Abstract
Articular cartilage is an important load-bearing tissue distributed on the surface of diarthrodial joints. Due to its avascular, aneural and non-lymphatic features, cartilage has limited self-regenerative properties. To date, the utilization of biomaterials to aid in cartilage regeneration, especially through the use of injectable scaffolds, has attracted considerable attention. Various materials, therapeutics and fabrication approaches have emerged with a focus on manipulating the cartilage microenvironment to induce the formation of cartilaginous structures that have similar properties to the native tissues. In particular, the design and fabrication of injectable hydrogel-based scaffolds have advanced in recent years with the aim of enhancing its therapeutic efficacy and improving its ease of administration. This review summarizes recent progress in these efforts, including the structural improvement of scaffolds, network cross-linking techniques and strategies for controlled release, which present new opportunities for the development of injectable scaffolds for cartilage regeneration.
Keywords: drug delivery, tissue engineering, injectable hydrogel, cartilage regeneration
Introduction
Articular cartilage is a highly organized tissue which has remarkable load-bearing and low friction properties that allow for smooth movement of diarthrodial joints [1, 2]. The cartilage component contains sparsely distributed chondrocytes which are embedded within the extracellular matrix (ECM), mainly comprised water, type II collagen and glycosaminoglycans that provide the tissue with sufficient mechanical properties for several biofunctions, such as load-bearing and low friction capabilities [3, 4]. Due to the avascular, aneural and non-lymphatic characteristics of cartilage, cartilage has limitations in its self-regeneration and intrinsic repair [3]. Thus, cartilage regeneration still remains a challenge in tissue engineering [1].
Currently, strategies of repairing cartilage defects include debridement and lavage, microfracture, as well as autografts (cell and tissue transplantation) [5–7]. Although these therapies have exhibited some efficacy in the repair of cartilage defects, there are still certain limitations, such as poor integration with healthy cartilage, lack of nutrients, and the formation of fibrous tissue instead of hyaline cartilage that has a consistent morphology and function in clinical applications [8]. Typically, the inadequately regenerated cartilage does not have normal mechanical properties and zonal organization, which could most likely result in further degeneration [9]. The limitations of current therapies for cartilage regeneration have hence led to cartilage tissue engineering, which aims to combine engineering with biological principles to induce the regeneration of cartilage and to treat osteoarthritis [10–12].
To further expand the utilization of biomaterials in cartilage regeneration, injectable hydrogel-based scaffolds have attracted considerable attention these years in cartilage tissue engineering [13, 14]. Hydrogels are notably swollen and porous with 3D polymeric networks, where various solutes and nutrients can be located and able to diffuse [15–18]. Furthermore, as illustrated in Fig. 1, injectable scaffolds can be delivered in a non-invasive or minimally invasive manner via either direct injection or arthroscopy. Injectable hydrogels can not only provide a biocompatible, biodegradable and highly hydrated 3D structure analogous to cartilaginous ECM and improve the supply of nutrients and cellular metabolites via elastic properties [19–22], but also encapsulate cells and deliver bioactive molecules efficiently and effectively through stimuli-responsive release mechanisms to targeted sites for cartilage regeneration [13, 23–26].
Figure 1.
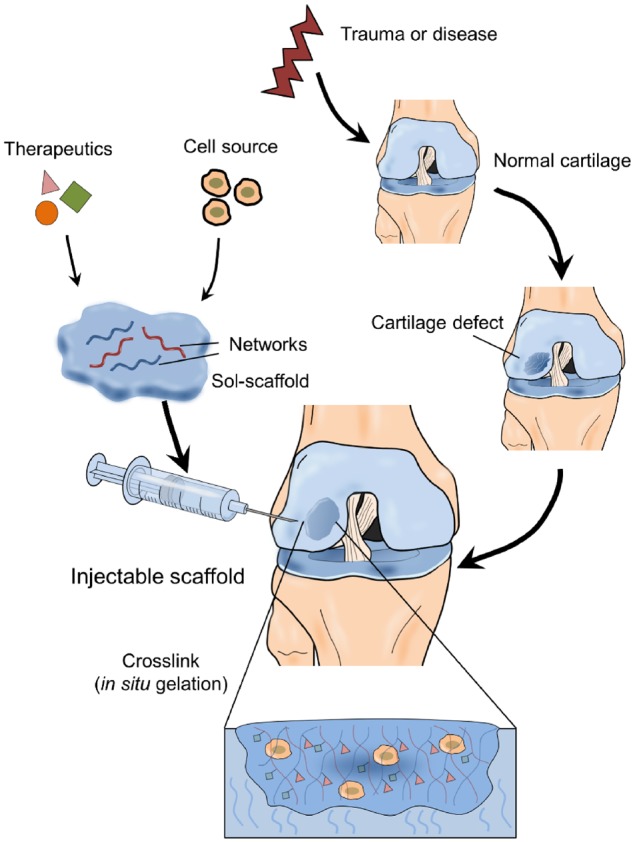
The schematic of the applications of injectable scaffolds for cartilage regeneration
An ideal injectable scaffold for cartilage regeneration should typically meet the following criteria: (i) ease of administration under physiological conditions, (ii) guaranteed injectability (gelation upon injection via either chemical or physical cross-linking), (iii) excellent biocompatibility and potential biodegradability, (iv) the ability to mimic cartilaginous ECM features and promote chondrogenic potential of cells, (v) the ability to easily fill defect sites inside the joint and integrate with the surrounding native cartilage tissue rather than shifting readily and (vi) a sustained release profile if associated with local drug delivery [13, 15, 27–29]. This review aims to provide an overview of the current advances of injectable scaffolds in cartilage regeneration with an emphasis on the components of scaffolds and cell sources.
The structure of injectable scaffolds
Hydrogels possess high water content and elastic properties with cross-linked, multiporous networks [30]. The potential of hydrogels as efficient biomaterials have been reported since the latter half of the 20th century, beginning with the use of nondegradable methacrylate gels to fabricate soft contact lenses [31, 32]. Thereafter, people have investigated hydrogels for various biomedical applications, including drug delivery, wound healing and tissue engineering [33]. Hydrogels can be broadly classified based on the source material (natural or synthetic) and biodegradability (biodegradable or non-biodegradable). Natural hydrophilic macromolecules used for hydrogel scaffold fabrication are often biodegradable and mainly consist of proteins and polysaccharides [34]. Natural polysaccharides used to prepare injectable hydrogels for tissue engineering include chitosan (CS), alginate, agarose and hyaluronic acid (HA) [35]. Protein-based materials, such as collagen, gelatin and fibrin, are also popular for engineering bioactive scaffolds because of their advantages in mimicking the extracellular environment [36–39]. Hydrogels derived from synthetic polymers are more chemically programmable and tunable to systematically determine their cell–matrix interactions [30]. Some examples of synthetic polymers that have been utilized in cartilage regeneration engineering include poly(ethylene glycol) (PEG), poly(vinyl alcohol) (PVA), polydioxanone as well as poly(lactic acid) [23]. For example, PEG has been extensively investigated for biomedical applications because of its good tissue compatibility, nontoxicity and hydrophilicity [30]. Notably, as aforementioned, because biodegradability is an essential characteristic of injectable scaffolds for cartilage tissue engineering, several degradable PEG-based synthetic polymeric systems have also been developed to form hydrogels, including copolymers of PEG with a diverse array of synthetic degradable polymers, such as poly(propylene fumarate), poly(lactic-co-glycolic) acid (PLGA), PVA, polyanhydrides, poly(propylene oxide) and polyphosphazenes [40]. In cartilage tissue engineering, these biomimetic polymers, either natural or synthetic, are designed to mimic crucial aspects of the native extracellular environment by distinctly adjusting mechanical, chemical and biological properties of hydrogels [41].
Multilayer structure of injectable scaffolds
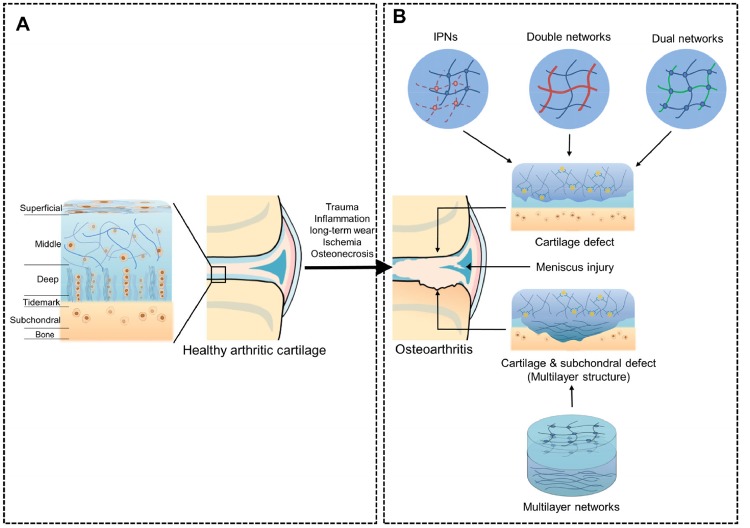
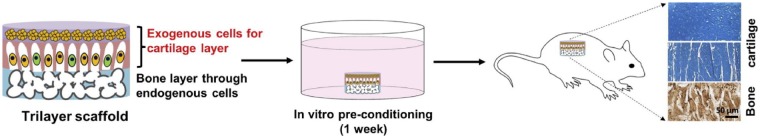
Articular cartilage in joints is divided into the superficial, middle, deep and calcified zones (Fig. 2A). These zones have different cell morphologies, compositions, structural arrangements of the ECM and mechanical properties [42]. Clinically, the most symptomatic cartilage damage is the osteochondral injury with involvement of both the cartilage and subchondral layers. To simulate the complex zonal architecture of cartilage and the zones likely damaged by osteochondral defects in the joint, as shown in Fig. 2B, multilayer injectable hydrogels attract particular interest for cartilage tissue engineering. In the early stages of work toward this goal, a bilayer hydrogel system was developed, which constituted a simple form of a multilayer matrix [43]. Cui et al. [44] and Sun et al. [45] investigated bilayer hydrogels by both 3D printing and projection of stereolithography. Nguyen et al. [46] designed a PEG-based tri-layer hydrogel with the first layer comprising chondroitin sulfate (CHS) and matrix metalloproteinase-sensitive peptides, the second layer comprising CHS and PEG, and the third layer comprising PEG and HA. It was demonstrated that this complex construct could encapsulate a single line of stem cells in all layers and could increase the production of type X collagen and proteoglycans. Kang et al. [47] fabricated a single-unit tri-layer scaffold to engineer osteochondral tissues in vivo, including a biomineralized bottom layer mimicking a calcium phosphate (CaP)-rich bone microenvironment, a middle hydrogel layer with anisotropic pore structure and a top hydrogel layer (Fig. 3). These tri-layer scaffolds contributed to the regeneration of osteochondral tissue with a lubricin-rich cartilage surface, which was similar to the native tissue. Furthermore, this scaffold significantly enhanced the sustained differentiation of transplanted cells to form neocartilage tissue and the recruitment of the surrounding endogenous cells to form bone tissues through the bottom layer. In addition to the osteochondral tissue repair, theoretically, it is also ineffective for monolayer scaffolds to be used for cartilage regeneration due to the complex hierarchical structure of cartilage. It remains to be seen how advances in the design of hydrogels will impact their ability to mimic the structure, properties, and arrangement of cells and collagen fibers of the native ECM.
Figure 2.
(A) The schematic of the anatomy, cell morphology and zonal features of articular cartilage, and its progression to different types of osteoarthritis. (B) The schematic of different structure scaffold networks utilized in the cartilage regeneration engineering
Figure 3.
The implantation of the cell-laden trilayer scaffold resulted in the formation of osteochondral tissue with a lubricin-rich cartilage surface. This figure was adapted with permission from Kang et al. [47]
Interpenetrating polymer network of scaffolds
Mechanical integrity is a crucial design criterion for hydrogels in cartilage regeneration. However, networks of traditional hydrogels are generally based on a single polymer, resulting in reduced mechanical properties, which are inferior to those of natural cartilage [10]. To enhance the mechanical properties of hydrogels to better mimic natural hyaline cartilage, as shown in Fig. 2B, the research focus is transitioning from conventional hydrogels that consist of a single polymer to the hydrogel systems integrated with two or more independent networks with superior functions [48, 49].
Interpenetrating polymer network (IPN) hydrogels were developed aiming to enhance its mechanical properties [50]. IPN, a type of unique mixture of polymers, is composed of two or more cross-linked networks, which are partially intertwined with each other rather than covalently linked [51]. According to recent reports, hydrogels with IPNs tend to exhibit superior mechanical properties compared with those formed by a single type of polymer. Snyder et al. [52] demonstrated that an IPN of cross-linked HA improved the mechanical strength of hydrogel constructs and increased the expression of the chondrogenic transcription factor Sox9 by the loaded human mesenchymal stem cells (MSCs). Gan et al. [50] investigated the incorporation of a primary network consisting of dextran and gelatin with a PEG-based secondary network. This IPN hydrogel showed high toughness and increased proliferation, clustering and matrix deposition of encapsulated nucleus pulposus cells when the quantity of the primary network was 4-fold greater than the secondary one. Chen et al. [51] investigated the combination of a sodium hyaluronate/sodium alginate (HA/SA)-based scaffold with berberine and found that this system could stimulate the regeneration of cartilage as well as subchondral bone. Furthermore, Guo et al. [53] investigated a tri-component IPN hydrogel (consisting of collagen, methacrylate-modified CHS and HA), which can better mimic the natural materials in articular cartilage.
Furthermore, double IPN networks and dual IPN networks have been investigated with the aim of developing injectable scaffolds. A double network consists of two polymers combined with different mechanical properties (rigid versus ductile), which leads to a hydrogel matrix with greater toughness than the corresponding single polymer network alone [54]. Therefore, double networks have attracted much interest in cartilage tissue engineering. A study comparing double-network hydrogels and traditional single-network hydrogels of either poly(2‐acrylamido‐2‐methylpropanesulfonic acid) (PAMPS) or poly(N,N′‐dimethyl acrylamide) (PDMAAm) demonstrated that the double-network hydrogel structure showed superior cartilage regeneration by histological and biochemical scoring [55]. Stagnaro et al. [56] built a porous scaffold based on alginate–polymethacrylate hybrid hydrogels and demonstrated that this matrix mimicked the microenvironment of the ECM in cartilage tissue by overcoming mechanical limitations. Levett et al. [57] generated a double-network hydrogel formed by gelatin and HA. Due to the high reactivity of methacrylate groups, this hydrogel matrix system exhibited advantages in terms of the compressive modulus and chondrification by encapsulating human chondrocytes [57]. In contrast to the double networks, the dual networks consist of two materials with similar cross-linking mechanisms. However, these two similar components have different and mutually beneficial properties. For example, a dual-network hydrogel consisting of HA with a high molecular weight (>1600 kDa) with PVA with a low molecular weight (27 kDa) was constructed by Pirinen et al. [58], which were further chemically cross-linked by the reaction between aldehydes and primary amines. The swelling properties can be tuned by varying the size of the PVA component. Enhanced cell viability of encapsulated bovine knee chondrocytes was observed with the addition of HA [58].
Nanocomposites integrated in scaffolds
In bone or cartilage tissue engineering, the load-bearing property of the material is a crucial feature. The stiffness of hydrogel scaffolds is 2 orders of magnitude lower than natural cartilage [59]. The high water content of hydrogels and their limited stiffness are the two main drawbacks of the progression of cartilage regeneration in vitro and in vivo [60]. Researchers have reported that these hindrances could be alleviated if nanomaterials were added into hydrogel scaffolds [61]. Thus, nanocomposite hydrogel systems have attracted increasing attention in recent years.
Hybrid hydrogels integrated with nanoscale composites are defined as hydrated polymeric networks which are either physically or chemically cross-linked with nanoparticles (NPs) or other nanostructures [62]. Nanoparticles can act as fillers to improve the mechanical properties of hydrogel scaffolds [63]. Different types of NPs, including carbon-based nanomaterials (such as carbon nanotubes, graphene and nanodiamonds), inorganic/ceramic NPs (such as hydroxyapatite, silica, silicates and calcium phosphate), polymeric NPs and metal/metal oxide NPs (such as gold, silver and iron oxide), can be incorporated in the polymeric network to form nanocomposite hydrogels [62]. Nanoscale composites with large surface area-to-volume ratios can not only improve the surface reactivity but also provide enhanced mechanical properties. In addition, because they can easily penetrate into the focal tissue via narrow or small capillaries or the epithelial lining, the efficacy of loaded therapeutic agents and bioactive agents can be enhanced [64–66].
Several nanomaterials have been developed as injectable scaffolds to mimic the ECM of cartilage. For example, Zhang et al. [67] synthesized a hybrid hydrogel (MagGel), composed of type II collagen, HA, PEG and magnetic NPs for cartilage regeneration. The MagGel showed similar microstructure and chemistry as hyaline cartilage and was cytocompatible with bone marrow mesenchymal stem cells (BMSCs) in vitro. Interestingly, MagGel could be used to direct the scaffold remotely to the cartilage defect site using an external magnet [67]. Radhakrishnan et al. [68] investigated a semi-interpenetrating network hydrogel scaffold formed by CHS NPs and nanohydroxyapatite used in chondral and subchondral hydrogel layers, respectively. The regeneration of subchondral bone and cartilage tissue was enhanced by this hybrid hydrogel [68]. Boyer et al. [69] developed a hybrid interpenetrating network mixed with laponites, known as a nano-reinforcing clay, and silated hydroxylpropylmethyl cellulose, which increased the hydrogel mechanical properties without compromising its oxygen diffusion capability, cytocompatibility, the self-organization of chondrogenic cells and generation of ECM components. Collectively, the functions of the added nanocomposite are as follows: (i) mechanical reinforcement, (ii) biological activity and biomimetic function, (iii) integration of cartilage with bone tissue and (iv) transport of drugs and growth factors [70, 71].
Formation of scaffolds
To guarantee the injectability of scaffolds, the scaffolds are typically in a solution state before administration and proceed to an in situ gelation after administration. Furthermore, while developing an ideal injectable hydrogel scaffold that can undergo in situ gelation for better usage in cartilage tissue engineering, it should also ideally meet the following criteria: (i) solubility in aqueous media with gelation occurring under physiological changes (such as temperature, pH and ionic concentration), (ii) no release of harmful byproducts during gelation and (iii) a suitable rate of gelation for practical use [30]. The process of hydrophilic polymers undergoing in situ gelation in response to various stimuli has been developed by either tuning the components of polymers or specifying the sensitive units in polymer chains. As shown in Table 1, the stimulation of gelation applied for cartilage tissue engineering includes functions of chemical agents, physiological stimuli and light.
Table 1.
Advances in formation of injectable scaffolds for cartilage regeneration
| Formation of hydrogels | Major materials | |
|---|---|---|
| Physically cross-linked hydrogels | Thermosensitive | Pluronics [72] |
| P(NIPAAm) [74] | ||
| PLGA-PEG-PLGA [76] | ||
| CS/GP [88–91] | ||
| Thermosensitive | Gelatin-Pluronic copolymer [79] | |
| CS/hydroxyethyl cellulose [81] | ||
| CS/HA [83] | ||
| pH-responsive | CHS–PEG [94] | |
| Poly(methacrylic acid) [95] | ||
| Ion-responsive | Hyaluronate-g-alginate [96] | |
| Chemically cross-linked hydrogels | Schiff base reaction | CS/aldehyde HA [99] |
| Glycol CS/poly(EO-co-Gly)-CHO [100] | ||
| Click Chemistry | Azadibenzocyclooctyne-modified and azide-modified Dextran [104] | |
| Michael addition reaction | Amino derivative of HA/divinylsulfone [110] | |
| Enzyme-catalyzed cross-linking | Heparin-tyramine/dextran-tyramine/HRP [115] | |
| Photo-cross-linking | Poly(ethylene glycol)dimethacrylate/UV [117] | |
| Sericin methacryloyl/UV [118] | ||
| Methacrylated glycol CS and HA/Visible light [116] |
Physically cross-linked hydrogels
Thermosensitive hydrogels are one of the most extensively studied injectable hydrogel systems for tissue engineering. The sol–gel transition occurs either above or below a critical temperature, termed as lower critical solution temperature or upper critical solution temperature. The most commonly used thermogels in cartilage tissue engineering include Pluronics [72], p(NIPAAm) [73, 74], poly(N-vinylcaprolactam) [75] and PLGA–PEG–PLGA [76–78]. For instance, Li et al. [76] loaded kartogenin (KGN) into PLGA–PEG–PLGA thermogel as an injectable scaffold for BMSCs, which exhibited good mechanical properties and effective cartilage regeneration in vivo. Moreover, these polymers are often mixed or conjugated with other natural polymers, such as gelatin [79, 80], cellulose [81] and HA [82, 83] to improve the biocompatibility and mechanical properties of the thermogels. [75, 84]. For example, Lynch et al. reported poly(N-vinylcaprolactam)-graft-HA with a lower critical solution temperature of around 33°C. This injectable thermosensitive hydrogel improved cell compatibility and promoted the generation of ECM proteins even under hypoxic conditions [75]. CS has attracted great attention as an injectable hydrogel scaffold for cartilage repair because of its structural similarity to glycosaminoglycan [81, 85, 86]. Formation of CS-based thermosensitive hydrogels can be achieved by mixing with β-glycerophosphate (GP), which can increase the pH of the CS solution to a range of 7.0–7.4 and allow for gel formation at body temperature [87]. The CS/GP thermogel has been widely applied to cartilage regeneration [88–91]. The mixture of CS with other polymers has also been investigated [92, 93]. For example, Qi et al. [92] designed a CS/PVA-based thermoresponsive hydrogel combined with rabbit BMSCs transfected with hTGFβ-1 to repair rabbit articular cartilage defects. The non-degradable PVA can postpone the degradation of the CS/PVA gel, thereby prolonging the self-repair duration. pH-responsive injectable scaffolds have also been investigated for cartilage tissue engineering. The formation of pH-responsive hydrogels mostly engages dissociation and association with H+ in response to the changes of environmental pH. This type of hydrogel is studied extensively for biomedical applications because the pH profiles at pathological tissues (such as inflammation, infection and cancer) differentiate from that of normal tissues. Strehin et al. [94] developed pH-responsive CHS–PEG adhesive hydrogels with potential applications in regenerative medicine including cartilage repair. The stiffness, swelling properties and gelation kinetics of the hydrogel can be tuned by adjusting the initial pH values of the precursor solutions. Halacheva et al. [95] reported a poly(methyl methacrylate-co-methacrylic acid)-based pH-sensitive hydrogel with high porosity, elasticity and ductility. The enhanced mechanical properties of the injectable hydrogel make it a good candidate for regenerative medicine.
Ion-sensitive injectable hydrogels have also been developed for cartilage regeneration. The hyaluronate-g-alginate solution can easily form a hydrogel by adding Ca2+ [96]. This ionically cross-linkable hydrogel provided an appropriate scaffold to transplant chondrocytes, resulting in efficient chondrogenic differentiation in cartilage regeneration.
Chemically cross-linked hydrogels
Chemically cross-linked hydrogels have been extensively utilized for tissue engineering [30]. Versatile chemistry enables the integration of functional groups into the polymers, allowing for in situ cross-linking. A variety of chemical reactions have been investigated to generate injectable hydrogels for cartilage regeneration, including Schiff base reaction, click chemistry, Michael addition, enzyme-catalyzed cross-linking and photo-cross-linking.
Gel formation by Schiff base reaction
Injectable hydrogels formed by Schiff base reaction between amine and carbonyl groups have been widely utilized for cartilage regeneration applications, owing to the high reaction rate, mild reaction conditions as well as good biocompatibility [97, 98]. CS, carrying abundant amino groups, is an excellent polymer candidate for synthesizing injectable hydrogels through Schiff base cross-linking. For example, Tan et al. [99] reported an injectable CS–HA hydrogel via Schiff base reaction for potential cartilage tissue engineering. Gelation time, degradation profile and mechanical properties can be controlled by adjusting S-CS/A-HA ratios. Cao et al. [100] designed a chemically cross-linked hydrogel via Schiff base reaction using glycol CS and aldehyde-functionalized PEG (poly(EO-co-Gly)-CHO) with a flexible capability to tune the properties of hydrogels.
Gel formation by click chemistry
Click chemistry involves a wide range of reactions, such as azide–alkyne cyclo-addition reactions, thiol–ene couplings, Diels–Alder reactions and tetrazine–norbornene chemistry [101]. These reactions have been widely used to fabricate injectable hydrogels, owing to their rapid reaction kinetics and low reactivity with cellular components [102, 103]. Wang et al. [104] reported dextran-based hydrogels formed by metal-free biorthogonal click chemistry. They used non-toxic metal-free azide–alkyne addition to form injectable hydrogels, which made it applicable for in vivo use. Yu et al. [105] prepared an in situ formed HA/PEG hydrogel via a two-step cross-linking method. The first step was the enzymatic cross-linking between tyramine groups of furylamine-grafted HA and the second step was the Diels–Alder click chemistry between unreacted furan-modified HA and dimaleimide PEG. The two-step cross-linking showed improved mechanical properties of the hydrogel.
Gel formation by Michael addition reaction
The Michael addition reaction has been commonly used to prepare injectable hydrogels, ascribed to its mild reaction condition and controllable reaction time [106–108]. Jin et al. [109] reported on an injectable hydrogel based on thiolated HA and PEG vinylsulfone via Michael addition. The gelation and degradation time can be adjusted by varying polymer concentrations and molecular weights of polymers. Fiorica et al. [110] prepared two kinds of HA-based injectable hydrogels by Michael addition, using the amino derivative of HA (HA-EDA), α-elastin-grafted HA-EDA and α,β-poly(N-2-hydroxyethyl)-dl-aspartamide derivatized with divinylsulfone. The controllable swelling and degradation kinetics and its ability to integrate articular chondrocytes of the hydrogel suggested that this scaffold processed desirable properties for cartilage regeneration.
Gel formation by enzyme-catalyzed cross-linking
The enzyme-catalyzed chemical cross-linking method has attracted increasing attention for hydrogelation, due to its fast gelation rate, high site specificity, ability to work at normal physiological conditions and low cytotoxicity [111]. There have been many attempts to produce enzymatically cross-linked hydrogels for cartilage tissue engineering, including transglutaminase, tyrosinase, phosphopantetheinyl transferase, lysyl oxidase, plasma amine oxidase and horseradish peroxidase (HRP) [112–114]. Particularly, Teixeira et al. showed that the HRP-mediated cross-linking systems can covalently bind the phenol-conjugated polymers (heparin-tyramine and dextran-tyramine conjugates) to the ECM proteins of the surrounding tissues, which is beneficial in maintaining the structural integrity for arthroscopic cartilage repair [115].
Gel formation by photo-cross-linking
Photo-cross-linking involves multiple steps, including initiation, propagation and termination, under light illumination. This method requires the introduction of free radical groups, such as vinyl and methacrylate residues together with photo-initiators. In recent years, the photo-cross-linking method has been widely applied to synthesize injectable hydrogels for cartilage tissue engineering owing to the flexible ability to control the timing and location of hydrogel cross-linking [116]. For example, Papadopoulos et al. [117] reported a swine auricular chondrocyte encapsulated poly(ethylene glycol)dimethacrylate copolymer-based hydrogel by photo-cross-linking for cartilage repair. Neocartilage resembled both composition of ECM and cellular population of the native cartilage, indicating the promise for cartilage regeneration. Qi et al. [118] designed a sericin methacryloyl (SerMA)-based UV cross-linking hydrogel, which was adhesive to chondrocytes and promoted the proliferation of attached chondrocytes even in a nutrition-deficient condition. In vivo implantation of chondrocyte-loaded SerMA hydrogels adequately formed artificial cartilages. Although UV-mediated cross-linking is characterized by low cytotoxicity, UV radiation may still have a negative influence on cells, proteins and tissues. Hence, considerable attempts in visible-light-initiated polymerization for cartilage repair have been investigated. For instance, Park et al. [116] reported a visible light-induced photo-cross-linking of methacrylated glycol CS and HA hydrogels. Choi et al. [119] also reported the incorporation of cartilaginous ECM components into an injectable CS hydrogel designed to undergo gelation upon exposure to visible light.
Incorporating cells into injectable scaffolds
Hydrogels are versatile and their various properties, such as high water content, biodegradability, porosity and biocompatibility, allow them to be used often for cell therapy [16, 120]. In cartilage tissue engineering, properly engineered hydrogel scaffolds are able to control cell proliferation and differentiation. Using advanced techniques, cell encapsulated hydrogels can also be fabricated with personalized geometries and compositions [30, 121, 122]. Over the last decade, various types of cell-loaded injectable hydrogel systems have been investigated for cartilage regeneration [30, 122]. Incorporation of cells into hydrogels can be realized by either seeding cells into the prefabricated porous scaffolds or encapsulating cells during scaffold formation. However, the cell lines that can be used for injectable scaffolds in cartilage regeneration are limited. Table 2 lists the examples of cells that have been incorporated in the injectable hydrogel scaffolds for cartilage regeneration [123–133].
Table 2.
Examples of incorporation of cells into injectable scaffolds for cartilage regeneration
| Cell source | Major materials | Advantages | |
|---|---|---|---|
| Chondrocytes (fully differentiated cells) | Chondrocytes | CS | Prolonged cell survival, retained cell morphology and improved chondrogenesis when cultured in vitro [137] |
| Chondrocytes | CS and type II collagen | Improved cellular condensation and chondrogenesis of embedded chondrocytes to promote cartilage regeneration [136] | |
| Chondrocytes | Oligo(lactic acid)-b-PEG- b-oligo(lactic acid) (PEG-LA) | Improved formation of cartilage matrix of aggrecan and collagen type II/VI [138] | |
| Stem cells | ESCs | PEG | Promoted ESC differentiation into chondrogenic cells and formation of neocartilage ECM [141] |
| MCS | Agarose, hyaluronan acid, PEG or alginate | Increased chondrogenic differentiation of the cells for the cartilage reconstruction [50, 144–146] | |
| iPSCs | Polylactic | Prompted cartilage regeneration of an osteochondral defect [150] | |
| PBMCs | Graphene oxide (GO)-polyethylenimine (PEI) | Easily obtained from peripheral blood and have a similar potential of chondrogenic differentiation and cartilage generation compared with MSCs [151] |
Fully differentiated chondrocyte-encapsulated hydrogels
Autologous chondrocyte implantation has been successfully used in clinic to treat cartilage defects [5]. However, it is still challenging to directly fix a chondrocyte graft in a focal cartilage defect site with a complex shape by invasive orthopedic surgeries [123]. Therefore, injectable scaffolds have been proposed to overcome this challenge. Many reports indicate that chondrocytes can proliferate well in hydrogels and express cartilage-related proteins or genes with well-maintained cell morphologies and phenotypes [124, 134–136]. Jin et al. [137] developed an injectable CS-based hydrogel and found that it could support long-term chondrocyte survival and retain cell morphology in vitro. During in vitro culture, chondrogenesis occurred with the formation of cartilage ECM, including type II collagen and aggrecan, which were homogenously distributed throughout the entire hydrogel. Roberts et al. [138] demonstrated that a chondrocyte-laden hydrogel consisting of oligo(lactic acid)-b-PEG-b-oligo(lactic acid) improved the formation of a cartilage matrix consisting of aggrecan and collagen types II/VI. Although chondrocyte-based biomaterial therapy has demonstrated promising in cartilage tissue engineering, two notable limitations should be considered. First, chondrocyte harvesting involves collecting healthy cartilage tissues from non-load-bearing areas and long-term in vitro culturing (∼1 month) [139, 140]. Because of the low quantity of chondrocytes, and because cartilage defects cannot regenerate, the donor area can become necrotic using this approach. Second, autologous chondrocyte therapy is nearly ineffective in elderly patients due to the low bioactivity and proliferation capacity of autologous primary chondrocytes.
Stem cells encapsulated in hydrogels
Biomedical therapies incorporating stem cells and hydrogels for cartilage regeneration commonly include ESCs, MSCs, induced pluripotent stem cells (iPSCs) and predifferentiated MSCs. ESCs, isolated from the tissues of early embryos, show an unlimited self-renewal capacity while maintaining a pluripotent differentiation potential [127]. However, stem cell pluripotency leads to difficult control over differentiation. Hwang et al. [141] have reported that combining these cells with biomimetic hydrogels and growth factors (such as transforming growth factor β1 and bone morphogenetic protein) created a synergistic environment for chondrogenesis. The encapsulated ESCs were able to differentiate into chondrogenic cells and promote the production of neocartilage ECM [141]. MSCs, derived from a variety of tissue sources, including bone marrow, adipose tissue, periodontal ligament, muscle, lung, liver, amnion, thymus, spleen, placenta, umbilical cord blood and corneal stroma, can interact with local biochemical stimuli to generate growth factors for multiple biofunctions for tissue regeneration [142, 143]. MSCs have become the most extensively used stem cells in biomedical applications due to their abundant cell sources, low immunogenicity, no ethical concerns and minimal teratoma risk [142]. Ample studies regarding the encapsulation of MSCs in chondrogenic 3D injectable hydrogels, such as agarose, hyaluronan acid, PEG and alginate, have been reported for the chondrogenic differentiation of cells and for the targeted reconstruction of cartilage [52, 144–147]. Notably, in vitro research has demonstrated that MSC proliferation and differentiation potential decreases with aging and with aging-related diseases [148], likely preventing their clinical applications in elderly individuals.
Recently, iPSCs have attracted significant attention because they exhibit pluripotency that is quite similar to ESCs in terms of multiple differentiation routes, thus resulting in increasingly widespread applications in regenerative medicine, which can be obtained from somatic cells including fibroblasts [129, 149]. Xu et al. [150] have demonstrated that human-derived iPSCs can maintain their pluripotency in a polylactic-based scaffold and are capable of regenerating cartilage in an osteochondral defect within 6 weeks in rabbits. Currently, it is feasible to produce iPSCs by using an integration-free approach with the development of cellular reprogramming techniques, which is safer and more amenable from a regulatory perspective for their future clinical applications [151]. Recently, predifferentiated MSCs, which can be easily obtained from peripheral blood with minimal invasiveness, have been reported to have a similar potential for chondrogenic differentiation and cartilage generation ability compared with MSCs [152]. However, their application potential in injectable scaffolds requires further study.
Controlled-release drug delivery scaffolds
Many therapeutics exhibit limited efficacy due to the rapid clearance of the drugs in joints. Injectable scaffolds, on the other hand, can sustain drug release and extend the drug retention time. Numerous studies have investigated natural and synthetic biomaterials to develop scaffolds with unique properties, such as improved joint articular dwelling time with sustained drug release while ameliorating the biodegradation of delivery systems. Strategies investigated for the release of biologics with biological activity for treating cartilage defects have developed from simple bolus injections into the focal cartilage defect to multifunctional delivery systems.
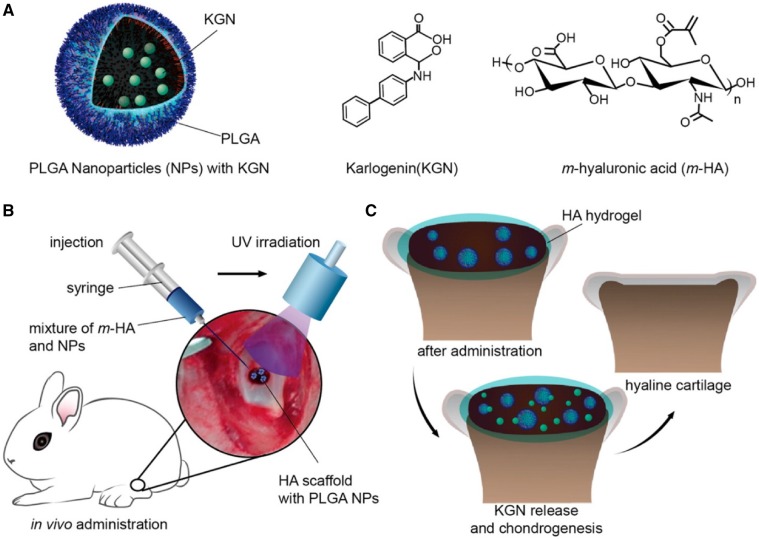
Microparticles (MPs) and NPs are desirable formulations for controlled drug release due to their high surface area to volume ratios, small dimensions, high drug encapsulating efficiencies and the capacity to quickly respond to surrounding environmental stimuli, such as temperature, pH, magnetic fields or ultrasound [153–155]. Recently, there have been many advancements in the application of MP and NP delivery vehicles for cartilage repair. One material that has received attention for the construction of MPs and NPs is the synthetic PLGA polymer, because of its controllable degradation profiles, ease of fabrication and safety in other FDA-approved applications [156]. Spiller et al. [157] have recently designed a hybrid scaffold system composed of PVA and PLGA loaded with insulin-like growth factor 1 (IGF-1). They used a double emulsion technique to form evenly dispersed PLGA MPs (11.3 ± 6.4 μm) containing IGF-1 throughout the PVA hydrogel, resulting in the release of IGF-1 in a linear and sustained manner for at least 45 days. Researchers have also designed NPs to deliver growth factors [158–160]. Recently, the Shi group reported that KGN can be encapsulated into biodegradable PLGA NPs through an emulsion-based formulation method, embedded in photocross-linked acrylated HA injectable hydrogels, and the release rate of KGN associated with the HA matrix integrated with KGN-NPs was nearly linear without an apparent burst release during the 2-month experimental period (Fig. 4) [161]. This injectable scaffold with a sustained release of KGN facilitated the filling of the defects and generation of hyaline cartilage. In another study, it has shown that NPs with a diameter of <10 nm can penetrate bovine cartilage explants, while NPs over 15 nm were limited to the superficial cartilage layer. Of note, a positive fixed charge density promoted the uptake of protein and enhanced protein retention to over 15 days, which was much longer compared with neutrally charged protein [162].
Figure 4.
(A) Schematic of KGN-loaded PLGA NPs, molecule structures of KGN and acrylated HA (m-HA). (B) Schematic of the surgical procedure for cartilage defect repair. (C) Schematic of the hyaline cartilage chondrogenesis with the photo-cross-linked HA scaffold encapsulated with KGN-loaded NPs. This figure was adapted with permission from Shi et al. [161]
Summary and future outlook
To date, injectable scaffolds have provided a promising therapeutic platform for cartilage regeneration. As surveyed above, a number of hydrogel-based scaffolds have been developed with inherent capabilities in cartilaginous tissue engineering, and sufficient mechanical properties for repairing cartilage defects to restore normal joint function. First, to enhance the mechanical properties of scaffolds, traditional single-network hydrogels have been supplemented with either additional networks or mixtures of polymers, and many nanocomposites have been utilized to vary the mechanical properties of scaffolds. These strategies have also been used to produce hydrogels which can improve the integration with surrounding cartilage while promoting chondrogenesis of stem cells encapsulated in hydrogels in vivo. Second, to enhance the efficiency and duration of the delivery of growth factors or other pharmaceuticals, advanced formulations such as MPs and NPs have been investigated in scaffolds for controlled drug delivery. These advances have also garnered interest in presenting biochemical cues in a controllable manner.
Looking ahead, there are still limitations of injectable scaffolds that restrict the complete regeneration of articular cartilage. First, it is essential that the injectable scaffolds can fill the defect area with a smooth interface that is similar to the native cartilage, without integrating into the surrounding healthy tissue. Second, the progressive degradation of hydrogels before they can be replaced by the de novo ECM could compromise their mechanical stability and long-term therapeutic efficacy. One option to overcome this issue is to incorporate appropriate exogenous cells, such as MSCs, within these scaffolds, which could potentially replace the scaffolds as they degrade with newly formed tissue. Third, signaling pathways and particular mechanisms from stem cells to specific cartilaginous cells need further in-depth understanding. It emphasizes more fundamental biological studies of cartilage development and regeneration, which could significantly contribute to the optimization of the injectable scaffolds in the long run. It is also essential to highlight the potential translation of the systems at the beginning of the design. Factors, such as biocompatibility of materials, ease of administration, feasibility of large-scale manufacturing and overall cost should be thoroughly evaluated. Lastly, the next generation of cartilage tissue engineering could be combined with noninvasive/minimally invasive diagnostic technologies to provide real-time assessment of the disease status and overall treatment performance, leading to personalized therapy.
Acknowledgements
This study was supported by the Projects of International Cooperation and Exchanges NSFC (81420108021), Key Program of NSFC (81730067), Excellent Young Scholars NSFC (81622033), Jiangsu Provincial Key Medical Center Foundation, Jiangsu Provincial Medical Outstanding Talent Foundation, Jiangsu Provincial Medical Youth Talent Foundation, Jiangsu Provincial Key Medical Talent Foundation and UCLA’s start-up package to Z.G.
Conflict of interest statement. None declared.
References
- 1. Madeira C, Santhagunam A, Salgueiro JB. et al. Advanced cell therapies for articular cartilage regeneration. Trends Biotechnol 2015;33:35–42. [DOI] [PubMed] [Google Scholar]
- 2. Krishnan Y, Grodzinsky AJ.. Cartilage diseases. Matrix Biol 2018;71:51–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huey DJ, Hu JC, Athanasiou KA.. Unlike bone, cartilage regeneration remains elusive. Science 2012;338:917–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chung C, Burdick JA.. Engineering cartilage tissue. Adv Drug Deliv Rev 2008;60:243–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brittberg M. Autologous chondrocyte implantation–technique and long-term follow-up. Injury 2008;39(Suppl 1):S40–9. [DOI] [PubMed] [Google Scholar]
- 6. Huang BJ, Hu JC, Athanasiou KA.. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 2016;98:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matsiko A, Levingstone T, O’Brien F.. Advanced strategies for articular cartilage defect repair. Materials 2013;6:637–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Temenoff JS, Mikos AG.. Review: tissue engineering for regeneration of articular cartilage. Biomaterials 2000;21:431–40. [DOI] [PubMed] [Google Scholar]
- 9. Natoli RM, Skaalure S, Bijlani S. et al. Intracellular Na(+) and Ca(2+) modulation increases the tensile properties of developing engineered articular cartilage. Arthritis Rheum 2010;62:1097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vega SL, Kwon MY, Burdick JA.. Recent advances in hydrogels for cartilage tissue engineering. Eur Cell Mater 2017;33:59–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kang Y, Yang J, Khan S. et al. A new biodegradable polyester elastomer for cartilage tissue engineering. J Biomed Mater Res 2006;77:331–9. [DOI] [PubMed] [Google Scholar]
- 12. Tran RT, Yang J, Ameer GA.. Citrate-based biomaterials and their applications in regenerative engineering. Annu Rev Mater Res 2015;45:277–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Balakrishnan B, Banerjee R.. Biopolymer-based hydrogels for cartilage tissue engineering. Chem Rev 2011;111:4453–74. [DOI] [PubMed] [Google Scholar]
- 14. Elisseeff J. Injectable cartilage tissue engineering. Exp Opin Biol Ther 2004;4:1849–59. [DOI] [PubMed] [Google Scholar]
- 15. Yu L, Ding J.. Injectable hydrogels as unique biomedical materials. Chem Soc Rev 2008;37:1473–81. [DOI] [PubMed] [Google Scholar]
- 16. Chen Z, Wang J, Sun W. et al. Synthetic beta cells for fusion-mediated dynamic insulin secretion. Nat Chem Biol 2018;14:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peppas NA, Van Blarcom DS.. Hydrogel-based biosensors and sensing devices for drug delivery. J Control Release 2016;240:142–50. [DOI] [PubMed] [Google Scholar]
- 18. Slaughter BV, Khurshid SS, Fisher OZ. et al. Hydrogels in regenerative medicine. Adv Mater 2009;21:3307–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park KM, Lee Y, Son JY. et al. Synthesis and characterizations of in situ cross-linkable gelatin and 4-arm-PPO-PEO hybrid hydrogels via enzymatic reaction for tissue regenerative medicine. Biomacromolecules 2012;13:604–11. [DOI] [PubMed] [Google Scholar]
- 20. Frith JE, Cameron AR, Menzies DJ. et al. An injectable hydrogel incorporating mesenchymal precursor cells and pentosan polysulphate for intervertebral disc regeneration. Biomaterials 2013;34:9430–40. [DOI] [PubMed] [Google Scholar]
- 21. Menzies DJ, Cameron A, Munro T. et al. Tailorable cell culture platforms from enzymatically cross-linked multifunctional poly(ethylene glycol)-based hydrogels. Biomacromolecules 2013;14:413–23. [DOI] [PubMed] [Google Scholar]
- 22. Jin R, Teixeira LS, Dijkstra PJ. et al. Enzymatically-crosslinked injectable hydrogels based on biomimetic dextran-hyaluronic acid conjugates for cartilage tissue engineering. Biomaterials 2010;31:3103–13. [DOI] [PubMed] [Google Scholar]
- 23. Li Y, Rodrigues J, Tomas H.. Injectable and biodegradable hydrogels: gelation, biodegradation and biomedical applications. Chem Soc Rev 2012;41:2193–221. [DOI] [PubMed] [Google Scholar]
- 24. Chen F, Yu S, Liu B. et al. An injectable enzymatically crosslinked carboxymethylated pullulan/chondroitin sulfate hydrogel for cartilage tissue engineering. Sci Rep 2016;6:20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lu Y, Aimetti AA, Langer R. et al. Bioresponsive materials. Nat Rev Mater 2017;2:16075. [Google Scholar]
- 26. Wang X, Sun X, Jiang G. et al. Synthesis of biomimetic hyperbranched zwitterionic polymers as targeting drug delivery carriers. J Appl Polym Sci 2013;128:3289–94. [Google Scholar]
- 27. Yao X, Peng R, Ding J.. Cell-material interactions revealed via material techniques of surface patterning. Adv Mater 2013;25:5257–86. [DOI] [PubMed] [Google Scholar]
- 28. Mujeeb A, Miller AF, Saiani A. et al. Self-assembled octapeptide scaffolds for in vitro chondrocyte culture. Acta Biomater 2013;9:4609–17. [DOI] [PubMed] [Google Scholar]
- 29. Rice JJ, Martino MM, De Laporte L. et al. Engineering the regenerative microenvironment with biomaterials. Adv Healthc Mater 2013;2:57–71. [DOI] [PubMed] [Google Scholar]
- 30. Amini AA, Nair LS.. Injectable hydrogels for bone and cartilage repair. Biomed Mater 2012;7:024105.. [DOI] [PubMed] [Google Scholar]
- 31. Wichterle O, Lim D.. Hydrophilic gels for biological use. Nature 1960;185:117. [Google Scholar]
- 32. Drury JL, Mooney DJ.. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 2003;24:4337–51. [DOI] [PubMed] [Google Scholar]
- 33. Peppas NA, Sahlin JJ.. Hydrogels as mucoadhesive and bioadhesive materials: a review. Biomaterials 1996;17:1553–61. [DOI] [PubMed] [Google Scholar]
- 34. Coviello T, Matricardi P, Marianecci C. et al. Polysaccharide hydrogels for modified release formulations. J Control Release 2007;119:5–24. [DOI] [PubMed] [Google Scholar]
- 35. Burdick JA, Prestwich GD.. Hyaluronic acid hydrogels for biomedical applications. Adv Mater Weinheim 2011;23:H41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stenzel KH, Miyata T, Rubin AL.. Collagen as a biomaterial. Annu Rev Biophys Bioeng 1974;3:231–53. [DOI] [PubMed] [Google Scholar]
- 37. Dreesmann L, Ahlers M, Schlosshauer B.. The pro-angiogenic characteristics of a cross-linked gelatin matrix. Biomaterials 2007;28:5536–43. [DOI] [PubMed] [Google Scholar]
- 38. Liu X, Smith LA, Hu J. et al. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials 2009;30:2252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spotnitz WD, Burks S.. Hemostats, sealants, and adhesives III: a new update as well as cost and regulatory considerations for components of the surgical toolbox. Transfusion 2012;52:2243–55. [DOI] [PubMed] [Google Scholar]
- 40. Lee PY, Cobain E, Huard J. et al. Thermosensitive hydrogel PEG-PLGA-PEG enhances engraftment of muscle-derived stem cells and promotes healing in diabetic wound. Mol Ther 2007;15:1189–94. [DOI] [PubMed] [Google Scholar]
- 41. Bryant SJ, Arthur JA, Anseth KS.. Incorporation of tissue-specific molecules alters chondrocyte metabolism and gene expression in photocrosslinked hydrogels. Acta Biomater 2005;1:243–52. [DOI] [PubMed] [Google Scholar]
- 42. Bhosale AM, Richardson JB.. Articular cartilage: structure, injuries and review of management. Br Med Bull 2008;87:77–95. [DOI] [PubMed] [Google Scholar]
- 43. Li X, Ding J, Wang J. et al. Biomimetic biphasic scaffolds for osteochondral defect repair. Regen Biomater 2015;2:221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cui X, Breitenkamp K, Finn MG. et al. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A 2012;18:1304.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun AX, Lin H, Beck AM. et al. Projection stereolithographic fabrication of human adipose stem cell-incorporated biodegradable scaffolds for cartilage tissue engineering. Front Bioeng Biotechnol 2015;3:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nguyen LH, Kudva AK, Saxena NS. et al. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials 2011;32:6946–52. [DOI] [PubMed] [Google Scholar]
- 47. Kang H, Zeng Y, Varghese S.. Functionally graded multilayer scaffolds for in vivo osteochondral tissue engineering. Acta Biomater 2018;78:365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jia S, Wang J, Zhang T. et al. Multilayered scaffold with a compact interfacial layer enhances osteochondral defect repair. ACS Appl Mater Interfaces 2018;10:20296–305. [DOI] [PubMed] [Google Scholar]
- 49. Zhu Y, Kong L, Farhadi F. et al. An injectable continuous stratified structurally and functionally biomimetic construct for enhancing osteochondral regeneration. Biomaterials 2019;192:149–58. [DOI] [PubMed] [Google Scholar]
- 50. Gan Y, Li P, Wang L. et al. An interpenetrating network-strengthened and toughened hydrogel that supports cell-based nucleus pulposus regeneration. Biomaterials 2017;136:12–28. [DOI] [PubMed] [Google Scholar]
- 51. Chen P, Xia C, Mo J. et al. Interpenetrating polymer network scaffold of sodium hyaluronate and sodium alginate combined with berberine for osteochondral defect regeneration. Mater Sci Eng C Mater Biol Appl 2018;91:190–200. [DOI] [PubMed] [Google Scholar]
- 52. Snyder TN, Madhavan K, Intrator M. et al. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J Biol Eng 2014;8:10.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Guo Y, Yuan T, Xiao Z. et al. Hydrogels of collagen/chondroitin sulfate/hyaluronan interpenetrating polymer network for cartilage tissue engineering. J Mater Sci Mater Med 2012;23:2267–79. [DOI] [PubMed] [Google Scholar]
- 54. Weng L, Gouldstone A, Wu Y. et al. Mechanically strong double network photocrosslinked hydrogels from N,N-dimethylacrylamide and glycidyl methacrylated hyaluronan. Biomaterials 2008;29:2153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ogawa M, Kitamura N, Kurokawa T. et al. Poly(2-acrylamido-2-methylpropanesulfonic acid) gel induces articular cartilage regeneration in vivo: comparisons of the induction ability between single- and double-network gels. J Biomed Mater Res A 2012;100:2244–51. [DOI] [PubMed] [Google Scholar]
- 56. Stagnaro P, Schizzi I, Utzeri R. et al. Alginate-polymethacrylate hybrid hydrogels for potential osteochondral tissue regeneration. Carbohydr Polym 2018;185:56–62. [DOI] [PubMed] [Google Scholar]
- 57. Levett PA, Hutmacher DW, Malda J. et al. Hyaluronic acid enhances the mechanical properties of tissue-engineered cartilage constructs. PLoS One 2014;9:e113216.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pirinen SK, Tiitu V, Suvanto M. et al. Control of swelling properties of polyvinyl alcohol/hyaluronic acid hydrogels for the encapsulation of chondrocyte cells. J Appl Polym Sci 2015;132:42272. [Google Scholar]
- 59. Levental I, Georges PC, Janmey PA. Soft biological materials and their impact on cell function. Soft Matter 2007;3:299–306. [DOI] [PubMed] [Google Scholar]
- 60. Asadi N, Alizadeh E, Salehi R. et al. Nanocomposite hydrogels for cartilage tissue engineering: a review. Artif Cells Nanomed Biotechnol 2018;46:465–71. [DOI] [PubMed] [Google Scholar]
- 61. Biondi M, Borzacchiello A, Mayol L. et al. Nanoparticle-integrated hydrogels as multifunctional composite materials for biomedical applications. Gels 2015;1:162–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gaharwar AK, Peppas NA, Khademhosseini A.. Nanocomposite hydrogels for biomedical applications. Biotechnol Bioeng 2014;111:441–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Song F, Li X, Wang Q. et al. Nanocomposite hydrogels and their applications in drug delivery and tissue engineering. J Biomed Nanotechnol 2015;11:40–52. [DOI] [PubMed] [Google Scholar]
- 64. Asghari F, Samiei M, Adibkia K. et al. Biodegradable and biocompatible polymers for tissue engineering application: a review. Artif Cells Nanomed Biotechnol 2017;45:185–92. [DOI] [PubMed] [Google Scholar]
- 65. Jayaraman P, Gandhimathi C, Venugopal JR. et al. Controlled release of drugs in electrosprayed nanoparticles for bone tissue engineering. Adv Drug Deliv Rev 2015;94:77–95. [DOI] [PubMed] [Google Scholar]
- 66. Eftekhari H, Jahandideh A, Asghari A. et al. Assessment of polycaprolacton (PCL) nanocomposite scaffold compared with hydroxyapatite (HA) on healing of segmental femur bone defect in rabbits. Artif Cells Nanomed Biotechnol 2017;45:961–8. [DOI] [PubMed] [Google Scholar]
- 67. Zhang N, Lock J, Sallee A. et al. Magnetic nanocomposite hydrogel for potential cartilage tissue engineering: synthesis, characterization, and cytocompatibility with bone marrow derived mesenchymal stem cells. ACS Appl Mater Interfaces 2015;7:20987–98. [DOI] [PubMed] [Google Scholar]
- 68. Radhakrishnan J, Manigandan A, Chinnaswamy P. et al. Gradient nano-engineered in situ forming composite hydrogel for osteochondral regeneration. Biomaterials 2018;162:82–98. [DOI] [PubMed] [Google Scholar]
- 69. Boyer C, Figueiredo L, Pace R. et al. Laponite nanoparticle-associated silated hydroxypropylmethyl cellulose as an injectable reinforced interpenetrating network hydrogel for cartilage tissue engineering. Acta Biomater 2018;65:112–22. [DOI] [PubMed] [Google Scholar]
- 70. Jeznach O, Kołbuk D, Sajkiewicz P.. Injectable hydrogels and nanocomposite hydrogels for cartilage regeneration. J Biomed Mater Res A 2018;106:2762–76. [DOI] [PubMed] [Google Scholar]
- 71. Coburn JM, Gibson M, Monagle S. et al. Bioinspired nanofibers support chondrogenesis for articular cartilage repair. Proc Natl Acad Sci USA 2012;109:10012–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yu H, Yang X, Cheng J. et al. Distraction osteogenesis combined with tissue-engineered cartilage in the reconstruction of condylar osteochondral defect. J Oral Maxillofac Surg 2011;69:e558–64. [DOI] [PubMed] [Google Scholar]
- 73. D'Este M, Sprecher CM, Milz S. et al. Evaluation of an injectable thermoresponsive hyaluronan hydrogel in a rabbit osteochondral defect model. J Biomed Mater Res 2016;104:1469–78. [DOI] [PubMed] [Google Scholar]
- 74. Choi SJ, Na K, Kim S. et al. Combination of ascorbate and growth factor (TGF β‐3) in thermo‐reversible hydrogel constructs embedded with rabbit chondrocytes for neocartilage formation. J Biomed Mater Res 2007;83:897–905. [DOI] [PubMed] [Google Scholar]
- 75. Lynch B, Crawford K, Baruti O. et al. The effect of hypoxia on thermosensitive poly (N‐vinylcaprolactam) hydrogels with tunable mechanical integrity for cartilage tissue engineering. J Biomed Mater Res 2017;105:1863–73. [DOI] [PubMed] [Google Scholar]
- 76. Li X, Ding J, Zhang Z. et al. Kartogenin-incorporated thermogel supports stem cells for significant cartilage regeneration. ACS Appl Mater Interfaces 2016;8:5148–59. [DOI] [PubMed] [Google Scholar]
- 77. Yan Q, Xiao LQ, Tan L. et al. Controlled release of simvastatin‐loaded thermo‐sensitive PLGA‐PEG‐PLGA hydrogel for bone tissue regeneration: in vitro and in vivo characteristics. J Biomed Mater Res 2015;103:3580–9. [DOI] [PubMed] [Google Scholar]
- 78. Zhang Y-B, Ding J-X, Xu W-G. et al. Biodegradable thermogel as culture matrix of bone marrow mesenchymal stem cells for potential cartilage tissue engineering. Chin J Polym Sci 2014;32:1590–601. [Google Scholar]
- 79. Kim DH, Heo S-J, Shin J-W. et al. Preparation of thermosensitive gelatin-pluronic copolymer for cartilage tissue engineering. Macromol Res 2010;18:387–91. [Google Scholar]
- 80. Ibusuki S, Fujii Y, Iwamoto Y. et al. Tissue-engineered cartilage using an injectable and in situ gelable thermoresponsive gelatin: fabrication and in vitro performance. Tissue Eng 2003;9:371–84. [DOI] [PubMed] [Google Scholar]
- 81. Hoemann C, Sun J, Legare A. et al. Tissue engineering of cartilage using an injectable and adhesive chitosan-based cell-delivery vehicle. Osteoarthritis Cartilage 2005;13:318–29. [DOI] [PubMed] [Google Scholar]
- 82. Lee H, Park TG.. Photo‐crosslinkable, biomimetic, and thermo‐sensitive pluronic grafted hyaluronic acid copolymers for injectable delivery of chondrocytes. J Biomed Mater Res 2009;88:797–806. [DOI] [PubMed] [Google Scholar]
- 83. Walker KJ, Madihally SV.. Anisotropic temperature sensitive chitosan‐based injectable hydrogels mimicking cartilage matrix. J Biomed Mater Res 2015;103:1149–60. [DOI] [PubMed] [Google Scholar]
- 84. Muramatsu K, Saito Y, Wada T. et al. Poly (N-isopropylacrylamide-co-N-tert-butylacrylamide)-grafted hyaluronan as an injectable and self-assembling scaffold for cartilage tissue engineering. J Biomed Sci Eng 2012;05:639. [Google Scholar]
- 85. Hao T, Wen N, Cao J-K. et al. The support of matrix accumulation and the promotion of sheep articular cartilage defects repair in vivo by chitosan hydrogels. Osteoarthritis Cartilage 2010;18:257–65. [DOI] [PubMed] [Google Scholar]
- 86. Oldenkamp HF, Vela Ramirez JE, Peppas NA.. Re-evaluating the importance of carbohydrates as regenerative biomaterials. Regen Biomater 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhou HY, Jiang LJ, Cao PP. et al. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr Polym 2015;117:524–36. [DOI] [PubMed] [Google Scholar]
- 88. Hoemann CD, Hurtig M, Rossomacha E. et al. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am 2005;87:2671–86. [DOI] [PubMed] [Google Scholar]
- 89. Chevrier A, Hoemann C, Sun J. et al. Chitosan–glycerol phosphate/blood implants increase cell recruitment, transient vascularization and subchondral bone remodeling in drilled cartilage defects. Osteoarthritis Cartilage 2007;15:316–27. [DOI] [PubMed] [Google Scholar]
- 90. Marchand C, Rivard G-E, Sun J. et al. Solidification mechanisms of chitosan–glycerol phosphate/blood implant for articular cartilage repair. Osteoarthritis Cartilage 2009;17:953–60. [DOI] [PubMed] [Google Scholar]
- 91. Sá-Lima H, Caridade SG, Mano JF. et al. Stimuli-responsive chitosan-starch injectable hydrogels combined with encapsulated adipose-derived stromal cells for articular cartilage regeneration. Soft Matter 2010;6:5184–95. [Google Scholar]
- 92. Qi B-W, Yu A-X, Zhu S-B. et al. Chitosan/poly (vinyl alcohol) hydrogel combined with Ad-hTGF-β1 transfected mesenchymal stem cells to repair rabbit articular cartilage defects. Exp Biol Med (Maywood) 2013;238:23–30. [DOI] [PubMed] [Google Scholar]
- 93. Rosalyn MA, Skylab RS.. Preparation of silk based hydrogel and sponges for tissue engineering application in cartilage repair/replacement. Int J Chemtech Res 2014;6:3328–31. [Google Scholar]
- 94. Strehin I, Nahas Z, Arora K. et al. A versatile pH sensitive chondroitin sulfate–PEG tissue adhesive and hydrogel. Biomaterials 2010;31:2788–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Halacheva SS, Freemont TJ, Saunders BR.. pH-responsive physical gels from poly (meth) acrylic acid-containing crosslinked particles: the relationship between structure and mechanical properties. J Mater Chem B 2013;1:4065–78. [DOI] [PubMed] [Google Scholar]
- 96. Park H, Woo EK, Lee KY.. Ionically cross-linkable hyaluronate-based hydrogels for injectable cell delivery. J Control Release 2014;196:146–53. [DOI] [PubMed] [Google Scholar]
- 97. Liu M, Zeng X, Ma C. et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res 2017;5:17014.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chen F, Ni Y, Liu B. et al. Self-crosslinking and injectable hyaluronic acid/RGD-functionalized pectin hydrogel for cartilage tissue engineering. Carbohydr Polym 2017;166:31–44. [DOI] [PubMed] [Google Scholar]
- 99. Tan H, Chu CR, Payne KA. et al. Injectable in situ forming biodegradable chitosan–hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009;30:2499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Cao L, Cao B, Lu C. et al. An injectable hydrogel formed by in situ cross-linking of glycol chitosan and multi-benzaldehyde functionalized PEG analogues for cartilage tissue engineering. J Mater Chem B 2015;3:1268–80. [DOI] [PubMed] [Google Scholar]
- 101. McKay CS, Finn M.. Click chemistry in complex mixtures: bioorthogonal bioconjugation. Chem Biol 2014;21:1075–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hermann CD, Wilson DS, Lawrence KA. et al. Rapidly polymerizing injectable click hydrogel therapy to delay bone growth in a murine re-synostosis model. Biomaterials 2014;35:9698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Anseth KS, Klok H-A. Click chemistry in biomaterials, nanomedicine, and drug delivery. Biomacromolecules, 2016;17:1–3. [DOI] [PubMed] [Google Scholar]
- 104. Wang X, Li Z, Shi T. et al. Injectable dextran hydrogels fabricated by metal-free click chemistry for cartilage tissue engineering. Mater Sci Eng C Mater Biol Appl 2017;73:21–30. [DOI] [PubMed] [Google Scholar]
- 105. Yu F, Cao X, Li Y. et al. An injectable hyaluronic acid/PEG hydrogel for cartilage tissue engineering formed by integrating enzymatic crosslinking and Diels–Alder “click chemistry”. Polym Chem 2014;5:1082–90. [Google Scholar]
- 106. Pritchard CD, O’Shea TM, Siegwart DJ. et al. An injectable thiol-acrylate poly (ethylene glycol) hydrogel for sustained release of methylprednisolone sodium succinate. Biomaterials 2011;32:587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rodell CB, MacArthur JW Jr, Dorsey SM. et al. Shear‐thinning supramolecular hydrogels with secondary autonomous covalent crosslinking to modulate viscoelastic properties in vivo. Adv Funct Mater 2015;25:636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Jukes JM, Van Der Aa LJ, Hiemstra C. et al. A newly developed chemically crosslinked dextran–poly (ethylene glycol) hydrogel for cartilage tissue engineering. Tissue Eng Part A 2010;16:565–73. [DOI] [PubMed] [Google Scholar]
- 109. Jin R, Teixeira LM, Krouwels A. et al. Synthesis and characterization of hyaluronic acid–poly (ethylene glycol) hydrogels via Michael addition: an injectable biomaterial for cartilage repair. Acta Biomater 2010;6:1968–77. [DOI] [PubMed] [Google Scholar]
- 110. Fiorica C, Palumbo FS, Pitarresi G. et al. Injectable in situ forming hydrogels based on natural and synthetic polymers for potential application in cartilage repair. RSC Adv 2015;5:19715–23. [Google Scholar]
- 111. Kobayashi S, Uyama H, Kimura S.. Enzymatic polymerization. Chem Rev 2001;101:3793–818. [DOI] [PubMed] [Google Scholar]
- 112. Bae JW, Choi JH, Lee Y. et al. Horseradish peroxidase-catalysed in situ-forming hydrogels for tissue-engineering applications. J Tissue Eng Regen Med 2015;9:1225–32. [DOI] [PubMed] [Google Scholar]
- 113. Ren K, He C, Xiao C. et al. Injectable glycopolypeptide hydrogels as biomimetic scaffolds for cartilage tissue engineering. Biomaterials 2015;51:238–49. [DOI] [PubMed] [Google Scholar]
- 114. Jin R, Lin C, Cao A.. Enzyme-mediated fast injectable hydrogels based on chitosan–glycolic acid/tyrosine: preparation, characterization, and chondrocyte culture. Polym Chem 2014;5:391–8. [Google Scholar]
- 115. Teixeira LSM, Bijl S, Pully VV. et al. Self-attaching and cell-attracting in-situ forming dextran-tyramine conjugates hydrogels for arthroscopic cartilage repair. Biomaterials 2012;33:3164–74. [DOI] [PubMed] [Google Scholar]
- 116. Park H, Choi B, Hu J. et al. Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomater 2013;9:4779–86. [DOI] [PubMed] [Google Scholar]
- 117. Papadopoulos A, Bichara DA, Zhao X. et al. Injectable and photopolymerizable tissue-engineered auricular cartilage using poly (ethylene glycol) dimethacrylate copolymer hydrogels. Tissue Eng Part A 2011;17:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Qi C, Liu J, Jin Y. et al. Photo-crosslinkable, injectable sericin hydrogel as 3D biomimetic extracellular matrix for minimally invasive repairing cartilage. Biomaterials 2018;163:89–104. [DOI] [PubMed] [Google Scholar]
- 119. Choi B, Kim S, Lin B. et al. Cartilaginous extracellular matrix-modified chitosan hydrogels for cartilage tissue engineering. ACS Appl Mater Interfaces 2014;6:20110–21. [DOI] [PubMed] [Google Scholar]
- 120. Chen Z, Hu Q, Gu Z.. Leveraging engineering of cells for drug delivery. Acc Chem Res 2018;51:668–77. [DOI] [PubMed] [Google Scholar]
- 121. Nuernberger S, Cyran N, Albrecht C. et al. The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials 2011;32:1032–40. [DOI] [PubMed] [Google Scholar]
- 122. Spiller KL, Maher SA, Lowman AM.. Hydrogels for the repair of articular cartilage defects. Tissue Eng Part B Rev 2011;17:281–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sharma B, Fermanian S, Gibson M. et al. Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci Transl Med 2013;5:167ra6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ko CY, Ku KL, Yang SR. et al. In vitro and in vivo co-culture of chondrocytes and bone marrow stem cells in photocrosslinked PCL-PEG-PCL hydrogels enhances cartilage formation. J Tissue Eng Regen Med 2016;10:E485–96. [DOI] [PubMed] [Google Scholar]
- 125. Ko JY, Kim KI, Park S. et al. In vitro chondrogenesis and in vivo repair of osteochondral defect with human induced pluripotent stem cells. Biomaterials 2014;35:3571–81. [DOI] [PubMed] [Google Scholar]
- 126. Chen K, Ng KS, Ravi S. et al. In vitro generation of whole osteochondral constructs using rabbit bone marrow stromal cells, employing a two-chambered co-culture well design. J Tissue Eng Regen Med 2016;10:294–304. [DOI] [PubMed] [Google Scholar]
- 127. Wang J, Rao S, Chu J. et al. A protein interaction network for pluripotency of embryonic stem cells. Nature 2006;444:364–8. [DOI] [PubMed] [Google Scholar]
- 128. Okita K, Ichisaka T, Yamanaka S.. Generation of germline-competent induced pluripotent stem cells. Nature 2007;448:313–7. [DOI] [PubMed] [Google Scholar]
- 129. Yu J, Vodyanik MA, Smuga-Otto K. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007;318:1917–20. [DOI] [PubMed] [Google Scholar]
- 130. Wang W, Li B, Yang J. et al. The restoration of full-thickness cartilage defects with BMSCs and TGF-beta 1 loaded PLGA/fibrin gel constructs. Biomaterials 2010;31:8964–73. [DOI] [PubMed] [Google Scholar]
- 131. Wang W, Li B, Li Y. et al. In vivo restoration of full-thickness cartilage defects by poly (lactide-co-glycolide) sponges filled with fibrin gel, bone marrow mesenchymal stem cells and DNA complexes. Biomaterials 2010;31:5953–65. [DOI] [PubMed] [Google Scholar]
- 132. Bian L, Zhai DY, Mauck RL. et al. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A 2011;17:1137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hu X, Wang Y, Tan Y. et al. A difunctional regeneration scaffold for knee repair based on aptamer‐directed cell recruitment. Adv Mater 2017;29:1605235.. [DOI] [PubMed] [Google Scholar]
- 134. Zeng L, Yao Y, Wang DA. et al. Effect of microcavitary alginate hydrogel with different pore sizes on chondrocyte culture for cartilage tissue engineering. Mater Sci Eng C Mater Biol Appl 2014;34:168–75. [DOI] [PubMed] [Google Scholar]
- 135. Chung JY, Song M, Ha CW. et al. Comparison of articular cartilage repair with different hydrogel-human umbilical cord blood-derived mesenchymal stem cell composites in a rat model. Stem Cell Res Ther 2014;5:39.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Choi B, Kim S, Fan J. et al. Covalently conjugated transforming growth factor-β1 in modular chitosan hydrogels for the effective treatment of articular cartilage defects. Biomater Sci 2015;3:742–52. [DOI] [PubMed] [Google Scholar]
- 137. Jin R, Moreira Teixeira LS, Dijkstra PJ. et al. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials 2009;30:2544–51. [DOI] [PubMed] [Google Scholar]
- 138. Roberts JJ, Nicodemus GD, Greenwald EC. et al. Degradation improves tissue formation in (un)loaded chondrocyte-laden hydrogels. Clin Orthop Relat Res 2011;469:2725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ruano-Ravina A, Jato DM.. Autologous chondrocyte implantation: a systematic review. Osteoarthritis Cartilage 2006;14:47–51. [DOI] [PubMed] [Google Scholar]
- 140. Peterson L, Vasiliadis HS, Brittberg M. et al. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med 2010;38:1117–24. [DOI] [PubMed] [Google Scholar]
- 141. Hwang NS, Varghese S, Zhang Z. et al. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Eng 2006;12:2695–706. [DOI] [PubMed] [Google Scholar]
- 142. Wei X, Yang X, Han ZP. et al. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin 2013;34:747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hass R, Kasper C, Bohm S. et al. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 2011;9:12.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Park KM, Lee SY, Joung YK. et al. Thermosensitive chitosan-Pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater 2009;5:1956–65. [DOI] [PubMed] [Google Scholar]
- 145. Cheng HW, Luk KD, Cheung KM. et al. In vitro generation of an osteochondral interface from mesenchymal stem cell-collagen microspheres. Biomaterials 2011;32:1526–35. [DOI] [PubMed] [Google Scholar]
- 146. Duan P, Pan Z, Cao L. et al. The effects of pore size in bilayered poly(lactide-co-glycolide) scaffolds on restoring osteochondral defects in rabbits. J Biomed Mater Res A 2014;102:180–92. [DOI] [PubMed] [Google Scholar]
- 147. Steinmetz NJ, Aisenbrey EA, Westbrook KK. et al. Mechanical loading regulates human MSC differentiation in a multi-layer hydrogel for osteochondral tissue engineering. Acta Biomater 2015;21:142–53. [DOI] [PubMed] [Google Scholar]
- 148. Payne KA, Didiano DM, Chu CR.. Donor sex and age influence the chondrogenic potential of human femoral bone marrow stem cells. Osteoarthritis Cartilage 2010;18:705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Haidar ZS, Hamdy RC, Tabrizian M.. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: current challenges in BMP delivery. Biotechnol Lett 2009;31:1817–24. [DOI] [PubMed] [Google Scholar]
- 150. Xu X, Shi D, Liu Y. et al. In vivo repair of full-thickness cartilage defect with human iPSC-derived mesenchymal progenitor cells in a rabbit model. Exp Ther Med 2017;14:239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Choi HY, Lee TJ, Yang GM. et al. Efficient mRNA delivery with graphene oxide-polyethylenimine for generation of footprint-free human induced pluripotent stem cells. J Control Release 2016;235:222–35. [DOI] [PubMed] [Google Scholar]
- 152. Lam J, Lu S, Meretoja VV. et al. Generation of osteochondral tissue constructs with chondrogenically and osteogenically predifferentiated mesenchymal stem cells encapsulated in bilayered hydrogels. Acta Biomater 2014;10:1112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Santo VE, Gomes ME, Mano JF. et al. Controlled release strategies for bone, cartilage, and osteochondral engineering–Part II: challenges on the evolution from single to multiple bioactive factor delivery. Tissue Eng Part B Rev 2013;19:327–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Horisawa E, Kubota K, Tuboi I. et al. Size-dependency of DL-lactide/glycolide copolymer particulates for intra-articular delivery system on phagocytosis in rat synovium. Pharm Res 2002;19:132–9. [DOI] [PubMed] [Google Scholar]
- 155. Butoescu N, Seemayer CA, Foti M. et al. Dexamethasone-containing PLGA superparamagnetic microparticles as carriers for the local treatment of arthritis. Biomaterials 2009;30:1772–80. [DOI] [PubMed] [Google Scholar]
- 156. Sheridan MH, Shea LD, Peters MC. et al. Bioabsorbable polymer scaffolds for tissue engineering capable of sustained growth factor delivery. J Control Release 2000;64:91–102. [DOI] [PubMed] [Google Scholar]
- 157. Spiller KL, Liu Y, Holloway JL. et al. A novel method for the direct fabrication of growth factor-loaded microspheres within porous nondegradable hydrogels: controlled release for cartilage tissue engineering. J Control Release 2012;157:39–45. [DOI] [PubMed] [Google Scholar]
- 158. Lim SM, Oh SH, Lee HH. et al. Dual growth factor-releasing nanoparticle/hydrogel system for cartilage tissue engineering. J Mater Sci Mater Med 2010;21:2593–600. [DOI] [PubMed] [Google Scholar]
- 159. Park JS, Yang HN, Woo DG. et al. In vitro and in vivo chondrogenesis of rabbit bone marrow–derived stromal cells in fibrin matrix mixed with growth factor loaded in nanoparticles. Tissue Eng Part A 2009;15:2163–75. [DOI] [PubMed] [Google Scholar]
- 160. Ertan AB, Yılgor P, Bayyurt B. et al. Effect of double growth factor release on cartilage tissue engineering. J Tissue Eng Regen Med 2013;7:149–60. [DOI] [PubMed] [Google Scholar]
- 161. Shi D, Xu X, Ye Y. et al. Photo-cross-linked scaffold with kartogenin-encapsulated nanoparticles for cartilage regeneration. ACS Nano 2016;10:1292–9. [DOI] [PubMed] [Google Scholar]
- 162. Bajpayee AG, Wong CR, Bawendi MG. et al. Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis. Biomaterials 2014;35:538–49. [DOI] [PMC free article] [PubMed] [Google Scholar]