Abstract
Palmoplantar tylosis is a focal non epidermolytic palmoplantar hyperkeratosis and is associated with a very high lifetime risk of developing squamous cell carcinoma of the esophagus (OSCC). It is generally inherited as an autosomal dominant trait with complete penetrance involving the RHBDF2 gene located on 17q25.1. The data regarding endoscopic appearance of the mucosa in patients with tylosis before development of cancer is limited. Surveillance endoscopy is recommended in family members which include annual esophagogastroscopy with biopsy of suspicious lesion with quadratic biopsies from upper, middle and lower esophagus. We describe characteristic endoscopy findings in a tylosis with no evidence of cancer. Prospective documentation of endoscopic findings of similar mucosal changes and disease process to establish a better screening protocol and supplemental intervention with agents like carotenoids (beta-carotene, alpha-carotene, lycopene, beta-cryptoxanthin, lutein, and zeaxanthin) may delay the progression and possibly revert to normal.
Keywords: Endoscopy, Esophageal cancer, Tylosis
Introduction
Palmoplantar tylosis is a focal non epidermolytic palmoplantar hyperkeratosis, which is inherited as an autosomal dominant condition. Tylosis with esophageal cancer was first reported in two large families from Liverpool [1, 2] and later found that both families are related distantly [3]. Smaller heritage lines were reported from Germany [4], United States [5], Finland [6], Spain [7] and Brazil [8]. We describe another family from USA with screening endoscopic findings of one of the family members.
Case Presentation
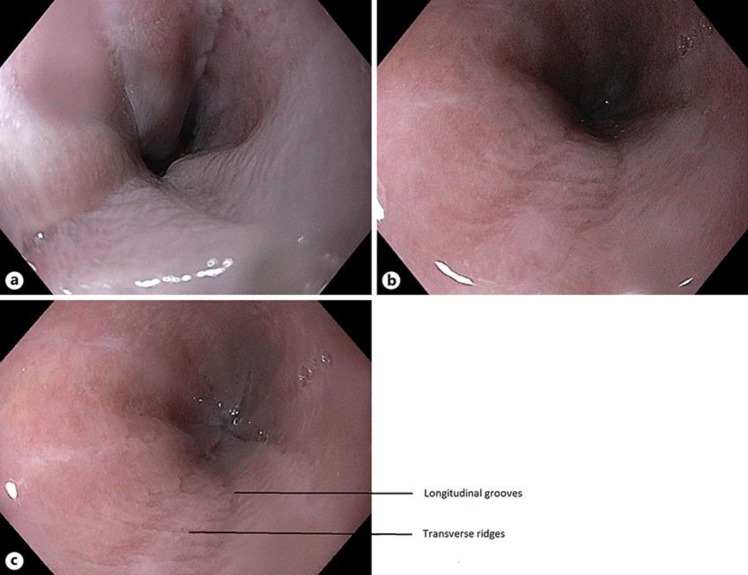
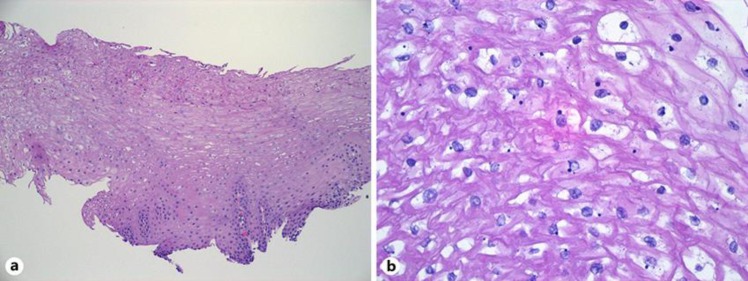
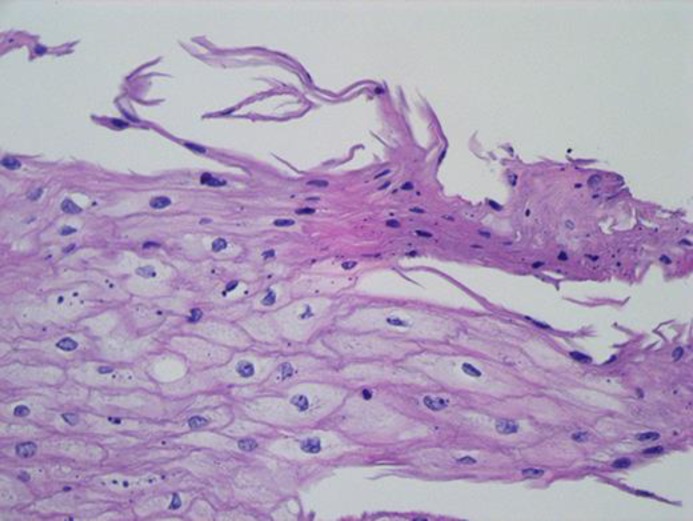
A 28-yo female presented with occasional complaints of GERD symptoms (heartburn, food regurgitation) approximately 3 times a week, sometimes related to diet which worsened during evening and nighttime. She denied nausea, vomiting, hematemesis, unexplained weight loss, night cough, abdominal pain or change in bowel habits. She had a family history of esophageal cancer (mother, maternal grandfather, great aunt) and a history of tylosis for which she had undergone genetic counselling in the past. Her last EGD was in 2015 and was visualized with white light, narrow banding imaging after chromoendoscopy with lugol's iodine and reported to be normal with no abnormalities. Cold forceps biopsy of the distal esophagus reported hyperplastic squamous mucosa with hyperkeratosis and parakeratosis but was negative for dysplasia or carcinoma. However, due to her significant family history she was advised to undergo a follow up surveillance endoscopy in 2 years. She also has a past medical history of tinea cruris, tinea pedis and obesity (BMI: 32). She consumes a probiotic 1 per oral daily and is on Mirena IUD birth control. She denies smoking and substance abuse. She drinks alcohol occasionally (wine, 1–2 times per month, 2 drinks/episode). On her recent endoscopy there was spontaneous mucosal sclerosis throughout the entire esophagus (Fig. 1a–c) with diffuse mild mucosal changes characterized by longitudinal grooves, transverse ridges, sloughing, smoothness and altered texture more prominent on lower third of esophagus (Fig. 1c). The stomach region was characterized by localized mild inflammation with congestion(edema), erythema and friability. Cold forceps biopsy was obtained from esophagus (20, 30, 35 and 40 cm) and stomach (gastric body and gastric antrum). Biopsy findings in esophagus was squamous mucosa with parakeratosis, clear cell acanthosis (thickening of the epithelium and increased glycogen content) and basophilic granular inclusions (Fig. 2a, b and Fig. 3). Gastric biopsy findings were inactive chronic gastritis with no Helicobacter pylori on immunostaining. Currently she has been recommended to undergo a screening surveillance endoscopy 2 years later with continued present diet and medications with symptomatic management of palmoplantar keratoderma.
Fig. 1.
a Upper Third of Esophagus shows diffuse spontaneous mucosal sclerosis characterized by longitudinal markings, loss of smoothness and altered texture. b Middle Third of Esophagus shows diffuse spontaneous mucosal sclerosis characterized by longitudinal markings, loss of smoothness and altered texture. c Lower Third of Esophagus shows diffuse spontaneous mucosal sclerosis characterized by longitudinal markings, loss of smoothness and altered texture. Endoscopic findings of Diffuse Hyperkeratosis along with linear and longitudinal grooves and transverse ridges.
Fig. 2.
a Low power view (100×) of one of the esophagus biopsies, with the basal layer at the bottom and the surface at the top. The epithelium is indistinguishable from normal esophagus, and shows no evidence of hyperkeratosis or dysplasia. There is mild acanthosis (thickening of the epithelium) relative to normal esophagus. b High power view (400×) showing the dark basophilic inclusions and patchy cytoplasmic clearing that can be seen in tylosis patients. These features are also seen in unaffected patients, however, and are of unclear significance.
Fig. 3.
Early parakeratosis is seen at the top of the epithelium, as squamous cells thin and flatten and begin to acquire mature keratin. This is a non-specific response to inflammation or irritation but may be a precursor to the hyperkeratotic or leukoplakic lesions seen in tylosis patients.
Discussion
Palmoplantar tylosis with is characterized by thickening of the skin of the hands and feet (focal, non-epidermolytic form of palmoplantar keratoderma) is associated with a very high lifetime risk of developing squamous cell carcinoma of the esophagus (OSCC) (13). The association of tylosis palmoplantaris with esophageal cancer is called Howel-Evans syndrome (8). It is generally inherited as an autosomal dominant trait with complete penetrance involving the RHBDF2 gene located on 17q25.1. This gene has been known to cause encoding of an inactive rhomboid protein, IRhom2 [10] leading to EGFR shedding [9] likely responsible for pathogenesis of esophageal cancer.
It is subclassified into Early onset and Late onset depending on age of presentation.
Late-onset tylosis (type A) is associated with a high prevalence of esophageal cancer (40–92% occurrence by 70 years) in three families in the UK, USA, and Germany [1, 2, 4, 5], whereas early-onset tylosis (type B) appears benign [14].
Diagnosis is made by a positive family history, characteristic clinical features like focal palmar and plantar hyperkeratosis, oral and esophageal lesions with mutations in RHBDF2. OSCC showed frequent genomic amplifications of CCND1, SOX2 and TP63, which were different from esophageal adenocarcinomas that showed amplifications in ERBB2, VEGFA, GATA4 and GATA6 [16]. Cao et al. [17] reported that mutations identified by whole-exome sequencing and array based comparative genomic hybridization in multiple regions from two OSCC patients showed mutational heterogeneity rate of 90% in all regions.
From observing data from the families in Liverpool, individuals are diagnosed of OSCC in the third-fourth decade of life with lesions usually found in the middle/distal third of the esophagus with age proportional to increased severity. Once detected in the family members genetic counselling report is advised. Surveillance endoscopy is recommended in family members which include annual esophagogastroscopy with biopsy of suspicious lesion with quadratic biopsies from upper, middle and lower esophagus [7, 11].
The data regarding endoscopic appearance of the mucosa in patients with Tylosis is limited. Endoscopic imaging performed on members of families with Tylosis have been documented to be advanced lesions which on biopsy revealed various degree of Dysplasia/Early malignancy. The members in the family from Liverpool were followed by screening endoscopy using White Light Imaging as well as Narrow Band Imaging to enhance the sensitivity. Although their findings did not report a significant advantage of using NBI, they categorized the patients into mild, moderate or severe endoscopic changes [12]. Endoscopic finding of Diffuse Hyperkeratosis along with linear and longitudinal grooves and transverse ridges, similar to the one present in our patient were documented in that family to be suggestive of “moderate” disease [12]. However, a correlation between endoscopic findings and disease progression has not been established, largely due to the fact that the study had a small sample size of five. Currently, the patients with a positive family history are followed up with annual screening EGD's and no interventions are available up until the first appearance of dysplastic changes.
We believe that further prospective documentation of endoscopic findings of similar mucosal changes and disease process to establish a better screening protocol for patients with tylosis is needed. This will help initiate early interventions and improve the long-term outcomes in patients with a positive family history.
Early supplemental interventions at this stage with carotenoids (beta-carotene, alpha-carotene, lycopene, beta-cryptoxanthin, lutein, and zeaxanthin) might prevent further worsening of the condition and on long term may revert to normal [15].
Statement of Ethics
The authors (or) their guardians and parents have given informed consent to publish this case. The research was conducted ethically in accordance with World Medical association declaration of Helsinki and there was no harm to the human subject(s) involved.
Disclosure Statement
The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Acknowledgements
The authors would like to thank Jain V, Monique Lubaton and Diana Molavi for helping us with the publication.
References
- 1.Howel-Evans W, McCONNELL RB, Clarke CA, Sheppard PM. Carcinoma of the oesophagus with keratosis palmaris et plantaris (tylosis): a study of two families. Q J Med. 1958 Jul;27((107)):413–29. [PubMed] [Google Scholar]
- 2.Ellis A, Field JK, Field EA, Friedmann PS, Fryer A, Howard P, et al. Tylosis associated with carcinoma of the oesophagus and oral leukoplakia in a large Liverpool family—a review of six generations. Eur J Cancer B Oral Oncol. 1994;30B((2)):102–12. doi: 10.1016/0964-1955(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 3.Harper PS, Harper RM, Howel-Evans AW. Carcinoma of the oesophagus with tylosis. Q J Med. 1970 Jul;39((155)):317–33. [PubMed] [Google Scholar]
- 4.Hennies HC, Hagedorn M, Reis A. Palmoplantar keratoderma in association with carcinoma of the esophagus maps to chromosome 17q distal to the keratin gene cluster. Genomics. 1995 Sep;29((2)):537–40. doi: 10.1006/geno.1995.9971. [DOI] [PubMed] [Google Scholar]
- 5.Stevens HP, Kelsell DP, Bryant SP, Bishop DT, Spurr NK, Weissenbach J, et al. Linkage of an American pedigree with palmoplantar keratoderma and malignancy (palmoplantar ectodermal dysplasia type III) to 17q24. Literature survey and proposed updated classification of the keratodermas. Arch Dermatol. 1996 Jun;132((6)):640–51. [PubMed] [Google Scholar]
- 6.Saarinen S, Vahteristo P, Lehtonen R, Aittomäki K, Launonen V, Kiviluoto T, et al. Analysis of a Finnish family confirms RHBDF2 mutations as the underlying factor in tylosis with esophageal cancer. Fam Cancer. 2012 Sep;11((3)):525–8. doi: 10.1007/s10689-012-9532-8. [DOI] [PubMed] [Google Scholar]
- 7.Varela AB, Blanco Rodríguez MM, Boullosa PE, Silva JG. Tylosis A with squamous cell carcinoma of the oesophagus in a Spanish family. Eur J Gastroenterol Hepatol. 2011 Mar;23((3)):286–8. doi: 10.1097/MEG.0b013e328344042d. [DOI] [PubMed] [Google Scholar]
- 8.de Souza CA, Santos AC, Santos LC, Carneiro AL. [Hereditary tylosis syndrome and esophagus cancer] An Bras Dermatol. 2009 Sep-Oct;84((5)):527–9. doi: 10.1590/s0365-05962009000500014. [DOI] [PubMed] [Google Scholar]
- 9.Brooke MA, Etheridge SL, Kaplan N, Simpson C, O'Toole EA, Ishida-Yamamoto A, et al. iRHOM2-dependent regulation of ADAM17 in cutaneous disease and epidermal barrier function. Hum Mol Genet. 2014 Aug;23((15)):4064–76. doi: 10.1093/hmg/ddu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaydon DC, Etheridge SL, Risk JM, Hennies HC, Gay LJ, Carroll R, et al. RHBDF2 mutations are associated with tylosis, a familial esophageal cancer syndrome. Am J Hum Genet. 2012 Feb;90((2)):340–6. doi: 10.1016/j.ajhg.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis A, Risk JM, Maruthappu T, Kelsell DP. Tylosis with oesophageal cancer: Diagnosis, management and molecular mechanisms. Orphanet J Rare Dis. 2015 Sep;10((1)):126. doi: 10.1186/s13023-015-0346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smart H, Kia R, Subramanian S, Khalid S, Campbell F, Ellis A. Defining the endoscopic appearances of tylosis using conventional and narrow-band imaging: a case series. Endoscopy. 2011 Aug;43((8)):727–30. doi: 10.1055/s-0030-1256338. [DOI] [PubMed] [Google Scholar]
- 13.Shahabi M, Noori Daloii MR, Langan JE, Rowbottom L, Jahanzad E, Khoshbin E, et al. An investigation of the tylosis with oesophageal cancer (TOC) locus in Iranian patients with oesophageal squamous cell carcinoma. Int J Oncol. 2004 Aug;25((2)):389–95. doi: 10.3892/ijo.25.2.389. [DOI] [PubMed] [Google Scholar]
- 14.Maillefer RH, Greydanus MP. To B or not to B: is tylosis B truly benign? Two North American genealogies. Am J Gastroenterol. 1999 Mar;94((3)):829–34. doi: 10.1111/j.1572-0241.1999.00954.x. [DOI] [PubMed] [Google Scholar]
- 15.Ge XX, Xing MY, Yu LF, Shen P. Carotenoid intake and esophageal cancer risk: a meta-analysis. Asian Pac J Cancer Prev. 2013;14((3)):1911–8. doi: 10.7314/apjcp.2013.14.3.1911. [DOI] [PubMed] [Google Scholar]
- 16.Cancer Genome Atlas Research Network, Analysis Working Group Integrated genomic characterization of oesophageal carcinoma. Asan University, BC Cancer Agency, et al. 2017;541:169–175. doi: 10.1038/nature20805. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao W, Wu W, Yan M, Tian F, Ma C, Zhang Q, et al. Multiple region whole-exome sequencing reveals dramatically evolving intratumor genomic heterogeneity in esophageal squamous cell carcinoma. Oncogenesis. 2015 Nov;4((11)):e175. doi: 10.1038/oncsis.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]