Abstract
Background and purpose
Recent studies suggest that thrombus imaging characteristics such as Hounsfield unit (HU) and perviousness assessed on noncontrast computed tomography (NCCT) and CT angiography (CTA) can predict successful recanalization. We assessed whether these thrombus imaging characteristics could predict successful first-pass recanalization.
Methods
We retrospectively reviewed cases of mechanical thrombectomy over a three-year period in which patients received a multiphase CTA and were treated with a stent retriever on first pass. Thrombus attenuation, thrombus enhancement on arterial- and delayed-phase CTA and percentage washout were calculated and their association with first-pass recanalization, successful recanalization and distal embolic complications was studied.
Results
Fifty-two mechanical thrombectomy patients were included. First-pass recanalization was achieved in 59.6% and complete revascularization (Thrombolysis in Cerebral Infarction scale 2b/3) was achieved in 84.6%. There was no correlation between first-pass recanalization with thrombus density on NCCT (p = 0.94), percentage enhancement on arterial (p = 0.61) and delayed-phase CTA (p = 0.23) or thrombus length (p = 0.16). There was no correlation between number of passes and either thrombus density on NCCT (p = 0.91) or percentage enhancement on arterial- (p = 0.79) and delayed-phase (p = 0.14) CTA or thrombus length (p = 0.34). Clot length was significantly higher in patients with distal embolic complications than in those without (18.5 ± 7.9 vs 11.4 ± 6.6 mm, p = 0.005).
Conclusions
Our data suggest that thrombus imaging characteristics on multiphase CTA cannot predict first-pass recanalization or successful revascularization in acute ischemic stroke patients treated with stent retrievers. Longer clot length was associated with higher risk of distal embolic complications.
Keywords: CTA, perviousness, stroke, thrombectomy, thrombus
Introduction
Recent randomized clinical trials have demonstrated the efficacy of mechanical thrombectomy using stent retrievers for patients with acute ischemic stroke (AIS) and large-vessel occlusions (LVOs).1,2 The achievement of successful recanalization (SR) of an LVO on first pass by a stent retriever (SRFP) is associated with higher rates of good clinical outcome.3 Certain imaging characteristics of the clot or thrombus have recently been demonstrated to be associated with successful recanalization of LVOs with mechanical thrombectomy and the use of intravenous thrombolysis (IVT). These include the clot density in Hounsfield units (HU) on noncontrast enhanced computed tomography (NCCT)4–6 and “perviousness” on CT angiography (CTA).7,8 Perviousness quantifies contrast penetration into a clot and has been recently described as a method of estimating thrombus permeability.7 Thrombus permeability describes how well blood flows into or around the clot. Highly permeable clots may not fully occlude the artery and allow perfusion of brain tissue distal to the clot; moreover, these clots may be more amenable to dissolution by intravenous tissue plasminogen activator (IV tPA) or have different mechanical properties that increase the chances of successful thrombectomy using stent retrievers. Understanding whether thrombus perviousness is associated with first-pass effect with stent retrievers could be clinically important for device selection prior to performing mechanical thrombectomy. To date, no studies have examined the correlation between thrombus perviousness and first-pass effect. If it is found that pervious thrombi have substantially higher rates of recanalization with stent retriever alone than impervious thrombi, then an SRFP-only thrombectomy strategy may be considered for such clots. Meanwhile, impervious clots could be pursued with combination techniques (i.e. Solumbra). We sought to assess the association of clot density and thrombus perviousness with SRFP and SR on multiphase CTA. We hypothesized that pervious thrombi would be more likely to be revascularized on first pass.
Materials and methods
Patient population
Following institutional board review approval, we retrospectively reviewed AIS patients treated with mechanical thrombectomy at our institution between January 2014 and October 2017. Inclusion criteria were 1) adult patients, 2) stent retriever used on first pass, 3) LVO involving the internal carotid artery (ICA) terminus, M1 or M2 segment of the middle cerebral artery (MCA), or the basilar artery and 4) a multiphase CTA with acceptable image quality on NCCT, arterial phase and venous phase. Occlusions of the proximal cavernous or extracranial ICA were excluded. Thrombectomies for which direct aspiration was the primary technique employed were also excluded. In addition, thrombectomies in which aspiration catheters were used in addition to stent retrievers during first pass were excluded.
Patient clinical and imaging data were reviewed. The primary outcome was rate of Thrombolysis in Cerebral Infarction scale (TICI) 2b/3 scores on SRFP. Secondary outcomes were successful revascularization, number of passes of stent retriever required for successful revascularization and distal embolic complications.
Imaging
All patients underwent 3 mm head NCCT (Aquilion ONE, Toshiba Medical Systems, Nasu, Japan). Cranial and cervical CTA was obtained following injection of 60 ml of 370 mg iodine per ml of contrast at a rate of 5 ml/s. CTA images were reconstructed at 0.5 mm thickness. NCCT, arterial-phase CTA and delayed-phase CTA images were reviewed by consensus on a picture archiving and communication workstation by two diagnostic and interventional neuroradiologists with at least two years of experience who were blinded to clinical data including reperfusion outcomes.
Thrombus attenuation and length
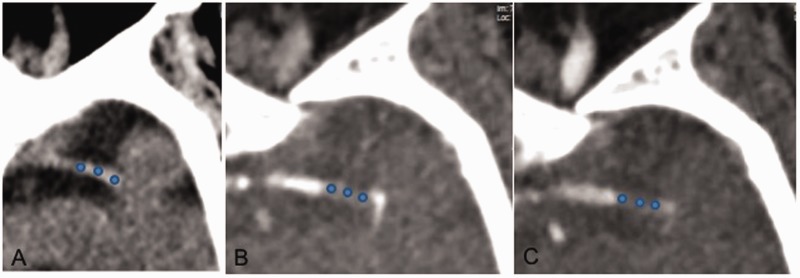
Thrombus HU value was calculated on NCCT using three circular regions of interest (proximal, middle and distal, Figures 1 and 2). HU values were calculated using regions of interest placed on three corresponding locations on the contralateral artery to correct for variability of hematocrit levels. The average of three measurements was used and the mean HU for the clot and contralateral artery calculated. Thrombus length was measured as the length of the nonenhancing portion of the vessel on delayed-phase images. Delayed phase images were chosen because they allowed the maximum amount of time to allow contrast to reach the distal face of the clot from leptomeningeal collaterals. In cases in which patients had poor collaterals, clot length was estimated as the length of the hyperdense artery. Mural calcifications were excluded from analysis. Thrombus perviousness was calculated using thrombus percentage enhancement on arterial-phase CTA and delayed-phase CTA and as well as percentage washout.
Figure 1.
(a) Example of measurement of thrombus attenuation increase using three regions of interest (circles) on a distal segment of a left middle cerebral artery on noncontrast computed tomography, (b) arterial-phase computed tomography angiography (CTA) and (c) delayed-phase CTA.
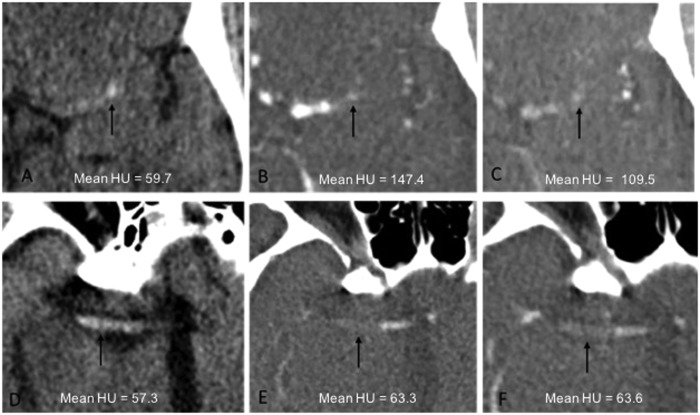
Figure 2.
Example of enhanced thrombus. (a) Noncontrast computed tomography (NCCT). (b) Arterial-phase computed tomography angiography (CTA). (c) Delayed-phase CTA. Example of thrombus that did not significantly enhance. (d) NCCT. (e) Arterial-phase CTA. (f) Delayed-phase CTA. Arrow: thrombus.
Estimation of perviousness
As an estimation of contrast penetration into the thrombus, the increase in mean HU of the thrombus on CTA was compared with the HU of the clot on NCCT. Percentage enhancement of the thrombus was calculated on the arterial- and delayed-phase CTA and percentage washout was calculated using the ratio of the mean HU on the arterial- and delayed-phase CTA. Three patterns of thrombus perviousness were identified. Pattern Type 1 indicated a situation in which there was no enhancement of the thrombus on postcontrast exams. Pattern Type 2 indicated a situation in which there was an increase in HU on arterial phase and then washout on the delayed phase. Pattern Type 3 indicated a situation in which there was an increase in HU on arterial phase and then no decrease on delayed phase or even an increase in attenuation on delayed phase.
Statistical analysis
Statistical analysis was undertaken using SPSS software (V.19, IBM Software, Chicago, IL, USA). Analysis of variables was performed using chi-squared testing for categorical data and linear regression and two-tailed t test for continuous data. For all analyses p < 0.05 was considered statistically significant.
Results
Patient population
Baseline characteristics are presented in Table 1. Over the course of the study time period there were 243 LVOs treated at our institution. Of these, 52 met inclusion criteria having undergone a multiphase CTA and having been treated with a stent-retriever on first pass. Solitaire (eV3, Irvine, CA, USA) or Trevo (Stryker Neurovascular, Fremont, CA, USA) stent retrievers were used for thrombectomy in the included patients. M1 occlusions were the most frequently observed LVO. SR (TICI 2b/3) was achieved in 44 patients (84.6%) and SRFP was achieved in 31 (59.6%) of patients. Three patterns of thrombus perviousness were identified: 1) “no change” (1.9%), 2) increase in HU then washout (28.8%) and 3) increase in HU and then either stable or increase in HU (69.2%).
Table 1.
Baseline characteristics.
| Characteristic | Outcome |
|---|---|
| Mean age (SD) | 69.6 (16.5) |
| N (%) Female | 27 (51.9) |
| Occlusion location N (%) | |
| M1 | 35 (67.3) |
| M2 | 11 (21.1) |
| ICA terminus | 1 (1.9) |
| Vertebrobasilar | 5 (9.6) |
| Thrombus characteristics | |
| Mean thrombus length in mm (SD) | 13.5 (7.6) |
| Mean thrombus attenuation NCCT (SD) | 49.2 (7.3) |
| Mean thrombus attenuation, arterial phase (SD) | 75.5 (19.9) |
| Mean thrombus attenuation, delayed phase (SD) | 80.9 (3.4) |
| Type of curve N (%) | |
| No change | 1 (1.9) |
| Increase and washout | 15 (28.8) |
| Increase and stable or increase | 36 (69.2) |
| Final TICI N (%) | |
| 0 | 3 (5.8) |
| 1 | 1 (1.9) |
| 2a | 4 (7.7) |
| 2b | 15 (28.8) |
| 3 | 29 (55.8) |
| Mean (SD) number of passes | 1.8 (1.1) |
| TICI 2b/3 on first pass N (%) | |
| Yes | 31 (59.6) |
| No | 21 (40.4) |
| IV tPA N (%) | 30 (57.7) |
ICA: internal carotid artery; IV: intravenous; NCCT: noncontrast computed tomography; TICI: Thrombolysis in Cerebral Infarction scale; tPA: tissue plasminogen activator.
Thrombus characteristics and outcomes
There was no correlation between first-pass recanalization and thrombus imaging characteristics, including clot density on NCCT (p = 0.94), percentage enhancement on arterial- (p = 0.61) or delayed-phase CTA (p = 0.23), percentage enhancement washout (p = 0.09) or thrombus length (p = 0.16) (Table 2).
Table 2.
Outcomes.
| First-pass recanalization |
Successful recanalization |
Number of stent-retriever passes |
Distal emboli |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Thrombus characteristics | Yes | No | p | Yes | No | p | R 2 | p | Yes | No | p |
| Mean HU on NCCT | 49.3 (7.5) | 49.1 (7.1) | 0.94 | 48.6 (7.0) | 52.7 (8.1) | 0.21 | 0 | 0.91 | 48.5 (5.3) | 49.5 (8.0) | 0.61 |
| % Enhancement on arterial phase | 52.9 (51.5) | 59.2 (36.7) | 0.61 | 54.2 (45.6) | 62.3 (50.1) | 0.68 | 0 | 0.79 | 55.6 (36.7) | 55.4 (49.5) | 0.99 |
| % Enhancement on delayed phase | 78.4 (97.7) | 54.5 (37.7) | 0.23 | 71.8 (83.9) | 51.5 (48.1) | 0.35 | 0.04 | 0.14 | 60.4 (50.1) | 72.1 (89.1) | 0.55 |
| % Washout | 25.1 (83.1) | –1.9 (16.6) | 0.09 | 17.8 (70.9) | –5.9 (14.1) | 0.05 | 0.03 | 0.21 | 4.8 (29.5) | 18.0 (75.9) | 0.37 |
| Thrombus length (mm) | 12.2 (6.8) | 15.4 (8.6) | 0.16 | 14.0 (7.2) | 10.2 (9.8) | 0.32 | 0.02 | 0.34 | 18.5 (7.9) | 11.4 (6.6) | 0.005 |
| Type of curve | |||||||||||
| 1 | 1 (3.2) | 0 (0.0) | 0.62 | 1 (2.3) | 0 (0.0) | 0.34 | 0.02 | 0.44 | 0 (0.0) | 1 (2.7) | 0.51 |
| 2 | 8 (25.8) | 7 (33.3) | 11 (25.0) | 4 (50.0) | 3 (20.0) | 12 (32.4) | |||||
| 3 | 22 (71.0) | 14 (66.7) | 32 (72.7) | 4 (50.0) | 12 (80.0) | 24 (64.9) | |||||
HU: Hounsfield unit; NCCT: noncontrast computed tomography.
There was no correlation between successful recanalization and thrombus imaging characteristics, including clot density on NCCT (p = 0.21), percentage enhancement on arterial- (p = 0.68) or delayed-phase CTA (p = 0.35), percentage enhancement washout (p = 0.05) or thrombus length (p = 0.32).
The number of stent-retriever passes was also not correlated with thrombus characteristics including clot density (p = 0.91), percentage enhancement on arterial CTA (p = 0.79), percent enhancement on delayed-phase CTA (p = 0.14), percentage washout (p = 0.21), and thrombus length (p = 0.34).
Longer clots were associated with a greater risk of distal embolic complications (no emboli: 11.4 ± 6.6 mm vs emboli: 18.5 ± 7.9 mm, p = 0.005). No other thrombus characteristics were associated with distal embolic complications.
Selection bias
To determine whether there was selection bias in our included cohort, we examined the 163 anterior circulation strokes that were excluded because of lack of multiphase CTA (67), motion artifact (23) or use of aspiration-first technique (73). First-pass recanalization occurred in 54.6% of cases (89/173) and the mean number of passes was 2.0 (SD = 0.7). There was no significant difference in first-pass recanalization or mean number of passes in our excluded cohort when compared with our included cohort.
Discussion
This retrospective study of 52 patients with AIS who were treated with stent-retriever devices and underwent multiphase CTA has a number of noteworthy findings. First, contrary to recent studies,7,8 we did not find an association between thrombus perviousness and rate of successful recanalization in our patients who were treated with mechanical thrombectomy using stent retrievers. Indeed, none of the quantifiable imaging characteristics, including density, percentage enhancement on CTA or percentage enhancement washout, were correlated with either successful first-pass recanalization, successful recanalization, number of passes required to recanalize the vessel or rate of distal emboli due to clot fragmentation. Only longer clots measured on preintervention imaging were associated with more risk of distal embolic complications. These findings are important as they suggest that clot imaging characteristics may not be as strongly correlated with angiographic and technical outcomes following stent-retriever thrombectomy as previously thought.
The primary aim of mechanical thrombectomy for AIS patients is to reopen the occluded vessel as quickly as possible, and the sooner this is achieved the more likely the patient will have a better functional outcome.9 Recanalization of LVO in AIS is influenced by collateral status, angioarchitecture (for instance, if a branch arises from the thrombosed vessel) as well as treatment modality (endovascular therapy with or without IVT).10 Recently there has been considerable interest in how thrombus composition may influence its response to IVT and mechanical thrombectomy; however, the literature is conflicting. Clots originating from cardiac sources may contain a higher proportion of fibrin and platelets than red blood cells compared with those due to noncardioembolic stroke.11 Fibrin-rich clots are often deemed more difficult to extract from a cerebral blood vessel requiring a greater number of passes than those rich in red blood cells.12 It has been demonstrated that the higher the density of the clot on NCCT the higher its proportion of red blood cells and the lower its proportion of fibrin. Additionally, clots with a higher proportion of fibrin demonstrate more pronounced contrast uptake than those rich in red blood cells after prolonged exposure; however, they could not be differentiated after initial contrast exposure.13 The correlation between thrombus density on NCCT and SR using IVT, intraarterial thrombolysis and mechanical thrombectomy has been reported,5,14,15 and a recent systematic review concluded that the hyperdense artery sign on NCCT is associated with red blood cell–rich thrombus and better successful recanalization rates.6 Other studies have failed to confirm these findings, however, and overall the literature has been conflicting with some studies showing no association between clot density and recanalization with stent retrievers or aspiration.16,17 However, when considering the use of stent retrievers only (as opposed to aspiration thrombectomy), Mokin et al. reported in their series a positive association between higher-density clots on NCCT and rate of SR using the Solitaire (eV3, Irvine, CA, USA) stent retriever.4
Thrombus perviousness has recently been described as a method of estimating how permeable a clot is using enhancement and washout measures using arterial- and delayed-phase CTA.7 More permeable (or pervious) clots as demonstrated by greater enhancement on arterial-phase CTA with either further enhancement or stable enhancement on the delayed phase with less contrast washout may be more amenable to dissolution by IVT and mechanical thrombectomy using stent retrievers.7,8 More recently in a post hoc analysis of the MR CLEAN trial, Borst et al. found that thrombus perviousness and thrombus length were independently associated with functional outcome while other variables including thrombus density were not.18 In another post hoc analysis of the Prove-IT study, Santos and colleagues found that thrombus attenuation increase on arterial-phase CTA was significantly associated with functional outcome while other measurements of thrombus attenuation and enhancement were not.7,8 The theory behind the idea that thrombus perviousness is associated with improved functional and angiographic outcomes is that clots that allow for contrast transit are more porous, less physically dense or subocclusive. Such clots would allow for improved penetration by IV tPA or perhaps improved engagement by a stent retriever. In our study using multiphase CTA, we found no association between any measures of clot perviousness and successful recanalization on first pass, final revascularization status, number of passes or distal emboli when thrombectomy was performed with stent retrievers. To date, there have been no studies examining the correlation between thrombus perviousness and recanalization rates with mechanical thrombectomy.
Clot length was not associated with either rate of successful revascularization on first pass or successful recanalization, and this is in line with most other published studies.4,14,19 However, we did find that clot length was associated with a greater likelihood of distal emboli, presumably due to greater risk of clot fragmentation during the thrombectomy. To our knowledge no studies have previously investigated the association of clot length (as assessed on preprocedural imaging) and rate of distal embolic complications. Chueh et al. demonstrated using an in vitro MCA occlusion model that thrombectomy technique influenced the risk of clot fragmentation and this was influenced by clot composition and thrombectomy technique. “Harder” clots (described as mimicking cholesterol-rich clots typically from cardioembolic sources) were extracted more successfully with fewer distal emboli using a Solumbra (stent retriever plus aspiration) technique whereas “softer” clots mimicking red blood cell–rich thrombi were less likely to fragment using a stent retriever plus a balloon-guided catheter.20 Further studies are required to correlate clot histology (from real patients) with imaging characteristics on NCCT and CTA to optimize treatment strategies that can take into consideration clot composition prior to undertaking thrombectomy.
Limitations
Our study has limitations. Our sample size is small, and data were reviewed retrospectively. We did not consider the adjunctive use of IV tPA and whether this influenced our outcomes. Furthermore, the use of balloon-guided catheters and the size and length of stent retriever used may have influenced our results. We considered the clot to be homogenous and conducted measures of density and perviousness using three regions of interest and averaged the values; however, it has been shown that composition may vary within a thrombus and measurement of the entire thrombus may be more correct. The use of 3-mm thick slices on NCCT did not directly correspond to 0.5-mm thick slices on CTA and for this reason some cases had to be excluded. It is clear from prior studies that the use of thin-section CT (<1 mm slice thickness) allows for improved evaluation of thrombus attenuation. In our study, only 3-mm slices were available. One of the reasons we failed to find a difference between groups could be the high rate of first-pass recanalization overall, which limited group size and thus power to detect differences between groups. We did not collect any clots for histopathological analysis, and thus we cannot make any statement regarding the correlation between thrombus perviousness and histopathology.
Conclusion
No association between clot density, clot length or clot perviousness and rate of successful recanalization, SRFP or number of passes was identified in this real-world study. Longer clots on preprocedural imaging are more prone to fragmentation and distal embolic complications. More research is required to accurately correlate clot histology with prethrombectomy imaging characteristics and clot behavior during thrombectomy to optimize treatment strategies.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: A meta-analysis. JAMA 2016; 316: 1279–1288. [DOI] [PubMed] [Google Scholar]
- 3.Zaidat OO, Castonguay AC, Linfante I, et al. First pass effect: A new measure for stroke thrombectomy devices. Stroke 2018; 49: 660–666. [DOI] [PubMed] [Google Scholar]
- 4.Mokin M, Morr S, Natarajan SK, et al. Thrombus density predicts successful recanalization with Solitaire stent retriever thrombectomy in acute ischemic stroke. J Neurointerv Surg 2015; 7: 104–107. [DOI] [PubMed] [Google Scholar]
- 5.Moftakhar P, English JD, Cooke DL, et al. Density of thrombus on admission CT predicts revascularization efficacy in large vessel occlusion acute ischemic stroke. Stroke 2013; 44: 243–245. [DOI] [PubMed] [Google Scholar]
- 6.Brinjikji W, Duffy S, Burrows A, et al. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: A systematic review. J Neurointerv Surg 2017; 9: 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos EM, Marquering HA, den Blanken MD, et al. Thrombus permeability is associated with improved functional outcome and recanalization in patients with ischemic stroke. Stroke 2016; 47: 732–741. [DOI] [PubMed] [Google Scholar]
- 8.Santos EM, Dankbaar JW, Treurniet KM, et al. Permeable thrombi are associated with higher intravenous recombinant tissue-type plasminogen activator treatment success in patients with acute ischemic stroke. Stroke 2016; 47: 2058–2065. [DOI] [PubMed] [Google Scholar]
- 9.Nogueira RG, Liebeskind DS, Sung G, et al. Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: Pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI Trials. Stroke 2009; 40: 3777–3783. [DOI] [PubMed] [Google Scholar]
- 10.Qazi EM, Sohn SI, Mishra S, et al. Thrombus characteristics are related to collaterals and angioarchitecture in acute stroke. Can J Neurol Sci 2015; 42: 381–388. [DOI] [PubMed] [Google Scholar]
- 11.Boeckh-Behrens T, Kleine JF, Zimmer C, et al. Thrombus histology suggests cardioembolic cause in cryptogenic stroke. Stroke 2016; 47: 1864–1871. [DOI] [PubMed] [Google Scholar]
- 12.Fennell VS, Setlur Nagesh SV, Meess KM, et al. What to do about fibrin rich ‘tough clots’? Comparing the Solitaire stent retriever with a novel geometric clot extractor in an in vitro stroke model. J Neurointerv Surg 2018; 10: 907–910. [DOI] [PubMed] [Google Scholar]
- 13.Borggrefe J, Kottlors J, Mirza M, et al. Differentiation of clot composition using conventional and dual-energy computed tomography. Clin Neuroradiol 2018; 28: 515–522. [DOI] [PubMed] [Google Scholar]
- 14.Mokin M, Levy EI, Siddiqui AH, et al. Association of clot burden score with radiographic and clinical outcomes following Solitaire stent retriever thrombectomy: Analysis of the SWIFT PRIME trial. J Neurointerv Surg 2017; 9: 929–932. [DOI] [PubMed] [Google Scholar]
- 15.Kim EY, Heo JH, Lee SK, et al. Prediction of thrombolytic efficacy in acute ischemic stroke using thin-section noncontrast CT. Neurology 2006; 67: 1846–1848. [DOI] [PubMed] [Google Scholar]
- 16.Spiotta AM, Vargas J, Hawk H, et al. Hounsfield unit value and clot length in the acutely occluded vessel and time required to achieve thrombectomy, complications and outcome. J Neurointerv Surg 2014; 6: 423–427. [DOI] [PubMed] [Google Scholar]
- 17.Baek JH, Yoo J, Song D, et al. Predictive value of thrombus volume for recanalization in stent retriever thrombectomy. Sci Rep 2017; 7: 15938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borst J, Berkhemer OA, Santos EMM, et al. Value of thrombus CT characteristics in patients with acute ischemic stroke. AJNR Am J Neuroradiol 2017; 38: 1758–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soize S, Batista AL, Rodriguez Regent C, et al. Susceptibility vessel sign on T2* magnetic resonance imaging and recanalization results of mechanical thrombectomy with stent retrievers: A multicentre cohort study. Eur J Neurol 2015; 22: 967–972. [DOI] [PubMed] [Google Scholar]
- 20.Chueh JY, Puri AS, Wakhloo AK, et al. Risk of distal embolization with stent retriever thrombectomy and ADAPT. J Neurointerv Surg 2016; 8: 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]