Abstract
Introduction
We report our experience with the novel stent-type clot-retrieval device EmboTrap II for the revascularization of large artery occlusions in acute ischaemic stroke.
Materials and methods
Twenty-nine patients with acute ischaemic stroke due to large artery occlusion underwent mechanical thrombectomy with the new EmboTrap II in two Italian centres. Clinical, procedural and radiological data were collected. Angiographic results and neurological outcomes were analysed.
Results
Only large vessel occlusions were included. Intravenous thrombolysis was administered in 72% of patients. Successful reperfusion (TICI 2b-3) was obtained in 76% of patients treated exclusively with EmboTrap II. No device-related permanent complications occurred.
Conclusion
In our experience, mechanical thrombectomy with EmboTrap II is safe and effective. Reperfusion rate was comparable to that obtained with other stent retrievers.
Keywords: Thrombectomy, stroke, stent, intervention, innovative biotechnology
Introduction
Mechanical thrombectomy is now considered the standard of care for acute ischaemic stroke (AIS) due to large vessel occlusion (LVO).1–8 Indeed, recent trials have demonstrated favourable outcomes in patients with LVO treated with stentrievers.1,3–5,9,10
Although similar in their overall function, different mechanical thrombectomy devices have distinct mechanisms of action that may lead to different results and clinical consequences.11 The rationale for using a stentriever is that its self-expanding mesh interacts with the clot entrapping it so that both can be withdrawn from the occluded artery. Although not changed in recent years, this underlying concept was integrated with the idea of restoring the flow while the stent is opened inside the clot.
The EmboTrap System (Neuravi/Cerenovus) is designed to achieve both flow restoration and clot retrieving, showing comparable results to those obtained with other stent retrievers.12 It is made of a two-layer Nitinol structure, with an inner closed cell stent, responsible for immediate flow restoration, and an outer low-profile cage. The external layer is based on units formed by open inlet windows, designed to engage the clot during the retraction of the device, and articulating petals, designed for clot trapping and retention. Also, the articulating petals and the distal-closed structure should limit distal embolism.
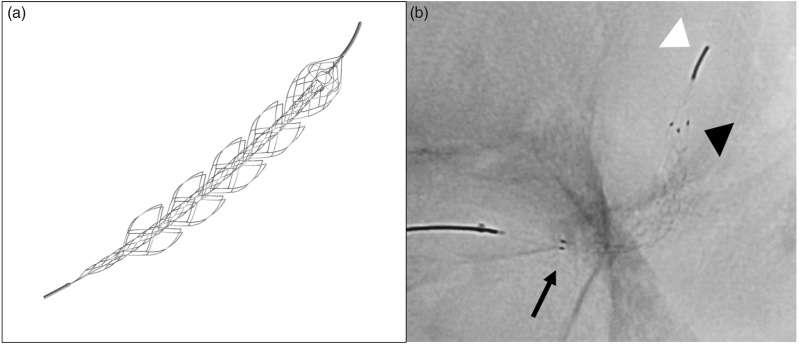
The new EmboTrap II (Neuravi/Cerenovus) is the evolution of the first version of the EmboTrap system. One of the most important improvements is the presence of a double marker at the proximal portion of the first articulating petal (Figure 1). This feature is essential for the correct positioning of the device which needs to be placed with its proximal end at the very beginning of the clot to achieve maximum effectiveness. Moreover, the number of units of the external cage has been increased from three to five; this feature potentially improves the clot capturing capability during the device retraction.
Figure 1.
(a) EmboTrap II revascularization device; (b) unsubtracted fluoroscopic image of EmboTrap II: proximal marker (arrow), distal marker (black arrow-head), distal tip (white arrow-head).
Our aim was to evaluate the safety and efficacy of the treatment of AIS due to LVO with the EmboTrap II.
Materials and methods
We retrospectively analysed the data of all consecutive patients with anterior circulation stroke due to LVO treated in two experienced centres with the EmboTrap II as the first-line choice. The collected data included: demographic data (age, sex, National Institutes of Health Stroke Scale (NIHSS) at admission and after the procedure), radiological findings (including Alberta stroke program early CT score (ASPECTS),13 site of occlusion, collateral circulation score (CCS)),14 angiographic procedure information, complications (symptomatic and asymptomatic) and timings (Time Last Known Good (TLKG)-to-groin time, reperfusion time). Immediate angiographic results were evaluated by each operator using Thrombolysis In Cerebral Infarction (TICI) score.15 We also collected post-procedural clinical information (post-procedural NIHSS) and 90 days clinical outcome with the modified Rankin scale (mRS).16
All patients underwent basal computed tomography (CT) head scan followed by multiphase CT angiogram and CT perfusion (CTP). If the patient had already undergone neuroradiological evaluation in another hospital before centralization, the basal CT scan was repeated only if the transfer took more than one hour or if the clinical conditions changed.
Patients underwent endovascular treatment if they had: (a) ASPECTS from 6–10; (b) moderate to good collaterals on the multiphase CT angiography; (c) ischaemic penumbra/infarct core mismatch larger than 50% of the total hypoperfused area identified by visual inspection on CTP maps; (d) baseline mRS < 2. There was no age limitation. We did not exclude from endovascular treatment those patients with unknown time-from-symptoms-onset but we evaluated each case on the basis of clinical history and neuroradiological findings. When the time-from-symptom-onset was unclear, or the arrival time was >6 h, the patients were considered eligible if immediate CTP imaging showed a large ischaemic penumbra of the brain, according to the criteria indicated in the trials.17,18 Moderate-to-good collateral circulation was defined as the filling of 50% or more of the middle-cerebral-artery pial arterial circulation; mean-transit-time (MTT) lesion represented total hypoperfusion (i.e. ischaemic penumbra), cerebral blood volume (CBV) lesion indicated infarct core and ischaemic penumbra was defined as the mismatch between MTT and CBV lesions. The NIHSS evaluation and the mRS assessment were performed by an experienced neurologist.
All patients with LVO underwent a full-dose intravenous thrombolysis (IVT) if indicated on the basis of national guidelines. The selection of proximal aspiration from the balloon guiding catheter or distal aspiration from a distal access catheter was up to each operator. No limit to the number of passages with the device were imposed. In case of failure in clot retrieving with the EmboTrap II, each operator could independently choose the technique and the device for the continuation of the treatment.
Successful reperfusion was defined as TICI 2b-3. All patients underwent a Magnetic Resonance (MR) examination 2–4 days after the procedure to evaluate the results of the treatment.
Three months clinical follow-up was performed by an experienced neurologist, not involved in the interventional management, by neurological examination and mRS assessment.
Continuous variables were analysed through mean and standard deviation (SD), and categorical variables have been expressed as frequency distribution.
Approval for data collection was based on local regulations. According to our national and institutional regulations, retrospective analysis of anonymised dataset does not require ethics committee review.
Most procedures were performed on patients with ischaemic stroke. Therefore, adequate written informed consent about mechanical thrombectomy could not be obtained due to poor clinical and psychological conditions. Informed consent was collected only for patients in suitable clinical and psychological conditions. All data have been entirely anonymised.
Results
Twenty-nine consecutive patients with AIS between June 2017–March 2018 treated with mechanical thrombectomy using the EmboTrap II system were included (patients’ demographics summarised in Table 1).
Table 1.
Population data.
| Basic demographics | |
| Patients | 29 |
| Mean age | 77.00 (SD ± 9.39) |
| Female | 18/29 (62%) |
| Mean baseline NIHSS | 18.48 (SD ± 5.37) |
| Mean basal ASPECTS | 8.10 (SD ± 1.45) |
| IVT | 21/29 (72%) |
| Site of arterial occlusion | |
| MCA (M1) | 22/29 (76%) |
| MCA (M2) | 3/29 (10%) |
| Terminal ICA | 4/29 (14%) |
ASPECTS: Alberta stroke program early CT score; ICA: internal carotid artery; MCA: middle cerebral artery; NIHSS: National Institutes of Health Stroke Scale; SD: standard deviation; IVT: intravenous thrombolysis.
Mean baseline NIHSS was 18.48 (SD ± 5.37). ASPECTS at basal CT head scan was 8.1 (SD ± 1.45). Target vessel occlusion were as follows: middle cerebral artery (MCA) in 90% (M1 in 80%, M2 in 10%) and terminal internal carotid artery (ICA) in 10%. Time of symptom onset was available in 25 of 29 patients (86%); four patients had wake-up stroke. The average time from symptom onset to groin puncture was 262 min (SD ± 153 min). The mean time from groin puncture to revascularization was 59 min (SD ± 46 min). The mean interval between symptoms onset and flow restoration was 306 min (SD ± 172 min). Of the patients 72% underwent IVT before mechanical thrombectomy.
Successful reperfusion was obtained in 25 patients (86%). Four patients required additional devices: Solitaire (eV3, Irvine, California, USA), Sophia Plus (MicroVention, Aliso Viejo, California, USA), Trevo (Stryker, Kalamazoo, Missouri, USA). Patients treated only with EmboTrap II had successful reperfusion in 76% of cases. The mean number of passes until achieving maximal revascularization only with EmboTrap II was 1.86 (SD ± 1.16). First-passage successful reperfusion was obtained in 10 patients (34%) while first-passage complete reperfusion (TICI 3) was obtained in eight patients (28%).
All the procedures were performed with a balloon guide catheter (BGC) except for six cases. Simultaneous aspiration was performed in all cases: 10 (34%) with proximal aspiration through the guide-catheter, 19 (66%) with both proximal and distal aspiration with the distal access catheter. In all cases, the delivery microcatheter was removed before thrombus extraction in order to increase the cross-sectional aspiration lumen.
Focal severe vasospasm of M1 was observed in one case; it was managed with intra-arterial injection of 2 mg of nimodipine. No other device-related complications, nor symptomatic intracranial haemorrhage, were encountered. No distal embolism occurred as documented by the final intra-procedural digital subtraction angiography (DSA) runs and by the MR performed the days after.
Sixteen patients (55%) were functionally independent at 90 days (mRS ≤ 2). Three patients (10%) died (mRS = 6), one secondary to the failure of the procedure, one to congestive heart failure and one to pulmonary oedema. One patient suffering from atrial fibrillation had a contralateral embolism during the night following treatment (mRS 4). The main results are summarised in Table 2.
Table 2.
Efficacy and safety results.
| EmboTrap II | ARISE II19 | ESCAPE3 | SWIFT PRIME20 | REVASCAT5 | Trevo221 | |
|---|---|---|---|---|---|---|
| Reperfusion rate (2b/3) | 76% | 80.2% | 72.4% | 83% | 65.70% | 68% |
| sICH | 0% | 5.3% | 3.60% | 0% | 1.90% | 4.50% |
| 90-days mRS 0-2 | 55.17% | 67.3% | 51.60% | 60% | 43.70% | 40% |
mRS: modified Rankin Scale; sICH: symptomatic intracerebral hemorrhage; ARISE: Analysis of Revascularization in Ischemic Stroke With EmboTrap; mRS: modified Rankin Scale; ESCAPE: Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times; SWIFT PRIME: Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment; REVASCAT: Randomized Trial of Revascularization with Solitaire FR Device versus Best Medical Therapy in the Treatment of Acute Stroke Due to Anterior Circulation Large Vessel Occlusion Presenting within Eight Hours of Symptom Onset.
Discussion
In 2015, five randomised trials showed the efficacy of endovascular thrombectomy over standard medical care in patients with acute ischaemic stroke caused by the occlusion of arteries of the proximal anterior circulation.6
Over the years, many stentrievers have been developed and many of them have been proven to be effective in capturing the clot responsible for an ischaemic stroke. Solitaire and Trevo, two stent-like self-expanding devices, dramatically improved mechanical thrombectomy’s reperfusion rates and clinical results.2,21
Recently, clot aspiration with dedicated large bore catheters gained wide diffusion because of an easier technique and good results. However, a recent study showed no significant advantages of this technique over stentrievers thrombectomy.10
Although frequent with modern techniques, a successful reperfusion rate cannot always be achieved. Moreover, clot fragments migration in distal territories is a possible, although uncommon, event. Therefore, industry and clinical researchers are still working on the improvement of thrombectomy devices in order to reduce these complications.
The EmboTrap was designed with the aim of overcoming the limitations of the other thrombectomy devices. The first-in-man multicentre study published online in 2014, showed that the device allows a technically safe thrombectomy in patients with AIS due to LVO with an equal rate of successful reperfusion compared to other types of stent retrievers.12 Moreover, the Analysis of Revascularization in Ischemic Stroke With EmboTrap (ARISE II trial) demonstrated high rates of substantial reperfusion and functional independence in patients with AIS secondary to LVO treated with the EmboTrap, although in most of patients the first generation of the device was used.19
The design of the EmboTrap II device, due to its new structure and to the addition of a proximal double marker, should both facilitate its correct positioning and improve its effectiveness. In fact, the proximal end of the first element of the EmboTrap II must be placed at the beginning of the clot in order to obtain the maximum capturing capability of the device. In fact, the inlet windows anchor the clot during its retraction. The absence of the double marker on the first version of the EmboTrap system could limit the efficacy of the device. Moreover, the addition of two more structural units in the 33 mm length version of the stent should further improve its capturing capability.
The EmboTrap II can be delivered through a 0.021-inch microcatheter, usually advanced over a 0.014-inch microwire. The microcatheter is navigated past the clot and then injected with contrast to prove the correct positioning. After the deployment, a flow channel of blood should be perceived in the middle of the stent after a contrast-media injection through the guiding catheter (Figure 2). This blood channel should ensure a temporary and fast reperfusion of the ischaemic brain tissue. After a waiting period of three minutes, which is thought to be useful to help stent-clot interaction, the device is removed into an intermediate catheter or into a proximal balloon guide catheter under flow arrest and aspiration.
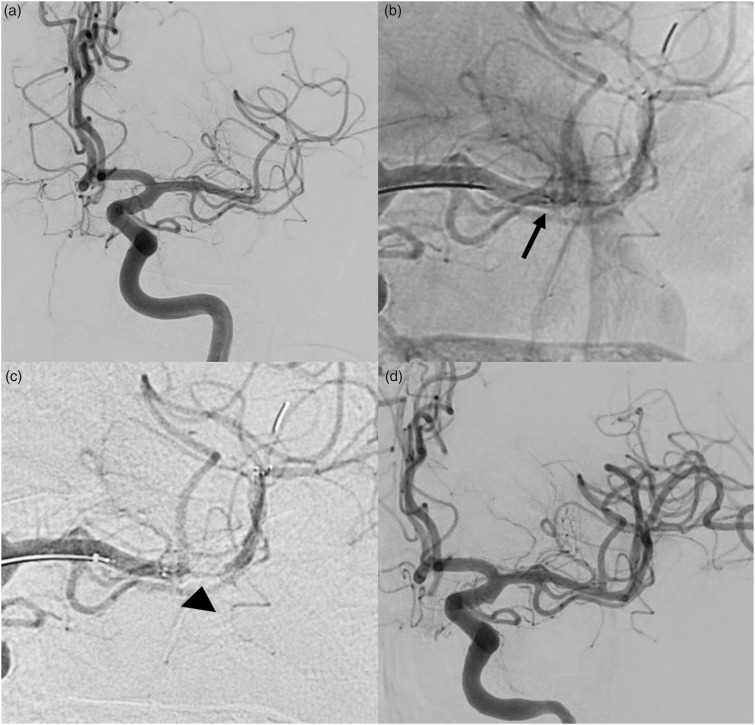
Figure 2.
(a) Left internal carotid artery (ICA) injection shows the embolic occlusion of the inferior branch (M2) of the left middle cerebral artery (MCA); (b) EmboTrap II opened with its proximal marker at the beginning of the clot (arrow); (c) left ICA injection shows a flow-channel within the EmboTrap II allowing immediate reperfusion of the ischaemic territory (arrow-head); (d) final angiogram showing the complete recanalization of the MCA.
In our series we aimed to assess both the safety and the effectiveness of the EmboTrap II revascularization device for mechanical thrombectomy of LVO in AIS. A total of 25 patients (86%) achieved a successful reperfusion (TICI 2b-3), 22 of them treated only with EmboTrap II. Therefore, with the EmboTrap II, the successful reperfusion rate was 76%.
When a successful reperfusion was not achieved with the EmboTrap, each operator could choose the device or technique to perform other attempts. In two cases a different stentriever (Trevo XP) had a better result (TICI 3 and 2b vs TICI 0 and 1 of the EmboTrap II). In one case the EmboTrap II did not achieve a successful reperfusion rate while the aspiration technique (Sofia Plus) achieved complete reperfusion (TICI 3). In this case a thrombus-aspiration attempt was made, probably because the operator was more confident with this technique; we do not know if using another stent retriever would have obtained the same result or not. In two cases, the additional stent or technique did not improve the result.
In most of the procedures, a flow channel could be appreciated inside the EmboTrap II after contrast media injection through the BGC.
In our experience, proper positioning of the proximal markers could not always be achieved as the length of the device limits sometimes a very distal access. In fact, the external cage of the five-elements-version (EmboTrap II 5 × 33) is 44 mm long and it has a 4 mm distal tip. Nevertheless, in our series no procedural complications such as distal perforation with subarachnoid haemorrhage or intracranial haemorrhage occurred. Vasospasm was a concern because of the stiff dual layer structure of the device. However, only one transient vasospasm occurred. No embolism in distal or unaffected territory occurred. The articulating-petals-structure is specifically designed to avoid this event22 that can be associated with severe clinical worsening.
The successful reperfusion rate was associated with good clinical outcomes (55% of mRS ≤ 2), in line with those shown in well-known trials.1,3,6
We are aware that the evaluation of a device performance has to be considered very prudently. Moreover, the small sample size of this study gives it a limited scientific value. Comparisons between the results obtained in our study and the results of larger studies would not be valid and this was not our intention. Although some differences in terms of angiographic results and clinical outcome may emerge from a comparison of our series with the ARISE II trial, a valid statistical comparison cannot be made due to the heterogeneity of the two cohorts. Furthermore, ARISE II does not seem to contemplate the exclusive use of the EmboTrap II stent, an exclusive feature of our study.
Brouwer et al. have published their long experience in mechanical thrombectomy using both EmboTrap and EmboTrap II systems showing slightly better results in terms of successful reperfusion rates (84.6%) but a comparable clinical outcome (52.8%).23
Anyhow, the use of EmboTrap II seemed technically feasible and safe and the reperfusion rates shown are comparable to that of other devices such as Trevo21 and Solitaire.1
Conclusion
According to this preliminary experience, mechanical thrombectomy with the EmboTrap II system is a feasible technique to treat AIS due to LVO. The reperfusion rate shown in our series appears to be comparable to those obtained with other stentrievers and we did not experience any device-related permanent complications. Further and more extensive studies are needed to more accurately assess both efficacy and safety in the use of this device.
Acknowledgements
All of the authors listed gave substantial contributions to the conception of the work, to the acquisition, analysis and interpretation of data, also revising it critically for important intellectual content. All of the authors gave their final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors have completed the International Committee of Medical Journal Editors (ICMJE) uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
References
- 1.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci retriever in patients with acute ischaemic stroke (SWIFT): A randomised, parallel-group, non-inferiority trial. Lancet 2012; 380: 1241–1249. [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 4.Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 5.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 6.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 7.Badhiwala JH, Nassiri F, Alhazzani W, et al. Endovascular thrombectomy for acute ischemic stroke ameta-analysis. JAMA 2015; 314: 1832–1843. [DOI] [PubMed] [Google Scholar]
- 8.Campbell BCV, Donnan GA, Lees KR, et al. Endovascular stent thrombectomy: The new standard of care for large vessel ischaemic stroke. Lancet Neurol 2015; 14: 846–854. [DOI] [PubMed] [Google Scholar]
- 9.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 10.Lapergue B, Blanc R, Gory B, et al. Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large vessel occlusion: The ASTER randomized clinical trial. JAMA 2017; 318: 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saber H, Rajah GB, Kherallah RY, et al. Comparison of the efficacy and safety of thrombectomy devices in acute stroke: A network meta-analysis of randomized trials. J Neurointerv Surg 2018; 10: 729–734. [DOI] [PubMed]
- 12.Kabbasch C, Mpotsaris A, Liebig T, et al. First-in-man procedural experience with the novel EmboTrap® revascularization device for the treatment of ischemic stroke–a European multicenter series. Clin Neuroradiol 2016; 26: 221–228 (first published online 2014). [DOI] [PubMed] [Google Scholar]
- 13.Pexman JHW, Barber PA, Hill MD, et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. Am J Neuroradiol 2001; 22: 1534–1542. [PMC free article] [PubMed] [Google Scholar]
- 14.Mangiafico S, Saia V, Nencini P, et al. Effect of the interaction between recanalization and collateral circulation on functional outcome in acute ischaemic stroke. Interv Neuroradiol 2014; 20: 704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higashida RT, Furlan AJ. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003; 34: e109–e137. [DOI] [PubMed] [Google Scholar]
- 16.Wilson JTL, Hareendran A, Grant M, et al. Improving the assessment of outcomes in stroke: Use of a structured interview to assign grades on the modified Rankin Scale. Stroke 2002; 33: 2243–2246. [DOI] [PubMed] [Google Scholar]
- 17.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; 378: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed]
- 19.Zaidat OO, Bozorgchami H, Ribó M, et al. Primary results of the multicenter ARISE II study (Analysis of Revascularization in Ischemic Stroke With EmboTrap). Stroke 2018; 49: 1107–1115. [DOI] [PubMed]
- 20.Goyal M, Menon BK, Van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 21.Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet 2012; 380: 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chueh J-Y, Marosfoi MG, Brooks OW, et al. Novel distal emboli protection technology: The EmboTrap. Interv Neurol 2017; 6: 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brouwer PA, Yeo LLL, Holmberg A, et al. Thrombectomy using the EmboTrap device: Core laboratory-assessed results in 201 consecutive patients in a real-world setting. J Neurointerv Surg 2018; 10: 964–968. [DOI] [PubMed] [Google Scholar]