Abstract
OBJECTIVE DATA
The purpose of this study was to perform a systematic review and metaanalysis of randomized controlled trials on oral progesterone compared with placebo or other interventions for preterm birth prevention in singleton pregnancies with previous spontaneous preterm birth. The primary outcome was preterm birth at <37 weeks gestation; the secondary outcomes included preterm birth rate at <34 weeks gestation, neonatal morbidity/death, and maternal side-effects.
STUDY
Searches were performed in PubMed, Scopus, ClinicalTrials.gov, PROSPERO, EMBASE, and the Cochrane Register with the use of a combination of words related to “preterm birth,” “preterm delivery,” “progesterone,” “progestogens,” and “oral” from inception of each database to April 2018. Additionally, systematic reviews on progesterone for preterm birth prevention that were identified in our search were also reviewed for additional studies. We included all randomized trials of asymptomatic singleton gestations with previous spontaneous singleton preterm birth that had been randomized to prophylactic treatment with oral progesterone vs placebo, no treatment, or other preterm birth intervention. Exclusion criteria included quasirandomized trials, trials that involved women with preterm labor/membrane rupture at the time of randomization or multiple gestations.
STUDY APPRAISAL AND SYNTHESIS METHODS
The risk of bias and quality of evidence were assessed for each study. All analyses were done with an intention-to-treat approach. The primary outcome was incidence of preterm birth at <37 weeks gestation; the secondary outcomes included preterm birth at <34 and <28 weeks gestation, maternal adverse events, maternal serum progesterone level, and neonatal morbidity and death. Summary measures were reported as relative risk or mean difference. I2 >30% was used to identify heterogeneity.
RESULTS
The search strategy identified 79 distinct studies. Three trials on oral progesterone vs placebo (involved 386 patients: 196 in oral progesterone and 190 in placebo) met the inclusion criteria; there were no studies on oral progesterone vs other intervention that met inclusion criteria. Metaanalysis demonstrated a significantly decreased risk of preterm birth at <37 weeks gestation (42% vs 63%; P=.0005; relative risk, 0.68; 95% confidence interval, 0.55–0.84), preterm birth at <34 weeks gestation (29% vs 53%; P<.00001; relative risk, 0.55; 95% confidence interval, 0.43–0.71), and increased gestational age of delivery (mean difference, 1.71 weeks; 95% confidence interval, 1.11–2.30) with oral progesterone compared with placebo. There was a significantly lower rate of perinatal death (5% vs 17%; P=.001; relative risk 0.32; 95% confidence interval, 0.16–0.63), neonatal intensive care admission (relative risk, 0.39; 95% confidence interval, 0.25–0.61), respiratory distress syndrome (relative risk, 0.21; 95% confidence interval, 0.05–0.93), and higher birthweight (mean difference, 435.06 g; 95% confidence interval, 324.59–545.52) with oral progesterone. There was a higher rate of maternal adverse effects with oral progesterone that included dizziness (relative risk, 2.95; 95% confidence interval, 1.47–5.90), somnolence (relative risk, 2.06; 95% confidence interval, 1.29–3.30), and vaginal dryness (relative risk, 2.37; 95% confidence interval, 1.10–5.11); no serious adverse effects were noted.
CONCLUSION
Oral progesterone appears to be effective for the prevention of recurrent preterm birth and a reduction in perinatal morbidity and mortality rates in asymptomatic singleton gestations with a history of previous spontaneous preterm birth compared with placebo. There were also increased adverse effects with oral progesterone therapy compared with placebo, although none were serious. Further randomized study on oral progesterone compared with other established therapies for the prevention of recurrent preterm birth are warranted.
Keywords: metaanalysis, preterm birth, progesterone, systematic review
Preterm birth is a leading cause of neonatal morbidity and death. History of previous spontaneous preterm birth is 1 of the major risk factors for preterm birth. Intramuscular 17-hydroxyprogesterone caproate (17OHPC), a synthetic progestin, has been shown to reduce the risk of recurrent preterm birth.1 However, because of issues that are related to access and side-effects, adherence to 17OHPC is not always ideal2,3 and may impact its effectiveness adversely in the real world.4 A recent metaanalysis also showed that daily vaginal natural progesterone, either suppository or gel, is a reasonable, if not better, alternative to weekly 17OHPC for the prevention of recurrent preterm birth.5
EDITOR’S CHOICE
Oral natural progesterone has not been as well-studied for recurrent preterm birth prevention. The advantages of oral micronized progesterone include increased patient acceptance and potential adherence and improved access because no specialty pharmacy is required. The efficacy of oral progesterone may be questioned because of a significant first-pass effect from hepatic metabolism,6 although studies outside of pregnancy have shown similar bioavailability as vaginal administration.7
The purpose of this study was to perform a systematic review and metaanalysis of randomized controlled trials about the use of oral progesterone for the prevention of recurrent preterm birth in singleton pregnancies with a history of spontaneous preterm birth.
Methods
Eligibility criteria, information sources, search strategy
This metaanalysis was performed according to a protocol that was recommended for systematic review.8 Before data extraction, the review was registered with the PROSPERO International Prospective Register of Systematic Reviews (CRD42018095246). The research protocol was designed a priori, defining methods for searching the literature, including examining articles, and extracting and analyzing data. Searches were performed in MEDLINE, OVID, Scopus, ClinicalTrials.gov, the PROSPERO International Prospective Register of Systematic Reviews, EMBASE, and the Cochrane Central Register of Controlled Trials with the use of a combination of keywords and text words related to “preterm birth,” “preterm delivery,” “progesterone,” “progestogens,” and “oral” from inception of each database to April 2018. No restrictions for language or geographic location were applied.
Study selection
We included all RCTs of asymptomatic singleton gestations with previous spontaneous singleton preterm birth who were randomized to prophylactic treatment with oral progesterone vs placebo, no treatment, or other preterm birth intervention (ie, intramuscular progesterone, cerclage). Exclusion criteria included quasirandomized trials (ie, trials in which allocation was done on the basis of a pseudo-random sequence, such as odd/even hospital number or date of birth, alternation) and trials that involved women with preterm labor/rupture at the time of randomization. Trials in women with multiple gestations were excluded.
Data extraction
All analyses were done with the use of aggregate data, as reported in original trials. Authors were contacted for additional data as needed. The primary outcome was incidence of preterm birth at <37 weeks gestation. Secondary outcomes were preterm birth at <34 weeks gestation, preterm birth <28 weeks gestation, maternal adverse events, serum progesterone levels at 20 and 28 weeks gestation, and neonatal outcomes that include birthweight (in grams), admission to neonatal intensive care unit (NICU), length of stay in NICU (days), respiratory distress syndrome (RDS; either transient tachypnea of the newborn infant or severe RDS), intraventricular hemorrhage (grade 3 or 4), necrotizing enterocolitis (grade 3 or 4), neonatal sepsis (culture-proven sepsis), and perinatal death. Perinatal death was defined as either fetal death (ie, fetal death after 20 weeks gestation) or neonatal death (ie, death of a live born baby within the first 28 days of life). All authors of the original trials were contacted to obtain missing data, if possible.
All review stages were conducted independently by 2 reviewers (R.C.B., L.D.D.) who assessed inclusion criteria, risk of bias, data extraction, and data analysis. Disagreements were resolved by discussion with a third reviewer (G.S.). Before data extraction, the review was registered with the PROSPERO International Prospective Register of Systematic Reviews (CRD42018095246).
Assessment of risk of bias
The risk of bias in each included study was assessed by use of the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions.8 Seven domains related to risk of bias were assessed in each included trial because there is evidence that these issues are associated with biased estimates of treatment effect: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting, and (7) other bias. Review authors’ judgments were categorized as “low risk,” “high risk,” or “unclear risk” of bias.7
For this review, the quality of evidence was assessed with the GRADE approach9 to assess the quality of the body of evidence that related to primary and selected secondary outcomes. GRADE-pro Guideline Development Tool https://gdt.gradepro.org/app/handbook/handbook.html) was used to import data from Review Manager (version 5.2; The Nordic Cochrane Center, Cochrane Collaboration, Copenhagen, Denmark) to create a “Summary of Findings” table. A summary of the intervention effect and a measure of quality for each of the aforementioned outcomes was produced with the GRADE approach. The evidence can be downgraded from “high quality” by 1 level for serious (or by 2 levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias. The quality of the evidence (and its interpretation) was judged in the following manner: high quality (further research is very unlikely to change our confidence in the estimate of effect), moderate quality (further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate), low quality (further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate), and very low quality (we are very uncertain about the estimate). The judgments about quality were justified, documented, and incorporated into the reporting of results for primary and secondary outcomes.
Data synthesis
The data analysis was completed with Review Manager software (version 5.3; The Nordic Cochrane Center, Cochrane Collaboration). The completed analyses was then compared and any difference was resolved with review of the entire data and independent analysis. The summary measures were relative risk (RR) or mean difference (MD) with 95% of confidence interval (CI) with the use of the fixed effects model. I-square (Higgins I2) >30% was used to identify heterogeneity, in such cases a random-effects model was used, as recommended by the Cochrane Handbook for Systematic Reviews. Potential publication bias was planned to be assessed with Begg’s and Egger’s tests. Probability value of <.05 was considered statistically significant.
Results
Study selection
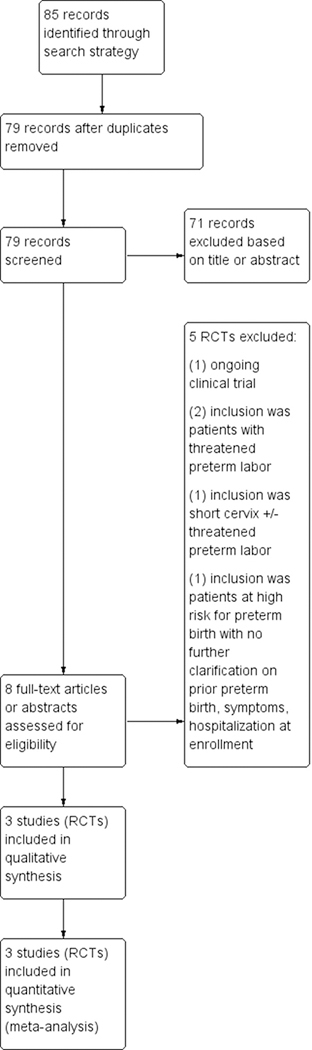
The search strategy identified 85 total reports that represented 79 distinct studies. Of these 79 studies, 3 met inclusion criteria for the review (Figure 1).10–12 Three trials (involving 386 patients: 196 in oral progesterone and 190 in placebo) met the inclusion criteria. There were no studies of oral progesterone compared with other interventions that met inclusion criteria.
FIGURE 1. Search strategy.
Flow diagram of studies identified in the systematic review.
RCT, randomized controlled trial.
Study characteristics
Characteristics of included studies are given in Table 1. All of the trials compared oral progesterone with placebo, although the specific dosing of oral progesterone varied by trial. All the trials included women with singleton gestation, previous spontaneous preterm birth, and randomization at <24 weeks gestation, although the definition of previous spontaneous preterm birth varied (Table 1). All included studies were double-blind placebo controlled randomized trials. Management of cervical length screening and short cervix varied by study. All of the studies performed at least 1 cervical length ultrasound scan; 1 study offered history-indicated cerclage and “rescue” cerclage for cervical length <15 mm, and approximately 70% of participants had a cerclage10; 1 study offered cerclage for cervical length <5 mm, and none of the participants had a cerclage11; and 1 study did cervical length ultrasound scan, but the criteria for cerclage were not reported, and only 3–4% of the participants had a cerclage.12 Baseline characteristics of study population are presented in Table 2. Regarding the 1 study with >70% of participants with cerclage, 60% of the participants had an elective cerclage placed, and approximately 10% of them had a “rescue” cerclage placed based on ultrasound scans.10
TABLE 1.
Characteristics of included randomized trials
| Randomized trial |
|||
|---|---|---|---|
| Characteristic | Ashoush et al, 201710 | Glover et al, 201111 | Rai et al, 200912 |
| Methods | Double blind randomized controlled trial | Double blind randomized controlled trial | Double blind randomized controlled trial |
| Location | Egypt | United States | India |
| Sample size | 205 | 33 | 148 |
| Oral progesterone dose | 100 mg every 6 hrs until 37 wks gestation (N=103) | 400 mg twice daily until 34 wks gestation (N=19) | 100 mg twice daily until 36 wks gestation (N=74) |
| Comparator | Placebo (N=102) | Placebo (N=14) | Placebo (N=74) |
| Gestational age range at randomization | 14–18 Wks | 16–20 Wks | 18–24 Wks |
| Inclusion criteria | Singleton gestation 14–18 wks, previous spontaneous preterm birth at <37 wks gestation | Singleton gestation <20 wks, previous spontaneous preterm birth at 20–36 wks 6 d | Singleton gestation 18–24 wks, previous spontaneous preterm birth at 16–36 wks 6 d |
| Assessment of cervical length | Cervical length ultrasound scan at 20 wks gestation | Cervical length ultrasound scan at least once at <24 wks gestation, every 2 wks if cervical length was 10–25 mm, weekly for cervical length <10 mm | Cervical length assessment in second trimester |
| Management of short cervix | Cerclage offered for cervical length <15 mm | Cerclage offered for cervical length <5 mm | Not reported |
| Primary outcome | Preterm birth at <37 wks gestation | Preterm birth at <37 wks gestation | Mean prolongation of pregnancy |
TABLE 2.
Baseline maternal characteristics
| Baseline characteristics | Trial | Oral progesterone | Placebo |
|---|---|---|---|
| Maternal age, ya | Ashoush et al, 2017 | 29.3±4.5 | 29.5±3.5 |
| Glover etal, 2011 | 29.3±4.7 | 27.2±4.9 | |
| Rai et al, 2009 | 26.07±3.24 | 25.72±3.42 | |
| Mean gestational age at randomization, wka | Ashoush et al, 2017b | 15.21 ±0.98 | 15.31 ±0.97 |
| Glover etal, 2011 | 16.9±2.6 | 18.2±2.7 | |
| Rai et al, 2009 | 20.69±2.83 | 20.73±1.78 | |
| Race (not white), % | Ashoush et al, 2017b | 100 % (all North African) | 100 % (all North African) |
| Glover etal, 2011 | 42.1 | 50 | |
| Rai et al, 2009b | 100% (all South Asian | 100% (all South Asian) | |
| Previous preterm births, na | Ashoush et al, 2017 | 1.65±0.63 | 1.72±0.65 |
| Glover etal, 2011 | 2.2±1.2 | 1.5±0.9 | |
| Rai et al, 2009 | 1.21 ±0.53 | 1.31 ±0.52 | |
| Baseline cervical length, mma | Ashoush et al, 2017 | 25.7±8.3 | 23.9±9.7 |
| Glover etal, 2011 | 34.9±6.9 | 34.0±4.5 | |
| Rai et al, 2009b | 28.99± 3.751 | 26.93±3.489 | |
| Cerclage, n (%) | Ashoush et al, 2017 | 70 (72.9) | 73 (80.2) |
| Glover etal, 2011 | 0 | 0 | |
| Rai et al, 2009b | 2(3) | 3(4) |
Data are given as mean±standard deviation
Indicates unpublished data provided by authors.
Risk of bias of included studies
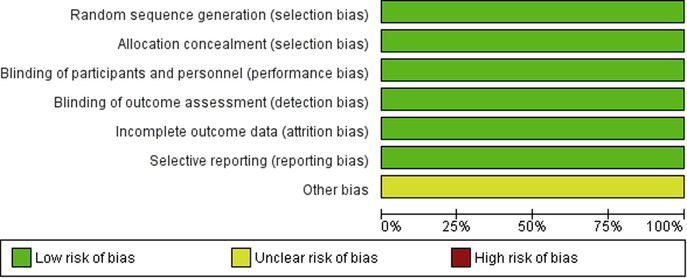
Risk of bias was overall low for all included studies; they were all double-blinded placebo-controlled prospective randomized trials (Figure 2).
FIGURE 2. Risk of bias graph.
Assessment of risk of bias graph: Each risk of bias item presented as percentages across all included studies.
Synthesis of results
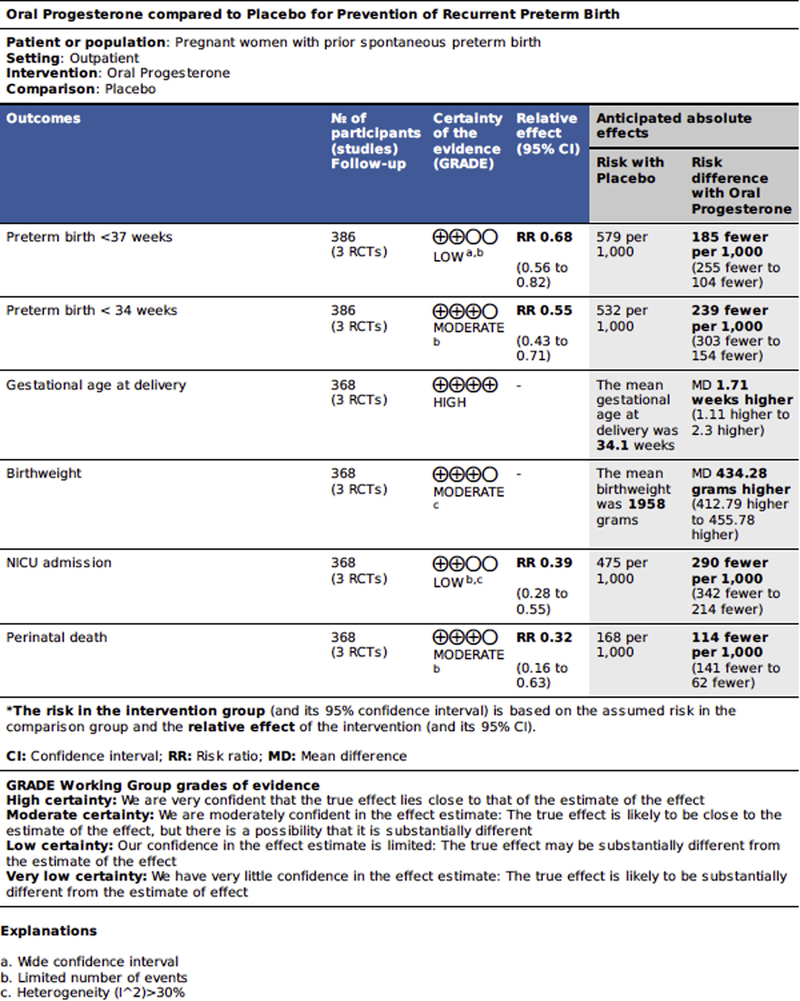
All outcomes are presented in Table 3, and key outcomes along with grade of evidence are presented in the summary of findings table (Figure 3). Unpublished data were provided by 3 of the authors (D.M., S.A., and S.R.).
TABLE 3.
Oral progesterone vs placebo in the prevention of recurrent preterm birth, metaanalysis, and summary of primary and secondary outcomes
| Outcome | Trial | Oral progesterone | Placebo | Relative risk or mean difference (95% confidence interval) Preterm birth |
|---|---|---|---|---|
| At <37 wks gestation, n/N (%) | Ashoush et al, 2017 | 50/103 (48) | 69/102 (67) | |
| Glover et al, 2011 | 5/19(26) | 8/14(57) | ||
| Rai et al, 2009 | 29/74 (39) | 44/74 (59) | ||
| Total | 84/196(42) | 121/190 (63) | 0.68 (0.55—0.84)a | |
| At <34 wks gestation, n/N (%) | Ashoush et al, 2017b | 32/103 (31) | 61/102 (59) | |
| Glover et al, 2011b | 3/19(15) | 3/14(21) | ||
| Rai et al, 2009 | 22/74 (29) | 37/74 (50) | ||
| Total | 57/196 (29) | 101/190(53) | 0.55 (0.43—0.71)a | |
| At <28 wks gestation, n/N (%) | Ashoush et al, 2017b | 7/103 (7) | 11/102 (11) | |
| Glover et al, 2011b | 0/19(0) | 0/14(0) | ||
| Rai et al, 2009 | 0/74 (0) | 0/74 (0) | ||
| Total | 7/196(4) | 14/196(7) | 0.51 (0.22—1.20) | |
| Gestational age at delivery, wkc | Ashoush et al, 2017 | 35.4±2.7 | 33.9±2.9 | |
| Glover et al, 2011 | 37±2.7 | 35.9±3.8 | ||
| Rai et al, 2009 | 36.1 ±2.7 | 34.0±3.3 | ||
| Mean difference | 1.71 (1.11—2.30)a | |||
| Birthweight, gc | Ashoush et al, 2017 | 2312±77 | 1878±74 | |
| Glover et al, 2011 | 2830±657 | 2839±923 | ||
| Rai et al, 2009 | 2400±650 | 1890±560 | ||
| Mean difference | 435.06 (324.59—545.52)a | |||
| Neonatal intensive care unit admission, n/N (%) | Ashoush et al, 2017 | 22/96 (23) | 42/91 (46) | |
| Glover et al, 2011b | 3/19(16) | 5/14(35) | ||
| Rai et al, 2009 | 10/74 (14) | 38/74(51) | ||
| Total | 35/189 (19) | 85/179(47) | 0.39 (0.28—0.55)a | |
| Length of neonatal intensive care unit stay, dc | Ashoush et al, 2017 | 15.4±5.5 | 19.5±5.8 | |
| Glover et al, 2011 | 6.5±10.5 | 7.5±9.0 | ||
| Rai et al, 2009bd | 2.10 (50.62) | 5.05 (121.34) | ||
| Mean difference | -3.93 (−5.50—−2.35) | |||
| Perinatal death, n/N (%) | Ashoush et al, 2017 | 7/96 (7) | 23/91 (25) | |
| Glover et al, 2011b | 0/19(0) | 0/14(0) | ||
| Rai et al, 2009 | 3/74 (4) | 7/74 (9) | ||
| Total | 10/189(5) | 30/179 (17) | 0.32 (0.16—0.63)a | |
| Respiratory distress syndrome, n/N (%) | Ashoush et al, 2017 | 21/96 (22) | 39/91 (43) | |
| Glover et al, 2011 | 0/19(0) | 3/14(21) | ||
| Rai et al, 2009 | 3/74 (4) | 31/74 (42) | ||
| Total | 24/189 (13) | 73/179(41) | 0.21 (0.05—0.93)a | |
| Intraventricular hemorrhage, n/N (%) | Ashoush et al, 2017 | 8/96 (8) | 11/91 (12) | 0.69 (0.29–1.64) |
| Necrotizing enterocolitis, n/N (%) | Ashoush et al, 2017 | 5/96 (5) | 9/91 (10) | 0.53 (0.18–1.51) |
| Neonatal sepsis, n/N (%) | Rai et al, 2009b | 0/74 (0) | 2/74 (3) | 0.20(0.01–4.10) |
| Serum progesterone, ng/mLc | Ashoush et al, 2017 | 34.5±3.8 | 17.6±3.3 | |
| Glover et al, 2011 | 122.6±61.8 | 90.1 ±38.7 | ||
| Mean difference | 16.91 (15.89–17.93)a | |||
| Dizziness, n/N (%) | Ashoush et al, 2017 | 28/96 (29) | 9/91 (10) | |
| Glover et al, 2011 | 0/19(0) | 0/14(0) | ||
| Total | 28/115 (24) | 9/105 (9) | 2.95 (1.47–5.90)a | |
| Constipation, n/N (%) | Ashoush et al, 2017 | 21/96 (22) | 13/91 (14) | |
| Glover et al, 2011 | 0/19(0) | 0/19(0) | ||
| Total | 21/115 (18) | 13/105 (12) | 1.53 (0.82–2.87) | |
| Somnolence, n/N (%) | Ashoush et al, 2017 | 40/96(41) | 18/91 (19) | |
| Glover et al, 2011 | 0/19(0) | 0/14(0) | ||
| Rai et al, 2009 | 1/74 (1) | 1/74 (1) | ||
| Total | 41/189 (22) | 19/179 (11) | 2.06 (1.29–3.30)a | |
| Vaginal dryness, n/N (%) | Ashoush et al, 2017 | 20/96 (21) | 8/91 (9) | |
| Glover et al, 2011 | 0/19(0) | 0/14(0) | ||
| Total | 20/115 (17) | 8/105 (8) | 2.37 (1.10–5.11)a | |
| Acne, n/N (%) | Glover et al, 2011 | 0/19(0) | 0/14(0) | |
| Rai et al, 2009 | 2/74 (3) | 1/74 (1) | ||
| Total | 2/93 (2) | 1/88 (1) | 2.00 (0.19–21.58) | |
| Esophageal reflux, n/N (%) | Glover et al, 2011 | 0/19(0) | 0/14(0) | |
| Rai et al, 2009 | 2/74 (3) | 0/74 (0) | ||
| Total | 2/93 (2) | 0/88 (0) | 5.00(0.24–102.40) | |
| Headache, n/N (%) | Glover et al, 2011 | 0/19(0) | 0/14(0) | |
| Rai et al, 2009 | 0/74 (0) | 1/74 (1) | ||
| Total | 0/93 (0) | 1/88 (1) | 0.33 (0.01–8.05) | |
| Depression, n/N (%) | Glover et al, 2011 | 0/19(0) | 0/14(0) | |
| Rai et al, 2009 | 0/74 (0) | 4/74 (5) | ||
| Total | 0/93 (0) | 4/88 (5) | 0.11 (0.01–2.03) |
FIGURE 3. Summary of findings.
These findings compare oral progesterone with placebo for the prevention of recurrent preterm birth.
Primary outcome
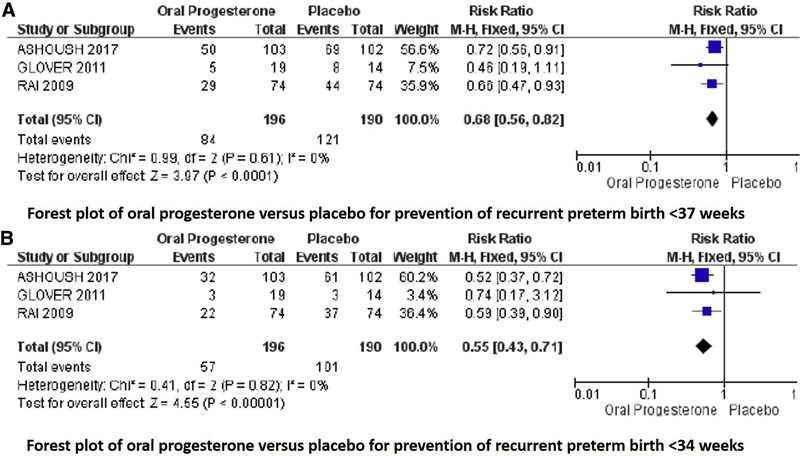
Regarding the primary outcome of this metaanalysis, all studies reported preterm birth at <37 weeks gestation. Two studies individually found a significantly decreased rate of preterm birth at <37 weeks gestation with oral progesterone compared with placebo, and meta-analysis demonstrated a significantly decreased risk of preterm birth at <37 weeks gestation with use of oral progesterone compared with placebo (42% vs 63%; P=.0005; RR, 0.68; 95% CI, 0.55–0.84; Figure 4).10,12
FIGURE 4. Oral progesterone vs placebo metaanalysis.
Forest plots of outcomes of preterm birth at A, <37 and B, <34 weeks gestation.
Cl, confidence interval; M-H, Mantel-Haenszel.
Secondary outcomes
Preterm birth
All included studies reported on preterm birth-related outcomes. Regarding secondary outcomes, metaanalysis demonstrated a significantly reduced risk of preterm birth at <34 weeks gestation (29% vs 53%; P<.00001; RR, 0.55; 95% CI, 0.43–0.71) and increased gestational age of delivery (MD, 1.71 weeks gestation; 95% CI, 1.11–2.30), but not of preterm birth at <28 weeks gestation (RR, 0.51; 95% CI, 0.22–1.20) with oral progesterone compared with placebo. Ashoush et al10 reported any delivery at <28 weeks gestation as “miscarriage” because the neonates are not able to be resuscitated, thus most analyses exclude those deliveries (n=187); however, the authors provided data on incidence of deliveries at 20–28, 28–34, 34–37, and >37 weeks gestation; thus, the number of participants for these outcomes is 205.
Neonatal outcomes
Regarding neonatal outcomes, I2 was >30% with certain outcomes. Oral progesterone therapy resulted in a significantly lower rate of perinatal death (5% vs 17%; P=.001; RR, 0.32; 95% CI, 0.16–0.63; studies=3; I2=0%), higher birth weight (MD, 435.06 g; 95% CI, 324.59–545.52; studies=3; I2=32%), lower rate of neonatal intensive care admission (RR, 0.39; 95% CI, 0.25–0.61; studies=3; I2=29%; P=.25), and shorter NICU stay (MD, 3.93 days; 95% CI, −5.50 to −2.35; studies=2; I2=0). Of note the Rai et al12 reported NICU stay in median; thus, it was not included in quantitative metaanalysis (Table 3). Regarding specific neonatal morbidities, there was a significantly reduced rate of RDS (RR, 0.21; 95% CI, 0.05–0.93; studies=3; I2=78%; P=.01) in oral progesterone compared with placebo. Regarding other neonatal morbidities, there was not a significant difference found in rates of intraventricular hemorrhage (RR, 0.69; 95% CI, 0.29–1.64; studies= 1), necrotizing enterocolitis (RR, 0.53; 95% CI, 0.18–1.51; studies=1), or neonatal sepsis (RR, 0.20; 95% CI, 0.01–4.10; studies=1).
Maternal effects
There was a significantly higher rate of maternal side-effects with oral progesterone compared with placebo, although no serious adverse effects were reported. These included a higher rate of dizziness (RR, 2.95; 95% CI, 1.47–5.90; studies=2), somnolence (RR, 2.06; 95% CI, 1.29–3.30; studies=3), and vaginal dryness (RR, 2.37; 95% CI, 1.10–5.11; studies=2; Table 3).
Serum progesterone
Use of oral progesterone was associated with a significantly higher maternal serum progesterone level at 28 weeks gestation compared with placebo (MD, 16.91 ng/mL; 95% CI, 15.89–17.93; studies=2; Table 3).
Sensitivity analyses
Given the high rate of concurrent cerclage use in 1 study,10 although equal in both groups, a post-hoc sensitivity analysis was performed for outcomes of preterm birth at <37 and <34 weeks gestation excluding this study. Oral progesterone remained associated with a reduced risk of preterm birth at <37 weeks gestation (RR, 0.62; 95% CI, 0.45–0.86) and preterm birth at <34 weeks gestation (RR, 0.61; 95% CI, 0.41–0.91).
Comment
Main findings
This review and metaanalysis suggests that oral progesterone is effective for the prevention of recurrent preterm birth and reduction in perinatal morbidity and mortality rates in asymptomatic singleton gestations with a history of spontaneous preterm birth. Specifically, we found a statistically significant reduction in preterm birth at <37 and <34 weeks gestation, perinatal deaths, NICU admission, and RDS. Notably, there were also increased adverse effects with oral progesterone therapy compared with placebo, although no serious adverse effects were noted.
Quality of evidence
Quality of evidence for primary outcome and key selected secondary outcomes was generally low to moderate, with some deduction for wide confidence interval or limited event number (Table 3). Quality of evidence for specific neonatal morbidities (ie, RDS, neonatal enterocolitis) was low to very low because of wide confidence interval, heterogeneity, and limited events. Similarly, quality of evidence for maternal adverse effects was low to very low because of wide confidence interval and limited number of events and limited studies.
Strengths and limitations
One of the strengths of this study is the assessment of a specific formulation of progesterone (oral) in a specific high-risk population (previous spontaneous preterm birth) that allows for the clinical application of these results. This is the only metaanalysis of oral progesterone compared with placebo in this specific population to have this many patients included. Other metaanalyses have either combined various progesterone formulations/routes of administration together13–16 or included studies with variable inclusion criteria/preterm birth risks,17 thus limiting the clinical utility of the results. Additionally, the quality of evidence for the reduction in early preterm birth (<34 weeks gestation) for some other key outcomes was moderate (Figure 3). Finally, the evaluation of serum progesterone and difference in treated vs placebo provides biologic plausibility to mechanism of action of oral progesterone in pregnancy and its ability to have a systemic effect.
There are a few limitations. This metaanalysis includes only 3 studies, 1 of which was a small pilot study. Because of the limited number of studies, publication bias was not assessed. The dosing of oral progesterone varied by each study, with the lowest regimen being 100 mg twice daily; the optimal dose for preterm birth prevention could not be concluded with this analysis. Because of limited sample size and event number, the quality of evidence for specific neonatal morbidities and maternal adverse events was low to very low. The study population between studies was heterogeneous that was related specifically to the incidence and management of short cervix. In the largest study, approximately 70% of participants had a cerclage, which were primarily placed due to obstetric history and even between groups; in the other 2 studies, cerclage use was zero to minimal. Thus, 1 randomized trial demonstrated benefit of oral progesterone compared with placebo even with 70% of participants with cerclage in each group; however, because this is not an individual patient data analysis, the specific efficacy of oral progesterone in normal vs short cervical length cannot be concluded. Oral progesterone remained beneficial in preterm birth prevention, even with the exclusion of this 1 study in our post hoc sensitivity analysis. Finally, neonatal outcomes depend on the resources and technology available; therefore, outcomes, such as perinatal death in other countries or in studies done over 5 or 10 years ago, may not be as applicable in the United States in the current day.
Comparison with existing literature
Although this analysis is unique in its focus on oral progesterone for a specific indication, our results are consistent with other reviews that have demonstrated efficacy in oral progesterone for preterm birth prevention in general. One recent review that included oral progesterone combined it with other progestogens to evaluate efficacy in different clinical scenarios.13 Another metaanalysis evaluated progesterone efficacy by route; thus, oral progesterone was evaluated separately, and it was found that oral progesterone was effective in preterm birth prevention. However, that analysis is limited because indication for therapy was varied (included symptomatic and asymptomatic patients), and the most recent and largest randomized study10 was not included.14 A recent metaanalysis evaluated progesterone by route and indication but did not include the most recent, large randomized study and did not examine the number of outcomes reported here.15 The conclusion of that metaanalysis that used just 2 trials11,12 similarly identified a reduction in preterm birth at <34 weeks gestation but not a reduction in preterm birth at <37 weeks gestation or in perinatal death.15 A Cochrane review on progesterone for preterm birth prevention found that progesterone overall was effective in preterm birth prevention compared with placebo and improved neonatal morbidity and mortality rates; however, all progesterone formulations were combined, and a conclusion on efficacy by route/formulation could not be made.16 The combination of progestogen formulations in metaanalyses is problematic because micronized progesterone that is administered vaginally and orally may have different systemic vs local uterine/cervical effects17,18 and because natural progesterone and 17OHPC are distinct in their mechanism of action and indication for use.19 The combination of indications for therapy is also problematic because clinically an asymptomatic patient in the second trimester has a different risk for preterm birth and different clinical treatment than a patient with preterm labor or preterm premature rupture of membranes.
There were a total of 7 completed randomized trials on oral progesterone for preterm birth prevention that were identified in our search strategy; Table 4 includes characteristics of excluded randomized studies. One study, in abstract form only, randomized “high-risk” participants in the second trimester to daily 17OHPC (n=52), oral progesterone (n=43), or placebo (n=26), which identified that both 17OHPC and oral progesterone were superior to placebo in the prevention of preterm labor and improvement of perinatal outcome, but no difference when oral progesterone is compared with 17OHPC.20 This study was not included in our analysis because the inclusion criteria were not specified, which included previous preterm birth or absence of symptoms, and the authors did not respond to our request for additional information; however, the findings are consistent with our review that identified the benefit of oral progesterone compared with placebo. There were 2 other randomized studies that evaluated oral progesterone in the setting of preterm labor or halted preterm labor; 1 study found that oral progesterone improved latency compared with placebo,21 and 1 study found that oral progesterone and placebo were equivalent in latency.22 Finally, 1 study evaluated oral progesterone, 17OHPC, and vaginal progesterone compared with cerclage in women with a short cervix and found that only vaginal progesterone was effective; this study is difficult to interpret because they included symptomatic and asymptomatic participants.23 The efficacy of progesterone in the setting of preterm labor or halted preterm labor is different from its efficacy in asymptomatic high-risk individuals16; therefore, we did not combine all randomized trials on oral progesterone in this review.
TABLE 4.
Description of 4 randomized studies with oral progesterone in preterm birth prevention that were excluded from metaanalysis
| Randomized trial |
||||
|---|---|---|---|---|
| Characteristic | Noblot et al, 199122 | Ndoni et al, 201020 | Choudhary et al, 201421 | Pustotina, 201823 |
| Methods | Double blind randomized controlled trial | Randomized control study | Double blind randomized controlled trial | Randomized trial, open label with crossover after 1 week of therapy in randomized assignment |
| Location | France | Albania | India | Russia |
| Sample size | 44 | 121 | 90 | 95 |
| Inclusion criteria | Pregnant patients (including multiples) underwent tocolytic therapy for threated preterm labor; patients with preterm premature rupture of membranes at <32 wks gestation or previous tocolytic therapy excluded | Pregnant patients hospitalized at high risk for preterm delivery | Singletons at 24–34 wks Singleton gestation, cervical length ≤25 mm with or without symptoms of preterm labor/miscarriage (60 symptomatic at randomization) | |
| Oral progesterone dose | 400 mg every 6 hrs for 24 hrs, every 8 hrs for 24 hrs, 300 mg every 8 hrs daily; micronized progesterone (Utrogestan) | Dose not specified, micronized oral progesterone (Utrogestan) | 200 mg micronized progesterone daily | Oral progesterone 400 mg daily |
| Comparator | Placebo | Daily 17 hydroxy-progesterone caproate and placebo | Placebo | 17OHP 250 mg intramuscularly weekly OR vaginal progesterone 400 mg daily OR dydrogesterone 30 mg daily |
| Gestational age range at randomization | <35 Wks | 15–22 Wks | 24–64 Wks | 15–24 Wks |
| Primary outcome | Latency to delivery: not different between groups | Not specified (abstract only), preterm labor and perinatal outcomes reported as improved in 17OHPC and oral progesterone vs placebo, but not compared with each other | Latency to delivery: improved in oral progesterone vs placebo | Not specified; oral progesterone not directly compared with other formulations |
There is currently 1 ongoing randomized controlled trial on oral progesterone for prevention of preterm birth (NCT03428685). The inclusion criteria includes all singletons, so it is not specific to those with previous preterm birth, and it also compares oral progesterone with placebo.
Our review is consistent with a previous randomized trials and systemic review by identifying that oral progesterone is effective in preterm birth prevention, although the inclusion criteria for those previous reports is distinct from ours.14 This study builds on existing literature with additional trial data and by identifying a specific patient population for which oral progesterone improves preterm birth rate and neonatal morbidity and mortality rates.
Implications
This review demonstrates the need for further head-to-head clinical trials on oral progesterone for the prevention of recurrent preterm birth. The mechanism of action of progesterone, in general, in preterm birth prevention is unclear. It likely plays a role in inflammatory pathways, uterine relaxation, and cervical modeling.24,25 Although oral progesterone does undergo an hepatic first-pass effect,6 based on this review, that does not preclude its efficacy; there is still a measurable increase in serum progesterone even with elevated endogenous progesterone in pregnancy and a reduction in preterm birth.
The use of serum progesterone as a potential pharmacokinetic and pharmacodynamics endpoint in oral progesterone allows for detailed studies into optimal dosing and assessment of threshold serum levels for therapeutic efficacy. Dosing in both vaginal progesterone and 17OHPC was derived empirically, and doses have yet to be modified based on pharmacologic principles to improve efficacy. The potential for oral progesterone to be studied in this way allows for a therapy that can be monitored with a standard laboratory assay and adjusted to maximize its benefit.
Head-to-head comparisons of oral progesterone against other formulations of progesterone in asymptomatic singleton pregnancies with previous spontaneous preterm birth are warranted to compare efficacy in prevention of preterm birth and neonatal morbidity/death, adherence, and side-effects. Currently, 17OHPC is the only medication approved for the prevention of recurrent preterm birth; however, its use is limited by patient characteristics,26,27 access,28 high rate of side-effect,1 and adherence.28,29 Oral progesterone is a more affordable and potentially more acceptable alternative for patients. Oral progesterone appears to be effective and well-accepted and warrants further study in prospective randomized trials.
EDITOR’S NOTE.
In women with a singleton gestation and a prior spontaneous preterm birth, this meta-analysis of RCTs shows that oral progesterone is associated with a statistically significant reductions in preterm birth <37 weeks, <34 weeks, perinatal mortality, NICU admission, and respiratory distress syndrome. Given recent results showing no benefit of 17-alpha-hydroxy-progesterone caproate (17-OHP) in this population (https://www.amagpharma.com/news/amag-pharmaceuticals-announces-topline-results-from-the-prolong-trialevaluating-makena-hydroxyprogesterone-caproate-injection/), oral progesterone as well as vaginal progesterone (shown in a prior meta-analysise–Saccone et al, Ultrasound Obstet Gynecol 2017; 49: 315–321–to be superior to 17-OHP) should be further studied as alternatives to 17-OHP.
AJOG at a Glance.
Why was this study conducted?
This systematic review and metaanalysis was conducted to evaluate the efficacy of oral progesterone for the prevention of recurrent preterm birth in randomized controlled trials. 17-hydroxyprogesterone caproate is currently the only Food and Drug Administration–approved medication for the prevention of recurrent preterm birth; however, its effectiveness is limited by access, adherence, and patient-specific characteristics; thus, alternatives should be explored.
Key findings
Our metaanalysis demonstrates that oral progesterone is effective in reducing the risk of preterm birth and perinatal morbidity and death, compared with placebo, in asymptomatic singleton pregnancies with previous spontaneous preterm birth.
What does this add to what is known?
Previous metaanalyses on progesterone have focused on vaginal progesterone or 17-hydroxyprogesterone caproate; reviews that have included oral progesterone therapy have either grouped progestogens together and/or grouped indications for therapy together, thereby limiting the external validity of the findings. This review and metaanalysis is unique in its examination of oral progesterone specifically for the prevention of recurrent preterm birth; our results suggest that randomized trials of oral progesterone compared with 17-hydroxyprogesterone caproate (17OHPC) and vaginal progesterone are warranted.
Acknowledgments
Supported in part by National Institutes of Health grant T32GM008562 (R.C.B.).
Footnotes
The authors report no conflict of interest.
Contributor Information
Rupsa C. Boelig, Maternal Fetal Medicine, Thomas Jefferson University, Philadelphia, PA.
Luigi Della Corte, Department of Neuroscience, Reproductive Sciences and Dentistry, University of Naples Federico II, Naples, Italy.
Sherif Ashoush, Department of Obstetrics and Gynecology, Faculty of Medicine, Ain Shams University, Cairo, Egypt.
David McKenna, Maternal Fetal Medicine, Wright State University, Dayton, OH.
Gabriele Saccone, Department of Neuroscience, Reproductive Sciences and Dentistry, University of Naples Federico II, Naples, Italy.
Shalini Rajaram, Department of Obstetrics and Gynecology, University College of Medical Sciences & Guru Teg Bahadur Hospital, Delhi, India.
Vincenzo Berghella, Maternal Fetal Medicine, Thomas Jefferson University, Philadelphia, PA.
REFERENCES
- 1.Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med 2003;348:2379–85. [DOI] [PubMed] [Google Scholar]
- 2.Yee LM, Liu LY, Sakowicz A, Bolden JR, Miller ES. Racial and ethnic disparities in use of 17-alpha hydroxyprogesterone caproate for prevention of preterm birth. Am J Obstet Gynecol 2016;214:374.e1–6. [DOI] [PubMed] [Google Scholar]
- 3.Feghali M, Venkataramanan R, Caritis S. Prevention of preterm delivery with 17-hydroxyprogesterone caproate: pharmacologic considerations. Semin Perinatol 2014;38: 516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson DB, McIntire DD, McDonald J, Gard J, Turrichi P, Leveno KJ. 17-alpha Hydroxyprogesterone caproate did not reduce the rate of recurrent preterm birth in a prospective cohort study. Am J Obstet Gynecol 2017;216:600. e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saccone G, Khalifeh A, Elimian A, et al. Vaginal progesterone versus intramuscular 17-alpha-hydroxyprogesterone caproate for prevention of recurrent spontaneous preterm birth in singleton gestations: a systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol 2017;49: 315–21. [DOI] [PubMed] [Google Scholar]
- 6.Stanczyk FZ. All progestins are not created equal. Steroids 2003;68:879–90. [DOI] [PubMed] [Google Scholar]
- 7.Norman T, Morse C, Dennerstein L. Comparative bioavailability of orally and vaginally administered progesterone. Fertil Steril 1991;56: 1034–9. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0. The Cochrane Collaboration. Published; 2011, www.cochrane-handbook.org. [Google Scholar]
- 9.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3, rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. [DOI] [PubMed] [Google Scholar]
- 10.Ashoush S, El-Kady O, Al-Hawwary G, Othman A. The value of oral micronized progesterone in the prevention of recurrent spontaneous preterm birth: a randomized controlled trial. Acta Obstet Gynecol Scand 2017;96: 1460–6. [DOI] [PubMed] [Google Scholar]
- 11.Glover MM, McKenna DS, Downing CM, Smith DB, Croom CS, Sonek JD. A randomized trial of micronized progesterone for the prevention of recurrent preterm birth. Am J Perinatol 2011;28:377–81. [DOI] [PubMed] [Google Scholar]
- 12.Rai P, Rajaram S, Goel N, Ayalur Gopalakrishnan R, Agarwal R, Mehta S. Oral micronized progesterone for prevention of preterm birth. Int J Gynecol Obstet 2009;104: 40–3. [DOI] [PubMed] [Google Scholar]
- 13.Likis FE, Edwards DRV, Andrews JC, et al. Progestogens for preterm birth prevention: a systematic review and meta-analysis. Obstet Gynecol 2012;120:897–907. [DOI] [PubMed] [Google Scholar]
- 14.Velez Edwards DR, Likis FE, Andrews JC, et al. Progestogens for preterm birth prevention: a systematic review and meta-analysis by drug route. Arch Gynecol Obstet 2013;287: 1059–66. [DOI] [PubMed] [Google Scholar]
- 15.Jarde A, Lutsiv O, Beyene J, McDonald SD. Vaginal progesterone, oral progesterone, 17OHPC, cerclage, and pessary for preventing preterm birth in at-risk singleton pregnancies: an updated systematic review and network metaanalysis. BJOG 2019;126:556–67. [DOI] [PubMed] [Google Scholar]
- 16.Dodd JM, Jones L, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. Cochrane Database Syst Rev 2013;7: CD004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tavaniotou A, Smitz J, Bourgain C, Devroey P. Comparison between different routes of progesterone administration as luteal phase support in infertility treatments. Hum Reprod Update 2000;6:139–48. [DOI] [PubMed] [Google Scholar]
- 18.Cicinelli E, de Ziegler D, Bulletti C, Matteo MG, Schonauer LM, Galantino P. Direct transport of progesterone from vagina to uterus. Obstet Gynecol 2000;95:403–6. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien JM. The safety of progesterone and 17-hydroxyprogesterone caproate administration for the prevention of preterm birth: an evidence-based assessment. Am J Perinatol 2012;29:665–72. [DOI] [PubMed] [Google Scholar]
- 20.Ndoni E, Bimbashi A, Dokle A, Kallfa E. Treatment with different types of progesterone in prevention of preterm delivery. J Matern Neonatal Med 2010;23(suppl):305. [Google Scholar]
- 21.Choudhary M, Suneja A, Vaid NB, Guleria K, Faridi MM. Maintenance tocolysis with oral micronized progesterone for prevention of preterm birth after arrested preterm labor. Obstet Gynecol Surv 2015;69:581–3. [DOI] [PubMed] [Google Scholar]
- 22.Noblot G, Audra P, Dargent D, Faguer B, Mellier G. The use of micronized progesterone in the treatment of menace of preterm delivery. Eur J Obstet Gynecol Reprod Biol 1991;40:203–9. [DOI] [PubMed] [Google Scholar]
- 23.Pustotina O Effectiveness of dydrogesterone, 17-OH progesterone and micronized progesterone in prevention of preterm birth in women with a short cervix. J Matern Neonatal Med 2018;31:1830–8. [DOI] [PubMed] [Google Scholar]
- 24.Wu SP, DeMayo FJ. Progesterone receptor signaling in uterine myometrial physiology and preterm birth. Curr Top Dev Biol 2017;125: 171–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talati AN, Hackney DN, Mesiano S. Pathophysiology of preterm labor with intact membranes. Semin Perinatol 2017;41:420–6. [DOI] [PubMed] [Google Scholar]
- 26.Manuck TA, Stoddard GJ, Fry RC, Esplin MS, Varner MW. Nonresponse to 17-alpha hydroxyprogesterone caproate for recurrent spontaneous preterm birth prevention: clinical prediction and generation of a risk scoring system. Am J Obstet Gynecol 2016;215:622.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heyborne KD, Allshouse AA, Carey JC. Does 17-alpha hydroxyprogesterone caproate prevent recurrent preterm birth in obese women? Am J Obstet Gynecol 2015;213:844. e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orsulak MK, Block-Abraham D, Gee RE. 17a-Hydroxyprogesterone caproate access in the Louisiana Medicaid population. Clin Ther 2015;37:727–32. [DOI] [PubMed] [Google Scholar]
- 29.Turitz AL, Bastek JA, Purisch SE, Elovitz MA, Levine LD. Patient characteristics associated with 17-alpha hydroxyprogesterone caproate use among a high-risk cohort. Am J Obstet Gynecol 2016;214:536.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. AMAG Pharmaceuticals announces topline results from the PROLONG trial evaluating Makena (hydroxyprogesterone caproate injection). AMAG Pharmaceuticals. Available at: https://www.amagpharma.com/news/amag-pharmaceuticals-announces-toplineresults-from-the-prolong-trial-evaluating-makena-hydroxyprogesterone-caproate-injection/. Accessed March 29, 2019.