Abstract
Background
Therapeutic strategies and outcomes vary with stroke subtypes for patients with acute vertebrobasilar occlusion (VBAO). This study aimed to compare characteristics and outcomes of VBAO due to intracranial atherosclerotic disease (ICAD) and embolisms and identify baseline predictors of ICAD.
Methods
Patients with VBAO who received endovascular therapy (EVT) were retrospectively analyzed. Participants fulfilling the criteria were classified as the ICAD group (focal stenosis of >70%, or fixed stenosis >50% in addition to either flow and perfusion impairment on angiography or an evident reocclusion tendency) and the embolism group (defined as no evidence of focal significant stenosis after thrombolysis or thrombectomy). Baseline characteristics and outcomes after EVT were compared between the two groups, and logistic regression was performed to explore the factors associated with ICAD.
Results
Among the 133 patients enrolled, 95 (71.4%) patients were categorized in the ICAD group, and 38 (28.6%) in the embolism group. A history of atrial fibrillation (odds ratio (OR) 0.142; 95% confidence interval (CI) (0.028–0.707), p = 0.017), distal basilar artery occlusion (OR 0.107; 95% CI (0.040–0.289), p < 0.001) and V4 segment occlusion (OR 3.423; 95% CI (1.172–9.999), p = 0.024) were independently associated with ICAD. Patients with VBAO due to ICAD had a lower rate of recanalization (81.1% vs 100%, p = 0.004), but the 90-day good clinical outcome was comparable (41.1% vs 50.0%, p = 0.347).
Conclusions
The occlusion sites and a history of atrial fibrillation might be helpful in predicting ICAD in patients with VBAO. Patients with ICAD who were treated by EVT had a lower rate of recanalization but comparable 90-day good outcomes.
Keywords: Endovascular therapy, intracranial atherosclerotic disease, vertebrobasilar artery occlusion
Introduction
Acute ischemic stroke caused by intracranial vertebrobasilar artery occlusion (VBAO) is rare, accounting for only 1% of all ischemic strokes.1 However, it is often associated with a high mortality and disability rate, even when intravenous thrombolysis (IVT) is given.2,3 Endovascular therapy (EVT) represented by thrombectomy can achieve a high proportion of revascularization and functional independence, while being relatively safe for patients with VBAO.4 Appropriate endovascular approaches, however, vary by stroke mechanisms.
In situ thrombosis superimposed on underlying intracranial atherosclerotic disease (ICAD) and embolism from a proximal artery or cardiac in origin are regarded as major pathomechanisms of acute VBAO.2 Coexistence with ICAD at the occlusion site is common in large-artery occlusion patients, especially in Asia.5 Evidence to predict ICAD before treatment is limited.
On account of the high incidence of ICAD in VBAO, it is helpful for therapeutic planning if we identify the stroke subtype before treatment. The purpose of this study was to clarify the discrepant characteristics of stroke subtypes for predictions of ICAD and to compare the outcomes of EVT between ICAD and embolic occlusion.
Methods
Patients
From prospectively collected clinical and imaging databases, we retrospectively identified consecutive acute ischemic stroke patients who presented with VBAO and were treated with EVT. A total of 136 patients with ischemic stroke related to VBAO were treated with EVT between April 2012 and October 2017 at our hospital. The enrollment criteria were as follows: (1) age ≥18 years; (2) presented with posterior circulation stroke symptoms and treated by EVT; (3) onset to puncture time <24 hours; (4) National Institutes of Health Stroke Scale (NIHSS) pretreatment ≥4; (5) modified Rankin Scale (mRS) ≤ 1 before the qualifying stroke and (6) occlusion of the V4 segment of the vertebral artery or basilar artery. Patients were excluded if they met the following criteria: (1) history of subarachnoid hemorrhage, intracranial hemorrhage, arteriovenous malformation, or tumors; (2) serum creatinine level >2.0 mg/dl (177 mmol/l) or glomerular filtration rate <30 ml/minutes/1.73 m2; (3) premorbid contraindication or laboratory findings suggestive against the use of contrast medium, aspirin or clopidogrel, or anesthesia; or (4) occlusion was caused by intracranial arterial dissection or vasculitis. This retrospective study was approved by the ethics committee of our hospital and the institutional review board, and the requirement for informed consent for study inclusion was waived because it was a retrospective study.
EVT
IVT was performed before EVT for patients who were eligible. Whether the EVT was performed under general anesthesia or local anesthesia was decided by the neurointerventionists based on patients’ clinical condition. Thrombectomy with Solitaire stent was recommended as the primary treatment. Rescued retrieval stent detachment (Solitaire stent), balloon angioplasty (Gateway (Boston Scientific Corp, Natick, MA, USA)) or stenting (balloon-mounted Apollo stent (MicroPort Medical, Shanghai, China), self-expandable Wingspan stent (Boston Scientific Corp, Stryker, USA)) could also be performed when severe residual stenosis resulting in inadequate distal perfusion or new thrombus formation at the target lesion was observed after thrombectomy. If acute thrombosis or a tandem lesion was observed at the occlusion site, glycoprotein IIb/IIIa inhibitor infusion (tirofiban) would be given to achieve successful reperfusion.
Patients would be transferred to neurologic intensive care unit after the procedure, and receive the standard postoperative management.
Baseline characteristics assessment
All of the information including demographic data, medical history, laboratory findings and procedural details were obtained from the neurointerventional database of the hospital. Time of symptom onset, mRS score before the qualifying stroke, and NIHSS score pretreatment were assessed and recorded by a stroke neurologist on admission at the emergency department. According to the angiographic definition by Lee et al.,6 the patients were categorized into an ICAD group or an embolism group after a thorough etiological investigation: The ICAD was defined as (1) significant focal stenosis >70% at the occlusion site, or (2) a degree of fixed stenosis >50% in addition to either flow and perfusion impairment on angiography or an evident reocclusion tendency even after sufficient treatment with stent retrievers. Patients without significant focal stenosis after thrombolysis or thrombectomy were categorized as embolic occlusion, which includes cardiogenic, artery-to-artery and cryptogenic embolism.
Pretreatment and follow-up image data were evaluated by two neuroradiologists who were blinded to the clinical information and conclusions were reached in consensus. The imagers assessed the images to determine the baseline postcirculation Alberta Stroke Program Early Computed Tomography Score (pc-ASPECTS) on diffusion-weighted imaging (b = 0 and b = 1000 seconds/mm2), based on the method described by Puetz and colleagues.7 The collateral status and site of occlusion were estimated on digital subtraction angiography, which was performed before EVT. The segments of the intracranial vertebrobasilar artery were defined as vertebral V4 (intracranial vertebral artery), proximal basilar artery (vertebrobasilar junction to the origins of the anterior inferior cerebellar arteries (AICAs)), midbasilar (between the AICAs and the origin of the superior cerebellar arteries) and distal basilar artery (BAD) (involvement of a segment distal to the superior cerebellar arteries), based on the study by Archer and Horenstein.8 The Basilar Artery on Computed Tomography Angiography (BATMAN) score9 was assessed, which evaluates both the extent of the occlusion and the presence of collaterals (range, 1–10: one point if either intracranial vertebral artery was patent, one point for each patent segment of the basilar artery, one point for each patent P1 segment of the posterior cerebral artery; two points for each posterior communicating artery (PCom), or three points for each fatal PCom).
Outcome assessment
Successful reperfusion was achieved when modified Thrombolysis in Cerebral Infarction (mTICI) score was 2b or 3.10 Computed tomography or magnetic resonance imaging was performed at 24 hours after therapy, or earlier if neurologic worsening was more than four points on the NIHSS, according to which we determine the existence of symptomatic intracranial hemorrhage (sICH) with Saver’s standard.11 The mRS score on follow-up at 90 days was recorded by neurologists also blinded to baseline characteristics, assessed via telephone or face-to-face interviews. A good neurofunctional outcome was regarded as mRS ≤ 2.
Statistical analysis
All statistical analyses were performed using SPSS Statistics, version 21.0 (IBM Corp, Armonk, NY, USA). The baseline and outcome data were described by means (SDs) or medians (25th and 75th percentiles) for continuous variables; frequencies or proportions were presented for categorical variables. The χ2 test, Fisher exact test, Student t test and Mann-Whitney U test were used to find the difference between the characteristics and outcomes between the two groups as appropriate. To find the predictors of the presence of ICAD, multivariable logistic regression analyses were performed. A p value less than 0.05 was considered to indicate a statistically significant difference.
Results
Patient population
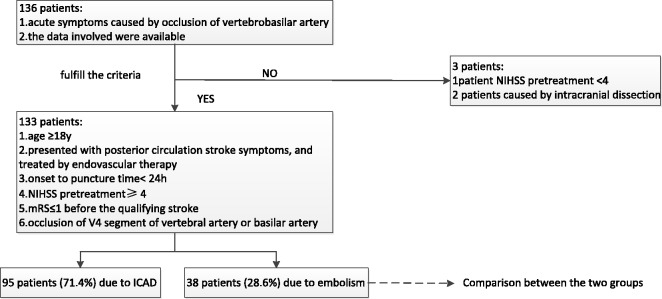
After patients diagnosed with intracranial dissection (n = 2) and pretreatment NIHSS < 4 (n = 1) were excluded, 133 patients who fulfilled the enrollment criteria were analyzed in our study (Figure 1).
Figure 1.
Flow chart of patient inclusion. h: hours; ICAD: intracranial atherosclerotic disease; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; y: years.
The mean age was 58.37 years (±9.92), and 83.5% of the participants were male. Median NIHSS pretreatment was 22.0 (interquartile range (IQR), 12.5–29.0). A total of 19.5% patients received intravenous recombinant tissue plasminogen activator (IV rt-PA) thrombolysis before EVT. Reperfusion was achieved in 115 patients (86.5%), and the median time from symptom onset to reperfusion was 505.0 minutes (IQR, 392.5–695.0 minutes). Fifty-eight patients (43.8%) achieved good outcomes at 90 days. The rate of sICH was 6.0%.
Differences in baseline characteristics between the ICAD and embolism groups
There were 95 patients in the ICAD group and 38 in the embolism group. Baseline characteristics including demographics, risk factors and imaging data are shown in Table 1. There was no difference between groups in demographic data or risk factors, except for the history of atrial fibrillation: three patients (3.2%) in the ICAD group, and 10 patients (26.3%) in the embolism group (p < 0.001). The pretreatment pc-ASCPECTS (6.0 vs 6.5, p = 0.368) and BATMAN score (5.0 vs 5.5, p = 0.805) were comparable. Occlusion in the segment of V4 (35.8% vs 15.8%, p = 0.023), proximal basilar artery (BAP) (50.5% vs 21.1%, p = 0.002) and midbasilar artery (BAM) (61.1% vs 36.8%, p = 0.011) was more prevalent in the ICAD group, but in contrast, BAD (81.6% vs 33.7%, p < 0.001) was more common in the embolism group.
Table 1.
Comparison of baseline characteristics between the ICAD group and the embolism group.
| Total (n = 133) | ICAD (n = 95) | Embolism (n = 38) | p value | |
|---|---|---|---|---|
| Age (years), mean ± SD | 58.37 ± 9.92 | 58.54 ± 8.70 | 57.95 ± 12.59 | 0.758 |
| Male (%) Risk factors | 111 (83.5%) | 81 (85.3%) | 30 (78.9%) | 0.376 |
| Hypertension (%) | 103 (77.4%) | 76 (80.0%) | 27 (71.1%) | 0.265 |
| Diabetes (%) | 46 (34.6%) | 32 (33.7%) | 14 (36.8%) | 0.729 |
| Dyslipidemia (%) | 32 (24.1%) | 26 (27.4%) | 6 (15.8%) | 0.158 |
| Atrial fibrillation (%) | 13 (9.8%) | 3 (3.2%) | 10 (26.3%) | <0.001 |
| Stroke/TIA (%) | 44 (33.1%) | 31 (32.6%) | 13 (34.2%) | 0.861 |
| Smoker (%) | 93 (69.9%) | 68 (71.6%) | 25 (65.8%) | 0.511 |
| pc-ASPECTS (IQR) | 6.0 (4.5–8.0) | 6.0 (4.0–8.0) | 6.5 (5.0–8.0) | 0.368 |
| BATMAN (IQR) | 5.0 (4.0–7.0) | 5.0 (4.0–7.0) | 5.5 (5.0–6.0) | 0.805 |
| NIHSS (IQR) | 22.0 (12.5–29.0) | 21.0 (12.0–28.0) | 24.5 (16.0–30.3) | 0.101 |
| SBP pretreatment (IQR) (mmHg) | 158.0 (140.0–180.0) | 160.0 (140.0–180.0) | 150.0 (138.0–171.8) | 0.215 |
| DBP pretreatment (IQR) (mmHg) | 90.0 (80.0–100.0) | 90.0 (80.0–100.0) | 85.0 (79.0–100.0) | 0.401 |
| Location of occlusion | ||||
| V4 (%) | 40 (30.1%) | 34 (35.8%) | 6 (15.8%) | 0.023 |
| BAP (%) | 56 (42.1%) | 48 (50.5%) | 8 (21.1%) | 0.002 |
| BAM (%) | 72 (54.1%) | 58 (61.1%) | 14 (36.8%) | 0.011 |
| BAD (%) | 63 (47.4%) | 32 (33.7%) | 31 (81.6%) | <0.001 |
BAD: distal basilar artery, segment distal to superior cerebellar arteries; BAM: midbasilar artery, between the anterior inferior cerebellar arteries (AICAs) and the origin of the superior cerebellar arteries; BAP: proximal basilar artery, vertebrobasilar junction to the origins of the AICAs; BATMAN: Basilar Artery on Computed Tomography Angiography; DBP: diastolic blood pressure; ICAD: intracranial atherosclerotic disease; IQR: interquartile range; pc-ASPECTS: postcirculation Alberta Stroke Program Early Computed Tomography Score; NIHSS: National Institutes of Health Stroke Scale; SBP: systolic blood pressure; TIA: transient ischemic attack; V4: intracranial vertebral artery.
The NIHSS pretreatment of embolic participants was shown to be higher than those with ICAD, albeit statistically insignificantly (24.5 vs 21.0, p = 0.101). A trend toward lower pretreatment systolic and diastolic blood pressure was observed in the ICAD group compared with the embolic group (160 vs 150 mmHg, p = 0.215; 90 vs 85 mmHg, p = 0.401).
Factors associated with ICAD occlusion
In multivariate binary logistic regression analysis (Table 2), a history of atrial fibrillation (odds ratio (OR) 0.142; 95% confidence interval (CI) (0.028–0.707), p = 0.017) and BAD segment occlusion (OR 0.107; 95% CI (0.040–0.289), p < 0.001) was significantly negatively associated with ICAD occlusion, while V4 occlusion (OR 3.423; 95% CI (1.172–9.999), p = 0.024) was positively associated with the presence of ICAD.
Table 2.
Logistic regression model for predictors of intracranial atherosclerotic disease.
| p | OR | 95% CI | |
|---|---|---|---|
| Atrial fibrillation | 0.017 | 0.142 | 0.028–0.707 |
| BAD | <0.001 | 0.107 | 0.040–0.289 |
| V4 | 0.024 | 3.423 | 1.172–9.999 |
BAD: distal basilar artery, segment distal to superior cerebellar arteries; CI: confidence interval; OR: odds ratio; V4: intracranial vertebral artery.
Differences in therapy and outcomes between the ICAD and embolism groups
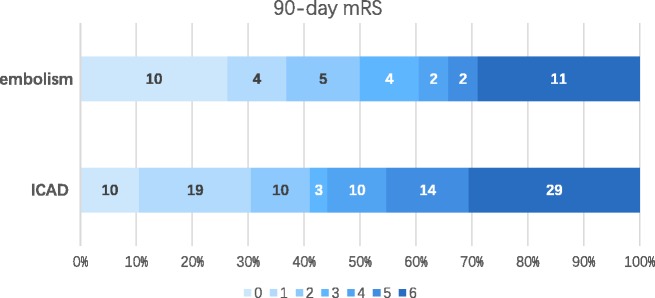
Data regarding therapy and outcomes are summarized in Table 3. There was no difference between the two groups regarding receiving IV rt-PA before EVT. During the procedure, general anesthesia (83.2% vs 60.5%, p = 0.005) and angioplasty including balloon and/or stenting at the occlusion site (60.0% vs 2.6%, p < 0.001) were performed more frequently in the ICAD group. Time from puncture to reperfusion tended to be longer in the ICAD group than in the embolism group (133.49 vs 113.87 minutes, p = 0.107). Finally, more embolismic occlusion patients achieved successful reperfusion (100% vs 81.1%, p = 0.004). However, the chance of achieving a 90-day good outcome (41.1% vs 50.0%, p = 0.347) was comparable between the two groups (Figure 2). There were no differences between the ICAD group and embolism group in the sICH (4.2% vs 10.5%; p = 0.327) and mortality rates (30.5% vs 28.9%, p = 0.858).
Table 3.
Comparison of procedures information and outcomes between the ICAD group and the embolism group.
| Total (n = 133) | ICAD (n = 95) | Embolism (n = 38) | p value | |
|---|---|---|---|---|
| IV rt-PA (%) | 26 (19.5%) | 22 (23.2%) | 4 (10.5%) | 0.097 |
| General anesthesia (%) | 102 (76.7%) | 79 (83.2%) | 23 (60.5%) | 0.005 |
| Onset-to-puncture time (minutes) (IQR) | 370.0 (274.0–557.7) | 390.0 (296.0–610.0) | 313.0 (258.8–452.8) | 0.026 |
| Retrieval times (IQR) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.25) | 0.188 |
| GPI (%) | 86 (64.7%) | 62 (65.3%) | 24 (63.2%) | 0.819 |
| Angioplasty (balloon and/or stenting) (%) | 58 (43.6%) | 57 (60.0%) | 1 (2.6%) | <0.001 |
| Puncture-to-reperfusion time (minutes), mean ± SD | 127.89 ± 63.32 | 133.49 ± 63.52 | 113.87 ± 61.40 | 0.107 |
| Onset-to-reperfusion time (minutes) (IQR) | 505.0 (392.5–695.0) | 535.0 (410.0–737.0) | 435.5 (358.0–549.3) | 0.012 |
| mTICI 2b/3 (%) | 115 (86.5%) | 77 (81.1%) | 38 (100.0%) | 0.004 |
| sICH (%) | 8 (6.0%) | 4 (4.2%) | 4 (10.5%) | 0.327 |
| 90-day favorable outcome (mRS ≤ 2) (%) | 58 (43.6%) | 39 (41.1%) | 19 (50.0%) | 0.347 |
| mortality (%) | 40 (30.1%) | 29 (30.5%) | 11 (28.9%) | 0.858 |
GPI: platelet glycoprotein IIb/IIIa receptor inhibitor; ICAD: intracranial atherosclerotic disease; IQR: interquartile range; IV rt-PA: intravenous recombinant tissue plasminogen activator; mRS: modified Rankin Scale; mTICI: modified Thrombolysis in Cerebral Infarction; sICH: symptomatic intracranial hemorrhage.
Figure 2.
Distribution of 90-day mRS of the two groups. ICAD: intracranial atherosclerotic disease; mRS: modified Rankin Scale.
Notably, the 60.0% of the patients with ICAD who had undergone angioplasty during the procedure achieved a higher rate of reperfusion (93.0% vs 63.2%, p < 0.001), whereas the sICH rate was comparable (3.5% vs 5.3%, p > 0.999).
Discussion
To our knowledge, our study is one of the largest-scale studies to explore the difference between stroke subtypes of VBAO patients undergoing EVT. In situ thrombosis is one of the most common stroke mechanisms in patients with large-vessel occlusion, especially in the Asian population.5 Several studies have demonstrated that 17%–60% of intracranial large-vessel occlusive strokes are due to ICAD, of which the highest rate was from China.12–14 Of the 133 patients enrolled in our study, an occlusion rate of 71.4% was attributed to ICAD, higher than the previous studies. Possible reasons accounting for the distinction included different populations, definitions of ICAD and inclusion criteria. The main findings of the study are as follows: (1) a history of atrial fibrillation and the distribution of the site of occlusion were independent factors to predict ICAD occlusion; and (2) VBAO relative to ICAD resulted in a low rate of reperfusion even with more frequent angioplasty during procedures; however, the 90-day clinical outcome was comparable.
For the EVT of vertebrobasilar occlusive stroke, we routinely choose and adjust the therapeutic strategy according to the etiology, so it is useful if we can identify the stroke subtype before treatment. Our data agreed with previous studies13,15 showing that a history of atrial fibrillation was more prevalent in the embolismic group than the ICAD group, and negatively associated with ICAD-related occlusion, for the reason that cardiac origin was a main cause of embolus.
Recent advances in predicting the existence of ICAD in VBAO have suggested that the occlusion site may be an important predictor of the stroke mechanism. The study by Kim and colleagues13 demonstrated that occlusions in the BAM were more frequent in the ICAD group, whereas occlusions in the BAD were more frequent in the embolic group. As shown in the multiple analysis results (Table 2), occlusion of the V4 segment was significantly positively associated with ICAD-related occlusion, whereas occlusion of the BAD was negatively associated with ICAD-related occlusion. The possible reason for these different results may lie in the higher prevalence of symptomatic atherosclerosis stenosis in the intracranial vertebral artery than in any segment of the basilar artery in the Chinese population.16 In addition, an embolus from the proximal artery or of cardiac origin can easily get stuck at the end of the vessel trunk when moving downstream with blood, so embolism patients have a high prevalence of BAD occlusion.
As to the therapy and outcomes of EVT, the presence of underlying ICAD can lead to a low rate of reperfusion, which is in accordance with the study by Al Kasab et al.,17 but contradicts the results by Lee and colleagues.12 The main reason for the discrepancy might be the different patient populations and adopted techniques. Angioplasty performed as the rescued strategy for patients with ICAD can improve the possibility of reperfusion without the increased risk of sICH.18 In addition, the procedure time tended to be longer in the ICAD group, but the difference was not significant, which is also different from prior research.13,17,18 According to our analysis, nine patients (23.7%) in the embolism group chose the balloon/stenting at the tandem stenosis as the prior attempt to acquire access to the occlusion site, which prolonged the time from puncture to reperfusion. The reperfusion strategy and classification method may account for these diverse results.
This study has several limitations inherent to its retrospective nature. Perioperative management (blood pressure, anticoagulant or antiplatelet drug) that may affect the outcomes was not analyzed. In addition, we assessed the characteristics of patients from a single Chinese center, for which the results may not be generalizable because of selection bias.
Conclusion
This retrospective study demonstrated that a history of atrial fibrillation and the distribution of the site of occlusion were independent factors for predicting ICAD occlusion. VBAO relative to ICAD resulted in a low rate of reperfusion even with more frequent angioplasty in procedures than in embolic patients; however, the 90-day clinical outcome was comparable.
Acknowledgements
We thank all the clinicians, imaging and laboratory technicians, and statisticians who contributed to the information collection and analysis of this study.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Israeli-korn SD, Schwammenthal Y, Yonash-Kimchi T, et al. Ischemic stroke due to acute basilar artery occlusion: Proportion and outcomes. Isr Med Assoc J 2010; 12: 671–675. [PubMed] [Google Scholar]
- 2.Mattle HP, Arnold M, Lindsberg PJ, et al. Basilar artery occlusion. Lancet Neurol 2011; 10: 1002–1014. [DOI] [PubMed] [Google Scholar]
- 3.Schonewille WJ, Wijman CA, Michel P, et al. Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): A prospective registry study. Lancet Neurol 2009; 8: 724–730. [DOI] [PubMed] [Google Scholar]
- 4.Phan K, Phan S, Huo YR, et al. Outcomes of endovascular treatment of basilar artery occlusion in the stent retriever era: A systematic review and meta-analysis. J Neurointerv Surg 2016; 8: 1107–1115. [DOI] [PubMed] [Google Scholar]
- 5.Gorelick PB, Wong KS, Bae HJ, et al. Large artery intracranial occlusive disease: A large worldwide burden but a relatively neglected frontier. Stroke 2008; 39: 2396–2399. [DOI] [PubMed] [Google Scholar]
- 6.Lee JS, Hong JM, Lee KS, et al. Endovascular therapy of cerebral arterial occlusions: Intracranial atherosclerosis versus embolism. J Stroke Cerebrovasc Dis 2015; 24: 2074–2080. [DOI] [PubMed] [Google Scholar]
- 7.Puetz V, Sylaja PN, Coutts SB, et al. Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke 2008; 39: 2485–2490. [DOI] [PubMed] [Google Scholar]
- 8.Archer CR, Horenstein S. Basilar artery occlusion: Clinical and radiological correlation. Stroke 1977; 8: 383–390. [DOI] [PubMed] [Google Scholar]
- 9.Alemseged F, Shah DG, Diomedi M, et al. The Basilar Artery on Computed Tomography Angiography prognostic score for basilar artery occlusion. Stroke 2017; 48: 631–637. [DOI] [PubMed] [Google Scholar]
- 10.Tomsick T, Broderick J, Carrozella J, et al. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am J Neuroradiol 2008; 29: 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): A randomised, parallel-group, non-inferiority trial. Lancet 2012; 380: 1241–1249. [DOI] [PubMed] [Google Scholar]
- 12.Lee YY, Yoon W, Kim SK, et al. Acute basilar artery occlusion: Differences in characteristics and outcomes after endovascular therapy between patients with and without underlying severe atherosclerotic stenosis. AJNR Am J Neuroradiol 2017; 38: 1600–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YW, Hong JM, Park DG, et al. Effect of intracranial atherosclerotic disease on endovascular treatment for patients with acute vertebrobasilar occlusion. AJNR Am J Neuroradiol 2016; 37: 2072–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao F, Lo WT, Sun X, et al. Combined use of mechanical thrombectomy with angioplasty and stenting for acute basilar occlusions with underlying severe intracranial vertebrobasilar stenosis: Preliminary experience from a single Chinese center. AJNR Am J Neuroradiol 2015; 36: 1947–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horie N, Tateishi Y, Morikawa M, et al. Acute stroke with major intracranial vessel occlusion: Characteristics of cardioembolism and atherosclerosis-related in situ stenosis/occlusion. J Clin Neurosci 2016; 32: 24–29. [DOI] [PubMed] [Google Scholar]
- 16.Miao Z, Zhang Y, Shuai J, et al. Thirty-day outcome of a multicenter registry study of stenting for symptomatic intracranial artery stenosis in China. Stroke 2015; 46: 2822–2829. [DOI] [PubMed] [Google Scholar]
- 17.Al Kasab S, Almadidy Z, Spiotta AM, et al. Endovascular treatment for AIS with underlying ICAD. J Neurointerv Surg 2017; 9: 948–951. [DOI] [PubMed] [Google Scholar]
- 18.Jia B, Feng L, Liebeskind DS, et al. Mechanical thrombectomy and rescue therapy for intracranial large artery occlusion with underlying atherosclerosis. J Neurointerv Surg 2018; 10: 746–750. [DOI] [PubMed] [Google Scholar]