Abstract
Ruptured arteriovenous malformations are a frequently encountered pathology with significant associated morbidity and mortality. Identifying and securing the rupture point is mandatory; however, this can often be difficult. Black blood vessel wall magnetic resonance imaging is a promising technique for identifying ruptured saccular aneurysms and has been used in cases of multiple aneurysms. Here we describe a case of using this imaging technique to identify the rupture point in a ruptured arteriovenous malformation with histopathological correlation.
Keywords: AVM, haemorrhage, vessel wall enhancement, histology
Introduction
Targeted embolisation of a ruptured arteriovenous malformation (AVM) is an accepted form of treatment aimed at minimising the risk of repeat haemorrhage.1 The annual risk of haemorrhage has been reported to be as high as 17.8% after the initial haemorrhagic event and this risk is highest in the first year after the initial haemorrhage.2 Identifying the likely site of rupture involves correlating the haemorrhagic pattern with angiographic high-risk features, e.g. intra-nidal aneurysms, venous ectasias and venous stenosis.3,4 Black blood vessel wall magnetic resonance imaging (MRI) has recently been used to identify the source of rupture in patients with multiple intracranial aneurysms,5–7 but there is limited published literature on the use of this advanced imaging technique with regard to ruptured brain AVMs.8,9
Here we present a case of a ruptured AVM with a solitary enhancing aneurysm that underwent embolisation and neurosurgical resection with histopathological analysis of the AVM.
Case report
A previously fit and healthy 33-year-old woman was admitted to our tertiary referral neurosurgical centre with acute headache, photophobia and acute onset vertigo. On admission her Glasgow coma scale (GCS) was 15 and she had no focal neurological deficits. A subarachnoid haemorrhage was suspected and she underwent an emergency computed tomography (CT) and CT angiogram that showed intraventricular haemorrhage and a likely AVM of the right frontal lobe. The CT angiogram demonstrated a small aneurysm projecting into the right frontal horn of the lateral ventricle, contiguous with the intraventricular blood that was believed to be the potential rupture point (Figure 1).
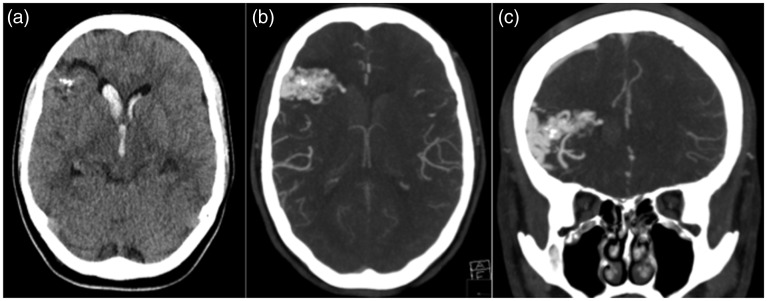
Figure 1.
(a) The unenhanced axial computed tomography (CT) scan demonstrates intraventricular haemorrhage with several foci of calcification within the right frontal lobe. A CT angiogram demonstrates a right frontal arteriovenous malformation with an aneurysm directed into the right frontal horn of the lateral ventricle that was believed to be the source of the haemorrhage (b and c).
Further imaging with black blood T1-weighted pre and post contrast vessel wall MRI was performed. The vessel wall MRI demonstrated thick circumferential wall enhancement of the aneurysm. There was no significant enhancement seen elsewhere (Figure 2). On the next day the patient underwent digital subtraction angiography in order to plan a potential endovascular targeted embolisation. This demonstrated arterial supply by way of the right ascending frontal arteries with superficial drainage (Spetzler–Martin grade 1). Several intra-nidal aneurysms were identified in addition to the likely ruptured aneurysm (Figure 3). After a multidisciplinary team meeting and discussion with the patient targeted embolisation was proposed.
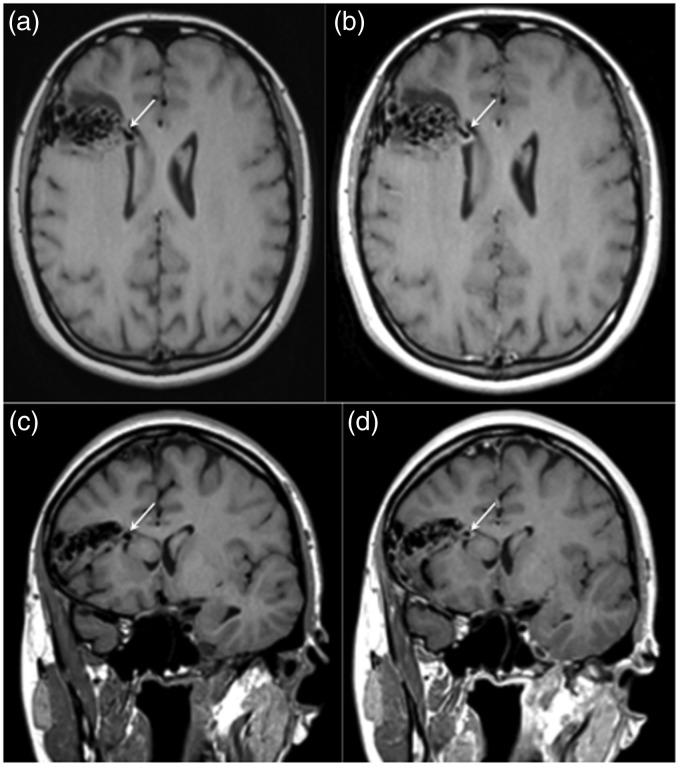
Figure 2.
A magnetic resonance image with pre and post-contrast T1-weighted sequences was performed. The aneurysm demonstrated on the computed tomography angiogram was clearly visible on the pre-contrast T1-weighted sequences (a and c, white arrow) with thick, circumferential enhancement of the aneurysmal wall seen on the post-contrast sequences (b and d, white arrow). There was no significant enhancement seen elsewhere within the arteriovenous malformation.
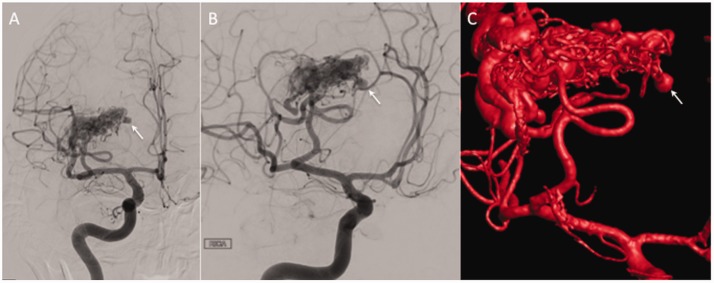
Figure 3.
Catheter angiography of the right internal carotid artery demonstrated supply to the arteriovenous malformation from branches of the middle cerebral artery (a and b) with a medially directed aneurysm that correlated with the enhancing aneurysm seen on magnetic resonance imaging that was clearly appreciated on intra-arterial rotational angiography (c).
MRI protocol
All MRI scans were acquired on a 1.5 T Avanto (Siemens, Erlangen, Germany) using a 12-channel head coil and volumetric 1.0 mm isotropic turbo spin echo sequences (SPACE sequence) with TR 700, TE 10 and flip angle 120° before and after the administration of gadolinium contrast (Dotarem; Guerbet, Paris, France). The field of view was 251 × 260 mm with an acquisition matrix of 256 × 248.
Embolisation and surgical resection
Under general anaesthesia and using right common femoral access a Neuron Max (Penumbra, Almeida, CA, USA) catheter was tracked into the right internal carotid artery. A Scepter XC balloon (Micovention, Aliso Viejo, CA, USA) was then tracked into the ascending frontal artery and the balloon inflated prior to embolisation with Onyx 18 (Medtronic, Dublin, Ireland). Although there was penetration of the AVM nidus with the Onyx there was significant reflux despite repeated re-inflation of the balloon, and therefore the procedure was terminated. Although a significant reduction in the flow was demonstrated on final angiography, a small branch continued to fill the superior aspect of the AVM, and therefore the patient underwent emergency neurosurgical resection (Figure 4).
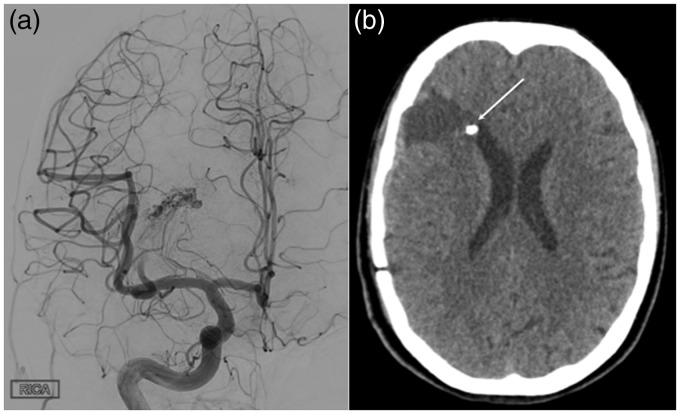
Figure 4.
At the end of the embolisation procedure persistent filling of the superior component of the arteriovenous malformation (AVM) was noted and further endovascular embolisation was not deemed feasible (a). The patient underwent resection for the residual AVM. A postoperative computed tomography scan showed successful resection of the AVM, with the embolised aneurysm still demonstrable adjacent to the right frontal horn.
Postoperative course
The patient awoke and was found to be neurologically intact. She was discharged home several days later. A CT scan performed postoperatively showed no residual AVM.
Histopathological findings
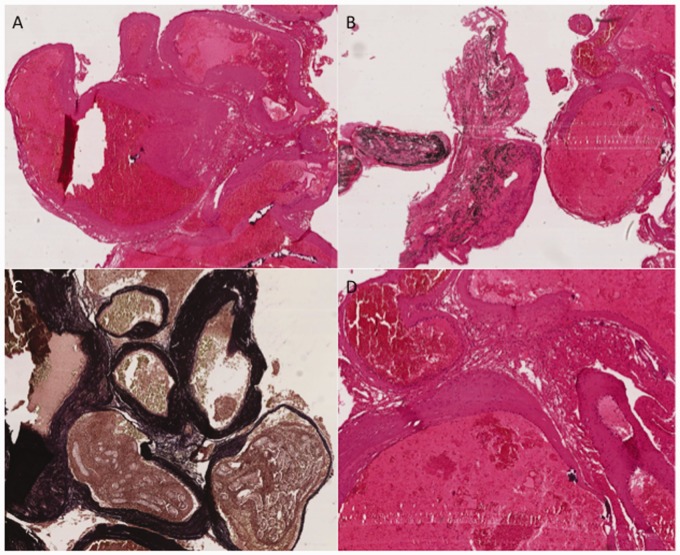
Histological analysis of the resected AVM demonstrated the typical appearance of an AVM with associated embolic material within the lumens of several constituent vessels. Examination of multiple regions of the AVM showed no morphological evidence of inflammatory cell infiltration within the vessel walls (Figure 5). This was also confirmed by immunohistochemistry (not shown in the figure). The ruptured aneurysm was not removed, and therefore could not be histologically assessed; however, within the examined material, there was no evidence of acute rupture arising from the resected AVM – suggesting that the enhancing aneurysm was the rupture point.
Figure 5.
Arteriovenous malformation demonstrating classic histological appearances of (a) closely associated, sometimes tortuous, ectatic vascular channels with walls of varying thickness and size. Occasional vessels contain black-staining embolisation material (Onyx) (b). Several vessel walls contain prominent internal elastic laminae, which are demonstrated on Masson trichrome staining as dark staining within the vessel walls (c). A higher power representative view demonstrating no histological evidence of mural inflammatory cell infiltrate within the vessel walls (d).
Discussion
Targeted embolisation of ruptured AVMs is an accepted treatment strategy.1,10,11 The aim of the treatment is to minimise the potential for repeat rupture from a focal weak point, which is often an aneurysm. However, identifying the exact rupture point is difficult and this difficulty is compounded when multiple aneurysms are present. High resolution black blood vessel wall MRI has recently been used to evaluate saccular intracranial aneurysms. This imaging technique has been used to identify the rupture point when multiple intracranial aneurysms are present.5,7 Vessel wall MRI has been used in this circumstance to guide treatment, and Matouk et al.12 were the first to publish the use of this technique with regard to multiple aneurysms. Of the five patients they presented, three had multiple aneurysms and enhancement was seen in only the ruptured aneurysms. In the series of Nagahata et al.13 15 patients were seen to have enhancement at the apex or on a bleb of the aneurysm. Seven of these 15 cases underwent surgical clipping and in all cases the rupture point correlated with the point of enhancement. Kondo et al.7 published a further case with two aneurysms associated with evenly distributed subarachnoid blood. In this case the aneurysms arose from the basilar tip and the anterior communicating artery. The basilar tip aneurysm measured 6 mm and was the larger of the two, therefore it was believed to be the most likely source of haemorrhage. Vessel wall MRI was performed and this revealed thick wall enhancement of the aneurysm arising from the anterior communicating artery, and at microsurgical clipping the anterior communicating artery aneurysm was seen to have ruptured. Similarly, Hu et al.14 recently reported their results of 25 patients with 30 aneurysms. In this series all six ruptured aneurysms demonstrated wall enhancement. Furthermore, there was significant lymphocyte and phagocyte invasion into the wall that corresponded with the enhancement. These results suggest that enhancement on vessel wall MRI scans may be related to inflammation within the aneurysm wall. Until recently a histological correlate of the aneurysmal enhancement in unruptured aneurysms has been lacking. Larsen et al.15 showed that of five resected unruptured middle cerebral artery bifurcation aneurysms that showed vessel wall enhancement there was positive staining for myeloperoxidase in four. Myeloperoxidase, an enzyme mainly secreted by neutrophilic granulocytes, is abundant in the walls of aneurysms with a higher estimated 5-year rupture risk, and it has been suggested that myeloperoxidase may serve as a biomarker for instability. Other studies have also suggested that vessel wall enhancement in unruptured aneurysms is associated with instability,16,17 with a recent meta-analysis18 of six studies including 505 aneurysms showing that enhancement had statistically significantly higher odds of being unstable (odds ratio (OR) 20, 95% confidence interval (CI) 6.4–62.1). The sensitivity, specificity, positive and negative predictive value of vessel wall imaging in identifying unstable aneurysms were 95.0% (90.4–97.8), 62.7% (57.1–67.9), 55.8% (52.2–59.4) and 96.2% (92.8–98.0), respectively. There is a statistically significant association between vessel wall enhancement and aneurysm instability, and the lack of aneurysmal wall enhancement is a strong predictor of aneurysm stability.
It is likely that similar processes occur in the rupture of aneurysms associated with AVMs, and therefore it is possible that vessel wall MRI may aid in the identification of the site of rupture and guide partial targeted embolisation. Furthermore, it is not inconceivable that areas of increased instability within an AVM can be detected prior to rupture.9,16–18 Hasan et al.19 investigated the feasibility of using ferumyxotol-enhanced MRI to image macrophages within brain AVMs in four patients. In two cases they showed increased intra-nidal uptake. One of these cases underwent resection and on histological analysis there was prominent staining of CD68-positive macrophages within the vessel wall of the nidus. The authors cautioned that they had not co-localised the MRI signal enhancement with the CD68 staining and that further studies were needed.
This is the first published case to correlate high-risk angioarchitectural characteristics, vessel wall enhancement and histopathology for site of rupture identification in a brain AVM. It builds on a single previously published report9 and suggests a potential application for partial targeted embolisation of ruptured brain AVMs.
Conclusion
Vessel wall MRI may be useful in site of rupture identification in ruptured brain AVMs. It may be useful to guide partial targeted embolisation. Further research on the use of vessel wall MRI in both ruptured and unruptured AVMs is warranted.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PB is a consultant for Phenox. The other authors report no conflicts of interest.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Krings T, Hans F-J, Geibprasert S, et al. Partial “targeted” embolisation of brain arteriovenous malformations. Eur Radiol 2010; 20: 2723–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mast H, Young WL, Koennecke HC, et al. Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. Lancet Lond Engl 1997; 350: 1065–1068. [DOI] [PubMed] [Google Scholar]
- 3.Meisel HJ, Mansmann U, Alvarez H, et al. Cerebral arteriovenous malformations and associated aneurysms: analysis of 305 cases from a series of 662 patients. Neurosurgery 2000; 46: 793–800. discussion 800–802. [DOI] [PubMed] [Google Scholar]
- 4.Hademenos GJ, Massoud TF. Risk of intracranial arteriovenous malformation rupture due to venous drainage impairment. A theoretical analysis. Stroke 1996; 27: 1072–1083. [DOI] [PubMed] [Google Scholar]
- 5.Bhogal P, Uff C, Makalanda HLD. Vessel wall MRI and intracranial aneurysms. J Neurointervent Surg 2016; 8: 1160–1162. [DOI] [PubMed] [Google Scholar]
- 6.Nagahata S, Nagahata M, Obara M, et al. Wall enhancement of the intracranial aneurysms revealed by magnetic resonance vessel wall imaging using three-dimensional turbo spin-echo sequence with motion-sensitized driven-equilibrium: a sign of ruptured aneurysm? Clin Neuroradiol 2016; 26: 277–283. [DOI] [PubMed] [Google Scholar]
- 7.Kondo R, Yamaki T, Mouri W, et al. [Magnetic resonance vessel wall imaging reveals rupture site in subarachnoid hemorrhage with multiple cerebral aneurysms]. No Shinkei Geka 2014; 42: 1147–1150. [DOI] [PubMed] [Google Scholar]
- 8.Omodaka S, Endo H, Fujimura M, et al. High-grade cerebral arteriovenous malformation treated with targeted embolization of a ruptured site: wall enhancement of an intranidal aneurysm as a sign of ruptured site. Neurol Med Chir (Tokyo) 2015; 55: 813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matouk CC, Cord BJ, Yeung J, et al. High-resolution vessel wall magnetic resonance imaging in intracranial aneurysms and brain arteriovenous malformations. Top Magn Reson Imaging TMRI 2016; 25: 49–55. [DOI] [PubMed] [Google Scholar]
- 10.Mjoli N, Le Feuvre D, Taylor A. Bleeding source identification and treatment in brain arteriovenous malformations. Interv Neuroradiol J Peritherapeut Neuroradiol Surg Proced Relat Neurosci 2011; 17: 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Feuvre D, Taylor A. Target embolization of AVMs: identification of sites and results of treatment. Interv Neuroradiol J Peritherapeut Neuroradiol Surg Proced Relat Neurosci 2007; 13: 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matouk CC, Mandell DM, Günel M, et al. Vessel wall magnetic resonance imaging identifies the site of rupture in patients with multiple intracranial aneurysms: proof of principle. Neurosurgery 2013; 72: 492–496; discussion 496. [DOI] [PubMed] [Google Scholar]
- 13.Nagahata S, Nagahata M, Obara M, et al. Wall enhancement of the intracranial aneurysms revealed by magnetic resonance vessel wall imaging using three-dimensional turbo spin-echo sequence with motion-sensitized driven-equilibrium: a sign of ruptured aneurysm? Clin Neuroradiol 2016; 26: 277–283. [DOI] [PubMed] [Google Scholar]
- 14.Hu P, Yang Q, Wang D-D, et al. Wall enhancement on high-resolution magnetic resonance imaging may predict an unsteady state of an intracranial saccular aneurysm. Neuroradiology 2016; 58: 979–985. [DOI] [PubMed] [Google Scholar]
- 15.Larsen N, von der Brelie C, Trick D, et al. Vessel wall enhancement in unruptured intracranial aneurysms: an indicator for higher risk of rupture? High-Resolution MR Imaging and Correlated Histologic Findings. AJNR Am J Neuroradiol 2018; 39: 1617–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edjlali M, Gentric J-C, Régent-Rodriguez C, et al. Does aneurysmal wall enhancement on vessel wall MRI help to distinguish stable from unstable intracranial aneurysms? Stroke J Cereb Circ 2014; 45: 3704–3706. [DOI] [PubMed] [Google Scholar]
- 17.Edjlali M, Guédon A, Ben Hassen W, et al. Circumferential thick enhancement at vessel wall MRI has high specificity for intracranial aneurysm instability. Radiology Epub ahead of print 3 July 2018. DOI: 10.1148/radiol.2018172879. [DOI] [PubMed] [Google Scholar]
- 18.Texakalidis P, Hilditch CA, Lehman V, et al. Vessel wall imaging of intracranial aneurysms: systematic review and meta-analysis. World Neurosurg 2018; 117: 453–458e. [DOI] [PubMed] [Google Scholar]
- 19.Hasan DM, Amans M, Tihan T, et al. Ferumoxytol-enhanced MRI to Image inflammation within human brain arteriovenous malformations: a pilot investigation. Transl Stroke Res 2012; 3(Suppl. 1): 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]