Abstract
Magnesium sulphate (MgSO4) given to women in preterm labor reduces cerebral palsy in their offspring but the mechanism behind this protection is unclear, limiting its effective, safe clinical implementation. Previous studies suggest that MgSO4 is not neuroprotective if administered during or after the insult, so we hypothesised that MgSO4 induces preconditioning in the immature brain. Therefore, we administered MgSO4 at various time-points before/after unilateral hypoxia-ischemia (HI) in seven-day-old rats. We found that MgSO4 treatment administered as a bolus between 6 days and 12 h prior to HI markedly reduced the brain injury, with maximal protection achieved by 1.1 mg/g MgSO4 administered 24 h before HI. As serum magnesium levels returned to baseline before the induction of HI, we ascribed this reduction in brain injury to preconditioning. Cerebral blood flow was unaffected, but mRNAs/miRNAs involved in mitochondrial function and metabolism were modulated by MgSO4. Metabolomic analysis (H+-NMR) disclosed that MgSO4 attenuated HI-induced increases in succinate and prevented depletion of high-energy phosphates. MgSO4 pretreatment preserved mitochondrial respiration, reducing ROS production and inflammation after HI. Therefore, we propose that MgSO4 evokes preconditioning via induction of mitochondrial resistance and attenuation of inflammation.
Keywords: Brain injury, neonatal, preconditioning, hypoxia-ischemia, magnesium
Introduction
Perinatal brain injury in term and preterm infants remains a paramount clinical problem causing death and neurological disabilities.1,2 Approximately 30% of infants born preterm (<28 gestational weeks) will suffer from cerebral palsy (CP), cognitive impairment or neuropsychiatric disorders.3–5 CP is the most common cause of severe disability in children (2/1000 births) and a major cost to sufferers, their families and society,6,7 underscoring the need for development of treatment and preventive strategies.
Magnesium administered as magnesium sulphate (MgSO4) has neuroprotective properties. Clinical cohort studies demonstrated that offspring of mothers treated with antenatal MgSO4 (given to inhibit preterm labor or as seizure prophylaxis in preeclampsia) had a lower risk of developing CP8 and intraventricular/periventricular hemorrhage.9 Randomized controlled trials have subsequently confirmed these results10–12 and a meta-analysis showed that the rate of CP or moderate/severe motor disability is reduced 30% by antenatal MgSO4.13 Additionally, recent discoveries demonstrate that the rates of echolucent/echodense brain lesions,14 as well as cerebellar hemorrhages,15 were attenuated. However, the mechanisms behind this protective effect remain obscure and the optimal dose for neuroprotection in preterm fetuses/neonates without serious side effects is unknown.
Preconditioning is a phenomenon whereby sub-threshold exposure or chemical agents confer resistance to a subsequent severe insult.16 The immature brain is responsive to a number of preconditioning exposures, e.g. sub-threshold hypoxia17,18 or pre-exposure to pharmacological agents such as xenon19 which reduce hypoxic-ischemic (HI) brain injury substantially. Clinically, MgSO4 is given antenatally to mothers in preterm labor well in advance of the expected critical period for development of brain injury. Indeed, the issue of timing is critical as there is no evidence of efficacy when MgSO4 is administered immediately before, during or after the insult.20–22 Here we investigate our hypothesis that MgSO4 induces preconditioning, thereby providing tolerance to insults such as HI or excitotoxicity occurring during the early neonatal period, and define the mechanisms underlying its neuroprotective effect. A better understanding of the mechanisms behind the neuroprotective actions of MgSO4 will optimize the efficacy and safety of treatment in preterms and widen its potential therapeutic applications in high-risk pregnancies to prevent asphyxial brain injury or prior to open heart surgery in neonates.
Materials and methods
Experimental design
Prior to data acquisition, a power analysis was performed for calculation of appropriate sample size for reliable measurements. Final endpoints/rules for stopping data collection were set in advance and all experiments, including potential outliers, were included. Pups were randomly allocated to different groups from a variety of litters to avoid litter-specific outcome and marked numerically rather than according to individual treatment. During analysis, samples for microtubule-associated protein-2 (MAP-2) and myelin basic protein (MBP), analyzed for serum Mg2+ content, proton nuclear magnetic resonance (H+-NMR), mRNA/miRNA, mitochondrial respiration, reactive oxygen species (ROS) formation, Bio Plex and cerebral blood flow (CBF) measurements were completely blinded to the researchers through numerical sample-marking with the researchers unaware of group belonging in order to avoid bias. Male and female Wistar rat pups from in house breeding at the facility of Experimental Biomedicine (EBM) were used along with Swiss mice from the Robert Debre hospital rodent facility. Animal experimental procedures conformed to guidelines established by the Swedish Board of Agriculture (SJVFS 2015: 38), were approved by the Gothenburg Animal Ethics Committee (ethical license 333-2012, 138-2013 and 01-2016) or by the Bichat-Robert Debre committee (ethical license 2011-14/646-048) and are reported in a manner consistent with the ARRIVE (Animal Research: Reporting in Vivo Experiments) guidelines. Animals were housed at EBM or at the Robert Debre hospital with access to food and water ad libitum. Mortality was low and not different between groups.
Preconditioning
Wistar rats were randomized into two groups and injected intraperitoneally (i.p.) with an MgSO4 bolus (Magnesium sulphate, 1 mmol/ml, Addex; Fresenius Kabi, Halden, Norway) (1.1 mg/g (n = 245), 0.55 mg/g (n = 46) or 0.37 mg/g (n = 41)) or as vehicle an equivalent volume of saline (Saline 9 mg/ml, B Braun Melsungen AG, Melsungen, Germany) prior to the induction of HI. The optimal dose (1.1 mg/g) was injected at different time-points prior to (six days (n = 43), 72 h (n = 36), 24 h (n = 41), 12 h (n = 33), 3 h (n = 35) and 30 min (n = 33)) or post (1 h (n = 31) HI. Brain injury was evaluated by macroscopic scoring and immunohistochemical staining seven days after HI.
Hypoxia-ischemia
At PND 7, pups were exposed to HI as previously described.23,24 Rats were anesthetized (isoflurane 1.5–5%) (IsoFlo vet 100%; Abbott Laboratories Ltd, Illinois, USA) in 1:1 oxygen-nitrogen mixture. The left common carotid artery was ligated with a 6.0 silk suture (Seide; Vömel, Germany), the incision closed and infiltrated with a local anesthetic (Xylocain 20 mg/ml, lidocain, hydrochloride; Astra Zeneca, Södertälje, Sweden). The total duration was <5 min. Pups were returned to their dams for recovery (1 h), then placed in a humidified chamber with air for 10 min, followed by 60 min hypoxia (8% oxygen in nitrogen), and 10 min in air (36℃).
Tissue collection and processing
On PND 14, rats were deeply anesthetized (0.1 ml of thiopental, Pentocur, Thiopental, 50 mg/ml i.p.; Abcur AB, Helsingborg, Sweden) and killed for histological processing; brains were fixed by transcardial perfusion (6% paraformaldehyde, Histofix; Histolab, Gothenburg, Sweden). Once dissected out, brains were photographed and graded macroscopically according to a previously published protocol25 (Table S1). After dehydration, brains were paraffin-embedded and cut (Meditome A550) into 7 µm thick sections at ∼3.3 mm from bregma.26
Immunohistochemistry
Sections were prepared for immunohistochemical staining by deparaffination in xylene followed by graded alcohol rehydration and boiling in 0.01 M citric acid buffer (pH 6.0) for antigen recovery followed by blocking of endogenous peroxidase/nonspecific binding (3% H2O2). Sections were incubated overnight with primary antibody: Mouse anti-MAP-2 (1:1000; M4403 Sigma-Aldrich, St. Louis, Missouri, USA) or Mouse anti-MBP (1:10,000; SMI-94 Covance, Princeton, New Jersey, USA), washed and incubated in appropriate secondary antibody. ABC elite was used for visualization of immunoreactivity followed by submersion into 0.5 mg/ml 3.3-diaminobenzidine, NiSO4.
Brain injury evaluation
Brain injury was quantified as area loss of MAP-2 immunoreactivity at the level of anterior hippocampus. Total MAP-2 positive area was measured in each hemisphere (MicroImage, 4.0, Olympus Optical) and tissue loss calculated as MAP-2 positive area in the ipsilateral hemisphere subtracted from the contralateral. Loss of MBP was used immunohistochemically to determine white matter injury. The MBP positive staining was measured (MicroImage, 4.0, Olympus Optical) in both hemispheres and the proportion of white matter injury was calculated by comparing the ipsilateral hemisphere to the contralateral.
Serum Mg2+ measurement
Pups (PND 6) were injected i.p. with MgSO4, (1.1 mg/g (n = 17), 0.55 mg/g (n = 13), 0.37 mg/g (n = 14)) or vehicle (n = 18) and decapitated after 3 or 24 h followed by immediate blood-collection. Serum was stored (−80℃) until analysis for Mg2+-content (Cobas 8000 Roche Diagnostics Scandinavia AB) by Centrallaboratoriet Klinisk Kemi, Sahlgrenska University Hospital, Gothenburg, Sweden.
RNA preparation
Pups (PND 6) were injected with MgSO4 (1.1 mg/g) or vehicle and deeply anesthetized (0.1 ml of thiopental, sodium pentotal, 50 mg/ml i.p.) after 3 or 24 h (n = 6/group), perfused with saline (0.9% Sodium Chloride Solution) and brains collected, snap-frozen and stored in −80℃. Total RNA including miRNA was prepared from whole cortex samples using miRNeasy Mini Kit (Qiagen). The RNA quality, (RQI-scores) was 9–10 as checked with Experion RNA STDsense chip (Biorad). Purity and amount were determined using Nano Drop (260/280 ratio of 1.9 - 2.1).
GeneChip expression analysis
Array expression analysis was performed according to the Affymetrix expression analysis technical manual, Genomics Centre, King's College London. Briefly, double-stranded cDNA was synthesized from total RNA and an in vitro transcription reaction was performed to produce biotin-labeled cRNA which was fragmented and hybridized to Affy mRNA array Gene ST v2.0, APT v1.15 (36,685 genes) and Affy miRNA 4.0. CEL files using the robust multi-array average algorithm27 performing three distinct operations: global background normalization, across array normalization, and log2 transformation of perfect match values (http://stat-www.berkeley.edu/users/bolstad/RMAExpress/RMAExpress.html). The robust multi-array average analysis, data management, statistical analysis, and gene ontology was performed using GeneSifter (http://login.genesifter.net/) and IPA (https://analysis.ingenuity.com).
RT-PCR
cDNA was prepared from 1 µg RNA using QuantiTect Reverse Transcription Kit (Qiagen). Each PCR (20 μL) ((2 μL cDNA, 10 μL Quanti Fast SYBR Green PCR Master Mix (Qiagen) and 2 μL PCR primer (QuantiTech Primer Assay, Qiagen)), was run on a LightCycler 480 (Roche, Sweden) (Table S2). Melting curve analysis ensured that only one PCR product was obtained and a standard curve generated using increasing concentrations of cDNA ensured quantification/estimation amplification efficiency. Amplified transcripts were quantified using the relative standard curve and normalized against the reference gene Ywhaz. Results were expressed as ΔCq; the difference in the quantification cycle (Cq) values between the target and reference genes.28 The difference in ΔCq for the different samples was then directly compared.
Mitochondrial isolation
Pups (PND 6) were injected with MgSO4 (1.1 mg/g) (n = 34) or vehicle (n = 37) and decapitated after 24 h (with or without HI). One hemisphere was immediately excised and homogenized in 3.8 ml 12% Percoll (GE Healthcare, Bio-Sciences AB, Uppsala, Sweden) in ice-cold MIB [225 mM mannitol, 75 mM sucrose, 1 mM EGTA, 5 mM HEPES–KOH, pH 7.2, 1 mg/mL fatty-acid-free bovine serum albumin (BSA)] using a Dounce Homogenizer (7 ml, #432-1271, VWR). Homogenate was pipetted onto 3.5 ml 26% Percoll laying on 3.5 ml 40% Percoll in centrifuge tubes, subsequently centrifuged at 30,000g for 10 min (4℃). The mitochondrial fraction was moved to a 2 ml tube containing 1500 µl MIB (ratio 1:4) and centrifuged at 15,000g for 5 min (4℃). Pellets were washed in 1 ml MIB, centrifuged at 15,000g for 5 min (4℃) and resuspended in 75 µl MIB containing BSA 1 mg/ml.
High-resolution respirometry and O2k-fluorometry
The Oxygraph-2k (O2k, OROBOROS Instruments, Innsbruck, Austria) was used for measurements of respiration and combined with the Fluorescence-Sensor Green, O2k-Fluo LED2-Module for H2O2 measurement. A protocol modified from our published methods29 was used for mitochondrial respiration measurements in the O2k. Measurements were performed on mitochondria (25℃) in 2.1 mL MIR05 respiration buffer [20 mM HEPES, 10 mM KH2PO4, 110 mM sucrose, 20 mM taurine, 60 mM K-lactobionate, 0.5 mM EGTA, 3 mM MgCl2·6H2O, 1 g/L BSA (fatty acid free)]. Pyruvate and malate (5 mM and 2.5 mM, respectively) or succinate (10 mM) with or without 0.5 µM Complex-I inhibitor rotenone (Rot) were used to determine Complex-I (CI) or Complex-II-(CII) linked LEAK respiration. ADP (f.c. 0.14 mM) was added which was saturating for oxygen flux to obtain oxidative phosphorylation (OXPHOS) capacity. FCCP (1 µM) was added to determine electron transfer system (ETS) capacity. Respiratory control ratio (RCR) was calculated as the ratio of the STATE 3 (ADP stimulated respiration of isolated coupled mitochondria in the presence of high ADP and Pi concentrations) to the resting respiration rate STATE 4 (respiratory state obtained in isolated mitochondria when ADP has been maximally phosphorylated to ATP).
Mitochondrial ROS formation can be detected with an H2O2 sensitive probe, here Amplex® UltraRed was used and a CII-linked protocol measuring respiration and H2O2 flux simultaneously in the O2k-Fluorometer was applied. For further details see O2k-Fluorometry in Supplementary Materials.
For ROS production measurements, mitochondria were stimulated with ADP followed by rotenone, blocking Complex-I mediated respiration, allowing for the study of ROS produced solely at Complex-II driven by succinate addition. To induce maximal respiration/ROS production, FCCP (1 µM) was added. Volume-specific H2O2 fluxes were calculated real-time (DatLab software, OROBOROS INSTRUMENTS, Innsbruck Austria). The stable portions of the H2O2 fluxes were selected and artifacts induced by addition of substrates/chemicals were excluded. Values were normalized to total protein content.30–32
Metabolomics
Brains were collected at 3 h (n = 10) or 24 h (n = 10) following injection (PND 6) with MgSO4 (1.1 mg/g) or vehicle (n = 18); 24 h after injection with MgSO4 (1.1 mg/g) (n = 9) or vehicle (n = 10), pups were exposed to HI (60 min) and brains collected immediately after hypoxia. Animals were decapitated and whole heads snap-frozen in −80℃ isopenthane. Portions (10–30 mg) of parietal cortex were dissected out (−20℃) and solubilized in 3 mol/L HClO433 (−10℃). After centrifugation at 5000g for 10 min, one volume of the supernatant was neutralized with 2.5 volumes of KHCO3 (2 mol/L) and centrifuged to remove precipitated KHCO3. Blood was collected at decapitation and serum prepared as described above and saved in 2 ml Micro tubes (Sarstedt Aktiengesellshaft & Co, D-51588 Nümbrecht) (−80℃). For detailed description of H+-NMR protocol see Metabolomics in Supplementary Materials.
CBF
CBF was measured using the iodoantipyrine method34 adapted for the immature rat.35,36 The measurements were performed in both hemispheres 24 h after MgSO4 or vehicle administration without exposure to HI (no HI; vehicle n = 11, MgSO4 n = 9), after 30 min of HI (30 min HI; vehicle n=7, MgSO4 n = 8) and after 60 min of HI (60 min HI; vehicle n = 9, MgSO4 n = 8). In all animals, 10 µCi, 100 µl 4-Iodo(N-metyl14C) antipyrine (ARC 0126A, ARC; USA) was subcutaneously injected in the neck followed by decapitation after 60 s. Blood, 10 µl, was collected directly and the hemispheres were dissected out, dissolved in Solveble™ (Perkin Elmer, Massachusetts; USA) and mixed with Ultima Gold ™ (Perkin Elmer, Massachusetts; USA). Samples were measured in a Beta-counter, Tricarb 3810tr (Perkin Elmer, Massachusetts, USA) and calculated as previously described.37
BioPlex
Pups were exposed to HI 24 h after MgSO4 injection (1.1 mg/g) (n = 20) or vehicle (n = 20). Brains were collected at 6 or 24 h following hypoxia, sonicated in PBS-buffer (5 mM EDTA, 1% Triton X-100, 1% Protease Inhibitor Cocktail (P8340, Sigma)) and centrifuged at 10,000g for 10 min (4℃). Supernatants were diluted in Sample Diluent (Bio-Plex kit, Bio-Rad) and run on a Bio-Plex Pro™ Rat Cytokine 24-plex Assay (#171K1001MSP, Bio-Rad) according to the manufacturer's instruction.
Statistical measurements
All statistical analyses (except for gene expression and metabolomic analysis described above) were performed using Graph Pad Prism. Statistical significance was determined by nonparametric unpaired Mann–Whitney U-test, paired or unpaired two-tailed t-tests or ANOVA with or without Tukey's multiple comparisons test as specified in each figure legend (Mean ± SEM). P values < 0.05 were considered statistically significant. The number of sampled units, n, is stated for each experiment in the methodologic section.
Results
MgSO4 pre-treatment reduces the extent of neonatal brain injury after HI
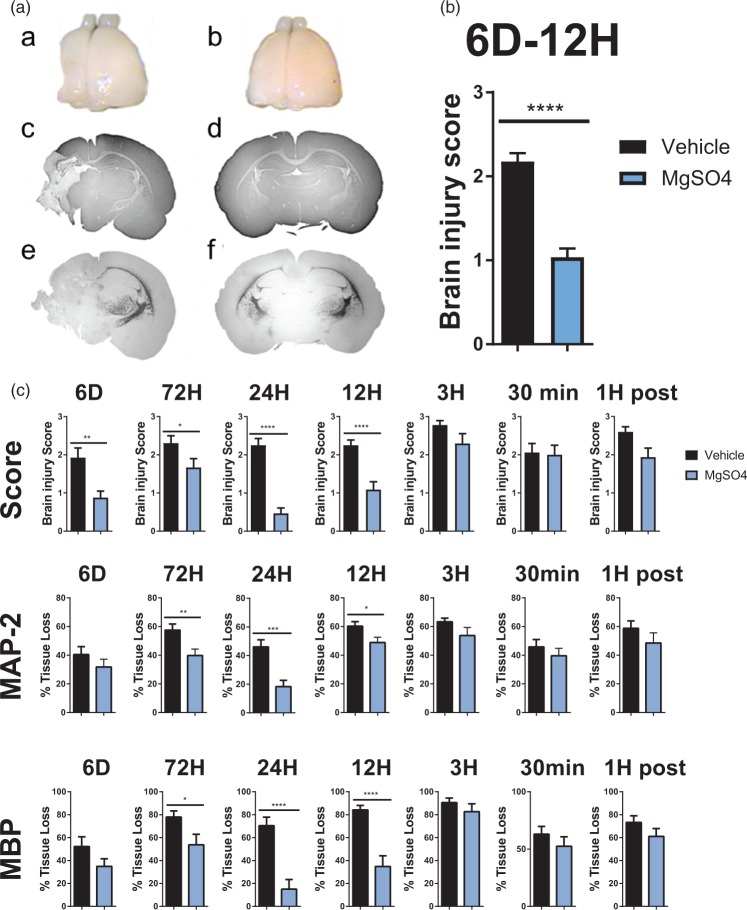
The effect of MgSO4 on neonatal brain injury was first explored in a well-established model of HI in seven-day-old rats.23 Macroscopic brain injury scoring (Table S1) showed that rats pre-treated with MgSO4 (1.1 mg/g) 24 h prior to HI had markedly reduced brain damage 0.47 ± 0.14 (n = 16) compared with 2.30 ± 0.18 (n = 18) in pups pre-treated with saline (p < 0.0001) (Figure 1(a) and (c)). Significant protection, was also seen when MgSO4 was administered six days prior to HI ( 0.9 ± 0.2 (n = 21) in MgSO4-treated pups vs. 1.9 ± 0.3 (n = 20) in controls (p < 0.005)), three days prior to HI (1.7 ± 0.2 (n = 18) in MgSO4-treated pups compared with 2.3 ± 0.2 (n = 18) in controls (p < 0.05)) and 12 h prior to HI: 1.1 ± 0.2 (n = 17) in MgSO4-treated pups vs. 2.3 ± 0.1 (n = 16) in controls (p < 0.0001). The overall effect of MgSO4 (1.1 mg/g) 6 days–12 h prior to HI is summarized in Figure 1(b) (n = 72/group). MgSO4 had no significant effect on brain injury when administered 3 h or 30 min prior to or 1 h after HI (Figure 1(c)). We also assessed the effect of MgSO4 pre-treatment on the loss of grey matter using immunoreactivity for MAP-2 and found a significant reduction in tissue loss when MgSO4 was given 72 h prior to HI (39.8 ± 4.6% (n = 17) vs. 57.5 ± 4.3% (n = 18) in controls (p = 0.01)), 24 h prior to HI (18.3 ± 4.4% (n = 15) in MgSO4-treated pups compared with 46.1 ± 4.8% (n = 19) in controls (p = 0.0003)) and at 12 h prior to HI (48.9 ± 3.7% (n = 18) in MgSO4-treated pups vs. 60.3 ± 3.2% (n = 17) in controls (p = 0.03); Figure 1(a) and(c)). For assessment of white matter loss, we estimated MBP immunoreactivity and found significant differences in the groups pre-treated with MgSO4 at 72 h prior to HI: loss of MBP positive immunoreactivity was 53.8 ± 9.2% (n = 18) in MgSO4-treated pups and 78.0 ± 5.3% (n = 18) in controls (p = 0.03). This was maintained in treatments at 24 h prior to HI (15.0 ± 8.4% (n = 15) in MgSO4-treated pups vs. 70.4 ± 7.4% (n = 19) in controls (p < 0.0001)) and 12 h prior to HI (34.8 ± 9.3% (n = 18) in MgSO4-treated pups compared with 84.3 ± 3.8% (n = 17) in controls (p = 0.03); Figure 1(a) and (c)).
Figure 1.
Evaluation of MgSO4 preconditioning protection.(a) Macroscopic brain injury assessment – brains were collected from rat pups preconditioned with (a) vehicle (n = 18) or (b) MgSO4 (n = 17) 24 h prior to HI, collected seven days post HI (p < 0.0001). Brain injury analyses were performed on MAP-2 stained sections (c and d) and MBP-stained sections (e and f) at the level of anterior hippocampus from brains shown in image (a) and (b). (B) Overall summary of macroscopic brain injury score at 6 days–12 h; n = 72/condition (p < 0.0001) (Mann–Whitney U test, Mean ± SEM). (c) Macroscopic brain injury score and measurement of grey (MAP-2) and white (MBP) matter injury at PND 14 after i.p. injection with NaCl (vehicle) or MgSO4 at different time points prior to and post HI, (p < 0.05) (Mann–Whitney U test, Mean ± SEM).
There are exposures other than HI that are believed to elicit preterm brain injury so we evaluated the neuroprotective effect of MgSO4 in a well-characterized excitotoxic model of preterm brain injury.38 MgSO4 (0.92 mg/g) 24 h prior to ibotenate injection (10 µg) reduced both grey and white matter injury (Figure S1) supporting the concept that MgSO4 offers efficacy in the treatment of immature brain injury regardless of its aetiology.
The neuroprotective dose of MgSO4 induces preconditioning
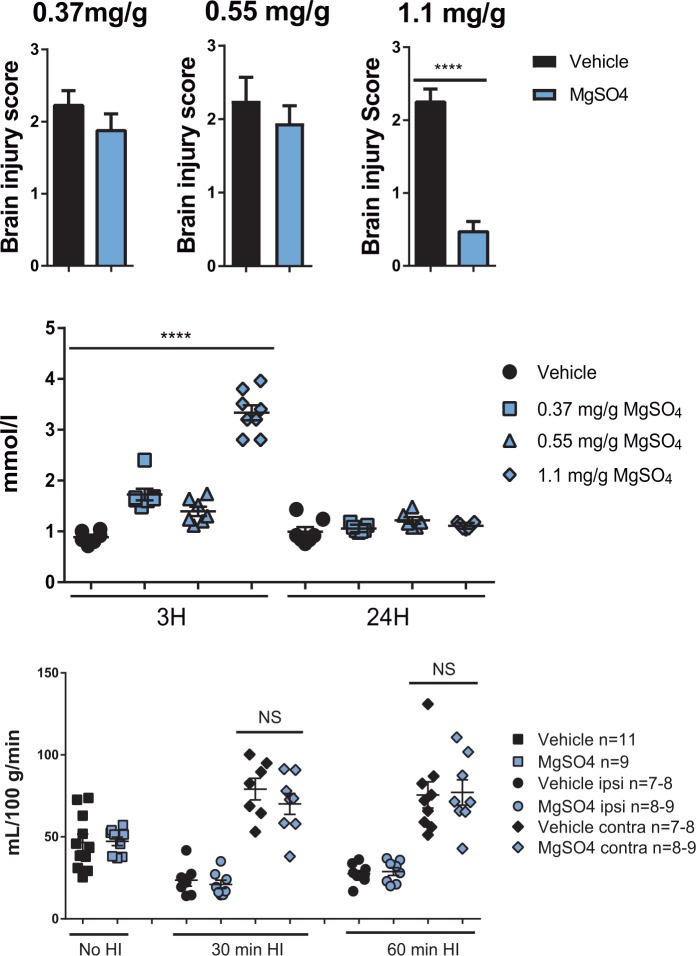
As the optimal interval between MgSO4 treatment and HI was 24 h, the next step was to identify the optimal dose required for maximal protection. We injected PND 6 rats with different doses of MgSO4 (0.37 mg/g, 0.55 mg/g or 1.1 mg/g) 24 h prior to HI. Brains were analyzed as described above and we found that only a dose of 1.1 mg/g provided the animals with significant protection (Figure 2(a)). We measured the concentration of Mg2+ in serum samples from pups after receiving vehicle or MgSO4 at 3 or 24 h after administration (Figure 2(b)). For all doses tested, serum Mg2+ levels increased transiently, peaking at 3 h after administration and returning to baseline within 24 h. Administration of the neuroprotective dose of MgSO4 (1.1 mg/g) resulted in a peak Mg2+concentration of 3.3 ± 0.2 mmol/L at 3 h later which is within the therapeutic window (2–4 mmol/L) when MgSO4 is given antenatally to women for seizure prophylaxis in preeclampsia or for neuroprotection. As pre-exposure with MgSO4 protects the brain in spite of the levels of magnesium being within the physiological range during/after HI, these data support our hypothesis that MgSO4 induces CNS tolerance (i.e. confers preconditioning) rather than conferring direct protection during/after the insult.
Figure 2.
MgSO4 dose-response, serum concentrations and effect on cerebral blood flow.(a) Preconditioning protection induced by different doses of MgSO4; 0.37 mg/g (vehicle n = 20, MgSO4 n = 20), 0.55 mg/g (vehicle n = 18, MgSO4 n = 28) or 1.1 mg/g (vehicle n = 18, MgSO4 n = 16) (p < 0.0001), unpaired t-test Mean ± SEM. (b) Concentration of Mg2+ in serum samples collected at 3 h or 24 h after injection with one of the three doses of MgSO4 (0.37 mg/g (n = 14), 0.55 mg/g (n = 13), or 1.1 mg/g (n = 17) or an equivalent volume of vehicle (n = 18) (p < 0.0001), unpaired t-test Mean ± SEM. (c) Cerebral blood flow (CBF) was measured in ipsi- and contralateral hemispheres 24 h after MgSO4 or vehicle administration in a group not subjected to HI, (no HI; vehicle n = 11, MgSO4 n = 9) and in groups exposed to 30 min (vehicle n = 7, MgSO4 n = 8) and 60 min (vehicle n = 9, MgSO4 n = 8)of HI. There were no significant differences in CBF between MgSO4 treated and vehicle groups (p > 0.05).
Preconditioning with MgSO4 does not change CBF
We then explored the possible mechanisms behind the profound preconditioning effect of MgSO4. It is well-known that MgSO4 has cerebrovascular effects, modulating the release of calcitonin gene-related peptide and nitric oxide,39 and could theoretically improve CBF during HI, explaining its protective action. However, no significant difference in CBF could be detected between MgSO4 and vehicle at 24 h after administration or during the subsequent period of HI (Figure 2(c)), implying that MgSO4 does not provide protection by affecting CBF.
MgSO4 regulates mitochondrial/metabolic mRNA and miRNA in the brain
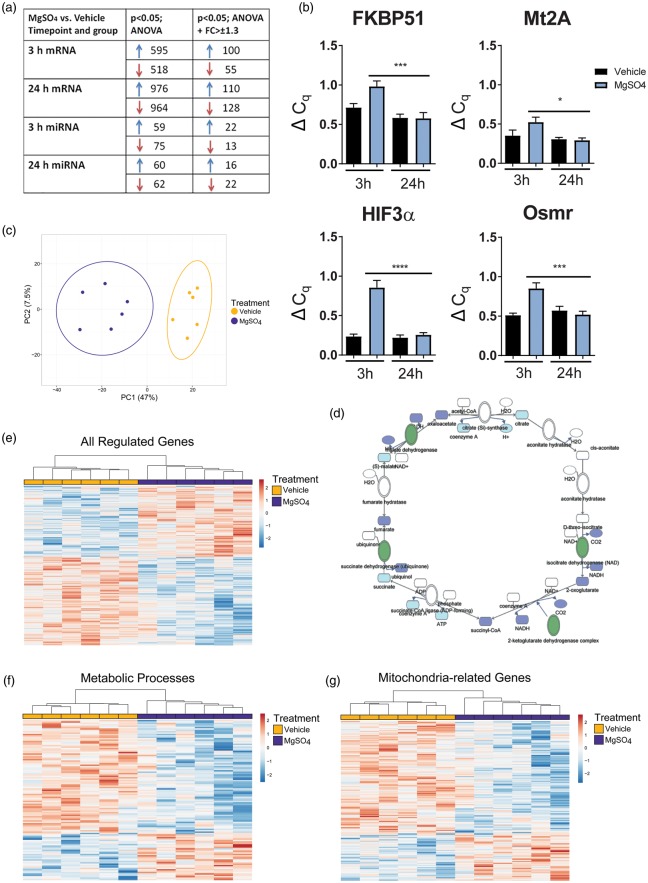
The development of CNS injury resistance required >12 h delay after MgSO4 injection (Figure 1). Previous studies have shown that preconditioning protection can depend on gene transcription and protein translation,16 therefore we analyzed the effect of MgSO4 on mRNA and miRNA expression in the cerebral cortex. Firstly, gene expression in MgSO4 and vehicle-treated groups was analyzed by microarray and compared with ANOVA (p < 0.05) alone or combined with the requirement of ±1.3 fold change. Multiple genes and miRNAs were altered (Figure 3(a)) and several of them have been found to be regulated in other preconditioning paradigms (Tables S3 and S4). Selective RT-PCR was performed to confirm differences observed by microarray, establishing that MgSO4 indeed modulates gene expression (Figure 3(b)).
Figure 3.
MgSO4 PC causes regulatory changes in mRNA and miRNA and downregulation of metabolic and mitochondrial pathways. (a) Summary of mRNA and miRNA findings at 3 h and 24 h after administration of MgSO4 or vehicle, ANOVA p < 0.05 with and without fold change > ± 1.3. (b) RT-PCR results for a selection of genes upregulated at 3 h post injection with MgSO4 or vehicle; FKBP51 (from 0.52 ± 0.04 ΔCq; n = 6 to 0.85 ± 0.07 ΔCq; n = 6) (p = 0.0004); Mt2A (from 0.29 ± 0.03 ΔCq; n = 6 to 0.52 ± 0.06; n = 6) (p = 0.02); HIF3α (from 0.26 ± 0.03 ΔCq; n = 6 to 0.86 ± 0.09 ΔCq; n = 6) (p < 0.0001); Osmr (from 0.52 ± 0.04 ΔCq; n = 6 to 0.85 ± 0.07 ΔCq; n = 6) (p = 0.0003) (ANOVA, p < 0.05). (c) Principal component analysis plot of all regulated genes 24 h after MgSO4/vehicle injection (n = 6/group). (d) Network analysis demonstrates that several genes for enzymes in the citric acid cycle were downregulated (marked in green) after MgSO4 injection, including succinate dehydrogenase, malate dehydrogenase, isocitrate dehydrogenase and 2-ketoglutarate complex at 24 h. (e) Heatmap cluster of all genes from (a). (f–g) Heatmaps visualizing the genes regulated in metabolic (f) and mitochondrial (g) pathways at 24 h post MgSO4 vs. vehicle, respectively.
Using ClustVis, a PCA plot40 was constructed using data from all 907 regulated genes at 24 h (Figure 3(c)) showing a clear separation between samples from MgSO4 pre-treated animals and vehicle offering further evidence that MgSO4 affects gene transcription in the brain. Many of the regulated genes relate to mitochondrial function and at 24 h after MgSO4 administration, both succinate dehydrogenase (SDH) and malate dehydrogenase were significantly downregulated (Figure 3(d)). Furthermore, pathway analysis demonstrated that metabolic pathways were significantly downregulated at 24 h after MgSO4 vs. vehicle (Z score: 2.98) which was further confirmed in the gene ontology analysis where 226/351 cellular metabolic process genes (Z score: 4.14) and 72/95 mitochondrial genes (Z score: 4.14) were downregulated (GeneSifter software, Geospiza). The gene data used for the PCA (above; 3(c)) was then used to create a heatmap showing up- or downregulation of multiple genes at 24 h after MgSO4 treatment compared to vehicle (Figure 3(e)). Heatmap clusters were constructed from all genes found to be regulated in metabolic pathways visualizing that 65% of genes in cellular metabolic processes (Figure 3(f)) and 76% of mitochondria-related genes were downregulated (Figure 3(g)). We also performed analyses of corresponding proteins utilizing an experimental dataset from the proteomic study of about 7300 mitochondrial proteins from MitProNet40,41 (a functional linkage network of mitochondrial proteins generated by integrating genomic features encoded by a wide range of datasets including genomic context, gene expression profiles, protein-protein interactions, functional similarity and metabolic pathways). We superimposed our microarray data on MitoProtNet dataset and performed IPA analyses and found a significant down-regulation of genes corresponding to proteins in complexes I, II, III and IV of the ETC at 24 h by MgSO4 (ANOVA; p < 0.05).
MgSO4 targets pathological succinate accumulation in the immature brain
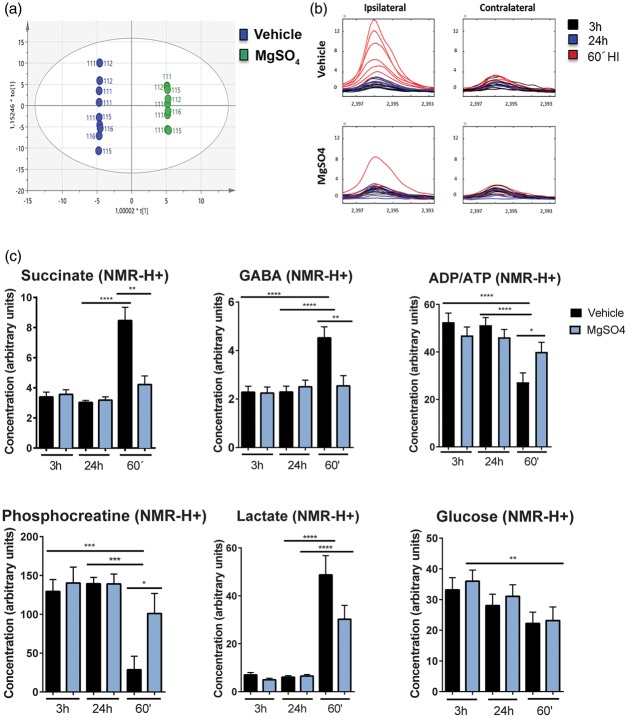
These results indicate that MgSO4 modulates mitochondrial pathways, including succinate metabolism, which is interesting but not surprising as magnesium is involved in regulation of >600 enzymatic reactions including those involved in energy metabolism.39,42 However, recently Chouchani et al.43 demonstrated that the extent of succinate increase during ischemia is critical as it triggers production of ROS and impairs mitochondrial function. Other groups42 suggest that succinate triggers a pro-inflammatory response which could be critical during post-HI recovery. Therefore, we explored whether MgSO4 modulates the metabolomic response before and during HI (Figure S2). The analysis revealed that the MgSO4-treated samples differ from vehicle in the 3 h group for cortex (Figure 4(a)), indicating that MgSO4 itself has an effect on brain metabolism. Furthermore, MgSO4 suppressed metabolic responses exacerbated in cerebral cortex during the HI insult. Succinate was significantly increased after HI in the vehicle-treated rats (n = 9) (from 4.1 ± 0.5 a.u. to 11.6 ± 2.4 a.u.; p < 0.01), whereas no such increase was detected following MgSO4 pre-treatment (n = 6) (from 4.2 ± 0.5 a.u. to 6.6 ± 2.0 a.u.; NS) (Figure 4(b) and (c)). An increase in GABA in the cerebral cortex was detected in the vehicle-treated group (n = 9) after 60 min of HI (from 2.3 ± 0.2 a.u. to 4.3 a.u.; p < 0.0001) whereas no increase was detected in the MgSO4 group (n = 6) (from 2.5 ± 0.3 a.u. to 2.5 ± 0.4 a.u.; NS). As expected, phosphocreatine and ATP/ADP levels decreased significantly in the vehicle group after HI (p < 0.0004 and p < 0.0001, respectively), whereas the levels were better maintained in the MgSO4 group. Additionally, a significant difference in phosphocreatine (p < 0.03) and ATP/ADP (p < 0.03) concentration was detected between the vehicle and MgSO4 pre-treated groups. Lactate was significantly increased in both the MgSO4 (from 6.5 ± 0.6 a.u. to 30.2 ± 5.7 a.u.; p < 0.0001) (n = 6) and vehicle group (from 6.1 ± 0.6 a.u. to 48.8 ± 8.0 a.u.; p < 0.0001) (n = 9) although with a tendency towards being higher in the latter (NS). Glucose concentrations were lower after HI with no difference between groups (Figure 4(c)). These metabolic data indicate that the potentially toxic succinate accumulation is attenuated by MgSO4 pre-treatment and this effect was paralleled by preservation of high-energy phosphates indicating protection of mitochondrial function.
Figure 4.
MgSO4 preconditioning prevents succinate and GABA accumulation as well as high-energy phosphate depletion in the brain after HI.(a) Principal component analysis (OPLS-DA) of the metabolomic analysis of cortex samples (ipsi- and contralateral) collected 3 h after i.p. injection with vehicle (n = 9) as shown in blue or MgSO4 (n = 6) shown in green. (b) Metabolic trace plots of succinate levels in cerebral cortex after 60 min of HI (red) in samples from rats injected with vehicle (n = 9) or MgSO4 (n = 6) 24 h prior to HI in contra- and ipsilateral hemisphere and in animals exposed to treatment without HI 3 h or 24 h prior to sampling. (c) Cerebro-cortical metabolites 3 h and 24 h after administration of MgSO4 or vehicle, and 24 h of MgSO4 or vehicle + 60 min of HI (60′). There was an accumulation of succinate (p < 0.05) and GABA (p < 0.001) after 60 min of HI in the vehicle group but not in MgSO4-treated group. Depletion of phosphocreatine (p < 0.0002) and ADP/ATP (p < 0.02) was attenuated during HI in the MgSO4 group compared to vehicle-treated group. The lactate concentration increased to the same extent in MgSO4 pre-treated animals (p < 0.0001) as in vehicle (p < 0.0001) after HI and glucose concentrations decreased in the cerebral cortex following HI in both groups (n = 6–10/condition), (one-way ANOVA with Tukey's multiple comparisons test).
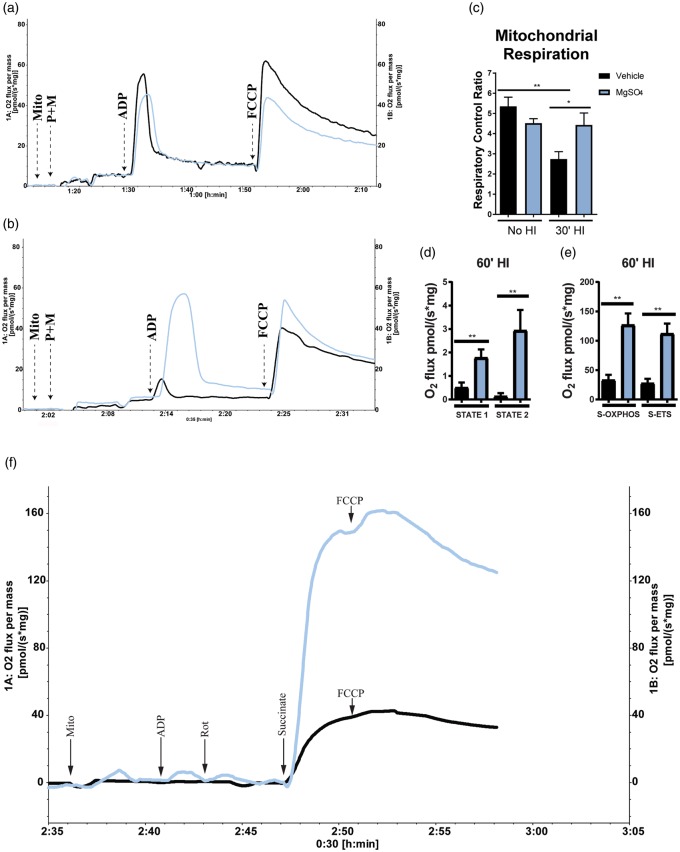
MgSO4 preserves brain mitochondrial respiratory capacity
The Complex-I-mediated respiratory capacity of isolated brain mitochondria was analyzed using high-resolution respirometry (O2k chamber). No significant difference in RCR, i.e. STATE 3/STATE 4 respiration, was detected between MgSO4 and vehicle at 24 h after injection (Figure 5(a) and (c)). We found, however, that the RCR decreased from 5.3 ± 0.5 pmol/(s*mg); n = 7 to 2.7 ± 0.4 pmol/(s*mg); n = 6; p < 0.002 after 30 min HI in the vehicle group, whereas it remained unchanged at 30 min HI in the MgSO4 group (4.5 ± 0.2 pmol/(s*mg); n = 6 before HI and 4.4 ± 0.6 pmol/(s*mg) after 30 min HI; n = 7) (Figure 5(b) and (c)). Hence, the RCR was significantly higher in the MgSO4 group compared with vehicle at 30 min HI induced 24 h after administration (p < 0.05).
Figure 5.
MgSO4 preconditioning preserved brain mitochondrial respiration after HI.(a) Respirogram showing vehicle (black) and MgSO4-(blue) treated samples analyzed 24 h post injection. (b) Respirogram showing vehicle (black) and MgSO4-(blue) treated samples analyzed after 30 min of HI. Respiration was significantly higher in the MgSO4-treated group compared to vehicle (p < 0.05) (Unpaired t-test, Mean ± SEM) (n = 6–7/group). (c) Mitochondrial respiration. A significantly higher RCR was found in brain mitochondria isolated from animals pretreated with MgSO4 compared to vehicle-treated animals after 30 min of HI (30′HI). No difference could be detected in RCR between MgSO4-treated animals with or without HI, whereas there was a ∼50% drop in RCR in response to HI in the vehicle-treated group (p < 0.002), (ANOVA, p < 0.05). (d) Basal mitochondrial respiration (STATE 1) as well as respiration in the presence of added ADP but absence of reducing substrates (STATE 2) in vehicle (black) vs. MgSO4 (blue; n = 4/group) (p < 0.03). (e) Complex-II-mediated respiration driven by succinate as reducing substrate during blockage of Complex-I by Rotenone in MgSO4 vs. vehicle (p < 0.03). Also, maximal ETS capacity induced by addition of FCCP (p < 0.03) (n = 5/group), (Mann–Whitney U test, Mean ± SEM). (f) Respirogram showing the respiratory activity of isolated mitochondria from MgSO4 (blue) compared to vehicle (black) group. The time-point for addition of each substrate is indicated by arrows in the figure.
To further characterize the effect of MgSO4 on mitochondrial function, we studied Complex-II-mediated mitochondrial respiration using succinate and rotenone in the O2k. The results showed a significant increase in succinate-driven OXPHOS (S-OXPHOS) in the MgSO4 pre-treated group compared to vehicle (p < 0.005) (Figure 5(e) and (f)) after 60 min HI. Further we analyzed the succinate driven ETS capacity (S-ETS) using the uncoupler FCCP to induce maximal Complex-II-mediated respiration, which was significantly higher in the MgSO4 group (p < 0.005) (Figure 5(e) and (f)). No significant increase could be detected in the Complex-II-mediated respiratory capacity after the addition of FCCP during these settings (compared to S-OXPHOS) which is in concordance with previous reports.44 A significant difference was also detected in the basal mitochondrial respiration (STATE 1) between the MgSO4 pre-treated group and vehicle (p < 0.009) as well as in STATE 2 respiration, (when ADP is added to mitochondria in the absence of reducing substrates) (p < 0.005, n = 6/group) (Figure 5(d)). In summary, these data indicate that MgSO4 preserves mitochondrial Complex-I and-II respiration after HI.
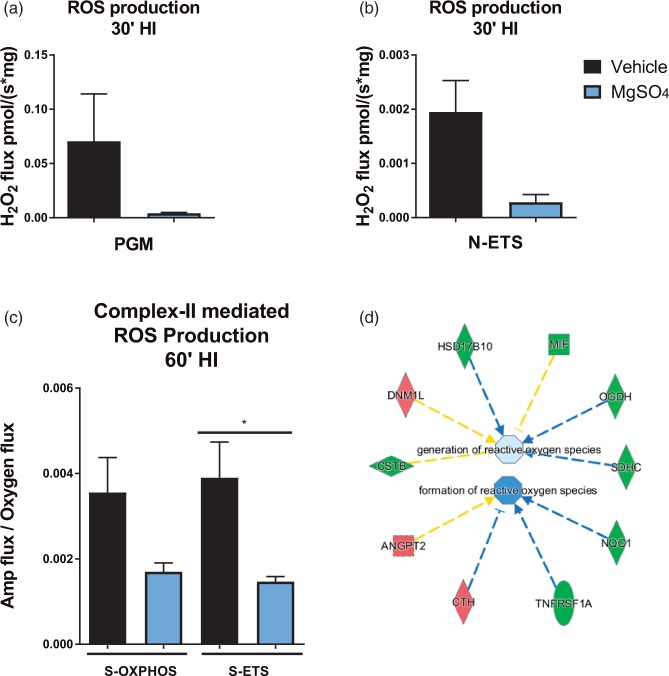
MgSO4 attenuates ROS production in the brain after HI
Next, we investigated whether this functional improvement was associated with alleviation of oxidative stress. Production of ROS in isolated brain mitochondria was measured using Amplex Red. We found that the Complex-I-linked production of ROS tended to decrease in the MgSO4 samples (n = 4) at 30 min HI compared to vehicle (n = 5) in the presence of NADH generating substrates (pyruvate, glutamate and malate) (Figure 6(a)) as well as when FCCP had been added (N-ETS) (Figure 6(b)). Furthermore, the production of ROS was measured using the O2k in the absence of exogenous NADH generating substrates with the purpose of detecting endogenous ROS production only. When succinate was added to the isolated brain mitochondria stimulated with ADP, the detected production of ROS was 0.004 ± 0.001 pmol/(s*mg); n = 6 in vehicle vs. 0.002 ± 0.001 pmol/(s*mg) (n = 6) in the MgSO4 group. Following the addition of FCCP, a significantly higher production of ROS was detected in the vehicle group (0.004 ± 0.001 pmol/(s*mg); n = 6) compared with the MgSO4 group (0.002 ± 0.0001 pmol/(s*mg); n = 6) (Figure 6(c)) (p = 0.02). We also used IPA to analyze our microarray data and detected downregulation of several genes involved in the pathway of ROS production in the MgSO4 group compared to vehicle, predicting inhibition of generation and formation of ROS (Figure 6(d)) corresponding to our findings using the O2k equipment.
Figure 6.
MgSO4 preconditioning attenuates brain mitochondrial ROS following HI. (a) ROS production at 30 min HI in the presence of the NADH substrates pyruvate, glutamate and malate in MgSO4 and vehicle group (n = 4/group). (b) ROS production at 30 min HI at maximal ETS induced by addition of FCCP (n = 4/group). (Mann–Whitney U test, Mean ± SEM; NS). (c) ROS production in MgSO4 vs. vehicle pre-treated samples collected at 60 min HI at the addition of succinate and FCCP (n = 6/group) controlled for level of mitochondrial respiration by dividing the total ROS production (the AMP-flux) by the mitochondrial respiration (O2 flux) (one-way ANOVA with Tukey's multiple comparisons test, p < 0.05). (d) IPA prediction of decreased ROS generation and formation in the MgSO4 group at 24 h after administration. Upregulated genes displayed in red and downregulated genes displayed in green. Downregulation as predicted by IPA is illustrated in blue.
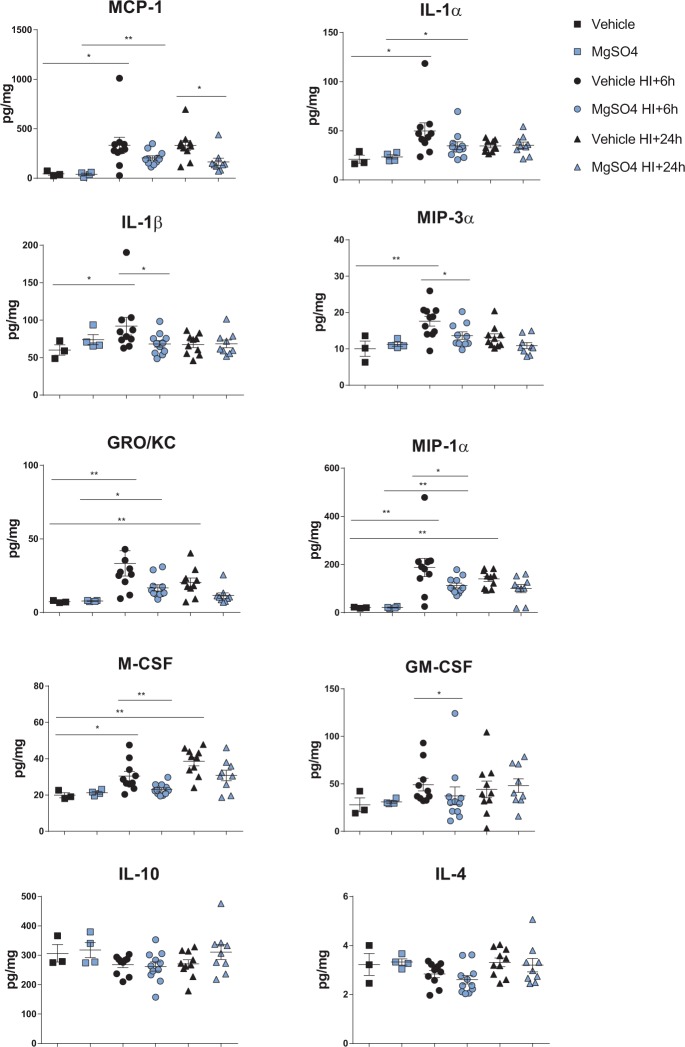
MgSO4 pre-treatment attenuates the post HI accumulation of chemokines and pro-inflammatory cytokines
Previous studies suggest that succinate activates the succinate receptor 1 (SUCNR1) leading to activation of pro-inflammatory responses via p38, NFAT (nuclear factor of activated T-cells) and NFκB (nuclear factor kappa B) signaling.42 Therefore, we speculated that MgSO4 could modify the cytokine/chemokine responses after HI due to the attenuation of succinate accumulation (above). Furthermore, mitochondrial ROS production has been suggested to enhance the pro-inflammatory response42 and as MgSO4 reduced ROS production, we expected that the inflammatory response would be affected. We prepared whole brain homogenate at 6 and 24 h after HI comparing the MgSO4 and vehicle groups. Indeed, pre-treatment with MgSO4 significantly attenuated the accumulation of MCP-1 (CCL2), IL-1 α, IL-1 β, MIP-3α (CCL20), GRO/KC (CXCL1), MIP-1 α (CCL3), M-CSF and GM-CSF at 6-24 h after neonatal HI (Figure 7(a) to (h)), whereas the anti-inflammatory cytokines IL-4 and IL-10 were not different between the groups (vehicle no HI; n = 3, MgSO4 no HI; n = 4, vehicle HI + 6 h; n = 10, MgSO4 HI + 6 h; n = 11, vehicle HI + 24 h; n = 10, MgSO4 HI + 24 h; n = 9) (Figure 7(i) to (j)).
Figure 7.
Attenuation of chemokines and pro-inflammatory cytokine accumulation in the brain by preconditioning with MgSO4. (a–h) Attenuation of MCP-1, IL-1α, IL-1β, MIP-3α, GRO-KC, MIP-1α, M-SCF and GM-CSF accumulation at 6 h and/or 24 h post HI in the MgSO4-treated group. No significant difference in IL-10 (I) or IL-4 (J) between the groups at either time-point. (one-way ANOVA with Tukey's multiple comparisons test, p < 0.05) (vehicle no HI; n = 3, MgSO4 no HI; n = 4, vehicle HI + 6 h; n = 10, MgSO4 HI + 6 h; n = 11, vehicle HI + 24 h; n = 10, MgSO4 HI + 24 h; n = 9) .
Discussion
We have demonstrated for the first time that MgSO4 conferred preconditioning to the immature rat brain if given 6 days–12 h prior to an HI or excitotoxic insult reducing brain injury in all areas, particularly cerebral cortex, by as much as 80%. Administration of MgSO4 induced mRNA/miRNA changes in the cerebral cortex previously shown to be regulated in response to other forms of preconditioning and many of them related to mitochondria.45–48 Metabolic pathways were generally downregulated including mitochondrial network genes, especially those corresponding to proteins in the electron transport chain. The metabolome was significantly modulated in both serum and brain at 3 h after MgSO4. Succinate accumulation, mitochondrial functional deterioration during HI and the increase of mitochondrial ROS production and tissue cytokines/chemokines after the insult were all attenuated by pre-treatment with MgSO4.
MgSO4 was given as an intraperitoneal bolus causing a temporary increase in serum magnesium concentration to ∼3.3 mmol/L at 3 h but it is important to point out that the levels were completely normalized at 24 h post injection, i.e. the concentration of magnesium was at physiological levels during and after the HI insult. According to previous studies, increases of serum MgSO4 elevate magnesium concentrations in the brain tissue49 as well as in the cerebrospinal fluid50 suggesting that extracellular and intracellular magnesium concentrations increase at least transiently in the brain. Magnesium modulates calcium hemostasis and the activity of multiple enzymes51 possibly explaining why the metabolome is altered in blood and brain at 3 h after MgSO4 administration. These metabolic/enzymatic changes may secondarily induce the subtle gene transcription changes detected in the brain at 3 and 24 h after treatment, probably contributing to development of mitochondrial and CNS resistance as well as the metabolism downregulation and attenuation of succinate accumulation in response to HI. Indeed, the gene ontogeny and pathway analysis indicating metabolic suppression by MgSO4 are supported by a recent clinical study demonstrating a drop in cerebral oxygen consumption and cerebral oxygen extraction in newborns pre-exposed to MgSO4 antenatally.52 These changes occurred in spite of unaffected CBF52 which agrees with our results showing that CBF was not different between the MgSO4 and vehicle group at 24 h after administration or even during the subsequent HI, supporting that both groups were suffering from a similar degree of primary HI insult.
Succinate is reported to accumulate in tissues, including the immature and adult brain,53,54 in response to HI leading to succinate-driven excessive mitochondrial production of ROS during reperfusion and subsequent aggravation of injury.51 The cause of the succinate increase is reversal of SDH activity, resulting in the conversion of fumarate to succinate, driven by fumarate overflow from purine nucleotide breakdown and partial reversal of the malate/aspartate shuttle.51 The SDH inhibitor dimethyl malonate prevents accumulation of succinate and ROS, conferring significant neuroprotection.53 Presently, we found that MgSO4 pre-treatment prevented the accumulation of succinate and improved mitochondrial function in the neonatal brain. Interestingly, the mRNA analysis of the rat brain tissue demonstrated that the genes for SDH and malate dehydrogenase were downregulated by MgSO4. When analyzing Complex-II-mediated succinate-driven respiration in isolated mitochondria, we detected an increase in the MgSO4 pre-treated group, whereas no such increase was detected in the vehicle group, further confirming our data on endogenous succinate accumulation in the vehicle group. GABA accumulation during HI was also attenuated by MgSO4. Speculatively, magnesium modulates both the reversal of SDH and the GABA-shunt, thereby preventing accumulation of toxic levels of succinate in the brain tissue after HI. Succinate accumulation during HI can also trigger a more profound inflammation42,55 during the secondary phase after HI which may worsen brain injury.1 In fact, several studies show that MgSO4 attenuates the pro-inflammatory reaction in experimental models.56,57 Additional experiments are needed to clarify the mechanisms behind succinate accumulation in the immature brain and to elucidate how these metabolic events and mitochondrial functions are modulated by magnesium preconditioning as well as their specific role in neuroprotection.
Many enzymes in intermediary metabolism are modulated by magnesium, affecting >600 biological reactions51 which could hypothetically shift the balance and attenuate succinate accumulation. Indeed, we found that genes related to proteins in the ETC were downregulated by MgSO4 which has been shown to occur also in preconditioning of the heart in parallel to improvement of mitochondrial respiratory recovery.48 Recently, it was discovered that when preventing degradation of components of the ETC, such as SDH, BAX dependent apoptotic cell death known to be critical in HI brain injury,58 was attenuated.59
This study offers mechanistic information indicating that succinate metabolism and mitochondrial function are involved in preconditioning by MgSO4. However, more detailed information (using 1H-NMR, 31P-NMR and 13C-NMR) on how the metabolic network is modulated by MgSO4 treatment is needed, having to be addressed in the future.
Concerning clinical implications, the present finding that a transient increase of serum MgSO4 induces tolerance in the developing brain rather than providing a direct neuroprotective effect during/after the insult is of great importance. Indeed, these data support the clinical findings that MgSO4 is an effective neuroprophylaxis if treatment starts antenatally, some-time before the critical period for brain insults during delivery and the first few days of life. However, our results also indicate that it may be sufficient to offer a MgSO4 bolus (high enough to reach a transient magnesium increase to >2.5 mmol/l which in most patients would correspond to 6 g of MgSO4) rather than administration of MgSO4 infusions during delivery up until birth. Today there is no consensus as to required MgSO4 dose/duration for neuroprophylaxis as all published randomized trials have followed different dosing regimens.10–13 Some studies even indicate that long-lasting infusions of MgSO4 may increase necrotizing colitis, gut perforations and perinatal mortality,60–62 problems that could be avoided by administration of only a bolus. In addition, MgSO4 infusions require much more extensive monitoring of the mother than if only a bolus dose is provided which affects compliance for clinical implementation. Therefore, clinical studies are warranted to investigate whether providing only a bolus of MgSO4 is an efficient mode of neuroprophylaxis with superior safety and compliance in women in preterm labor.
Furthermore, the understanding that MgSO4 induced preconditioning protection of the immature CNS could lead to development of prophylactic treatment of other patients at high risk such as cases with high risk of intrapartum asphyxia or cases undergoing open heart surgery.
In conclusion, MgSO4 induces strong preconditioning of the immature brain which provides resistance to HI- and excitotoxicity-mediated injury in rats and mice. Magnesium increases in the CNS and have previously been shown to modulate enzymatic activities, calcium homeostasis and NMDA receptor activity. These alterations are anticipated to provoke transcriptomal changes with modulation of neuroprotective and metabolic mRNAs and miRNAs. The metabolism will be downregulated and altered leading to reduced succinate toxicity, mitochondrial protection and slower loss of high energy phosphate reserves in the brain tissue after HI. Speculatively, these reactions contribute to attenuated ROS production and inflammation (Figure S3).
Supplementary Material
Acknowledgments
We wish to thank Dr. Pernilla Svedin for excellent technical assistance and we gratefully acknowledge the support of the Department of Perinatal Imaging and Health. Furthermore, Anders Pedersen and Daniel Malmodin from the Swedish NMR centre at the University of Gothenburg are acknowledged for their support.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We gratefully acknowledge the financial support from Wellcome Trust (WT094823) (https://wellcome.ac.uk/), the Swedish Medical Research Council (VR 2015-02493) (https://www.vr.se/), Swedish Governmental Grant to Researchers at University Hospitals (ALFGBG-426401) (http://www.fou.nu/is/alfgbg/), Action Medical Research (https://www.action.org.uk/), ERA-net (EU;VR 529-2014-7551), Hjärnfonden (Brain Foundation 2015-0004) (http://www.hjarnfonden.se/), Project Grant awarded by the Research Foundation, Cerebral Palsy Alliance (PG4416) and the Leducq Foundation (DSRRP34404) (https://www.fondationleducq.org/) to enable this study to be completed. In addition, the authors acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) (https://www.nihr.ac.uk/) comprehensive Biomedical Research Centre Award to Guy's & St Thomas' NHS Foundation Trust (http://www.guysandstthomas.nhs.uk/home.aspx) in partnership with King's College London and King's College Hospital NHS Foundation Trust (https://www.gov.uk/government/groups/kings-college-hospital-nhs-foundation-trust). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions
Gabriella Koning performed the animal experiments and tissue preparations, carried out the analyses, performed the statistical analysis and drafted the initial manuscript together with Henrik Hagberg. Anna-Lena Leverin participated in the design of the study, assisted in some of the experimental work, performed some of the tissue preparations, carried out analyses and reviewed and revised the manuscript. Syam Nair provided technical support and performed the measurements of mitochondrial respiration and ROS accumulation, assisted in interpretation of data, performed some of the bioinformatic evaluations and reviewed the manuscript. Leslie Schwendimann performed all experiments in the ibotenate model in PND 5 mice, participated in data interpretation and reviewed the manuscript. Joakim Ek interpreted data, performed some of the bioinformatic evaluations and reviewed the manuscript. Ylva Carlsson participated in data interpretation and reviewed and revised the manuscript.Pierre Gressens participated in data interpretation and reviewed and revised the manuscript.Claire Thornton participated in data interpretation, offered professional support in writing the manuscript and reviewed and revised it. Xiaoyang Wang participated in data interpretation and reviewed and revised the manuscript. Carina Mallard took part in designing the study, participated in data interpretation and reviewed and revised the manuscript. Henrik Hagberg conceptualized and designed the study, obtained funding, interpreted data and reviewed and revised the manuscript. All authors approved the final manuscript as submitted. None of the material included in this manuscript has been published or is under consideration elsewhere, including the Internet.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Hagberg H, Mallard C, Ferriero DM, et al. The role of inflammation in perinatal brain injury. Nat Rev Neurol 2015; 11: 192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferriero DM. Neonatal brain injury. N Engl J Med 2004; 351: 1985–1995. [DOI] [PubMed] [Google Scholar]
- 3.Marlow N, Wolke D, Bracewell MA, et al. Neurologic and developmental disability at six years of age after extremely preterm birth. New Engl J Med 2005; 352: 9–19. [DOI] [PubMed] [Google Scholar]
- 4.Brown NC, Inder TE, Bear MJ, et al. Neurobehavior at term and white and gray matter abnormalities in very preterm infants. J Pediatr 2009; 155: 32–38. 8 e1. [DOI] [PubMed] [Google Scholar]
- 5.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 2009; 8: 110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson KB. Can we prevent cerebral palsy? New Engl J Med 2003; 349: 1765–1769. [DOI] [PubMed] [Google Scholar]
- 7.Hayden EC. Neuroscience: the most vulnerable brains. Nature 2010; 463: 154–156. [DOI] [PubMed] [Google Scholar]
- 8.Nelson KB, Grether JK. Can magnesium-sulfate reduce the risk of cerebral-palsy in very-low-birth-weight infants. Pediatrics 1995; 95: 263–269. [PubMed] [Google Scholar]
- 9.Kuban KC, Leviton A, Pagano M, et al. Maternal toxemia is associated with reduced incidence of germinal matrix hemorrhage in premature babies. J Child Neurol 1992; 7: 70–76. [DOI] [PubMed] [Google Scholar]
- 10.Crowther CA, Hiller JE, Doyle LW, et al. Effect of magnesium sulfate given for neuroprotection before preterm birth – a randomized controlled trial. JAMA 2003; 290: 2669–2676. [DOI] [PubMed] [Google Scholar]
- 11.Rouse DJ, Hirtz DG, Thom E, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. New Engl J Med 2008; 359: 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marret S, Marpeau L, Zupan-Simunek V, et al. Magnesium sulphate given before very-preterm birth to protect infant brain: the randomised controlled PREMAG trial*. BJOG 2007; 114: 310–318. [DOI] [PubMed] [Google Scholar]
- 13.Doyle LW, Crowther CA, Middleton P, et al. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev 2009. 1: CD004661. DOI: 10.1002/14651858.CD004661.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Hirtz DG, Weiner SJ, Bulas D, et al. Antenatal magnesium and cerebral palsy in preterm infants. J Pediatr 2015; 167: 834–839 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gano D, Ho ML, Partridge JC, et al. Antenatal exposure to magnesium sulfate is associated with reduced cerebellar hemorrhage in preterm newborns. J Pediatr 2016; 178: 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meller R, Simon RP. Tolerance to ischemia – an increasingly complex biology. Transl Stroke Res 2013; 4: 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gidday JM, Fitzgibbons JC, Shah AR, et al. Neuroprotection from ischemic brain injury by hypoxic preconditioning in the neonatal rat. Neurosci Lett 1994; 168: 221–224. [DOI] [PubMed] [Google Scholar]
- 18.Gustavsson M, Anderson MF, Mallard C, et al. Hypoxic preconditioning confers long-term reduction of brain injury and improvement of neurological ability in immature rats. Pediatr Res 2005; 57: 305–309. [DOI] [PubMed] [Google Scholar]
- 19.Shu Y, Patel SM, Pac-Soo C, et al. Xenon pretreatment attenuates anesthetic-induced apoptosis in the developing brain in comparison with nitrous oxide and hypoxia. Anesthesiology 2010; 113: 360–368. [DOI] [PubMed] [Google Scholar]
- 20.Galvin KA, Oorschot DE. Postinjury magnesium sulfate treatment is not markedly neuroprotective for striatal medium spiny neurons after perinatal hypoxia/ischemia in the rat. Pediatr Res 1998; 44: 740–745. [DOI] [PubMed] [Google Scholar]
- 21.de Haan HH, Gunn AJ, Williams CE, et al. Magnesium sulfate therapy during asphyxia in near-term fetal lambs does not compromise the fetus but does not reduce cerebral injury. Am J Obstet Gynecol 1997; 176: 18–27. [DOI] [PubMed] [Google Scholar]
- 22.Greenwood K, Cox P, Mehmet H, et al. Magnesium sulfate treatment after transient hypoxia-ischemia in the newborn piglet does not protect against cerebral damage. Pediatr Res 2000; 48: 346–350. [DOI] [PubMed] [Google Scholar]
- 23.Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 1981; 9: 131–141. [DOI] [PubMed] [Google Scholar]
- 24.Vannucci RC, Vannucci SJ. A model of perinatal hypoxic-ischemic brain damage. Ann N Y Acad Sci 1997; 835: 234–249. [DOI] [PubMed] [Google Scholar]
- 25.Bona E, Hagberg H, Loberg EM, et al. Protective effects of moderate hypothermia after neonatal hypoxia-ischemia: short- and long-term outcome. Pediatr Res 1998; 43: 738–745. [DOI] [PubMed] [Google Scholar]
- 26.Nijboer CH, Heijnen CJ, Groenendaal F, et al. Strong neuroprotection by inhibition of NF-kappaB after neonatal hypoxia-ischemia involves apoptotic mechanisms but is independent of cytokines. Stroke 2008; 39: 2129–2137. [DOI] [PubMed] [Google Scholar]
- 27.Bolstad BM, Irizarry RA, Astrand M, et al. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003; 19: 185–193. [DOI] [PubMed] [Google Scholar]
- 28.Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 2009; 55: 611–622. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Leverin AL, Han W, et al. Isolation of brain mitochondria from neonatal mice. J Neurochem 2011; 119: 1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured filroblasts. Nat Protoc 2007; 2: 287–295. [DOI] [PubMed] [Google Scholar]
- 31.Kuznetsov AV, Veksler V, Gellerich FN, et al. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc 2008; 3: 965–976. [DOI] [PubMed] [Google Scholar]
- 32.Puka-Sundvall M, Wallin C, Gilland E, et al. Impairment of mitochondrial respiration after cerebral hypoxia-ischemia in immature rats: relationship to activation of caspase-3 and neuronal injury. Dev Brain Res 2000; 125: 43–50. [DOI] [PubMed] [Google Scholar]
- 33.Gilland E, Puka-Sundvall M, Hillered L, et al. Mitochondrial function and energy metabolism after hypoxia-ischemia in the immature rat brain: involvement of NMDA-receptors. J Cereb Blood Flow Metab 1998; 18: 297–304. [DOI] [PubMed] [Google Scholar]
- 34.Sakurada O, Kennedy C, Jehle J, et al. Measurement of Local Cerebral Blood-Flow with Antipyrine-Iodo-C-14. Am J Physiol 1978; 234: H59–H66. [DOI] [PubMed] [Google Scholar]
- 35.Gilland E, Hagberg H. NMDA receptor-dependent increase of cerebral glucose utilization after hypoxia-ischemia in the immature rat. J Cerebr Blood Flow Metab 1996; 16: 1005–1013. [DOI] [PubMed] [Google Scholar]
- 36.Lyons DT, Vasta F, Vannucci RC. Autoradiographic determination of regional cerebral blood flow in the immature rat. Pediatr Res 1987; 21: 471–476. [DOI] [PubMed] [Google Scholar]
- 37.Eklind S, Mallard C, Leverin AL, et al. Bacterial endotoxin sensitizes the immature brain to hypoxic-ischaemic injury. Eur J Neurosci 2001; 13: 1101–1106. [DOI] [PubMed] [Google Scholar]
- 38.Marret S, Mukendi R, Gadisseux JF, et al. Effect of ibotenate on brain development: an excitotoxic mouse model of microgyria and posthypoxic-like lesions. J Neuropathol Exp Neurol 1995; 54: 358–370. [DOI] [PubMed] [Google Scholar]
- 39.de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev 2015; 95: 1–46. [DOI] [PubMed] [Google Scholar]
- 40.Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res 2015; 43: W566–W570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Yang J, Mao S, et al. MitProNet: a knowledgebase and analysis platform of proteome, interactome and diseases for mammalian mitochondria. PLoS One 2014; 9: e111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mills E, O'Neill LA. Succinate: a metabolic signal in inflammation. Trends Cell Biol 2014; 24: 313–320. [DOI] [PubMed] [Google Scholar]
- 43.Chouchani EE, Pell V, Gaude E, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Eur J Heart Fail 2015; 17: 29–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makrecka-Kuka M, Krumschnabel G, Gnaiger E. High-resolution respirometry for simultaneous measurement of oxygen and hydrogen peroxide fluxes in permeabilized cells, tissue homogenate and isolated mitochondria. Biomolecules 2015; 5: 1319–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gustavsson M, Mallard C, Vannucci SJ, et al. Vascular response to hypoxic preconditioning in the immature brain. J Cereb Blood Flow Metab 2007; 27: 928–938. [DOI] [PubMed] [Google Scholar]
- 46.Gustavsson M, Wilson MA, Mallard C, et al. Global gene expression in the developing rat brain after hypoxic preconditioning: involvement of apoptotic mechanisms? Pediatr Res 2007; 61: 444–450. [DOI] [PubMed] [Google Scholar]
- 47.Bernaudin M, Tang Y, Reilly M, et al. Brain genomic response following hypoxia and re-oxygenation in the neonatal rat. Identification of genes that might contribute to hypoxia-induced ischemic tolerance. J Biol Chem 2002; 277: 39728–39738. [DOI] [PubMed] [Google Scholar]
- 48.McLeod CJ, Jeyabalan AP, Minners JO, et al. Delayed ischemic preconditioning activates nuclear-encoded electron-transfer-chain gene expression in parallel with enhanced postanoxic mitochondrial respiratory recovery. Circulation 2004; 110: 534–539. [DOI] [PubMed] [Google Scholar]
- 49.Hallak M, Cotton DB. Transfer of maternally administered magnesium sulfate into the fetal compartment of the rat: assessment of amniotic fluid, blood, and brain concentrations. Am J Obstet Gynecol 1993; 169: 427–431. [DOI] [PubMed] [Google Scholar]
- 50.Reed DJ, Yen MH. The role of the cat choroid plexus in regulating cerebrospinal fluid magnesium. J Physiol 1978; 281: 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Golshani-Hebroni S. Mg(++) requirement for MtHK binding, and Mg(++) stabilization of mitochondrial membranes via activation of MtHK & MtCK and promotion of mitochondrial permeability transition pore closure: a hypothesis on mechanisms underlying Mg(++)'s antioxidant and cytoprotective effects. Gene 2016; 581: 1–13. [DOI] [PubMed] [Google Scholar]
- 52.Stark MJ, Hodyl NA, Andersen CC. Effects of antenatal magnesium sulfate treatment for neonatal neuro-protection on cerebral oxygen kinetics. Pediatr Res 2015; 78: 310–314. [DOI] [PubMed] [Google Scholar]
- 53.Chouchani ET, Pell VR, Gaude E, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014; 515: 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamel D, Sanchez M, Duhamel F, et al. G-protein-coupled receptor 91 and succinate are key contributors in neonatal postcerebral hypoxia-ischemia recovery. Arterioscler Thromb Vasc Biol 2014; 34: 285–293. [DOI] [PubMed] [Google Scholar]
- 55.O'Neill LA. Biochemistry: succinate strikes. Nature 2014; 515: 350–351. [DOI] [PubMed] [Google Scholar]
- 56.Beloosesky R, Khatib N, Ginsberg Y, et al. Maternal magnesium sulfate fetal neuroprotective effects to the fetus: inhibition of neuronal nitric oxide synthase and nuclear factor kappa-light-chain-enhancer of activated B cells activation in a rodent model. Am J Obstet Gynecol 2016; 215: 382.e1–6. [DOI] [PubMed] [Google Scholar]
- 57.Tam Tam HB, Dowling O, Xue X, et al. Magnesium sulfate ameliorates maternal and fetal inflammation in a rat model of maternal infection. Am J Obstet Gynecol 2011; 204: 364: e1–8. [DOI] [PubMed] [Google Scholar]
- 58.Hagberg H, Mallard C, Rousset CI, et al. Mitochondria: hub of injury responses in the developing brain. Lancet Neurol 2014; 13: 217–232. [DOI] [PubMed] [Google Scholar]
- 59.Jiang X, Li L, Ying Z, et al. A small molecule that protects the integrity of the electron transfer chain blocks the mitochondrial apoptotic pathway. Mol Cell 2016; 63: 229–239. [DOI] [PubMed] [Google Scholar]
- 60.Rattray BN, Kraus DM, Drinker LR, et al. Antenatal magnesium sulfate and spontaneous intestinal perforation in infants less than 25 weeks gestation. J Perinatol 2014; 34: 819–822. [DOI] [PubMed] [Google Scholar]
- 61.Kamyar M, Clark EA, Yoder BA, et al. Antenatal magnesium sulfate, necrotizing enterocolitis, and death among neonates < 28 weeks gestation. AJP Rep 2016; 6: e148–e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borja-Del-Rosario P, Basu SK, Haberman S, et al. Neonatal serum magnesium concentrations are determined by total maternal dose of magnesium sulfate administered for neuroprotection. J Perinat Med 2014; 42: 207–211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.