Abstract
We describe a new, elegant, two-phase, microsurgical method that minimizes the surgical preparation time for different complex vascular lesions in a swine model. In the first phase, the model is prepared microsurgically in the experimental laboratory using arterial or/and venous grafts. In the second phase, the model is implanted in the experimental animal. This two-fold method allows for increasing the complexity and accuracy of the model while reducing preparation time on the day of the training session.
Keywords: Endovascular training, animal experimentation, aneurysm model, arteriovenous fistula model, experimental surgery
Introduction
Since the introduction and development of endovascular techniques, the profile of patients that become candidates for surgery has changed dramatically. Continuous research and development efforts have resulted in more numerous and sophisticated endovascular devices that achieve safe treatments in those would have formerly been neurosurgical patients. Therefore, there are fewer patient candidates, yet for open neurosurgery each is more complex than the last. In addition, the inherent learning curve in mastering endovascular procedures is becoming more arduous due to the rising number and complexity of these devices. This nexus, where both techniques and devices are becoming more complex, can benefit from the surgical development of animal models that allow for dual training in microsurgical and endovascular techniques.
The aim of this report is to present a new two-phase microsurgical method that elegantly minimizes intraoperative preparation times for various vascular lesions in a swine model that resembles actual complex situations in human patients. In this scenario, the neurosurgeon and the endovascular surgeon can each benefit by familiarization and training with microsurgical anastomosis and the new endovascular devices.
Description of the technique
The experimental protocol described in this study was approved by the animal experimentation ethics committee of the Vall d’Hebron Research Institute (protocol number CEEA 24/14) and was conducted in compliance with Spanish legislation and the directives of the European Union for animal research (2010/63/EU). All experiments were performed in 2.5- to 3-month-old female hybrid pigs (Large White × Landrace) weighing 30 to 40 kg. Animals were sourced from Specipig (Barcelona, Spain) and recorded in the registry of breeding centres, suppliers and users of experimental animals with reference number B9900041. The transport company was authorized and certified to perform this service. Animals were previously acclimated to our facilities and housed in conventional pens for at least 1 week with free access to water and twice-daily feedings using a conventional diet. Animals underwent fasting for 12 hours before surgery but had free access to water with diluted sucrose. Only specimens with a satisfactory examination were included in the study. The pigs were kept under general anesthesia for the entire duration of the experiment, and they did not experience any pain or distress. To avoid graft thrombosis, 450 mg of lysine acetylsalicylate (Inyesprin, Aristo Pharma Iberia, S.L, Madrid, Spain) was administrated after anesthesia induction.
Surgical technique
First phase: Microsurgical in vitro preparation
Adhering to the principle of reduction, preparation of the vascular lesions was carried out using carotid arteries and jugular veins previously extracted from other experimental animals used for other scientific purposes, reducing the use of additional animals. This system allows the preparation of vascular lesions even 1 week prior to the implantation of the graft performed for educational purposes. In this process, all obtained samples are kept in a conventional refrigerator at 4℃ and immersed in 5% glucose solution with 20% albumin (500 ml of 5% glucose solution + 20% albumin 100 ml).
The preparation of the models is carried out in the microsurgery laboratory under magnification of a surgical microscope, utilizing micro forceps to immobilize the tissue. The elaboration of the model requires having previously made a draft in collaboration with the neuroradiologist in which the characteristics and measurements are identified to make sure the model is adapted to the needs of the training session. From this, the final design is constructed, such as those presented in Figures 2, 4 and 5, in which the necessary arterial and/or venous structures and distribution are developed. Previously harvesting vein and artery grafts allows time to create any vascular model with microsurgical techniques, regardless of complexity, anastomosing venous and arterial configurations, as there is no pressure to prepare the lesion during the same procedure. Likewise, this system allows us to calculate with high accuracy the measurements of the vascular lesions specified.
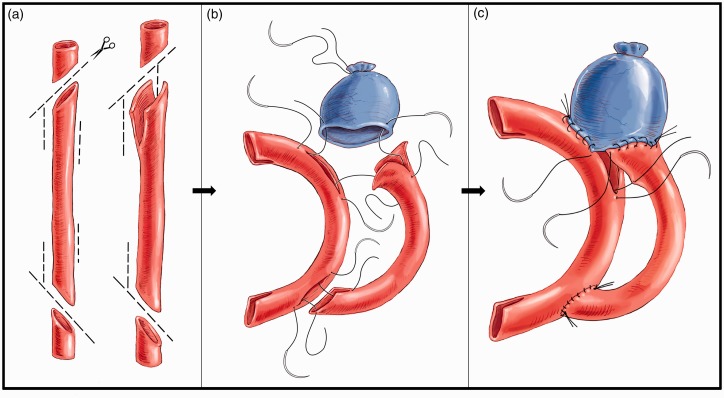
Figure 2.
illustrates the construction of a complex wide-neck bifurcation aneurysm. The purpose of this figure is to display schematically how we perform the aneurysm reconstruction represented in Figure 3. For the preparation of this complex model, we require the use of two arterial grafts and one venous graft (image 2a). The first arterial graft is used as a recipient of the second arterial graft and part of the venous graft. For its subsequent implementation, two oblique cuts are made at each end, as well as two lateral slits (fish mouth). In this same artery two lateral slits are made for the suturing of the second arterial graft. In the second arterial graft, two oblique cuts and two lateral slits are made to facilitate anastomosis to the first graft. However, at the proximal end of this second graft additional slits are made, the length of which determines the neck of the aneurysm and the location of the base on one or both branches. In other words, the shorter the upper groove in relation to the lower one, the lower the aneurysm will be in relation to this vessel. To carry out the anastomosis, the usual technique is followed, in which anchorage is made at the margins and a continuous suture is put under tension once all points have been correctly passed (images 2b and 2c).
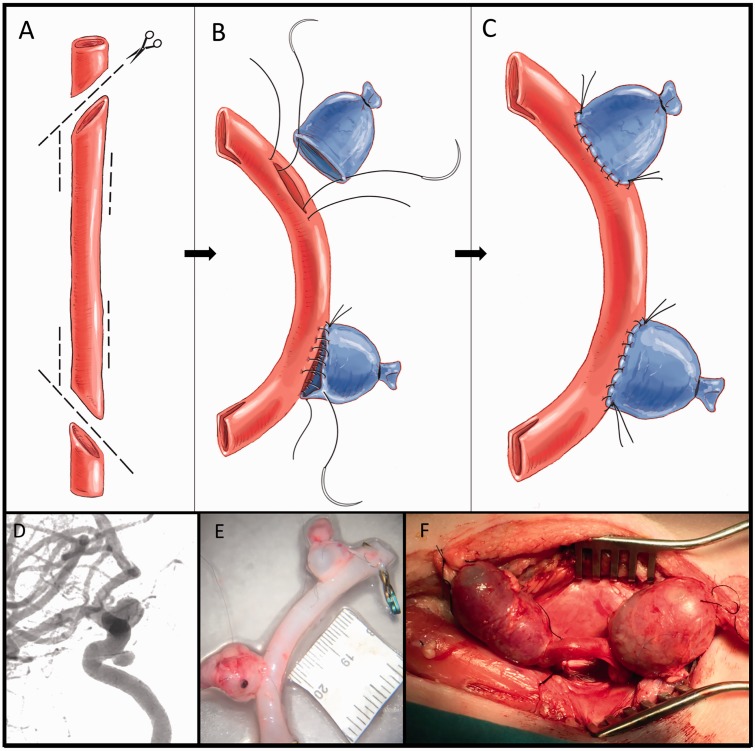
Figure 4.
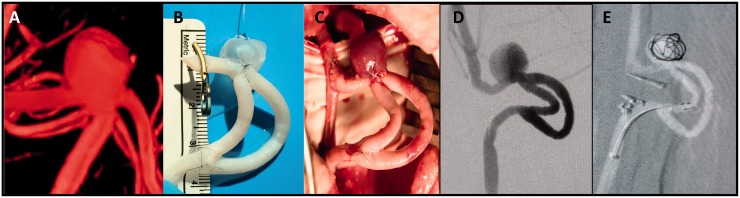
The figure shows the construction of two wide-neck aneurysms in a cavernous carotid artery model. The object of the model is to represent two aneurysms in an artery with the precise curvature typical of the cavernous artery. This requires the use of one arterial graft and one venous graft (image 4a). The arterial graft is used as a recipient of the venous graft. For its subsequent implementation in the carotid artery, two oblique cuts with lateral slits (fish mouth) are made at each end. In this same artery, two lateral slits are made for the implantation of the venous grafts. The size of these lateral slits determines the neck of the aneurysm and the size of the selected vein dictates the size of the aneurismal dome. To carry out the anastomosis, the usual technique is followed, anchoring first at the margins and tensing a continuous suture once all points have been correctly passed (images 4b and c). Image 4d corresponds to a typical angiography of a wide-neck aneurysm of the intracavernosal carotid artery. In image 4e the created model is shown, ready for later implantation in the experimental animal. Image 4f shows a surgical image of the model after implantation.
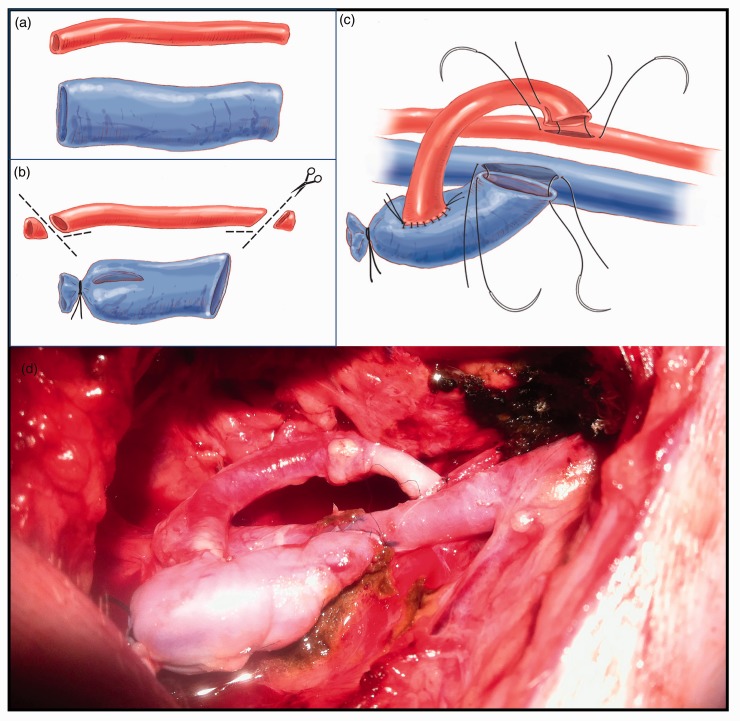
Figure 5.
The figure shows the construction of an arteriovenous fistula. This requires the use of one arterial graft and one venous graft (image 5a). As per protocol, two oblique cuts with lateral slits (fish mouth) are made at each end. For the preparation of the model, one end of the vein is occluded. A slit that matches the diameter of the artery to be anastomosed is made (image 5b). The arterial graft is connected laterally to the venous graft for subsequent implementation on the carotid artery and internal jugular vein (image 5c). To carry out the anastomosis, the usual technique is followed: anchorage at the margins and tension of a continuous suture once all tissue has been correctly passed. Image 5d shows a surgical view of the arteriovenous model after implementation.
Second phase: Microsurgical implantation
Once the graft is prepared, it is kept in refrigeration, in the same solution, until the day of the training session. Now, from the neurosurgical point of view, we require only the dissection of the common carotid artery (Figure 1) and the preparation of two anastomoses (proximal and distal) to the carotid artery or jugular vein, depending the model needed.
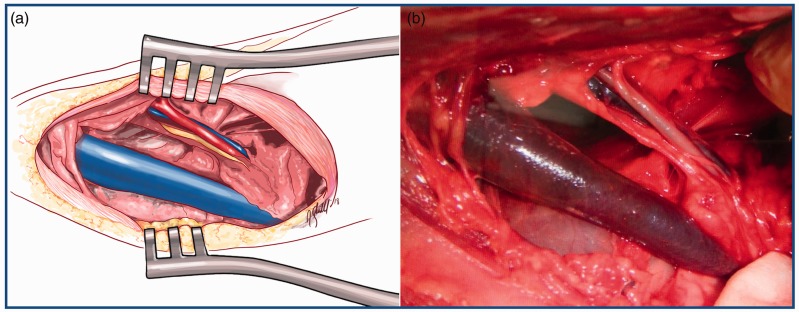
Figure 1.
displays a schematic drawing (image a) and a surgical picture (image b) of a cervical dissection and exposure of the neurovascular bundle of the neck. This is the site of implantation of the models. It includes the common carotid artery, the vagosympathetic trunk and the internal jugular vein. Of greater thickness, the external jugular vein is also exposed in the dissection.
To better understand the model, we present three examples.
Examples
Complex wide-neck bifurcation aneurysms (Figure 2 and 3)
Figure 3.
Image 3a corresponds to three-dimensional reconstruction of a typical aneurysm at the bifurcation of the middle cerebral artery. Note the amplitude of the neck of the aneurysm and its preferential relationship with one of the two branches. In image 3b the created model is shown, ready for later implantation in the experimental animal. Image 3c shows a surgical view of the model just after implantation and image 3d corresponds to an angiographic image of the final implanted model. Note the model has all the angiographic characteristics of the desired aneurysm. The created aneurysm has a wide base of implantation with a preferential relationship to one of the branches. Image 3e shows an image of the practice in which partial embolization of the aneurysm is observed. Note the two clips placed distally to the first end-to-side anastomosis and proximally to the second end-to-side anastomosis to occlude the carotid artery.
For the creation of this model, we use two arterial grafts and one venous graft. First, we microsurgically constructed a bifurcation with the arterial grafts. The venous graft was then sutured to the prepared arterial bifurcation creating the wide-neck aneurysm. Having the grafts on the preparation table allowed us to create the aneurysm with exact measurements in terms of the arterial aneurysm relationship, wide-neck diameter and aneurysm size. Likewise, it allowed us to define the relationship to each of the two related arteries; we can position the aneurysm symmetrically between the two branches or, if preferred, extend it specifically towards one.
At the moment of the training session, we performed a lateral cervical dissection for complete exposure of the carotid artery. We made two end-to-side anastomoses to adapt the graft to the carotid artery. After testing the patency of the anastomoses, we occluded the carotid artery distally to the first end-to-side anastomosis and proximally to the second end-to-side anastomosis using two aneurismal clips.
This model will provide endovascular training for treating the aneurysm from three different access points, allowing application of remodeling or stenting techniques according to the desired purpose.
Wide-neck aneurysm in a cavernous carotid artery model (Figure 4)
The objective of this model is to prepare two wide-neck aneurysms in an artery that maintains a curvature similar to that of the cavernous carotid artery. To create this, we used one arterial graft and one venous graft. Again, in the arterial graft we microsurgically constructed an arterial bifurcation. We then added the venous grafts to construct the wide-neck aneurysm on the created arterial bifurcation. Having the separate grafts at the laboratory table of experimental surgery allows for precision measurements of the aneurysm as well as specific placement in the surgical field.
As with the previous example, we performed two end-to-side anastomoses to adhere the graft to the carotid artery. Patency of the anastomosis was confirmed, and we occluded the carotid artery distally to the first anastomosis and proximally to the second, with two aneurismal clips.
This model allows endovascular training on two wide-neck aneurysms mimicking two cavernous carotid artery aneurysms.
Arteriovenous fistula model (Figure 5)
For the preparation of this model, an artery and vein graft are necessary. Both are anastomosed microsurgically so that there is a clear change in caliber from one structure to the other. On the day of the endovascular training session, the dissection of the vasculo-nervous package of the neck is performed, and two end-to-side anastomoses are performed, one between the graft artery with the carotid artery and the other between the vein of the graft with the jugular vein. This procedure will put into practice the treatment of high-flow arteriovenous fistulas.
Discussion
With the progressive development of new complex endovascular devices, it has become increasingly necessary to use strategies that allow the evaluation of these new devices, while additionally training operating physicians to minimize the burden of the learning curve. In this context, animal models continue to offer a consistent and invaluable role. Swine are widely used in models such as these because the vasculature is suitable in size for testing endovascular devices. The main problem of the experimental models described so far is that the neurosurgeon requires extensive time to prepare them, which increases with direct correlation to the complexity of the model being simulated.
The search of models that replicate this complexity has generated new sophisticated models of silicone created with three-dimensional printers, some so realistic that they even incorporate fluids of a viscosity and temperature similar to the blood and pumps that replicate the cardiac cycle.1
Previous work in large animals such as dogs, sheep and pigs has been developed to create sidewall aneurysm models or bifurcations aneurysm models for testing and training in neuroendovascular therapies.2–7 Despite attempts to minimize the time for preparation, these previous models required considerable preparation time; the more complex the model, the more time required. Unfortunately, this results in surgeons tending to build less complex models that are not adequate for the increasing intricacy involved in modern neurosurgical practice.
Our methods described above are intended to offer a timely solution for the creation of highly complex vascular lesion models. The pre-preparation allows for focus on the production of complicated models, a critical point in designing the highest standard of training for increasingly complex cases. This two-phase microsurgical method has the additional advantage of utilizing biological tissue that mimics human anatomy and tissue consistency.
The lesions created with this model all share morphological features with those we found in humans. In both aneurysm models presented, we can predetermine the fundus-to-neck ratio and relationships of the aneurysm to parent vessels. This allows the surgeon to create a variety of aneurysm structures necessary to use in testing endovascular technology and techniques. For example, the wide-neck bifurcation aneurysms made in this model should be used to practice balloon or stent remodelling, which will allow endovascular surgeons to develop additional strategies and more complex technical maneuvers for the treatment of aneurysms in humans. In the case of the fistula model, our two-phase method allows us to determine the size of the varix, as well as the distance of the afferent vessel and the size of the fistula between the artery and the vein.
A potential limitation of our technique is the necessity of an additional animal to donate the vessels for the creation of the vascular lesions. Although donors in our two-phase microsurgical method were animals used for other experimental purposes, a concern is that this method might not be followed in some environments where access to other previously used research animals is limited. Another limitation of our model is that it was only designed for training purposes. Being an allotransplant model, it is not intended to be used for other scientific purposes, especially those for which follow-up studies are required.
Conclusion
We present a new two-phase microsurgical method that elegantly minimizes surgical preparation times for a variety of vascular lesions in a swine model. We have demonstrated this model is reproducible and closely resembles actual complex situations in humans.
These methods will be beneficial for training and investigating endovascular techniques and devices before human clinical trials and at the same time allow the neurosurgeon to become familiar and train in microsurgical anastomosis.
Acknowledgments
We are grateful for the assistance of Jessica Shull in preparing the drawings in this study, as well as the collaboration of all Albert Cored and Angel Lorente of the Animal Facility Unit, who carefully assisted with animal management.
Ethics statement
The experimental protocol described in this study was approved by the animal experimentation ethics committee of the Vall d’Hebron Research Institute (protocol number CEEA 24/14) and was conducted in compliance with Spanish legislation and the directives of the European Union for animal research (2010/63/EU).
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Neurotraumatology and Neurosurgery Research Unit is supported by a grant from the Departament d'Universitats, Recerca i Societat de la Informació de la Generalitat de Catalunya (SGR 2014-844).
References
- 1.Arthur A, Hoit D, Coon A, et al. Physician training protocol within the WEB Intrasaccular Therapy (WEB-IT) study. J Neurointerventional Surg 2018; 10: 500–504. [DOI] [PubMed] [Google Scholar]
- 2.Bavinzski G, al-Schameri A, Killer M, et al. Experimental bifurcation aneurysm: A model for in vivo evaluation of endovascular techniques. Minimally Invasive Neurosurg 1998; 41: 129–132. [DOI] [PubMed] [Google Scholar]
- 3.Boulos AS, Deshaies EM, Fessler RD, et al. A triple bifurcation aneurysm model for evaluating complex endovascular therapies in dogs. J Neurosurg 2005; 103: 739–744. [DOI] [PubMed] [Google Scholar]
- 4.da Silva SL, Jr, Pitta GB, Pereira AH, et al. Stable experimental model of carotid artery saccular aneurysm in swine using the internal jugular vein. Rev Col Bras Cir 2013; 40: 130–136. [DOI] [PubMed] [Google Scholar]
- 5.Olabe J, Olabe J, Roda J. Microsurgical cerebral aneurysm training porcine model. Neurol India 2011; 59: 78–81. [DOI] [PubMed] [Google Scholar]
- 6.Spetzger U, Reul J, Weis J, et al. Endovascular coil embolization of microsurgically produced experimental bifurcation aneurysms in rabbits. Surgical Neurology 1998; 49: 491–494. [DOI] [PubMed] [Google Scholar]
- 7.Yapor W, Jafar J, Crowell RM. One-stage construction of giant experimental aneurysms in dogs. Surgical Neurology 1991; 36: 426–430. [DOI] [PubMed] [Google Scholar]