Key Points
Question
In young adults aged 18 to 49 years, what is the age- and sex-specific case fatality and long-term mortality associated with stroke?
Findings
In this Dutch register-based cohort study that included 15 527 patients who in the years 1998-2010 had a first stroke at age 18 to 49 years, cumulative 15-year mortality among 30-day survivors was 13.3 per 1000 person-years compared with an expected mortality of 2.4 per 1000 person-years in the general population, an excess mortality of 10.9 per 1000 person-years.
Meaning
Mortality risk 15 years after stroke among young adults aged 18 to 49 years who were 30-day survivors remained elevated.
Abstract
Importance
Stroke remains the second leading cause of death worldwide. Approximately 10% to 15% of all strokes occur in young adults. Information on prognosis and mortality specifically in young adults is limited.
Objective
To determine short- and long-term mortality risk after stroke in young adults, according to age, sex, and stroke subtype; time trends in mortality; and causes of death.
Design, Setting, and Participants
Registry- and population-based study in the Netherlands of 15 527 patients aged 18 to 49 years with first stroke between 1998 and 2010, and follow-up until January 1, 2017. Patients and outcomes were identified through linkage of the national Hospital Discharge Registry, national Cause of Death Registry, and the Dutch Population Register.
Exposures
First stroke occurring at age 18 to 49 years, documented using International Classification of Diseases, Ninth Revision, and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, codes for ischemic stroke, intracerebral hemorrhage, and stroke not otherwise specified.
Main Outcomes and Measures
Primary outcome was all-cause cumulative mortality in 30-day survivors at end of follow-up, stratified by age, sex, and stroke subtype, and compared with all-cause cumulative mortality in the general population.
Results
The study population included 15 527 patients with stroke (median age, 44 years [interquartile range, 38-47 years]; 53.3% women). At end of follow-up, a total of 3540 cumulative deaths had occurred, including 1776 deaths within 30 days after stroke and 1764 deaths (23.2%) during a median duration of follow-up of 9.3 years (interquartile range, 5.9-13.1 years). The 15-year mortality in 30-day survivors was 17.0% (95% CI, 16.2%-17.9%). The standardized mortality rate compared with the general population was 5.1 (95% CI, 4.7-5.4) for ischemic stroke (observed mortality rate 12.0/1000 person-years [95% CI, 11.2-12.9/1000 person-years]; expected rate, 2.4/1000 person-years; excess rate, 9.6/1000 person-years) and the standardized mortality rate for intracerebral hemorrhage was 8.4 (95% CI, 7.4-9.3; observed rate, 18.7/1000 person-years [95% CI, 16.7-21.0/1000 person-years]; expected rate, 2.2/1000 person-years; excess rate, 16.4/1000 person-years).
Conclusions and Relevance
Among young adults aged 18 to 49 years in the Netherlands who were 30-day survivors of first stroke, mortality risk compared with the general population remained elevated up to 15 years later.
This cohort study uses Dutch registry and population data to estimate associations between incident stroke occurring between ages 18 and 49 years and short- and long-term all-cause mortality stratified by age, sex, and stroke subtype.
Introduction
Stroke is the second leading cause of death worldwide. In 2013, more than 10 million people experienced a stroke, and more than 6 million died.1,2,3 Approximately 10% to 15% of all strokes occur in young adults aged 18 to 49 years.4,5 Information on the risk of death in this subgroup is limited.6
Previous studies of mortality after stroke in young adults were often small, hospital based, had limited periods of follow-up, or included patients who had their stroke between 1980 and 2000.7,8,9 Over the past decades, both acute treatment and secondary prevention have improved. Because of the small number of patients younger than age 50 years with stroke included in previous studies, it has not been possible to estimate mortality according to age, sex, and stroke subtypes.7,10,11,12,13,14,15
This study aimed to investigate case fatality and cumulative 1-year, 5-year, 10-year, and 15-year mortality and trends over time of first stroke in young adults aged 18 to 49 years, stratified by age, sex, and stroke subtype; excess mortality after stroke compared with the general population; and causes of death.
Methods
The Medical Ethics Review Committee Arnhem/Nijmegen assessed the protocol and waived the requirement for ethical review and for patient consent due to the use of deidentified data. All analyses were performed in a secured environment of Statistics Netherlands according to Dutch privacy legislation.
Exposure
We constructed a nationwide cohort of patients aged 18 to 49 years with first stroke (ischemic stroke, intracerebral hemorrhage, or stroke not otherwise specified) through linkage of the Dutch nationwide hospital registry (Hospital Discharge Registry [HDR]) and Dutch population registry from January 1, 1998, to January 1, 2011, and the National Cause of Death Registry (CDR) from January 1, 1998, to January 1, 2017, by using International Classification of Diseases, Ninth Revision (ICD-9) and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes for stroke (ischemic stroke, intracerebral hemorrhage, and stroke not otherwise specified; World Health Organization International Classification of Diseases) (eTable 1 in the Supplement). We did not include transient ischemic attacks (TIAs) and subarachnoid hemorrhage (SAH). Details of these registries and linkage procedures have been previously described.14,16,17
ICD-9 and ICD-10 codes for the identification of stroke have been proven to be reliable in studies of patients with stroke of all ages.18,19 For this study, we specifically assessed the accuracy of ICD-10 codes for young adults (aged 18-49 years). We checked final diagnoses in medical records against the attached ICD-10 code at discharge of 569 patients admitted in 2 university medical centers and 1 large general hospital between 1995 and 2017. For ischemic stroke, the ICD code was correct in 90.4% of cases (n = 301), for intracerebral hemorrhage in 86.3% of cases (n = 183), and for stroke not otherwise specified in 87.1% of cases (n = 85) (eTable 2 in the Supplement).
For all individuals, the Charlson Comorbidity Index (CCI) score at the time of the index event was estimated based on previously documented hospital admissions. The CCI is a score from 1 to 6 based on 19 primary medical conditions, such as congestive heart failure, diabetes, malignancies, and other organ dysfunction, and has previously been shown to be a valid tool to predict outcome.20
Outcomes
The primary outcome was all-cause cumulative mortality at the end of follow-up, stratified for age, sex, and stroke subtype in 30-day survivors. The secondary outcomes were case fatality and cumulative 1-year, 5-year, 10-year, and 15-year mortality in the 30-day survivors. Other secondary outcomes were the annual risk of death, excess mortality after stroke compared with the general population, time trends of case fatality, 1-year and 5-year mortality in 30-day survivors after first stroke in young adults, and causes of death, all stratified by age, sex, and stroke subtype.
Out-of-hospital deaths were identified if an ICD-10 code for stroke was registered in the CDR without previous hospitalizations in the HDR for this individual. Individuals who emigrated were censored. The number of hospitals participating in the HDR declined from 2005 to 2010, leading to an increasing number of missing records, varying from 1.1% to 14%, resulting in some missing index strokes. Date and cause of death were retrieved from the CDR. In the Netherlands, all deaths are recorded in the CDR by Statistics Netherlands. Cause of death was missing for 1 patient (0.05%); this patient was excluded from the analysis. Data regarding other variables, age, sex, CCI, and length of stay were complete for all patients. Follow-up after first stroke between 1998 and 2010 was defined as the time until death or the end of follow-up (January 1, 2017), whichever occurred first. Case fatality was defined as death occurring within 30 days after stroke. For survival analysis, only survivors beyond these 30 days were included. Causes of death were analyzed by ICD-10 codes.
Statistical Analysis
We used Kaplan-Meier analysis to estimate the risk of death for all-cause stroke, ischemic stroke, intracerebral hemorrhage, and stroke not otherwise specified for men and women separately. We calculated the number of person-years at risk for each individual patient from date of stroke to the date of death or until January 1, 2017, whichever occurred first. We censored the Kaplan-Meier curves at 16 years after the index event because thereafter, the number of person-years at risk became too small to provide reliable estimates of mortality. We calculated 1-year, 5-year, 10-year, and 15-year cumulative mortality by sex and stroke subtype. Survival curves were compared between men and women using log-rank analysis.
For comparison of mortality in young adults with stroke vs mortality in the general population, we used mortality data of the general Dutch population matched by sex, age, and calendar year.17 The annual risk of the observed mortality after stroke, as well as the expected mortality in the general population, was calculated using the formula: 1 − ([1 − Ic][1/n]), where n is the number of years after the index event and Ic is the cumulative mortality at n years, obtained by Kaplan-Meier analysis.
We calculated standardized mortality ratios (SMRs) by dividing the observed deaths in the cohort by the expected deaths of their peers from the general population for each stroke subtype, for both sexes and for different age categories (18-29, 30-39, and 40-49 years). The expected matched mortality rates were retrieved from the worldwide Human Mortality Database (http://www.mortality.org).21 The 95% CIs were calculated by assuming a Poisson distribution. Additionally, for each subgroup, we calculated an absolute excess number of deaths by taking the difference between the observed and expected deaths, divided by the person-years at risk. Differences of the SMRs by sex and stroke subtype were evaluated by tests of interactions through bivariable and multivariable linear regression modeling.
We assessed the association of age at index event, sex, CCI score, and length of stay with the risk of long-term mortality, stratified for stroke subtypes, through Cox proportional hazard models, and expressed the associations as hazard ratios (HRs) with 95% CIs. After bivariable analysis, all 4 variables were simultaneously entered to obtain a multivariable model. Assumption of proportionality in the Cox regression model was evaluated graphically assessing the log(−log[Survival]) plots for all covariates. We found no indication of violating the assumption. In addition, we plotted the scaled Schoenfeld residuals against time, which confirmed proportionality. We calculated time trends in case fatality and 1-year and 5-year mortality rates in yearly average percentage change (APC) and tested change over time with linear regression analysis. We used R2 to assess the goodness of fit of the linear regression analyses. Time trends regarding 10-year and 15-year mortality were not calculated because we did not have a complete 10-year and 15-year follow-up period for all inclusion years. Also, time trends were calculated for mean length of stay and tested through linear regression.
In the Netherlands, cause of death is established among all patients who die (both inside or outside the hospital) by a coroner or physician who is required by law to complete the death certificate. This death certificate is then sent to coders employed by Statistics Netherlands who assign an ICD-10 code for the primary (underlying disease) and secondary (possible complications, such as pneumonia) causes of death accordingly. Studies on the reliability of these ICD-10 codes found an intercoder agreement of 78% and intracoder agreement of 89%. The highest reliability was found for major causes of death (malignancy and acute myocardial infarction).22 We categorized causes of death based on the ICD-10 codes for primary cause in 30-day survivors (eTable 3 in the Supplement). The proportion of causes of death were compared through χ2 analyses. We tested for interactions between stroke subtype and different causes of death separately using a Poisson regression model, with adjustment for sex through adding this as a covariate to a multivariable Poisson model.
Two-sided P values of .05 were considered statistically significant. For the SMR analyses, we set the threshold for significance to a P value of .005 after Bonferroni adjustment for 10 subgroup analyses.
Data were analyzed with SPSS Software version 22 (IBM), R version 3.22 (packages rateratio.test, survival, survminer; R Project for Statistical Computing), Stata version 12 (StataCorp), and Microsoft Office Excel 2007.
Results
We identified 15 257 young adults with a stroke (53.3% women; median age, 44 years [interquartile range, 38-47 years]). A total of 8444 (55.3%) had ischemic stroke, 3077 (20.2%) had intracerebral hemorrhage, and 3736 (24.5%) had a stroke not otherwise specified. Less than 1% of strokes were out-of-hospital deaths, and most had no comorbidity (n = 12 803, 83.9%) (Table 1). The median duration of follow-up was 9.3 years (interquartile range, 5.9-13.1 years; Table 1).
Table 1. Demographics Including Age, Sex, Comorbidity, and Follow-up of Patients With Stroke Aged 18-49 Years.
| Stroke, No. (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any | Ischemic | Intracerebral Hemorrhage | Not Otherwise Specified | |||||||||
| Total | Men | Women | Total | Men | Women | Total | Men | Women | Total | Men | Women | |
| Patients | 15 257 (100) | 7127 (46.7) | 8130 (53.3) | 8444 (100) | 3851 (45.6) | 4593 (54.4) | 3077 (100) | 1585 (51.5) | 1492 (48.5) | 3736 (100) | 1691 (45.3) | 2045 (54.7) |
| Age, mean (SD), y | 41.8 (6.8) | 42.3 (6.5) | 41.4 (7.0) | 42.0 (6.6) | 42.6 (6.1) | 41.4 (7.0) | 40.7 (7.5) | 40.7 (7.5) | 40.8 (7.5) | 42.2 (6.4) | 42.9 (6.0) | 41.6 (6.7) |
| Charlson Comorbidity Index scorea | ||||||||||||
| 0 | 12 803 (83.9) | 5951 (83.5) | 6852 (84.3) | 7178 (85.0) | 3262 (84.7) | 3916 (85.2) | 2539 (82.5) | 1307 (82.4) | 1232 (82.6) | 3086 (82.6) | 1382 (81.7) | 1704 (83.3) |
| 1 | 1694 (11.1) | 810 (11.4) | 884 (10.8) | 879 (10.4) | 397 (10.3) | 482 (10.5) | 353 (11.5) | 189 (11.9) | 164 (10.9) | 462 (12.4) | 223 (13.2) | 238 (11.6) |
| 2 | 574 (3.8) | 275 (3.9) | 299 (3.7) | 299 (3.5) | 152 (3.9) | 147 (3.2) | 137 (4.5) | 63 (4.0) | 74 (5.0) | 138 (3.7) | 69 (4.1) | 78 (3.8) |
| ≥3 | 186 (1.2) | 91 (1.3) | 95 (1.2) | 88 (1.0) | 40 (1.0) | 48 (1.0) | 48 (1.6) | 26 (1.6) | 22 (1.4) | 50 (1.3) | 25 (1.5) | 25 (1.2) |
| Duration of follow-upb | ||||||||||||
| Follow-up, median (IQR), y | 9.3 (5.9-13.1) | 9.2 (5.8-12.9) | 9.5 (6.0-13.3) | 9.5 (6.3-13.2) | 9.3 (6.2-12.9) | 9.7 (6.4-13.4) | 6.9 (0.0-11.9) | 7.1 (0.0-11.9) | 6.7 (0.0-11.8) | 10.3 (7.0-13.5) | 10.2 (6.9-13.3) | 13.7 (7.2-13.7) |
| >5 y | 12 541 (82.2) | 5789 (81.2) | 6752 (83.0) | 7355 (87.1) | 3314 (86.1) | 4041 (88.0) | 1842 (59.9) | 969 (61.1) | 873 (58.5) | 3344 (89.5) | 1509 (89.2) | 1838 (89.9) |
| >10 y | 6928 (45.4) | 3138 (44.0) | 3790 (46.6) | 3903 (46.2) | 1716 (44.6) | 2187 (47.6) | 1059 (34.4) | 549 (34.6) | 510 (34.2) | 1966 (52.6) | 873 (51.6) | 1093 (53.4) |
Abbreviation: IQR, interquartile range.
Charlson Comorbidity Index score ranges from 0 to 6, with higher scores indicating more comorbidity.
Duration of follow-up is defined as the time, in years, between event and death or end of study, whichever occurred first.
Following any stroke, 1776 patients (11.6%) died in the first 30 days. Case fatality after ischemic stroke was 7.4% (n = 629) (7.6% [n = 291] in men, 7.4% [n = 338] in women); after intracerebral hemorrhage, 32.3% (29.8% [n = 991] in men, 34.8% [n = 519] in women); and after stroke not otherwise specified, 4.2% (3.8% [n = 65] in men, 4.4% [n = 91] in women). This resulted in a cohort of 13 481 30-day survivors of any stroke (7815 ischemic stroke, 2086 intracerebral hemorrhage, and 3580 stroke not otherwise specified; eTable 4 in the Supplement).
Cumulative Mortality
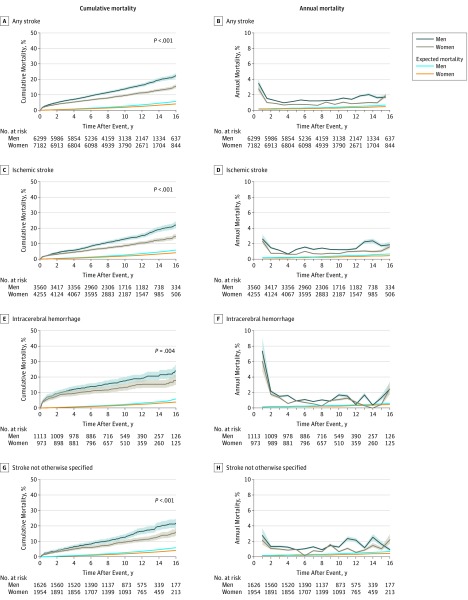
At end of follow-up, a total of 3540 patients (23.2%) had died. In 30-day survivors, the cumulative mortality after any stroke increased from 3.1% (95% CI, 2.8%-3.4%) after 1 year to 7.0% (95% CI, 6.6%-7.4%) at 5 years, 11.5% (95% CI, 11.0%-12.1%) at 10 years, and 17.0% (95% CI, 16.2%-17.9%) after 15 years (Figure, A, C, E, and G; eTable 5 in the Supplement).
Figure. Cumulative Mortality and Annual Mortality Over Time in 30-Day Stroke Survivors.
For men and women, the median (IQR) observation time was 10.0 years (6.7-13.4) and 10.3 years (7.3-13.8), respectively, for stroke (A); 9.8 years (7.8-13.3) and 10.2 years (7.2-13.7) for ischemic stroke (C); 9.9 years (6.6-13.6) and 10.4 years (6.9-14.3) for intracerebral hemorrhage (E); and 10.5 years (7.3-13.7) and 10.7 years (7.6-13.8) for stroke not otherwise specified (G). The log-rank test was used for differences between men and women. Shaded regions indicate 95% CIs.
Sex-Specific Annual Risk of Death by Stroke Subtype
The annual risk of death of 30-day survivors was highest in the first years after stroke and then stabilized (Figure, B, D, F, and H). After ischemic stroke, the risk of death after 1 year was 2.0% (95% CI, 1.8%-2.3%) for men and 1.6% (95% CI, 1.4%-1.8%) for women, and after 10 years decreased to an annual risk of 1.4% (95% CI, 1.2%-1.6%) for men and 0.9% (95% CI, 0.7%-1.1%) for women.
For intracerebral hemorrhage, the annual risk of death was 4.8% (95% CI, 4.4%-5.1%) for men and 3.9% (95% CI, 3.6%-4.3%) for women after 1 year and after 10 years decreased to an annual risk of 1.9% (95% CI, 1.7%-2.1%) for men and 1.4% (95% CI, 1.2%-1.6%) for women (Figure, B, D, F, and H).
Long-term Mortality in 30-Day Survivors of Stroke Compared With the General Population
The SMR for young adults with any stroke compared with peers from the general population matched by sex, age, and calendar year was 5.6 (95% CI, 5.3-5.9). The observed mortality rate was 13.3 per 1000 person-years (95% CI, 12.6-14.0) vs an expected rate of 2.4 per 1000 person-years, with an excess rate of 10.9 deaths per 1000 person-years. The SMR for ischemic stroke was 5.1 (95% CI, 4.7-5.4), with an observed mortality rate of 12.0 per 1000 person-years (95% CI, 11.2-12.9) and expected mortality rate of 2.4 per 1000 person-years and an excess rate of 9.6 per 1000 person-years. For intracerebral hemorrhage, the SMR was 8.4 (95% CI, 7.4-9.3; observed mortality rate, 18.7/1000 person-years [95% CI, 16.7-21.0]; expected rate, 2.2/1000 person-years; excess rate, 16.5/1000 person-years). The SMR for stroke not otherwise specified was 5.2 (95% CI, 4.7-5.8; observed rate, 12.9 [95% CI, 11.7-14.3]; expected rate, 2.5/1000 person-years; excess rate, 10.5/1000 person-years). There were no significant differences in SMR between men and women (bivariable linear regression model, P = .74; multivariable model including stroke subtype and age groups, P = .07). Results stratified for age groups and sex are summarized in Table 2.
Table 2. Long-term Mortality in 30-Day Stroke Survivors Compared With Mortality of the General Population.
| Total | Patient-Years at Risk | Observed Deaths | Observed Deaths per 1000 Person-Years (95% CI) | Expected Deathsa | Expected Deaths per 1000 Person-Years | Excess Rate per 1000 Person-Yearsb | Standardized Mortality Rate (95% CI)c | |
|---|---|---|---|---|---|---|---|---|
| Any Stroke | ||||||||
| Total | 13 481 | 102 184 | 1356 | 13.3 (12.6-14.0) | 242.8 | 2.4 | 10.9 | 5.6 (5.3-5.9) |
| Men | 6299 | 46 937 | 756 | 16.1 (15.0-17.3) | 131.1 | 2.8 | 13.3 | 5.8 (5.4-6.2) |
| Women | 7182 | 55 247 | 600 | 10.9 (10.0-11.8) | 111.8 | 2.0 | 8.8 | 5.4 (4.9-5.8) |
| 18-29 y | 928 | 7477 | 52 | 7.0 (5.3-9.1) | 3.3 | 0.4 | 6.5 | 15.8 (11.6-20.4) |
| Men | 340 | 2635 | 28 | 10.6 (7.4-15.4) | 1.6 | 0.6 | 10.0 | 18.1 (11.7-25.2) |
| Women | 588 | 4842 | 24 | 5.0 (3.3-7.4) | 1.7 | 0.4 | 4.6 | 13.8 (8.6-19.5) |
| 30-39 y | 2951 | 23 423 | 228 | 9.7 (8.6-11.1) | 25.2 | 1.1 | 8.7 | 9.1 (7.9-10.3) |
| Men | 1285 | 9958 | 128 | 12.9 (10.8-15.3) | 12.3 | 1.2 | 11.6 | 10.4 (8.6-12.3) |
| Women | 1666 | 13 465 | 100 | 7.4 (6.1-9.0) | 12.9 | 1.0 | 6.5 | 7.8 (6.3-9.3) |
| 40-49 y | 9602 | 71 284 | 1076 | 15.1 (14.2-16.0) | 214.4 | 3.0 | 12.1 | 5.0 (4.7-5.3) |
| Men | 4674 | 34 344 | 600 | 17.5 (16.1-18.9) | 117.2 | 3.4 | 14.1 | 5.1 (4.7-5.5) |
| Women | 4928 | 36 939 | 476 | 12.9 (11.8-14.1) | 97.1 | 2.6 | 10.3 | 4.9 (4.5-5.3) |
| Ischemic Stroke | ||||||||
| Total | 7815 | 58 668 | 704 | 12.0 (11.2-12.9) | 139.1 | 2.4 | 9.6 | 5.1 (4.7-5.4) |
| Men | 3560 | 26 228 | 382 | 14.6 (13.2-16.1) | 73.5 | 2.8 | 11.8 | 5.2 (4.7-5.7) |
| Women | 4255 | 32 440 | 322 | 9.9 (8.9-11.1) | 65.6 | 2.0 | 7.9 | 4.9 (4.4-5.5) |
| 18-29 y | 487 | 3903 | 26 | 6.7 (4.5-9.8) | 1.7 | 0.4 | 6.2 | 15.6 (10.2-21.6) |
| Men | 162 | 1247 | 10 | 8.0 (4.3-14.9) | 0.7 | 0.6 | 7.4 | 13.7 (5.5-23.2) |
| Women | 325 | 2656 | 16 | 6.0 (3.7-9.8) | 0.9 | 0.4 | 5.7 | 17.2 (9.7-25.7) |
| 30-39 y | 1667 | 13 414 | 100 | 7.5 (6.1-9.1) | 14.5 | 1.1 | 6.4 | 6.9 (5.6-8.3) |
| Men | 696 | 5510 | 52 | 9.4 (7.2-12.4) | 6.8 | 1.2 | 8.2 | 7.6 (5.6-9.8) |
| Women | 971 | 7904 | 48 | 6.1 (4.6-8.1) | 7.7 | 1.0 | 5.1 | 6.3 (4.6-8.1) |
| 40-49 y | 5661 | 41 351 | 578 | 14.0 (12.9-15.2) | 123.0 | 3.0 | 11.0 | 4.7 (4.3-5.1) |
| Men | 2702 | 19471 | 320 | 16.4 (14.7-18.3) | 66.0 | 3.4 | 13.1 | 4.9 (4.3-5.4) |
| Women | 2959 | 21 880 | 258 | 11.8 (10.4-13.3) | 57.0 | 2.6 | 9.2 | 4.5 (4.0-5.1) |
| Intracerebral Hemorrhage | ||||||||
| Total | 2086 | 15 560 | 291 | 18.7 (16.7-21.0) | 34.8 | 2.2 | 16.5 | 8.4 (7.4-9.3) |
| Men | 1113 | 8158 | 174 | 21.3 (18.4-24.7) | 20.5 | 2.5 | 18.8 | 8.5 (7.3-9.8) |
| Women | 973 | 7402 | 117 | 15.8 (13.2-18.9) | 14.4 | 1.9 | 13.9 | 8.2 (6.7-9.7) |
| 18-29 y | 228 | 1835 | 17 | 9.3 (5.8-14.9) | 0.8 | 0.5 | 8.8 | 20.4 (10.8-31.1) |
| Men | 109 | 866 | 11 | 12.7 (7.1-22.9) | 0.5 | 0.6 | 12.1 | 22.0 (10.0-35.9) |
| Women | 119 | 969 | 6 | 6.2 (2.8-13.8) | 0.3 | 0.4 | 5.9 | 17.9 (6.0-32.9) |
| 30-39 y | 515 | 3864 | 72 | 18.6 (14.8-23.4) | 4.1 | 1.1 | 17.6 | 17.7 (13.8-21.9) |
| Men | 279 | 2042 | 46 | 22.5 (16.9-30.0) | 2.4 | 1.2 | 21.4 | 19.1 (13.7-25.0) |
| Women | 236 | 1822 | 26 | 14.3 (9.7-20.9) | 1.7 | 0.9 | 13.4 | 15.6 (10.2-21.7) |
| 40-49 y | 1343 | 9861 | 202 | 20.5 (17.9-23.5) | 29.9 | 3.0 | 17.5 | 6.8 (5.9-7.7) |
| Men | 725 | 5250 | 117 | 22.3 (18.6-26.7) | 17.6 | 3.3 | 18.9 | 6.7 (5.5-7.9) |
| Women | 618 | 4611 | 85 | 18.4 (14.9-22.8) | 12.4 | 2.7 | 15.8 | 6.9 (5.4-8.4) |
| Stroke Not Otherwise Specified | ||||||||
| Total | 3580 | 27 956 | 361 | 12.9 (11.7-14.3) | 68.9 | 2.5 | 10.5 | 5.2 (4.7-5.8) |
| Men | 1626 | 12 551 | 200 | 15.9 (13.9-18.3) | 37.1 | 3.0 | 13.0 | 5.4 (4.7-6.2) |
| Women | 1954 | 15 405 | 161 | 10.5 (9.0-12.2) | 31.8 | 2.1 | 8.4 | 5.1 (4.3-5.9) |
| 18-29 y | 213 | NDd | NDd | NDd | NDd | NDd | NDd | NDd |
| Men | 69 | NDd | NDd | NDd | NDd | NDd | NDd | NDd |
| Women | 144 | NDd | NDd | NDd | NDd | NDd | NDd | NDd |
| 30-39 y | 769 | 6145 | 56 | 9.1 (7.0-11.8) | 6.6 | 1.1 | 8.0 | 8.5 (6.4-10.7) |
| Men | 310 | 2406 | 30 | 12.5 (8.7-17.8) | 3.1 | 1.3 | 11.2 | 9.8 (6.6-13.5) |
| Women | 459 | 3739 | 26 | 7.0 (4.7-10.2) | 3.6 | 1.0 | 6.0 | 7.3 (4.8-10.1) |
| 40-49 y | 2598 | 20 072 | 296 | 14.8 (13.2-16.5) | 61.5 | 3.1 | 11.7 | 4.8 (4.3-5.4) |
| Men | 1247 | 9624 | 163 | 16.9 (14.6-19.7 | 33.7 | 3.5 | 13.4 | 4.8 (4.1-5.6) |
| Women | 1351 | 10449 | 133 | 12.7 (10.8-15.1) | 27.8 | 2.7 | 10.1 | 4.8 (4.0-5.6) |
Abbreviation: ND, not disclosed.
Expected deaths retrieved from mortality data of the Dutch population matched for age, sex, and calendar year characteristics (Human Mortality Database).
The excess mortality rate was calculated as (observed deaths − expected deaths) / person-years at risk expressed per 1000 person-years.
Standardized mortality rate is the ratio of the observed mortality rate divided by expected mortality rate, assuming the observed deaths follow a Poisson distribution. For subgroup analyses within any stroke and the different stroke subtypes, the significance threshold was set to a Bonferroni-adjusted P value of .005. P < .001 for all rows that have data.
Subgroup was too small in number of patients to adequately protect privacy according to legislation regarding the use of this register-based data set of Statistics Netherlands.
Association of Age, Sex, Comorbidity, and Length of Stay With the Risk of Death After Index Stroke
In bivariable Cox regression analysis for any stroke, age was associated with mortality during follow-up of 30-day survivors (35-39 years: hazard ratio [HR], 2.4 [95% CI, 1.5-3.8], P for interaction < .001; 40-44 years: HR, 2.7 [95% CI, 1.7-4.2], P for interaction < .001; 45-49 years: HR, 3.8 [95% CI, 2.4-5.9], P for interaction < .001; all compared with the reference group aged 18-24 years). Age groups 25-29 and 30-34 were not significantly associated with a higher risk of mortality. Male sex was associated with a higher risk of mortality, with an HR of 1.5 (95% CI, 1.4-1.7; P for interaction < .001), as well as a CCI score above 0 (CCI of 1: HR 2.1 [95% CI, 1.9-2.4], P for interaction < .001; CCI of 2: HR, 6.5 [95% CI, 5.6-7.5], P for interaction < .001; CCI of 3 or higher: HR, 9.4 [95% CI, 7.5-11.7], P for interaction < .001). In addition, length of hospital stay longer than 14 days was associated with mortality (HR, 2.2 [95% CI, 1.9-2.6]; P for interaction < .001). In the multivariable Cox regression model, all of these associations remained significant. Table 3 shows HRs of the bivariable and multivariable Cox regressions models specified per stroke subtype.
Table 3. Factors Associated With Mortality Among 30-Day Stroke Survivors According to Stroke Subtype.
| Analysis, Hazard Ratio (95% CI) | ||
|---|---|---|
| Bivariablea | Multivariableb | |
| Any Stroke | ||
| Age at index event, yc | ||
| 25-29 | 1.5 (0.9-2.6) | 1.5 (0.9-2.7) |
| 30-34 | 1.5 (0.9-2.5) | 1.4 (0.9-2.4) |
| 35-39 | 2.4 (1.5-3.8) | 2.3 (1.4-3.6) |
| 40-44 | 2.7 (1.7-4.2) | 2.5 (1.6-4.0) |
| 45-49 | 3.8 (2.4-5.9) | 3.4 (2.1-5.3) |
| Mend | 1.5 (1.4-1.7) | 1.4 (1.3-1.6) |
| Charlson Comorbidity Index scoree | ||
| 1 | 2.1 (1.9-2.4) | 2.1 (1.8-2.4) |
| 2 | 6.5 (5.6-7.5) | 5.9 (5.1-6.9) |
| ≥ 3 | 9.4 (7.5-11.7) | 7.7 (6.2-9.7) |
| Length of stay, df | ||
| 3-7 | 1.1 (0.9-1.3) | 1.2 (1.0-1.4) |
| 8-14 | 1.2 (1.0-1.4) | 1.3 (1.1-1.5) |
| ≥15 | 2.2 (1.9-2.6) | 2.2 (1.9-2.6) |
| Ischemic Stroke | ||
| Age at index event, yc | ||
| 25-29 | 1.5 (0.7-3.1) | 1.4 (0.6-2.9) |
| 30-34 | 0.9 (0.4-1.8) | 0.8 (0.4-1.7) |
| 35-39 | 1.9 (1.0-3.7) | 1.9 (1.0-3.7) |
| 40-44 | 2.4 (1.3-4.5) | 2.2 (1.2-4.2) |
| 45-49 | 3.5 (1.9-6.5) | 3.1 (1.7-5.8) |
| Mend | 1.5 (1.4-1.8) | 1.4 (1.3-1.6) |
| Charlson Comorbidity Index scoree | ||
| 1 | 2.1 (1.7-2.5) | 2.0 (1.7-2.4) |
| 2 | 6.3 (5.1-7.7) | 5.5 (4.5-6.8) |
| ≥ 3 | 9.7 (7.1-13.4) | 7.6 (5.5-10.4) |
| Length of stay, df | ||
| 3-7 | 1.2 (0.9-1.5) | 1.3 (1.0-1.7) |
| 8-14 | 1.2 (1.0-1.6) | 1.3 (1.0-1.7) |
| ≥15 | 2.3 (1.8-2.9) | 2.4 (1.9-3.0) |
| Intracerebral Hemorrhage | ||
| Age at index event, yc | ||
| 25-29 | 2.7 (0.9-7.5) | 3.2 (1.1-9.1) |
| 30-34 | 3.0 (1.2-7.9) | 2.8 (1.1-7.2) |
| 35-39 | 4.5 (1.8-11.2) | 3.6 (1.4-9.0) |
| 40-44 | 4.4 (1.8-10.7) | 4.0 (1.6-9.9) |
| 45-49 | 5.1 (2.1-12.4) | 4.6 (1.9-11.3) |
| Mend | 1.4 (1.1-1.7) | 1.4 (1.1-1.7) |
| Charlson Comorbidity Index scoree | ||
| 1 | 1.7 (1.3-2.2) | 1.7 (1.3-2.3) |
| 2 | 8.2 (6.1-11.2) | 8.3 (6.1-11.2) |
| ≥3 | 9.5 (6.1-15.0) | 9.3 (5.9-14.8) |
| Length of stay, df | ||
| 3-7 | 1.2 (0.8-1.8) | 1.3 (0.8-2.0) |
| 8-14 | 1.2 (0.8-1.9) | 1.3 (0.8-1.9) |
| ≥15 | 2.0 (1.4-2.9) | 2.1 (1.4-3.0) |
| Stroke Not Otherwise Specified | ||
| Age at index event, yc | ||
| 25-29 | 0.7 (0.2-2.5) | 0.7 (0.2-2.7) |
| 30-34 | 1.7 (0.6-5.0) | 1.7 (0.6-4.8) |
| 35-39 | 2.0 (0.7-5.5) | 1.7 (0.6-4.8) |
| 40-44 | 2.2 (0.8-6.0) | 2.0 (0.7-5.3) |
| 45-49 | 3.4 (1.3-9.0) | 2.6 (1.0-7.1) |
| Mend | 1.5 (1.2-1.8) | 1.4 (1.2-1.7) |
| Charlson Comorbidity Index scoree | ||
| 1 | 2.5 (2.0-3.1) | 2.4 (1.9-3.0) |
| 2 | 5.5 (4.1-7.4) | 5.0 (3.7-6.8) |
| ≥3 | 7.7 (5.0-11.9) | 6.3 (4.1-9.8) |
| Length of stay,df | ||
| 3-7 | 1.0 (0.7-1.3) | 1.1 (0.8-1.4) |
| 8-14 | 1.1 (0.8-1.5) | 1.2 (0.9-1.4) |
| ≥15 | 2.1 (1.6-2.7) | 2.1 (1.6-2.8) |
Hazard ratios (95% CI) were computed separately for age at index event, sex, Charlson Comorbidity Index score, and length of stay.
Age at index event, sex, Charlson Comorbidity Index score, and length of stay were entered simultaneously in a Cox proportional hazards model.
Reference category for age at index event is 18-24 years.
Reference category for sex is women.
Reference category for Charlson Comorbidity Index is a score of 0. Charlson Comorbidity Index score ranges from 0 to 6, with higher scores indicating more comorbidity.
Reference category for length of stay is 0-2 days.
Time Trends in Case Fatality, 1-Year and 5-Year Mortality, and Length of Stay for Any Stroke and for Stroke Subtypes
Case fatality after any stroke decreased from 15.2% (n = 160) in 1998 to 6.9% (n = 86) in 2010 (yearly APC, −5.5% [95% CI, −13.1% to 2.0%]; P < .001; R2 = 0.9), from 8.4% (n = 47) in 1998 to 4.9% (n = 38) in 2010 for ischemic stroke (yearly APC, −1.3% [95% CI, −17.1% to 14.6%]; P < .001; R2 = 0.6), from 37.9% (n = 89) in 1998 to 21.1% (n = 44) in 2010 for intracerebral hemorrhage (yearly APC, −3.8% [95% CI, −11.9% to 4.4%]; P < .001; R2 = 0.8), and from 4.6% (n = 12) in 1998 to 1.2% (n = 3) in 2010 for stroke not otherwise specified (yearly APC, −0.4% [95% CI, −36.3% to 35.5%; P = .006; R2 = 0.5).
One-year mortality in 30-day survivors after any stroke decreased from 3.7% (n = 39) in 1998 to 2.3% (n = 29) in 2010 (yearly APC, −2.2% [95% CI, −13.0% to 8.6%]; P = .002; R2 = 0.6). In ischemic stroke, 1-year mortality decreased from 3.3% (n = 18) in 1998 to 1.2% (n = 9) in 2010 (yearly APC, −2.8% [95% CI, −20.2% to 14.7%]; P = .005; R2 = 0.5), whereas in intracerebral hemorrhage, it was 7.8% (n = 18) in 1998 and 6.1% (n = 13) in 2010 (yearly APC, 5.4% [95% CI, −18.6% to 29.4%]; P = .07; R2 = 0.3) and in stroke not otherwise specified, 1-year mortality remained stable (3.6% [n = 9] in 1998 and 2.8% [n = 7] in 2010; yearly APC, 24.1% [95% CI, −18.8% to 67.0%]; P = .97; R2 = 0.0).
Cumulative 5-year mortality in 30-day stroke survivors decreased significantly over time in any stroke from 8.3% (n = 89) in 1998 to 5.2% (n = 64) in 2010 (yearly APC, −3.1% [95% CI, −10.2% to 4.0%]; P for interaction < .001; R2 = 0.8), in ischemic stroke from 8.0% (n = 45) to 3.0% (n = 23) (yearly APC, −6.2% [95% CI, −17.2% to 4.8%; P for interaction < .001; R2 = 0.8), and in stroke not otherwise specified from 8.0% (n = 22) to 7.2% (n = 18) (yearly APC, 2.8% to [95% CI, −11.7% to 23.4%]; P for interaction = .046; R2 = 0.3). Cumulative 5-year mortality remained stable in intracerebral hemorrhage (9.6% [n = 23] in 1998 and 12.1% [n = 25] in 2010; yearly APC, 5.9% [95% CI, −13.7% to 19.3%]; P for interaction = .98; R2 = 0.0) (eTable 6 in the Supplement).
From 1998 to 2010, length of stay after any stroke decreased significantly from a mean of 18.2 days to a mean of 8.6 days, resulting in a yearly decrease of −0.8 days (95% CI, −0.2 to 1.3 days; P < .001; R2 = 0.4). This was also true for ischemic stroke, intracerebral hemorrhage, and stroke not otherwise specified.
Causes of Death
Of the 30-day survivors, a total of 1764 patients died during follow-up (13.1%), of which 267 (15.1%) were due to recurrent stroke or were stroke related and 302 (17.1%) due to other cardiovascular diseases. The main cause of death was malignancy (n = 577, 32.7%). The remaining patients (n = 617, 34.9%) died as a result of infection, trauma, and miscellaneous causes. The proportion of deaths attributable to malignancies was higher in the intracerebral hemorrhage group than the group with an ischemic stroke as the index event (41.5% [n = 145] vs 28.8% [n = 273]; χ2 = 18.9; P < .001). The proportion of cardiovascular-related deaths was higher in the ischemic stroke group than the intracerebral hemorrhage group (19.4% [n = 184] vs 7.2% [n = 25]; χ2 = 28.4; P < .001). Also, the Poisson regression model showed significant interaction between stroke subtype and malignancies, as well as cardiovascular-related deaths (P < .001), even after correcting for sex in this model (P < .001). The different categories of causes of death stratified by stroke subtype are listed in Table 4.
Table 4. Causes of Death in 30-Day Survivors, Stratified by Stroke Subtype and Sex.
| Causes of Deatha | Index Event, No. (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any Stroke | Ischemic Stroke | Intracerebral Hemorrhage | Stroke Not Otherwise Specified | |||||||||
| Total | Men | Women | Total | Men | Women | Total | Men | Women | Total | Men | Women | |
| Total | 1763 (100) | 987 (100) | 776 (100) | 947 (100) | 524 (100) | 423 (100) | 349 (100) | 210 (100) | 139 (100) | 467 (100) | 253 (100) | 214 (100) |
| Stroke related | 267 (15.1) | 147 (14.9) | 120 (15.5) | 129 (13.6) | 72 (13.7) | 57 (13.5) | 79 (22.6) | 42 (20.0) | 37 (26.6) | 59 (12.6) | 33 (13.0) | 26 (12.1) |
| Ischemic stroke | 35 (2.0) | 20 (2.0) | 15 (1.9) | 28 (3.0) | NDb | NDb | NDb | NDb | NDb | NDb | NDb | NDb |
| Intracerebral hemorrhage | 49 (2.8) | 26 (2.6) | 23 (3.0) | 12 (1.3) | NDb | NDb | NDb | NDb | NDb | NDb | NDb | NDb |
| Other stroke-related deaths | 183 (10.4) | 101 (10.2) | 82 (10.6) | 89 (9.4) | 50 (9.5) | 39 (9.2) | 50 (14.3) | 26 (12.4) | 24 (17.3) | 44 (9.4) | 25 (9.9) | 19 (8.9) |
| Cardiac and other vascular events | 302 (17.1) | 194 (19.7) | 108 (13.9) | 184 (19.4) | 113 (21.6) | 71 (16.8) | 25 (7.2) | NDb | NDb | 93 (19.9) | 63 (24.9) | 30 (14.0) |
| Malignancies | 577 (32.7) | 273 (27.7) | 304 (39.2) | 273 (28.8) | 127 (24.2) | 146 (34.5) | 145 (41.5) | 80 (38.1) | 65 (46.8) | 159 (34.0) | 92 (26.1) | 126 (43.5) |
| Lung cancer | 145 (25.1) | 53 (19.4) | 92 (30.3) | 83 (30.4) | 31 (24.4) | 52 (35.6) | 14 (9.7) | NDb | NDb | 48 (30.2) | NDb | NDb |
| Brain tumor | 86 (14.9) | 56 (20.5) | 30 (9.9) | 29 (10.6) | 16 (12.6) | 13 (8.9) | 44 (30.3) | 32 (40.0) | 12 (18.5) | 13 (8.2) | 34 (51.5) | 38 (40.9) |
| Hematological malignancies | 47 (8.1) | 22 (8.1) | 25 (8.2) | NDb | NDb | NDb | NDb | NDb | NDb | NDb | NDb | NDb |
| Breast cancer | 40 (6.9) | NDb | NDb | 22 (8.1) | NDb | NDb | NDb | NDb | NDb | 12 (7.5) | NDb | NDb |
| Melanoma | 28 (4.9) | NDb | NDb | NDb | NDb | NDb | 21 (14.5) | 10 (12.5) | 11 (16.9) | NDb | NDb | NDb |
| Other forms of malignancies | 232 (40.2) | 128 (46.9) | 103 (33.9) | 115 (42.1) | 71 (55.9) | 44 (30.1) | 46 (31.7) | 24 (30.0) | 22 (33.8) | 70 (44.0) | 33 (50.0) | 37 (39.8) |
| Infections | 87 (4.9) | 50 (5.1) | 37 (4.8) | 52 (5.5) | 32 (6.1) | 20 (4.7) | 19 (5.4) | NDb | NDb | 16 (3.4) | NDb | NDb |
| Trauma | 78 (4.4) | 53 (5.4) | 25 (3.2) | 42 (4.4) | 29 (5.5) | 13 (3.1) | 12 (3.4) | NDb | NDb | 24 (5.1) | NDb | NDb |
| Miscellaneous | 452 (25.6) | 270 (27.4) | 182 (23.5) | 267 (28.2) | 151 (28.8) | 116 (27.4) | 69 (19.8) | 50 (23.8) | 19 (13.7) | 116 (24.8) | 69 (27.3) | 47 (22.0) |
Abbreviation: ND, not disclosed.
International Classification of Diseases codes used to define the categories of causes of death are listed in eTable 3 in the Supplement.
Subgroup was too small in the number of patients to adequately protect privacy according to legislation regarding the use of this register-based data set of Statistics Netherlands.
Discussion
Among young adults aged 18 to 49 years in the Netherlands with first stroke, mortality risk compared with the general population remained elevated up to 15 years later.
Major strengths of this study include the population-based setting with a large number of young patients with stroke (15 257 patients <50 years), whereas in other large studies, patients were included up to 55 years (with substantially fewer patients <50 years). In addition, this population-based setting increased the likelihood of complete ascertainment of (cause of) death, whereas referral bias has occurred in previous other studies with a hospital-based setting because the more severely affected patients are more likely to die already at home and will not be included in hospital-based populations.
This study reports, to our knowledge, for the first time the risk and causes of death, cumulative mortality, and annual mortality after intracerebral hemorrhage at young age in large numbers that allow for sufficient power to perform age- and sex-stratified analysis. Another strength of this study is that ICD codes for all stroke subtypes were validated in young age groups specifically.
Furthermore, due to the availability of longitudinal data, in combination with the very large numbers, this study was able to report on differential time trends of mortality including case fatality after ischemic stroke and intracerebral hemorrhage.
Additionally, this study reports on outcomes of patients who were treated with stroke unit care, intravenous thrombolysis, and hemicraniectomy and secondary preventive treatment, whereas earlier studies included patients who had their stroke years before the implementation of these therapies.23,24,25
In addition, within this large cohort, it was possible to analyze specific causes of death in more detail than previous studies, with information available on subtypes of malignancies. Previous studies have presented this only for patients with ischemic stroke and not for patients with intracerebral hemorrhage, and causes of death were limited to recurrent stroke, other cardiovascular disease, malignancies, and other causes.
A limited number of earlier studies with a comparable duration of follow-up has shown comparable mortality rates of 4.6% (3 year) and 16% (16 year).7,9,10,15 Most previous studies on long-term mortality after stroke in young adults were hospital based, had varying inclusion criteria, or also included SAH or TIA. These disorders may have a different prognosis than ischemic stroke and intracerebral hemorrhage and may, therefore, bias the mortality rates after stroke. In young patients with TIA, the 1-year cumulative mortality was shown to be only 0.7% and in the years thereafter, 0.2% per year.7,9,10 In contrast, patients with an SAH had a 17% excess mortality rate compared with the general population after 20 years of follow-up.26
The excess long-term mortality in young adults following stroke compared with age- and sex-matched individuals in the general population may suggest that even after treatment for stroke and treatment of associated risk factors according to current standards, the risk of death of young patients with stroke remained increased compared with their population peers. From 1998 to 2017, the already low risk of death in young adults in the general population has continued to decrease, possibly because of improved treatment of other life-threatening diseases, such as malignancies, and because of fewer traffic crashes.27
The observed decreasing trend observed in mortality after ischemic stroke in young adults could be partially due to improved diagnosis of ischemic stroke with better and more imaging techniques available. In this way, minor and resolved syndromes can be increasingly diagnosed as stroke (by magnetic resonance imaging), which would partially explain decreasing rates of poststroke mortality only for ischemic stroke. This hypothesis is supported by the fact that the 1- and 5-year mortality rates of intracerebral hemorrhage did not decrease significantly over the study period, which can be readily diagnosed purely by use of a computed tomographic scan, which was already available in the earlier years of the study period.
A previous study also found male sex as a risk factor for long-term mortality.9 The increased risk of death in men may be due to differences in risk factors and etiology between men and women (eg, higher prevalence of traditional vascular risk factors and more large-artery disease in men28,29,30) as the incidence of stroke in the study period was higher in young women.4 When compared with the general population, no significant differences between men and women in their risk of death were found.
In Sweden, a decline in case fatality of ischemic stroke was seen in men aged 30 to 84 years, but not in women. A similar difference with significant decrease of case fatality only in men was seen in the Framingham study.31,32 The decrease in case fatality of ischemic stroke this study found for both young men and women might be attributable to both the change in the organization of stroke care with better education, introduction of stroke units in the early 1990s, the introduction of hemicraniectomy for space-occupying infarctions,23,33 and the higher detection of more minor and resolved syndromes as mentioned here. The reason that case fatality of intracerebral hemorrhage has remained stable over time may be the limited treatment options for this type of stroke compared with ischemic stroke.33,34,35,36
This study explored the causes of death in young adults after stroke, which may provide evidence for possible underlying disease mechanisms. For example, a higher percentage of patients who died of malignancies and cardiovascular disease was found compared with the general population. Malignancies were responsible for 32.7% of deaths in this cohort, whereas in corresponding age groups in published records of Statistics Netherlands, 25.6% died of malignancy in the period from 1998 to 2010.37 A total of 17.1% of patients died of cardiovascular diseases, whereas in corresponding age groups from the general population, this percentage was 10.6%, according to Statistics Netherlands.37 This may suggest that the underlying risk factors and causes of stroke continue to expose patients to new events throughout the rest of their lives.13 However, results of causes of death that are based on small patient numbers should be interpreted with caution.
Limitations
This study has several limitations. First, due to the registry-based study design, there was an inability to control for possible confounders (eg, stroke severity, family history, medication) and comorbidity could only be assessed with the CCI, which provides a reliable measure of someone’s comorbidity, but without information about actual risk factors and underlying etiology.20 Specifically for young patients with stroke, with a wide variety of risk factors and causes, more detailed information would have been desirable. Because the CCI is composed of comorbidities defined by a previous hospital admission before the time of index event, this may underestimate the effect of comorbidity on long-term mortality when comorbidity increases between index stroke and death. Conversely, the adjustment for baseline CCI score may result in overestimation when comorbidity decreases after the index stroke until death. This possible bias is expected to be very low because young adults are less likely to have major comorbidity than elderly patients.
Second, because fewer hospitals contributed to the HDR register from 2006 onwards, the incidence of stroke could have been underestimated. If these records were not missing completely at random, some bias may have been introduced into the analysis.
Third, strokes between 1998 and 2010 were defined as first if no earlier admission for stroke was registered between 1995 and 2010. A limitation of this method is that a very small proportion of incident strokes may have been misclassified as “first,” if these patients would have had a stroke before 1995.38 However, given the 2% yearly risk of recurrent stroke 3 years after index stroke, this risk of misclassification is considered very low.38
Fourth, using the general population as a control group includes young adults with stroke, which could possibly bias the study toward the null. However, because only a very small proportion of the controls will have had a stroke (in the order of a few cases per thousands of controls), this is unlikely to have had a major effect on the findings.
Fifth, given that recent advances in the management of ischemic stroke with mechanical thrombectomy occurred largely after the end date of the data included in this study, the risk of mortality after ischemic stroke may not entirely be generalizable to reflect contemporary management.
Conclusions
Among young adults aged 18 to 49 years in the Netherlands who were 30-day survivors of first stroke, mortality risk compared with the general population remained elevated up to 15 years later.
eTable 1. Overview of Used ICD-9 and ICD-10 Codes
eTable 2. Results of Validation Procedure of ICD Codes in Young Patients With Stroke
eTable 3. ICD Codes of Causes of Death
eTable 4. Demographics Including Age, Sex, Comorbidity and Follow-up of Stroke of 30-Day Survivors
eTable 5. Sex-Specific Mortality for Any Stroke and Stroke Subtypes
eTable 6. Annual Case Fatality, 1-Year Mortality and 5-Year Mortality Stratified for Stroke Subtypes, and Sex
References
- 1.Barker-Collo S, Bennett DA, Krishnamurthi RV, et al. ; GBD 2013 Writing Group; GBD 2013 Stroke Panel Experts Group . Sex differences in stroke incidence, prevalence, mortality and disability-adjusted life years: results from the Global Burden of Disease Study 2013. Neuroepidemiology. 2015;45(3):203-214. doi: 10.1159/000441103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120(3):439-448. doi: 10.1161/CIRCRESAHA.116.308413 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Cardiovascular diseases (CVDs). https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed May 10, 2019.
- 4.Ekker MS, Verhoeven JI, Vaartjes I, van Nieuwenhuizen KM, Klijn CJM, de Leeuw FE. Stroke-incidence in young adults according to age, subtype, sex, and time-trends: a nationwide registry-based study. Neurology. 2019;92:1-11. doi: 10.1212/WNL.0000000000007533 [DOI] [PubMed] [Google Scholar]
- 5.Béjot Y, Daubail B, Jacquin A, et al. Trends in the incidence of ischaemic stroke in young adults between 1985 and 2011: the Dijon Stroke Registry. J Neurol Neurosurg Psychiatry. 2014;85(5):509-513. doi: 10.1136/jnnp-2013-306203 [DOI] [PubMed] [Google Scholar]
- 6.Sultan S, Elkind MS. The growing problem of stroke among young adults. Curr Cardiol Rep. 2013;15(12):421. doi: 10.1007/s11886-013-0421-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waje-Andreassen U, Naess H, Thomassen L, Eide GE, Vedeler CA. Long-term mortality among young ischemic stroke patients in western Norway. Acta Neurol Scand. 2007;116(3):150-156. doi: 10.1111/j.1600-0404.2007.00822.x [DOI] [PubMed] [Google Scholar]
- 8.Putaala J, Curtze S, Hiltunen S, Tolppanen H, Kaste M, Tatlisumak T. Causes of death and predictors of 5-year mortality in young adults after first-ever ischemic stroke: the Helsinki Young Stroke Registry. Stroke. 2009;40(8):2698-2703. doi: 10.1161/STROKEAHA.109.554998 [DOI] [PubMed] [Google Scholar]
- 9.Naess H, Waje-Andreassen U. Review of long-term mortality and vascular morbidity amongst young adults with cerebral infarction. Eur J Neurol. 2010;17(1):17-22. doi: 10.1111/j.1468-1331.2009.02868.x [DOI] [PubMed] [Google Scholar]
- 10.Koivunen RJ, Tatlisumak T, Satopää J, Niemelä M, Putaala J. Intracerebral hemorrhage at young age: long-term prognosis. Eur J Neurol. 2015;22(7):1029-1037. doi: 10.1111/ene.12704 [DOI] [PubMed] [Google Scholar]
- 11.Koton S, Schneider AL, Rosamond WD, et al. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA. 2014;312(3):259-268. doi: 10.1001/jama.2014.7692 [DOI] [PubMed] [Google Scholar]
- 12.Marini C, Totaro R, De Santis F, Ciancarelli I, Baldassarre M, Carolei A. Stroke in young adults in the community-based L’Aquila registry: incidence and prognosis. Stroke. 2001;32(1):52-56. doi: 10.1161/01.STR.32.1.52 [DOI] [PubMed] [Google Scholar]
- 13.Rutten-Jacobs LC, Arntz RM, Maaijwee NA, et al. Long-term mortality after stroke among adults aged 18 to 50 years. JAMA. 2013;309(11):1136-1144. doi: 10.1001/jama.2013.842 [DOI] [PubMed] [Google Scholar]
- 14.Nieuwkamp DJ, Vaartjes I, Algra A, Bots ML, Rinkel GJ. Age- and gender-specific time trend in risk of death of patients admitted with aneurysmal subarachnoid hemorrhage in the Netherlands. Int J Stroke. 2013;8(suppl A100):90-94. doi: 10.1111/ijs.12006 [DOI] [PubMed] [Google Scholar]
- 15.Giang KW, Björck L, Nielsen S, et al. Twenty-year trends in long-term mortality risk in 17,149 survivors of ischemic stroke less than 55 years of age. Stroke. 2013;44(12):3338-3343. doi: 10.1161/STROKEAHA.113.002936 [DOI] [PubMed] [Google Scholar]
- 16.Vaartjes I, Reitsma JB, de Bruin A, et al. Nationwide incidence of first stroke and TIA in the Netherlands. Eur J Neurol. 2008;15(12):1315-1323. doi: 10.1111/j.1468-1331.2008.02309.x [DOI] [PubMed] [Google Scholar]
- 17.Vaartjes I, O’Flaherty M, Capewell S, Kappelle J, Bots M. Remarkable decline in ischemic stroke mortality is not matched by changes in incidence. Stroke. 2013;44(3):591-597. doi: 10.1161/STROKEAHA.112.677724 [DOI] [PubMed] [Google Scholar]
- 18.Jolink WMT, Klijn CJ, Brouwers PJ, Kappelle LJ, Vaartjes I. Time trends in incidence, case fatality, and mortality of intracerebral hemorrhage. Neurology. 2015;85(15):1318-1324. doi: 10.1212/WNL.0000000000002015 [DOI] [PubMed] [Google Scholar]
- 19.Tirschwell DL, Longstreth WT Jr. Validating administrative data in stroke research. Stroke. 2002;33(10):2465-2470. doi: 10.1161/01.STR.0000032240.28636.BD [DOI] [PubMed] [Google Scholar]
- 20.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676-682. doi: 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 21.Barbieri M, Wilmoth JR, Shkolnikov VM, et al. Data resource profile: the Human Mortality Database (HMD). Int J Epidemiol. 2015;44(5):1549-1556. doi: 10.1093/ije/dyv105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harteloh P, de Bruin K, Kardaun J. The reliability of cause-of-death coding in the Netherlands. Eur J Epidemiol. 2010;25(8):531-538. doi: 10.1007/s10654-010-9445-5 [DOI] [PubMed] [Google Scholar]
- 23.Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB; HAMLET investigators . Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol. 2009;8(4):326-333. doi: 10.1016/S1474-4422(09)70047-X [DOI] [PubMed] [Google Scholar]
- 24.Shepherd J, Blauw GJ, Murphy MB, et al. ; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk . Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623-1630. doi: 10.1016/S0140-6736(02)11600-X [DOI] [PubMed] [Google Scholar]
- 25.Mihaylova B, Emberson J, Blackwell L, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaborators . The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581-590. doi: 10.1016/S0140-6736(12)60367-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huhtakangas J, Lehto H, Seppä K, et al. Long-term excess mortality after aneurysmal subarachnoid hemorrhage: patients with multiple aneurysms at risk. Stroke. 2015;46(7):1813-1818. doi: 10.1161/STROKEAHA.115.009288 [DOI] [PubMed] [Google Scholar]
- 27.National Agency for Waterways and Public Works Number of road deaths stable in 2014. https://www.cbs.nl/en-gb/news/2015/18/number-of-road-deaths-stable-in-2014. Published July 5, 2015. Accessed November 14, 2018.
- 28.Béjot Y, Bailly H, Durier J, Giroud M. Epidemiology of stroke in Europe and trends for the 21st century. Presse Med. 2016;45(12 pt 2):e391-e398. doi: 10.1016/j.lpm.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 29.Putaala J, Metso AJ, Metso TM, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki Young Stroke Registry. Stroke. 2009;40(4):1195-1203. doi: 10.1161/STROKEAHA.108.529883 [DOI] [PubMed] [Google Scholar]
- 30.Tibæk M, Dehlendorff C, Jørgensen HS, Forchhammer HB, Johnsen SP, Kammersgaard LP. Increasing incidence of hospitalization for stroke and transient ischemic attack in young adults: a registry-based study. J Am Heart Assoc. 2016;5(5):e003158. doi: 10.1161/JAHA.115.003158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vangen-Lønne AM, Wilsgaard T, Johnsen SH, Carlsson M, Mathiesen EB. Time trends in incidence and case fatality of ischemic stroke: the tromsø study 1977-2010. Stroke. 2015;46(5):1173-1179. doi: 10.1161/STROKEAHA.114.008387 [DOI] [PubMed] [Google Scholar]
- 32.Carandang R, Seshadri S, Beiser A, et al. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA. 2006;296(24):2939-2946. doi: 10.1001/jama.296.24.2939 [DOI] [PubMed] [Google Scholar]
- 33.Schreuder FH, Sato S, Klijn CJ, Anderson CS. Medical management of intracerebral haemorrhage. J Neurol Neurosurg Psychiatry. 2017;88(1):76-84. doi: 10.1136/jnnp-2016-314386 [DOI] [PubMed] [Google Scholar]
- 34.Brainin M, Olsen TS, Chamorro A, et al. ; EUSI Executive Committee; EUSI Writing Committee; European Stroke Initiative . Organization of stroke care: education, referral, emergency management and imaging, stroke units and rehabilitation. Cerebrovasc Dis. 2004;17(suppl 2):1-14. doi: 10.1159/000074816 [DOI] [PubMed] [Google Scholar]
- 35.Asadi H, Dowling R, Yan B, Wong S, Mitchell P. Advances in endovascular treatment of acute ischaemic stroke. Intern Med J. 2015;45(8):798-805. doi: 10.1111/imj.12652 [DOI] [PubMed] [Google Scholar]
- 36.Aarnio K, Haapaniemi E, Melkas S, Kaste M, Tatlisumak T, Putaala J. Long-term mortality after first-ever and recurrent stroke in young adults. Stroke. 2014;45(9):2670-2676. doi: 10.1161/STROKEAHA.114.005648 [DOI] [PubMed] [Google Scholar]
- 37.Population; generation, gender, age and origin. Statistics Netherlands. CBS Statline. http://statline.cbs.nl/statweb/publication/?DM=SLNL&PA=37325&D1=0&D2=a&D3=0&D4=a&D6=18-21&VW=T. Updated July 18, 2017. Accessed January 26, 2017.
- 38.Rutten-Jacobs LC, Maaijwee NA, Arntz RM, et al. Long-term risk of recurrent vascular events after young stroke: the FUTURE study. Ann Neurol. 2013;74(4):592-601. doi: 10.1002/ana.23953 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Overview of Used ICD-9 and ICD-10 Codes
eTable 2. Results of Validation Procedure of ICD Codes in Young Patients With Stroke
eTable 3. ICD Codes of Causes of Death
eTable 4. Demographics Including Age, Sex, Comorbidity and Follow-up of Stroke of 30-Day Survivors
eTable 5. Sex-Specific Mortality for Any Stroke and Stroke Subtypes
eTable 6. Annual Case Fatality, 1-Year Mortality and 5-Year Mortality Stratified for Stroke Subtypes, and Sex