a post-stroke memory assessment. Neuropsychiatr Dis Treat 2014; 10: 1677–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine DA, Galecki AT, Langa KM, et al. Trajectory of cognitive decline after incident stroke. JAMA 2015; 314: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tatemichi TK, Desmond DW, Stern Y, et al. Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. J Neurol Neurosurg Psychiatr 1994; 57: 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaapsmeerders P, Maaijwee NA, van Dijk EJ, et al. Long-term cognitive impairment after first-ever ischemic stroke in young adults. Stroke 2013; 44: 1621–1628. [DOI] [PubMed] [Google Scholar]
- 8.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 2009; 8: 1006–1018. [DOI] [PubMed] [Google Scholar]
- 9.Kofler J, Hattori K, Sawada M, et al. Histopathological and behavioral characterization of a novel model of cardiac arrest and cardiopulmonary resuscitation in mice. J Neurosci Methods 2004; 136: 33–44. [DOI] [PubMed] [Google Scholar]
- 10.Allen D, Nakayama S, Kuroiwa M, et al. SK2 channels are neuroprotective for ischemia-induced neuronal cell death. J Cereb Blood Flow Metab 2011; 31: 2302–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Huang R, Shetty RA, et al. Transient focal cerebral ischemia induces long-term cognitive function deficit in an experimental ischemic stroke model. Neurobiol Dis 2013; 59: 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia J, Verma S, Nakayama S, et al. Sex differences in neuroprotection provided by inhibition of TRPM2 channels following experimental stroke. J Cereb Blood Flow Metab 2011; 31: 2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimizu T, Dietz RM, Cruz-Torres I, et al. Extended therapeutic window of a novel peptide inhibitor of TRPM2 channels following focal cerebral ischemia. Exp Neurol 2016; 283: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orfila JE, Shimizu K, Garske AK, et al. Increasing small conductance Ca2+-activated potassium channel activity reverses ischemia-induced impairment of long-term potentiation. Eur J Neurosci 2014; 40: 3179–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudy JW, O'Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn Affect Behav Neurosci 2001; 1: 66–82. [DOI] [PubMed] [Google Scholar]
- 16.Debanne D, Guerineau NC, Gahwiler BH, et al. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol 1996; 491(Pt 1): 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudhof TC. The synaptic vesicle cycle. Ann Rev Neurosci 2004; 27: 509–547. [DOI] [PubMed] [Google Scholar]
- 18.Atack JR, Bayley PJ, Seabrook GR, et al. L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for alpha5-containing GABAA receptors. Neuropharmacology 2006; 51: 1023–1029. [DOI] [PubMed] [Google Scholar]
- 19.Dietz RM, Deng G, Orfila JE, et al. Therapeutic hypothermia protects against ischemia-induced impairment of synaptic plasticity following juvenile cardiac arrest in sex-dependent manner. Neuroscience 2016; 325: 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng G, Orfila JE, Dietz RM, et al. Autonomous CaMKII activity as a drug target for histological and functional neuroprotection after resuscitation from cardiac arrest. Cell Rep 2017; 18: 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jo S, Yarishkin O, Hwang YJ, et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer's disease. Nat Med 2014; 20: 886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon BE, Woo J, Chun YE, et al. Glial GABA, synthesized by monoamine oxidase B, mediates tonic inhibition. J Physiol 2014; 592: 4951–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schedin-Weiss S, Inoue M, Hromadkova L, et al. Monoamine oxidase B is elevated in Alzheimer disease neurons, is associated with gamma-secretase and regulates neuronal amyloid beta-peptide levels. Alzheimers Res Ther 2017; 9: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]