Abstract
Aims and Background
Only few patients with cirrhosis and hepatocellular carcinoma (HCC) larger than 5 cm are amenable to resection or straight liver transplantation, and in such circumstances, multibipolar radiofrequency ablation (mbp-RFA) could be a reliable alternative. This study was aimed to assess the long-term outcome in patients treated with mbp-RFA for unresectable HCC > 5 cm.
Methods
Eighty-three consecutive patients with cirrhosis (median age 70 years [37–93 years], 67 males, BCLC A/B/C: 54/21/8, 74 naive) with up to three HCCs, the largest > 5 cm in diameter (median: 6.2 cm, 5.1–9 cm, 22 infiltrative forms, 12 with segmental portal invasion of which 10 were infiltrative forms) were treated with mbp-RFA. Overall (OS) and recurrence-free (RFS) survival and their associated predictive factors were assessed.
Results
Complete ablation was observed in 78/83 (94%) patients. Thirty-one side effects occurred, including 6 (7%) severe complications. After a median follow-up of 26.1 months (1–112 months), in naive patients the 3- and 5-year OS was 51% (38–62) and 24% (13–36), 63 and 30% for mass-forming and 25 and 6% for infiltrative form, respectively. Infiltrative form (HR: 2.5 [1.33–4.69], p = 0.004) was the only independent OS predictor. In naive patients with mass-forming and infiltrative form, the 3- and 5-year RFS were 47 and 17 and 18 and 18%, respectively. Alpha-fetoprotein (HR: 2.86 [1.32–6.21], p = 0.008), multinodular form (HR: 2.74 [1.4–5.38], p = 0.003) and infiltrative form (HR: 3.43 [1.67–7.01], p = 0.0007) were independent RFS predictors.
Conclusions
mbp-RFA offers good OS in inoperable patients with cirrhosis and large HCC, with acceptable safety profile. For infiltrative forms, although mbp-RFA leads to complete responses in more than 80% cases, few only remain tumor progression-free in long-term.
Keywords: Hepatocellular carcinoma, Large tumor, Multibipolar technology, No touch technique, Radiofrequency ablation
Introduction
Liver resection (mostly major hepatectomy when feasible) or liver transplantation (after downstaging) are the gold standard treatments of large hepatocellular carcinoma (HCC) > 5 cm [1]. Major resections are not recommended even in compensated cirrhosis in case of significant portal hypertension or if the remnant liver is considered to be insufficient after ipsilateral portal vein embolization due to the risk of postoperative liver failure [2].
Thus, a significant proportion of patients with compensated cirrhosis and large (≥5 cm) HCC are not suitable for surgical treatment due to (1) severity of portal hypertension, (2) inadequate remnant liver, (3) poor response to preoperative procedures performed to increase liver volume, (4) advanced age, (5) and/or significant comorbidities. Moreover, only a patient downstaged within the Milan criteria could benefit from liver transplantation with an acceptable long-term survival [3]. As a consequence, most patients with HCC > 5 cm receive palliative care such as transarterial chemoembolization (TACE) or sorafenib, these approaches being characterized by poorly predictable partial response [4, 5]. Consequently, alternative curative approaches need to be proposed to such patients.
Multibipolar radiofrequency ablation (mbp-RFA) consists of inserting into the tumor several linear electrodes (up to six) that function sequentially in bipolar mode and aiming to control precisely the margin of ablation in order to safely perform treatment of large volume of tumors. It has previously been demonstrated that mbp-RFA using no-touch concept could ensure an efficient tumor ablation with a very low rate of local recurrence in HCC < 5 cm [6]. A small pilot study of 26 inoperable patients with HCC > 5 cm in diameter has reported the safety of the method with a local complete response rate of 70% [7]. Indeed, mbp-RFA could be a potential approach for HCC > 5 cm in a wide spectrum of patients, reserving TACE only to those with tumor load clearly beyond curative limits. The aim of our study was to assess the long-term outcome of 83 consecutive patients treated with mbp-RFA for HCC > 5 cm.
Materials and Methods
Selection of Patients
This retrospective study was in accordance with ethical principles of the Declaration of Helsinki and was approved by the local committee on human research. In our tertiary center, the therapeutic management of primary malignant liver tumors is decided by consensus during a weekly multidisciplinary tumor board gathering hepatologists, oncologists, radiologists, and surgeons involved in liver transplantation. For HCC, noninvasive diagnosis and the initial staging of the tumors relied on at least standard triphasic cross-sectional imaging examinations with either CT or MR. Thus, patients with up to three HCC, the largest > 5 cm in diameter and < 10 cm without main portal vein invasion or evidence of extrahepatic spread are evaluated first for a potentially straight curative treatment including liver resection and mbp-RFA. Liver transplantation was considered secondary as salvage treatment in patients who experience recurrence or liver failure after a first-line local curative treatment (liver resection or mbp-RFA). When liver resection was contraindicated for at least one of the following reasons: (1) cirrhosis Child-Pugh score > 6, (2) significant portal hypertension: platelet count < 100 g/L or portohepatic vein gradient pressure > 12 mm Hg, (3) bilirubin serum level > 50 µmol/L, (4) assumed insufficient remnant hepatic volume after hepatectomy, (5) severe comorbidities, (6) performance status > 1, if tumors were located at least 1 cm from major bile ducts, prothrombin activity > 40%, platelet count > 40 g/L, and the Child-Pugh score ≤B7, mbp-RFA was considered. Small amount of intraperitoneal fluid not detected on physical examination (radiological ascites) and/or subcapsular or dome locations, as well as presence of large vessel abutting the tumor were not regarded as contraindication to mbp-RFA. For the patients not amenable to any local curative treatments, transarterial embolization (TAE) was considered with the aim to downstage the tumor load within the Milan criteria and with the prospect of liver transplantation when possible.
Ablation Procedures
All ablative procedures were performed percutaneously under ultrasound guidance and general anesthesia. Radiofrequency (RF) energy was provided by a 3 × 2 channel 250-W (maximal output power), 470-kHz RF generator (CelonLabPower®; Olympus-Celon, Teltow Germany), sequentially supplying every 2 s, up to 15 possible dipoles. The generator fed 15-G electrodes terminated by bipolar coaxial 40-mm active part (CelonProsurg®; Olympus-Celon) internally cooling with a triple peristaltic pump (CelonAquaflow®; Olympus-Celon).
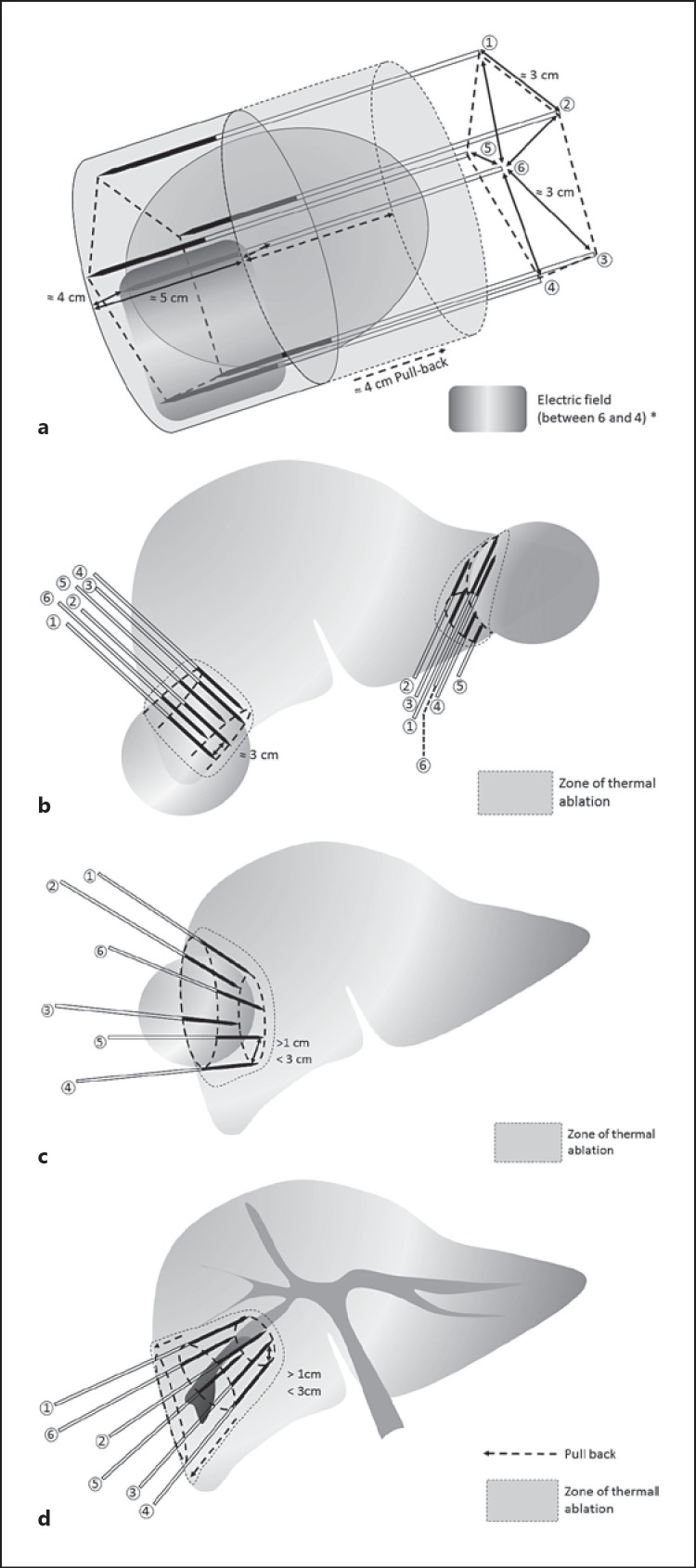
The maximum distance between two adjacent electrodes was 3 cm. They were inserted into the liver parenchyma in a roughly parallel course (±30°). Up to six electrodes could be connected via adaptors plugged each on a single channel of generator [6]. Specific strategies of electrode placement have been designed with the aim to achieve safe complete ablation of large tumor resorting to the lowest as possible intratumorous pass and electrode repositioning whatever the location (intrahepatic, superficial, or exophytic) and form subtype of tumor (mass forming defined as nodular form including at least one tumor > 5 cm or infiltrative defined as poorly demarcated tumor tending to grow and invade portal vascularization along segmental distribution) (Fig. 1). The rise in impedance leading to shutdown between each electrode combination was the endpoint energy cycle deposition for a given arrangement of electrodes [6]. Tract ablation was systematically performed during electrode withdrawal to prevent postprocedure hemorrhage. From January 2010, to protect the diaphragm, artificial ascites with saline water solution at room temperature was performed in patients who had a main tumor located in the dome [8]. Antibiotic prophylaxis was performed only in case of history of transpapillary biliary endoscopic procedure or bilioenteric anastomosis.
Fig. 1.
Strategies for the placement of six electrodes (40 mm active part) according to the location and the form of hepatocellular carcinoma (HCC) to treat with multibipolar radiofrequency ablation (with adaptors) tumors > 5 cm by limiting as much as possible full intraprocedural repositioning and intratumorous punctures. a Basic pentagonal configuration planning for the insertion of six electrodes according to roughly parallel courses (±30°) according as much as possible to the long axis of the tumor. b The cutting technique for the ablation of large tumors located at the periphery of the right or left lobe. Using the cutting strategy, the effect of the ablation extends from pure thermal effect for the safety margin and the proximal side of the tumor to ischemic effect for its opposite exo-hepatic side. c The no touch retronodular convergent technique is the safer strategy for the ablation of subcapsular tumor having a large liver side. This technique allows a strict extratumorous (no touch) ablation of subcapsular tumor up to 6 cm. Distances between the tips of two adjacent electrodes have to be within the 1–3 cm range. d The wedged ablation technique consisting of inserting electrodes according to the convergent course to the apex of the liver subsegment or a segment where the tumor is located is especially designed to treat poorly delineated infiltrative HCC (assumed to be not diffuse).
Treatment Response
Radiological response was assessed 1 month after mbp-RFA, then every 3 months for 2 years, and thereafter every 6 months with a triple-phase contrast-enhanced CT or MRI. Alpha-fetoprotein serum level measurement accompanied each follow-up imaging examination. Any tumor progression described during follow-up was reviewed for validation at our weekly multidisciplinary tumor board.
Technical success (defined as the ability to complete the mbp-RFA procedure as planned) and primary, secondary, and tertiary treatment effectiveness (defined as the ability to achieve complete response after one, two, or three mbp-RFA procedures, respectively) were assessed. A tumor was considered completely ablated if no nodular or irregular enhancement adjacent to the ablation zone was visible during the arterial phase. In cases of incomplete ablation, an additional mbp-RFA procedure was performed using the number of electrodes required regarding the volume of residual tumor remaining to be treated according to no touch concept as previously described [6]. If additional procedures could not be attempted or did not achieve complete ablation, the case was regarded as a failure of mbp-RFA treatment. The number of applications, the amount of energy delivered, the time of energy deposition, and the duration of the original mbp-RFA procedures performed for the treatment of the main tumor of each patient were recorded.
Complications
Complications were assessed according to the Clavien-Dindo classification [9]. The duration of hospital stay was recorded.
Recurrence and Survival
After complete ablation was achieved, local and distant recurrences were assessed during follow-up as previously defined [10]. Additional mbp-RFA procedure was considered when recurrence was detected. For recurrence, not amenable to percutaneous ablation, other treatments were considered: TAE, sorafenib, best conservative management. At the end of follow-up, overall local tumor progression rate included primary treatment failures, and local recurrences were recorded. In naive patients, the overall (local and distant tumor recurrences) recurrence-free survival (RFS) was computed. In cases of liver transplantation (for tumor progression within the Milan criteria or liver failure), all explants were submitted to a liver pathologist for gross and microscopic examination of ablated tumors as previously reported [11]. For patients who died during the follow-up period, the cause of death was recorded.
Statistical Analysis
Continuous data were expressed as median and interquartile range, and categorical variables were expressed as frequency. Overall survival (OS) was defined from the date of original mbp-RFA to the patient's death, or the last date of follow-up, the last visit. Follow-up was recorded until June 1, 2015. Patients who underwent liver transplantation during follow-up were censored at the date of transplantation. Tumor RFS (including local tumor progression and distant recurrence) was measured from the date of original mbp-RFA to the date of the first tumor progression or death or last date of follow-up without tumor progression.
Survival curves were estimated using the Kaplan-Meier method. Univariate and multivariate Univariate Cox proportional hazards regressions were performed to determine the parameters associated with OS and RFS in naive patients. All variables with a p < 0.2 were entered into a stepwise Cox proportional hazards regression model. All statistical analyses were conducted using SAS software 9.4.1.
Results
Selection of Patients
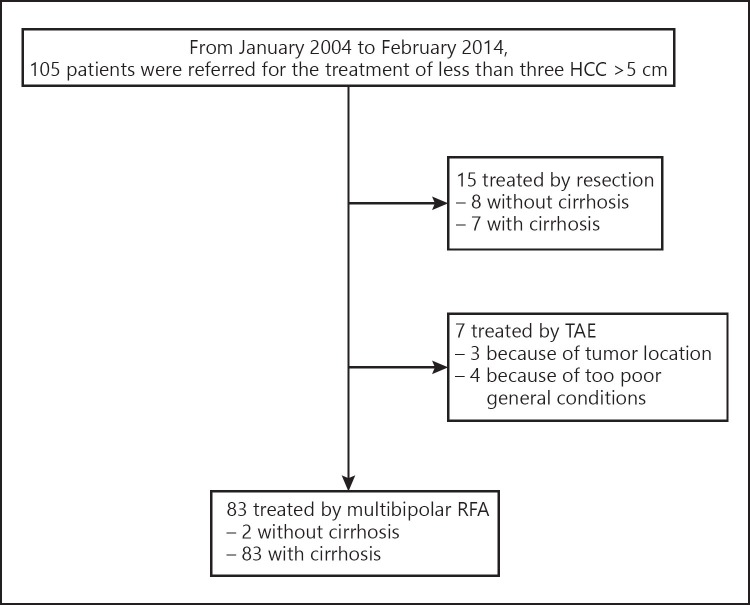
Between January 2004 and February 2014, among the 105 patients referred to our Center for the treatment of HCC larger than 5 cm, not exceeding 10 cm, 83 (79%) received mbp-RFA as first-line treatment (Fig. 2). Eighty (96.4%) patients had biopsy-proven cirrhosis, 61 (78.3%) mass-forming and 22 (21.7%) infiltrative forms including 12 (14.5%) cases of segmental portal invasion at imaging, 10 of which were infiltrative forms. Eighteen had a serum AFP level > 200 ng/ml. The median size of the largest nodule was 6.2 cm (5.1–9), and the diagnosis of HCC was made on the basis of histological findings at percutaneous biopsy for 56 patients and on the basis of noninvasive criteria [12] for the remaining 27 patients. Patient and tumor baseline characteristics are provided in detail in Table 1.
Fig. 2.
Flow chart of patient selection for treatment by multibipolar radiofrequency ablation (RFA) of up to three hepatocellular carcinomas (HCC) with a main nodule > 5 cm in the largest diameter. TAE, transarterial embolization.
Table 1.
Characteristics of 83 patients with HCC >5 cm in diameter treated with multipolar RFA
| Age, years | 71 [37-93] |
| Male gender | 67 (80.7) |
| Child-Pugh score | |
| <6 | 64 (77.1) |
| =7 a | 16 (19.3) |
| Main etiology of liver disease | |
| Alcohol | 34 (41) |
| HCV | 27 (32.5) |
| HBV-HDV | 11 (13) |
| NASH | 7 (8.5) |
| Hemochromatosis | 4 (5) |
| Esophageal varices | 36 (43.5) |
| Platelet count, g/L | 149 [53-440] |
| Platelet count <100 g/L | 22 (26.5) |
| Prothrombin activity, % | 79 [42-100] |
| Prothrombin activity <75% | 30 (36) |
| Serum bilirubin level, μπιοI/L | 16 [4-78] |
| Bilirubin >20 μπιI/L | 15 (18.1) |
| Naive | 74 (89.1) |
| Nonnaive | 9 (8.9%) |
| Serum AFP level, ng/mL | 1,404 [1-60,500] |
| AFP >200 ng/mL | 18 (21.7) |
| Tumor | |
| Mass forming | 61 (73.5) |
| Infiltrative | 22 (26.5) |
| Portal invasion at imaging | 12 (14.5) b |
| Diameter of the main tumor, mm | 62 [51-90] |
| Tumor diameter >70 mm | 22 (26.5) |
| Number of tumors/patient | |
| 1 | 62 (74.7) |
| 2 | 17 (20.5) |
| 3 | 4 (4.8) |
| Intrahepatic portal vein invasion | 12 (14.5) |
Data are presented as mean [range] or n (%). HCC, hepatocellular carcinoma; RFA, radiofrequency ablation; HCV, hepatitis C virus; HBV, hepatitis B virus; HDV, hepatitis D virus; NASH, nonalcoholic steatosic hepatitis; AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer.
Excluded 3 patients without established cirrhosis.
Of which 10 were infiltrative forms.
Treatment Response
Technical success was 100% (Fig. 3, 4). The original mbp-RFA procedures for the treatment of the main tumor required a mean number of 2.14 ± 1.4 [1, 2, 3, 4, 5, 6, 7, 8, 9, 10] of RFA applications, a mean total amount of energy of 200 ± 100 kJ, a mean total deposition time of 39.7 ± 15 min, and a mean total procedure duration of 104 ± 30 min. Whole treatment course consisted in one, two, and three procedures (the second and third procedures were performed within 2 months after a previous one regarded as incomplete) in 75, 6, and 2 patients, respectively. After the last mbp-RFA procedures, CT or MRI showed complete ablation at 1 month in 78/83 (94%) patients resulting in primary, secondary, and tertiary effectiveness rates of 84, 91, and 94%, respectively. Thus, mbp-RFA failed to achieve complete ablation in 5 patients, 4 with infiltrative and 1 with mass-forming HCC.
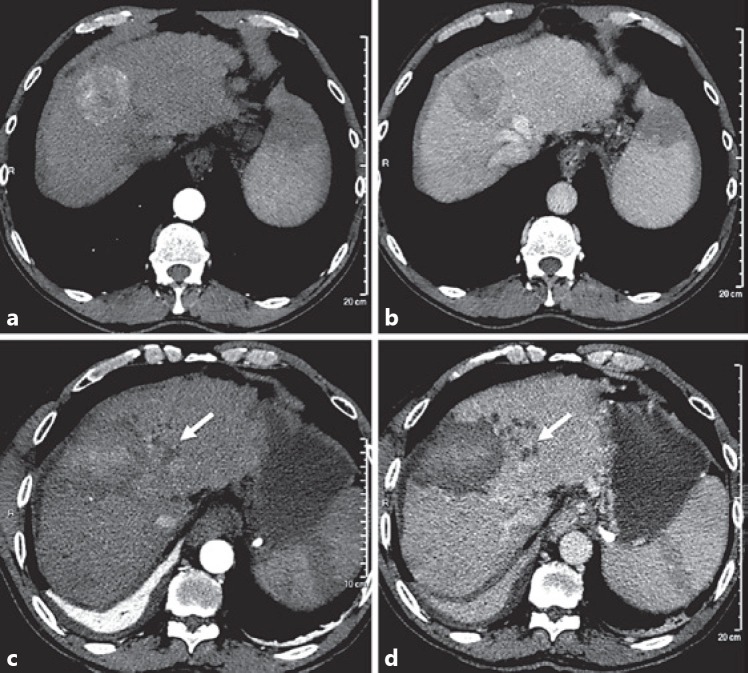
Fig. 3.
Complete ablation of 55-mm nodular hepatocellular carcinoma (HCC) of segment 4 in a 61-year-old man with Child-Pugh A cirrhosis. Axial CT scan at arterial phase (a) and portal phase (b) of intravenous iodinate contrast medium injection showing well-demarcated subcapsular mass of segment 4 with typical wash-in wash-out of HCC. Anterior hypoattenuating area of the spleen is related to history of embolization for hypersplenism. One month after multibipolar radiofrequency ablation, axial CT scan at arterial phase (c) and portal phase (d) of intravenous iodinate contrast medium injection shows unenhanced mass encompassing the limits of the tumor. Note the presence intrasegmental bile duct dilatation upstream the ablation area (arrow) and basal right lung atelectasis due to reactive pleural effusion.
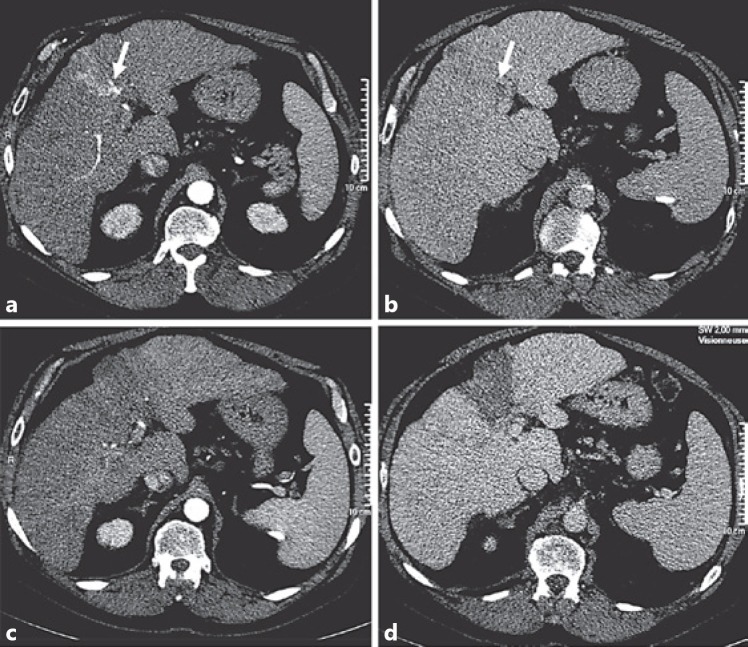
Fig. 4.
Complete ablation of 52-mm infiltrative hepatocellular carcinoma of segment 4 in a 69-year-old man with Child-Pugh A cirrhosis. Axial CT scan at arterial phase (a) and portal phase (b) of intravenous iodinate contrast medium injection showing a subsegmental infiltrative tumor of segment 4 with portal invasion reaching the left branch (arrow). One month after multibipolar radiofrequency ablation, axial CT scan at arterial phase (c) and portal phase (d) of intravenous iodinate contrast medium injection shows wedged unenhanced area extending beyond the limits of infiltrative tumor.
Complications
The median duration of hospital stay after original mbp-RFA procedure was 4.3 days (2–45). The 31 observed complications were graded I (n = 25, 30%), II (n = 0, 0%), IIIa (n = 3, 3.6%), IIIb (n = 1, 1.2%), IVa (n = 1, 1.2%), IVb (n = 0, 0%), and V (n = 1, 1.2%) according to Clavien-Dindo (Table 2). Six (7%) severe complications occurred, including one (1.2%) lethal septicemia. One patient treated for an infiltrative recurrence of HCC developed tract seeding and another one, with past history of sphincterotomy identified only after the treatment using six electrodes, developed hepatic abscess with biliopleural fistula successfully treated by drainage, antibiotic, and surgery to close the disruption of diaphragm. Three patients developed right pleural effusion requiring thoracocentesis. Twenty-five (30%) patients experienced at least one minor complication including (20.5%) postablation syndrome that persisted up to 7 days. Most of the pleural effusion (4/5) had an early onset when artificial ascites was not systematically performed for HCC located in the dome.
Table 2.
Complications encountered after single original multibipolar RFA procedure for the treatment of HCC >5 cm in 83 patients according to the Clavien-Dindo classification
| Grade | Events |
|---|---|
| I | 17 (20.5%) postablation syndrome persisting beyond 7 days* |
| 1 (1.2%) paralytic ileus* | |
| 1 (1.2%) hemobilia+ | |
| 2 (2.4%) pleural effusion* | |
| 2 (2.4%) ascites 1 | |
| 2 (2.4%) icterus 1 | |
| II | No event |
| IIIa | 3 (3.6%) pleural effusion requiring thoracentesis 1 |
| IIIb | 1 (1.2%) tumor tract seeding 1 |
| IVa | 1 (1.2%) liver abscess and biliopleural fistula 1 |
| IVb | No event |
| V | 1 (1.2%) septicemia 1 |
RFA, radiofrequency ablation; HCC, hepatocellular carcinoma.
+ Regarded as minor and major according to the Society of Interventional Radiology classification, respectively.
Recurrence and Survival
The median follow-up was 26.1 months (1–112) months. After complete ablation (n = 78), local recurrence occurs in 14 patients (17.9%). Adding the 5 patients with mbp-RFA failure, the overall local-tumor progression was 22.9% (19/83). At multivariate analysis, infiltrative form (HR 5.18 [2.01–13.2], p = 0.0006), multinodular form (HR 5.29 [2.14–13.1], p = 0.003), and serum AFP >200 ng/mL (HR 3.45 [1.29–9.64], p = 0.02) were predictive of overall local tumor progression (Table 3).
Table 3.
Factors associated with tumor progression-free survival (local and overall) and overall survival in patients treated with multibipolar radiofrequency ablation for hepatocellular carcinoma >5 cm
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| hazards ratio | p | hazards ratio | p | |
| Overall local tumor progression-free survival (83 patients) | ||||
| Age (>65 years) | 0.95 (0.31-2.85) | 0.92 | ||
| Male gender | 2.29 (0.53-9.91) | 0.26 | ||
| Prothrombin activity (<75%) | 1.66 (0.68-4.01) | 0.26 | ||
| Serum bilirubin level (>20 μιτιοΙ/L] | 0.67 (0.15-2.94) | 0.62 | ||
| Platelet count (>100 g/L) | 95 (0.34-2.61) | 0.29 | ||
| Child-Pugh score <6/=7 | 1.11 (0.32-3.87) | 0.86 | ||
| Esophageal varices | 1.40 (0.58-3.37) | 0.45 | ||
| Serum AFP level (>200 ng/mL) | 2.17 (0.82-5.67) | 0.11 | 3.45 (1.29-9.64) | 0.02 |
| Multinodular form | 3.88 (1.60-9.37) | 0.0025 | 5.29 (2.14-13.1) | 0.003 |
| Tumor diameter (>70 mm) | 1.66 (0.66-4.18) | 0.27 | ||
| Infiltrative tumor | 3.37 (1.37-8.24) | 0.00745 | 5.18 (2.01-13.2) | 0.0006 |
| Vascular invasion | 3.83 (1.45-10.1) | 0.006 | ||
| Number of electrodes (<4) | 1.3767 (0.59-3.29) | 0.48 | ||
| | ||||
| Overall tumor progression-free survival (74 naive patients) | ||||
| Age (>65 years) | 0.69 (0.34-1.41) | 0.31 | ||
| Male gender | 0.59 (0.29-1.17) | 0.13 | ||
| Prothrombin activity (<75%) | 1.01 (0.54-1.88) | 0.96 | ||
| Serum bilirubin level (>20 μιτιI/L] | 0.74 (0.29-1.90) | 0.54 | ||
| Platelet count (>100 g/L) | 0.63 (0.33-1.21) | 0.16 | ||
| Child-Pugh score <6/=7 | 1.25 (0.55-2.83) | 0.59 | ||
| Esophageal varices | 1.46 (0.81-2.61) | 0.20 | ||
| Serum AFP level (>200 ng/mL) | 2.05 (0.98-4.28) | 0.06 | 2.86 (1.32-6.21) | 0.008 |
| Multinodular form | 2.05 (1.08-3.89) | 0.03 | 2.74 (1.4-5.38) | 0.003 |
| Tumor diameter (>70 mm) | 2.15 (1.11-4.16) | 0.03 | ||
| Infiltrative tumor | 2.28 (1.19-4.38) | 0.01 | 3.43 (1.67-7.01) | 0.0007 |
| Vascular invasion | 2.28 (1.04-4.99) | 0.04 | ||
| Number of electrodes (<4) | 0.96 (0.54-1.73) | 0.91 | ||
| | ||||
| Overall survival (74 naive patients) | ||||
| Age (>65 years) | 0.56 (0.28-1.11) | 0.09 | ||
| Male gender | 1.00 (0.48-2.07) | 0.99 | ||
| Prothrombin activity (<75%) | 1.13 (0.62-2.07) | 0.68 | ||
| Serum bilirubin level (>20 μιτιI/L] | 1.43 (0.69-2.97) | 0.34 | ||
| Platelet count (>100 g/L) | 0.93 (0.47-1.83) | 0.83 | ||
| Child-Pugh score <6/=7 | 1.97 (0.99-3.91) | 0.05 | ||
| Esophageal varices | 1.24 (0.69-2.20) | 0.46 | ||
| Serum AFP level (>200 ng/mL) | 1.45 (0.70-3.01) | 0.31 | ||
| Multinodular form | 0.99 (0.51-1.93) | 0.99 | ||
| Tumor diameter (>70 mm) | 1.21 (0.62-2.40) | 0.57 | ||
| Infiltrative tumor | 2.50 (1.33-4.70) | 0.004 | 2.5 (1.33-4.69) | 0.004 |
| Macrovascular invasion | 2.45 (1.21-4.97) | 0.01 | ||
| Number of electrodes (>4) | 1.04 (0.58-1.85) | 0.88 | ||
Figures in parentheses indicate confidence intervals. AFP, alpha fetoprotein.
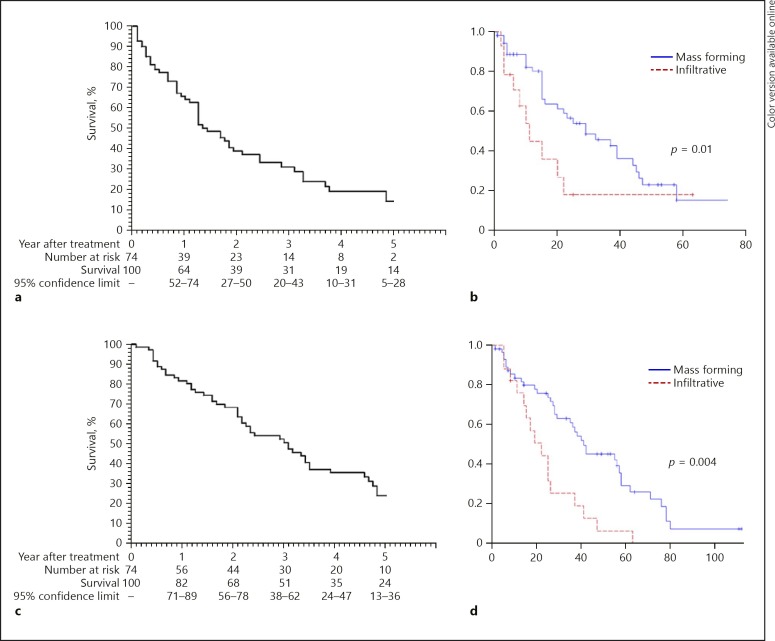
Distant recurrence occurred in 43 out of the 78 (55.1%) patients with an imaging pattern of complete ablation after the initial cycle of mbp-RFA treatment, including 4 patients for whom extrahepatic metastasis appeared. Adding the local tumor progression, in the 74 naive patients, the estimated 3- and 5-year RFS were 31% (20–43) and 14% (5–28), respectively (Fig. 3). The median time of tumor progression was 14 months. Serum AFP > 200 ng/mL (HR = 2.86 [1.32–6.21], p = 0.008), multinodular form (HR 2.74 [1.4–5.38], p = 0.003), and infiltrative form (HR 3.43 [1.67–7.01], p = 0.0007) were independent risk factor of overall tumor progression in multivariate analysis (Table 3). The 3-year RFS was 47% in the 57 naive patients with mass-forming HCC, compared to 18% in the 17 naive patients with infiltrative HCC (Fig. 5). Compared with the mass-forming form, infiltrative HCC were associated with lower 3-year local and distant RFS: 85 versus 22%, and 48 versus 18%, respectively.
Fig. 5.
Survival figures. a Probabilities of tumor progression-free survival in 74 naïve patients treated with multibipolar radiofrequency ablation (RFA) for hepatocellular carcinomas (HCC) > 5 cm (median: 17.4 months). b Probabilities of tumor progression-free survival in naïve patients after multibipolar RFA for the treatment of HCC > 5 cm according to the tumor type: mass-forming (n = 57) (median: 11.5 months) vs. infiltrative form (n = 17) (median: 29.7 months). c Probabilities of overall survival in 74 naïve patients treated with multibipolar RFA for HCC > 5 cm (median: 37.2 months). d Probabilities of overall survival in naïve patients after multibipolar RFA for the treatment of HCC > 5 cm according to the tumor type: mass forming (n = 57) (median: 21.8 months) vs. infiltrative form (n = 17) (median: 41.8 months).
Among the 74 naive patients, 53 (71.6%) patients died during follow-up and 2 patients were lost at follow-up. Known causes of deaths (n = 52) were: HCC progression (n = 31, 41.9%), liver failure or digestive hemorrhage (n = 9, 12.2%), and non-liver related (n = 12, 16.2%). Twenty-one (28.4%) patients were alive, including transplanted patients (censored at the date of transplantation). Five patients were listed for transplantation, 3 for tumor progression, 1 for liver failure, and 1 for both causes. Two patients were finally not transplanted, 1 without tumor recurrence refused and died from liver failure; 1 died of severe sepsis on the waiting list 21 months after mbp-RFA. The 3 patients transplanted were alive without recurrence with a median follow-up since transplantation of 53 months (9.7–137 months). In these patients, three nodules ≥5 cm previously treated with mbp-RFA appeared completely ablated at systematic pathological examination of the explanted livers. Among the 19 nontransplanted living patients, 10 had no detectable tumor. The estimated 3- and 5-year OS in naive 74 patients were 51% (38–62) and 24% (13–36), respectively. The median OS was 38 months, (Fig. 5). In univariate analysis, the variables associated with OS in naïve patients were age, Child-Pugh B class, macrovascular vascular invasion and infiltrative form (Table 3). In multivariate analysis, infiltrative form was the only independent risk factor for death (HR: 2.5 [1.33–4.69]; p = 0.004). In the 57 naive patients with mass-forming HCC, the estimated 3- and 5-year OS was 63.4 and 30% compared to 25 and 6% in 17 patients with infiltrative HCC, respectively (Fig. 3).
Discussion
In this study, we reported the long-term outcomes of patients after mbp-RFA for the treatment of up to three unresectable HCC arising on compensated cirrhosis, the largest nodule measuring more than 5 cm in diameter. From the original cohort of 105 patients referred to our center with similar HCC staging, this strategy could be performed for 83 (79%), while less than 10% (n = 7, 6.7%) received TAE including only 3 (2.8%) because the tumors were technically not suitable for percutaneous RFA. The fact is that mbp-RFA was highly effective to obtain complete response (78/83, 94%) in various challenging situations regarding the size and location of the tumor. Thus, mbp-RFA allows to maintain in upfront curative strategy a large portion of patients with large HCC and cirrhosis mostly not amenable to resection.
Preoperative ipsilateral portal vein embolization can increase marginally the overall resectability of these patients. However, at least 20% of originally planned liver resections are cancelled after portal vein embolization because of insufficient growth of future remnant lobe or tumor progression [13]. In our study, mbp-RFA was associated with 3- and 5-year OS of 51 and 24%, respectively. Although these results were in the lower range of 5-year OS reported after resection of HCC > 5 cm: 34% [14] and 33.8% [15], it must be pointed out that in our patients resection was contraindicated mainly because of too advanced cirrhosis and/or insufficient general condition. For instance, in comparison with western HCC patients resected by Kluger et al.[14], in our study twice more had cirrhosis, and their median age was 10 years older. That emphasized the main advantages of mbp-RFA over resection in the setting of cirrhosis by offering a curative option in a larger number of less selected patients. In our study, while fewer patients were amenable to salvage transplantation, 39% died of competitive causes of tumor progression (77.1% patients without local tumor progression at the endpoint of the study). The high efficacy of mbp-RFA to induce complete ablation of large HCC was confirmed in 3 patients transplanted since no viable tumor tissue in the treatment sites was found at histopathological analysis of liver specimens. Complications of the cirrhosis were nonnegligible cause of death in our cohort, emphasizing the need to improve the management of the underlying liver disease, and particularly alcohol abstinence. Thus, careful comparison of results achieved by resection and mbp-RFA for the treatment of HCC 5–10 cm in diameter in patients sharing similar characteristics, shows a comparable or even better (in intention to treat) rate of OS [14].
Recently, in more selected patients (n = 17) bearing large mass-forming HCC ranging from 5 to 7 cm in maximum diameter without intrahepatic major vessel invasion, similar 94% complete response rate has been achieved with the overlapping RFA technique using simultaneously only three electrodes [16]. The 5.7% major complication rate reported was also remarkably comparable to the 7% of our study, while a higher mean of 9 ± 2.2 intratumorous electrode placements and 3 ± 0.8 sessions were required.
For the treatment of large HCC in inoperable patients, combined treatments consisting of TACE followed by intratumorous mono-applicator ablation (mostly with RFA and more recently with microwave ablation) have gained popularity [17, 18, 19, 20]. Although these strategies led to 65–94% response rates for HCC > 5 cm when all therapeutic schedule was completed [17, 18, 19], the main limitation of this approach is that all patients with such large HCC respond sufficiently to TACE to benefit from the subsequent mono-applicator ablation procedure. Like the mono-applicator ablative procedure either performed with RFA or microwave ablation, response to TACE drops dramatically with the tumor diameter [21]. In a recent study, while the superselective cone beam CT-guided technique was used to perform lipiodol TACE, 35% of patients still had no objective response according to m-RECIST criteria [22, 23]. These results suggest that a nonnegligible portion of patients bearing solitary large HCC could not be treated with curative intent using TACE combined with the mono-applicator ablative procedure.
Infiltrative HCC was associated with treatment failure with impaired RFS and OS. Infiltrative HCC is a morphological subtype of primary liver cancer identified by imaging and is characterized by a more aggressive phenotype than the classical and more frequent “mass-forming” subtype [24]. This aggressive behavior was confirmed in our study as these tumors were characterized by a high rate of vascular invasion at imaging (10/12), elevated level of AFP and poor survival. In addition, even if the feasibility of ablation of infiltrative tumor was improved with mbp-RFA thanks to the wedged ablation strategy for the placement of electrodes especially when six are used (18/22 complete ablation), results were still impeded by a high and early recurrence rate likely due in part to the difficulty to ensure a safety margin in these poorly demarcated tumors. Infiltrative form was also a negative feature in other types of treatments including surgery that is frequently not considered or not possible in this setting. Thus, nonsurgical approaches like percutaneous intraarterial ethanol injection, radioembolization, TACE, or combined treatments also led to poorly sustained favorable outcomes even in few cases displaying initial complete response [25, 26, 27, 28]. Thus, for the treatment of infiltrative but not diffuse HCCs, further strategies remain to be studied, including a better selection of patients who might benefit from local aggressive treatment such as mbp-RFA.
mbp-RFA appeared as a safe option for the treatment of large HCC. The mortality rate was 1.2% (n = 1), and major complications occurred in 7% (n = 6). Most complications except one biliopleural fistula and one tumor seeding were easily manageable. The increasing number of electrode insertions needed to perform mbp-RFA was not associated with more frequent hemorrhagic events. However, the rate of complications reported in our study was clearly higher than that reported in the treatment of small HCCs [29], but was still in the range of those reported by other series of percutaneous ablation of large HCCs [30]. We observed especially a relatively high incidence of pleural effusion and postablation syndrome due to treatment in HCC located in the dome. These complications mostly occurred during the first period of the study and dramatically decreased since artificial ascites were systematically used during the treatment of HCC located in the dome. Interestingly, according to the Clavien-Dindo grading of surgical complications, the safety of mbp-RFA of large HCC still compares favorably with hepatectomy, while patients treated were definitely in the higher risk group than those commonly resected [31].
The limitations of our study were related to its retrospective, monocentric, and noncomparative design. In addition, the small size of our population and the number of events may have limited the ability of our multivariable model to study more than 2 association factors. The direct comparison with surgical series was not possible as most of our patients were considered as nonresectable. Comparison with combined TACE and monopolar RFA could be interesting if the analysis includes patients for whom RFA is not attempted because of insufficient response to previous TACE. Finally, we acknowledge that mbp-RFA is skill demanding and that slows down its development. However, regarding the stake to offer curative strategy to the numerous patients bearing large HCC not upfront resectable or transplantable, we believe that mbp-RFA is worth being an available option in expert centers.
In conclusion, our study showed that mbp-RFA treatment has an acceptable safety profile, offers good OS in HCC > 5 and < 10 cm and should be considered as a potential curative approach when surgical resection is not possible. Conversely, for infiltrative HCC, although complete response rate to mbp-RFA, even lower, remained favorable, the worst RFS and OS associated with this form confirmed its aggressive behavior. Beyond a more stringent strategy for selecting patients with infiltrative form to benefit from mbp-RFA, new or combined treatment approaches are needed to improve survival of patients with these subtypes of tumors.
Disclosure Statement
Olivier Seror is consultant for Olympus/Celon, Angiodynamics, General Electric Healthcare, and Bayer Healthcare.
Funding Sources
No author received financial support for completing the study or writing the manuscript.
References
- 1.Belghiti J, Kianmanesh R. Surgical treatment of hepatocellular carcinoma. HPB (Oxford) 2005;7:42–49. doi: 10.1080/13651820410024067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi SB, Kim HJ, Song TJ, Ahn HS, Choi SY. Influence of clinically significant portal hypertension on surgical outcomes and survival following hepatectomy for hepatocellular carcinoma: a systematic review and meta-analysis. J Hepatobiliary Pancreat Sci. 2014;21:639–647. doi: 10.1002/jhbp.124. [DOI] [PubMed] [Google Scholar]
- 3.Pompili M, Francica G, Ponziani FR, Iezzi R, Avolio AW. Bridging and downstaging treatments for hepatocellular carcinoma in patients on the waiting list for liver transplantation. World J Gastroenterol. 2013;19:7515–7530. doi: 10.3748/wjg.v19.i43.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bargellini I, Bozzi E, Campani D, Carrai P, De Simone P, Pollina L, Cioni R, Filipponi F, Bartolozzi C. Modified RECIST to assess tumor response after transarterial chemoembolization of hepatocellular carcinoma: CT-pathologic correlation in 178 liver explants. Eur J Radiol. 2013;82:e212–e218. doi: 10.1016/j.ejrad.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Sacco R, Mismas V, Romano A, Bertini M, Bertoni M, Federici G, Metrangolo S, Parisi G, Tumino E, Bresci G, Giacomelli L, Marceglia S, Bargellini I. Assessment of clinical and radiological response to sorafenib in hepatocellular carcinoma patients. World J Hepatol. 2015;7:33–39. doi: 10.4254/wjh.v7.i1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seror O, N'Kontchou G, Nault JC, Rabahi Y, Nahon P, Ganne-Carrie N, Grando V, Zentar N, Beaugrand M, Trinchet JC, Diallo A, Sellier N. Hepatocellular carcinoma within Milan criteria: no-touch multibipolar radiofrequency ablation for treatment-long-term results. Radiology. 2016;280:611–621. doi: 10.1148/radiol.2016150743. [DOI] [PubMed] [Google Scholar]
- 7.Seror O, N'Kontchou G, Ibraheem M, Ajavon Y, Barrucand C, Ganne N, Coderc E, Trinchet JC, Beaugrand M, Sellier N. Large (> or = 5.0-cm) HCCs: multipolar RF ablation with three internally cooled bipolar elec trodes – initial experience in 26 patients. Radiology. 2008;248:288–296. doi: 10.1148/radiol.2481071101. [DOI] [PubMed] [Google Scholar]
- 8.Song I, Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol. 2009;19:2630–2640. doi: 10.1007/s00330-009-1463-x. [DOI] [PubMed] [Google Scholar]
- 9.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed M, Solbiati L, Brace CL, Breen DJ, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. Radiology. 2014;273:241–260. doi: 10.1148/radiol.14132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seror O, N'Kontchou G, Van Nhieu JT, Rabahi Y, Nahon P, Laurent A, Trinchet JC, Cherqui D, Vicaut E, Beaugrand M, Sellier N. Histopathologic comparison of monopolar versus no-touch multipolar radiofrequency ablation to treat hepatocellular carcinoma within Milan criteria. J Vasc Interv Radiol. 2014;25:599–607. doi: 10.1016/j.jvir.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TM, van Delden OM. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol. 2013;36:25–34. doi: 10.1007/s00270-012-0440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kluger MD, Salceda JA, Laurent A, Tayar C, Duvoux C, Decaens T, Luciani A, Van Nhieu JT, Azoulay D, Cherqui D. Liver resection for hepatocellular carcinoma in 313 Western patients: tumor biology and underlying liver rather than tumor size drive prognosis. J Hepatol. 2015;62:1131–1140. doi: 10.1016/j.jhep.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Cucchetti A, Djulbegovic B, Tsalatsanis A, Vitale A, Hozo I, Piscaglia F, Cescon M, Ercolani G, Tuci F, Cillo U, Pinna AD. When to perform hepatic resection for intermediate-stage hepatocellular carcinoma. Hepatology. 2015;61:905–914. doi: 10.1002/hep.27321. [DOI] [PubMed] [Google Scholar]
- 16.Lin CC, Cheng YT, Chen MW, Lin SM. The effectiveness of multiple electrode radiofrequency ablation in patients with hepatocellular carcinoma with lesions more than 3 cm in size and Barcelona clinic liver cancer stage A to B2. Liver Cancer. 2016;5:8–20. doi: 10.1159/000367755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang ZJ, Wang MQ, Duan F, Song P, Liu FY, Chang ZF, Wang Y, Yan JY, Li K. Transcatheter arterial chemoembolization followed by immediate radiofrequency ablation for large solitary hepatocellular carcinomas. World J Gastroenterol. 2013;19:4192–4199. doi: 10.3748/wjg.v19.i26.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin X, Zhang L, Wang YH, Zhang BH, Gan YH, Ge NL, Chen Y, Li LX, Ren ZG. Transcatheter arterial chemoembolization combined with radiofrequency ablation delays tumor progression and prolongs overall survival in patients with intermediate (BCLC B) hepatocellular carcinoma. BMC Cancer. 2014;14:849. doi: 10.1186/1471-2407-14-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Yin X, Gan YH, Zhang BH, Zhang JB, Chen Y, Xie XY, Ge NL, Wang YH, Ye SL, Ren ZG. Radiofrequency ablation following first-line transarterial chemoembolization for patients with unresectable hepatocellular carcinoma beyond the Milan criteria. BMC Gastroenterol. 2014;14:11. doi: 10.1186/1471-230X-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ni JY, Sun HL, Chen YT, Luo JH, Chen D, Jiang XY, Xu LF. Prognostic factors for survival after transarterial chemoembolization combined with microwave ablation for hepatocellular carcinoma. World J Gastroenterol. 2014;20:17483–17490. doi: 10.3748/wjg.v20.i46.17483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allard MA, Sebagh M, Ruiz A, Guettier C, Paule B, Vibert E, Cunha AS, Cherqui D, Samuel D, Bismuth H, Castaing D, Adam R. Does pathological response after transarterial chemoembolization for hepatocellular carcinoma in cirrhotic patients with cirrhosis predict outcome after liver resection or transplantation? J Hepatol. 2015;63:83–92. doi: 10.1016/j.jhep.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 22.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 23.Duan F, Wang EQ, Lam MG, Abdelmaksoud MH, Louie JD, Hwang GL, Kothary N, Kuo WT, Hofmann LV, Sze DY. Superselective chemoembolization of HCC: comparison of short-term safety and efficacy between drug-eluting LC beads, QuadraSpheres, and conventional ethiodized oil emulsion. Radiology. 2016;278:612–621. doi: 10.1148/radiol.2015141417. [DOI] [PubMed] [Google Scholar]
- 24.Yopp AC, Mokdad A, Zhu H, Mansour JC, Balch GC, Choti MA, Singal AG. Infiltrative hepatocellular carcinoma: natural history and comparison with multifocal, nodular hepatocellular carcinoma. Ann Surg Oncol. 2015;22((suppl 3)):1075–1082. doi: 10.1245/s10434-015-4786-7. [DOI] [PubMed] [Google Scholar]
- 25.Golfieri R, Mosconi C, Cappelli A, Giampalma E, Galaverni MC, Pettinato C, Renzulli M, Monari F, Angelelli B, Pini P, Terzi E, Ascanio S, Garzillo G, Piscaglia F, Bolondi L, Trevisani F. Efficacy of radioembolization according to tumor morphology and portal vein thrombosis in intermediate-advanced hepatocellular carcinoma. Future Oncol. 2015;11:3133–3142. doi: 10.2217/fon.15.267. [DOI] [PubMed] [Google Scholar]
- 26.Han K, Kim JH, Yoon HM, Kim EJ, Gwon DI, Ko GY, Yoon HK, Ko HK. Transcatheter arterial chemoembolization for infiltrative hepatocellular carcinoma: clinical safety and efficacy and factors influencing patient survival. Korean J Radiol. 2014;15:464–471. doi: 10.3348/kjr.2014.15.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kokabi N, Camacho JC, Xing M, El-Rayes BF, Spivey JR, Knechtle SJ, Kim HS. Open-label prospective study of the safety and efficacy of glass-based yttrium 90 radioembolization for infiltrative hepatocellular carcinoma with portal vein thrombosis. Cancer. 2015;121:2164–2174. doi: 10.1002/cncr.29275. [DOI] [PubMed] [Google Scholar]
- 28.Nault JC, Nkontchou G, Nahon P, Grando V, Bourcier V, Barge S, Ziol M, Sellier N, Ganne-Carrie N, Seror O. Percutaneous treatment of localized infiltrative hepatocellular carcinoma developing on cirrhosis. Ann Surg Oncol. 2016;23:1906–1915. doi: 10.1245/s10434-015-5064-4. [DOI] [PubMed] [Google Scholar]
- 29.Koda M, Murawaki Y, Hirooka Y, Kitamoto M, Ono M, Sakaeda H, Joko K, Sato S, Tamaki K, Yamasaki T, Shibata H, Shimoe T, Matsuda T, Toshikuni N, Fujioka S, Ohmoto K, Nakamura S, Kariyama K, Aikata H, Kobayashi Y, Tsutsui A. Complications of radiofrequency ablation for hepatocellular carcinoma in a multicenter study: an analysis of 16 346 treated nodules in 13 283 patients. Hepatol Res. 2012;42:1058–1064. doi: 10.1111/j.1872-034X.2012.01025.x. [DOI] [PubMed] [Google Scholar]
- 30.Yin XY, Xie XY, Lu MD, Xu HX, Xu ZF, Kuang M, Liu GJ, Liang JY, Lau WY. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer. 2009;115:1914–1923. doi: 10.1002/cncr.24196. [DOI] [PubMed] [Google Scholar]
- 31.Harimoto N, Shirabe K, Ikegami T, Yoshizumi T, Maeda T, Kajiyama K, Yamanaka T, Maehara Y. Postoperative complications are predictive of poor prognosis in hepatocellular carcinoma. J Surg Res. 2015;199:470–477. doi: 10.1016/j.jss.2015.06.012. [DOI] [PubMed] [Google Scholar]