Abstract
Aim:
To examine associations between periodontitis and developmental trajectories of glycated haemoglobin (HbA1c) during the third and fourth decades in an initially healthy sample.
Materials and methods:
HbA1c data collected at ages 26, 32 and 38 in the prospective Dunedin Multidisciplinary Health and Development Study were used to assign study members (n=893) to trajectories applying group-based trajectory modelling (GBTM). The model allowed the statistical linking of baseline demographic, smoking and waist-height ratio covariates to group membership probability; and added a time-varying covariate (periodontitis) to the trajectories themselves to examine whether events that occurred during the course of the trajectory altered its course.
Results:
Three HbA1c trajectory groups were identified: “Low” (n=98, 11.0%); “Medium” (n=482, 54.0%); and “High” (n=313, 35.0%) with mean HbA1c of 29.6 mmol/mol, 34.1 mmol/mol, and 38.7 mmol/mol respectively at age 38. Having periodontitis at 32 and 38 was associated with an upward shift in the trajectories. However, none of the associations were statistically significant.
Conclusions:
Periodontitis was not found to be associated with dysglycaemia over 12 years from early adulthood into early middle age. This suggests that any influence periodontitis may have on dysglycaemia develops later in life.
Keywords: Periodontitis, Glycated hemoglobin, Type 2 diabetes, Longitudinal, Waist-height ratio, Smoking
Introduction
The possibility of periodontitis being a risk factor for dysglycaemia has been recognised for some time (Borgnakke et al., 2013; Demmer et al., 2010; Morita et al., 2012; Preshaw et al., 2012; Saito et al., 2004; Taylor et al., 1996). Most studies have focused on: participants who were already diabetic (or the study had diabetes incidence as an outcome) (Morita et al., 2012; Taylor et al., 1996); middle-aged or older people (Morita et al., 2012; Saito et al., 2004); or population-based samples with the full range of adult age groups (Demmer et al., 2010; Taylor et al., 1996). Only two studies have reported a temporal association whereby periodontitis preceded dysglycaemia or deterioration in glycated haemoglobin (HbA1c) levels (Demmer et al., 2010; Morita et al., 2012).
Moreover, what little research has been conducted to date on the associations between periodontitis and early dysglycaemia/prediabetes has all been cross-sectional (Arora et al., 2014; Kowall et al., 2015; Lamster et al., 2014; Saito et al., 2004). None has specifically examined associations in an initially healthy sample during their third and fourth decades (a potentially important time for intervention aimed at preventing progression to type 2 diabetes).
In addition, only a few studies have used glycated haemoglobin (HbA1c) to define or quantify dysglycaemia (Demmer et al., 2010; Lamster et al., 2014; Morita et al., 2012). This is a clear gap in the research, as HbA1c is increasingly being recommended and used for the screening and diagnosis of diabetes (American Diabetes Association, 2014; The International Expert Committee, 2009; World Health Organization, 2011).
Accordingly, the objective of this study was to examine the influence of periodontitis on developmental trajectories of HbA1c identified using group-based trajectory modelling (GBTM) during the third and fourth decades of life in an initially healthy sample.
Material and Methods
This study used HbA1c and periodontal data collected during the age-26, age-32 and age-38 assessments of the Dunedin Multidisciplinary Health and Development Study (DMHDS). The DMHDS is a longitudinal epidemiological study of a birth cohort of 1,037 children born at the Queen Mary Hospital, Dunedin, New Zealand between 1 April 1972 and 31 March 1973 (Poulton et al., 2015). These 1037 children represent 91% of the 1139 eligible children; the 9% not included did not differ from the 91% with respect to sociodemographic and perinatal characteristics. The study has a very high retention rate (over 95% of the surviving cohort at each age), and a wealth of physical, mental and psychosocial data. Ethics approval for the study was granted by the Otago Research Ethics Committee, and participants gave informed consent.
HbA1c was measured during assessments at 26, 32, and 38 (Shearer et al., 2016). Blood samples collected at 26 and 32 were assayed using ion-exchange high performance liquid chromatography on a BioRad Variant II, and at 38 on a BioRad Variant II Turbo (Bio-Rad, Hercules, California). No HbA1c data were collected for pregnant women, and four individuals with known Type 1 diabetes were excluded from all analyses. In New Zealand, HbA1c is now exclusively reported using the International Federation of Clinical Chemistry (IFCC) SI units of mmol/mol (Braatvedt et al., 2012). Therefore we have reported findings in IFCC units and converted them to National Glycohaemoglobin Standardization Program (NGSP) % units using the master equation: NGSP-HbA1c (%) = [0.09148*IFCC-HbA1c (mmol/mol)] + 2.152.
Periodontal examinations were conducted at ages 26, 32 and 38, with half-mouth examinations at age 26, but full-mouth examinations at ages 32 and 38. Third molars and implants were excluded from examination, and Study members with a history of cardiac valvular abnormalities or rheumatic fever were not examined. Three sites (mesiobuccal, buccal, and distolingual) per tooth were examined using an NIDR probe (the Hu-Friedy PCP-2). All measurements were rounded down to the nearest whole millimeter. Two measures were recorded: gingival recession (GR; the distance in millimetres from the gingival margin to the cemento-enamel junction) and probing depth (PD; the distance from the gingival margin to the tip of the probe). GR was recorded as a negative where the gingival margin was situated more than 1mm coronally to the cemento-enamel junction. The attachment loss (AL) for each site was computed by summing the GR and PD measurements. At age 26, dental examinations were conducted by three examiners who had been previously calibrated. Due to time limitations at age 26, periodontal measurements were made in only two quadrants; quadrants 1 and 3 for those whose study ID number was odd, and quadrants 2 and 4 for those whose study ID number was even. The mix of odd and even numbers was approximately even. At ages 32 and 38, the clinical procedures were identical, except that a full-mouth examination was now possible. Full-mouth data at ages 32 and 38 were converted to half-mouth data to allow longitudinal comparisons with the age 26 data. Two and three calibrated examiners were used at ages 32 and 38 respectively. Replicate examinations were not carried out at age 26 due to time constraints, but intra- and inter-examiner reliability at ages 32 and 38 were acceptable (Thomson et al., 2006; Zeng et al., 2014). At each age, two different case definitions for the prevalence of periodontal disease were determined by identifying Study members with 1+ sites with 4+mm AL and with 2+ sites with 4+mm AL (Thomson et al., 2007).
Measures of socioeconomic status (SES) at 26, 32 and 38 were obtained from Study members (Milne et al., 2013). Each scale applied a six-interval, occupationally-based classification of SES whereby a doctor scores “1” and a labourer scores “6”. Study members with a score of “1” or “2” were allocated to the “High” SES group; those with a score of “3” or “4” were assigned to the “Medium” SES group; and those with a score of “5” or “6” were assigned to the “Low” SES group.
Study members were questioned on their smoking history at 26, 32 and 38. Those who gave a positive response to the question “Have you smoked every day for one month or more of the previous 12 months?” were categorised as current smokers.
Anthropometric parameters were assessed at 26, 32, and 38. Height was determined to the nearest 1 mm using calibrated scales. Waist circumference (WC) was recorded by measuring girth to the nearest 1 mm at the level of the noticeable waist narrowing (approximately halfway between the costal border and the iliac crest). Measurements were taken twice and the mean of two readings calculated. Waist-height ratio was recorded as the ratio of the WC to that of the person’s height and was dichotomized according to established guidelines for greater risk of cardiometabolic complications with ≥0.50 generally regarded as being of higher cardiometabolic risk for both sexes (Ashwell et al., 2012; Browning et al., 2010). We used the waist-height ratio because a previous paper (Shearer et al., 2016) found it to be strongly associated with high HbA1c trajectory group membership, independently of sex, SES and smoking at age 26.
Statistical analysis
Analyses were undertaken using Stata IC 12.0 for Windows (StataCorp 2011, Stata Statistical Software: Release 12, College Station, Tx, USA). The HbA1c data collected at ages 26, 32 and 38 were used to assign study members to trajectories applying GBTM. GBTM identifies groups of distinctive trajectories by gathering individuals into a small number of groups that show statistically similar trajectories; individuals are assigned a probability of group membership (Nagin, 2005).
The HbA1c GBTM analysis—along with a description of the sample—has been described in detail elsewhere (Shearer et al., 2016) and in an online supplement. Briefly, three HbA1c trajectory groups were identified: “Low” (n=98, 11.0%); “Medium” (n=482, 54.0%); and “High” (n=313, 35.0%) with mean HbA1c of 29.6 mmol/mol (4.8%), 34.1 mmol/mol (5.3%), and 38.7 mmol/mol (5.7%) respectively at age 38 (Figure 1).
Fig 1.
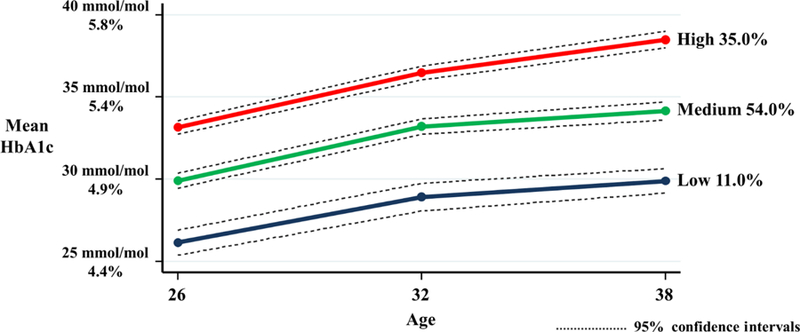
GBTM HbA1c trajectory groups using mean HbA1c (mmol/mol and %) measures at ages 26, 32 and 38.
Bivariate associations between HbA1c trajectories and demographic, periodontal, anthropometric and smoking covariates were tested for statistical significance using Chi-square tests for proportions, and Kruskal-Wallis tests for means. An attrition analysis was conducted for the time-varying covariate GBTM sample. Statistical tests were two-tailed and the threshold for statistical significance was set at p<0.05.
Two generalisations of the GBTM were used to handle covariates. The first linked time-invariant or baseline characteristics (demographic, smoking and waist-height ratio covariates) to the probability of trajectory group membership (Nagin, 2005; Nagin and Odgers, 2010); the coefficients produced can be interpreted in terms of odds ratio (Shearer et al., 2016). The second added a time-varying covariate (periodontitis) to the trajectories themselves to examine whether events that occurred during the course of the trajectory altered its course (Nagin, 2005; Nagin and Odgers, 2010); the coefficients produced are interpreted in terms of how much higher or lower a trajectory is for each unit increase in the time varying covariate. Further details of the model generalisations are in the online supplement.
Results
A total 893 Study members were included in the HbA1c GBTM analysis, and 799 were included in the HbA1c GBTM analysis and had a periodontal examination at all three ages 26, 32 and 38 (Figure 2). Bivariate analyses found that higher proportions of males, those of low SES at 26 and 38, and those who were smokers at 26, 32 and 38, were in the “High” HbA1c trajectory group (Table 1). Mean HbA1c at each age showed clear upward gradients across the three groups from “Low” to “High”. A higher proportion of Study members with 1+ sites with 4+mm AL at 26 and 32, and with 2+ sites with 4+mm AL at 26 and 32, were in the “High” HbA1c trajectory group. Those in the “High” trajectory group had higher mean waist-height ratio at 38, and a higher proportion of high waist-height group members at 26 and 38.
Fig 2.
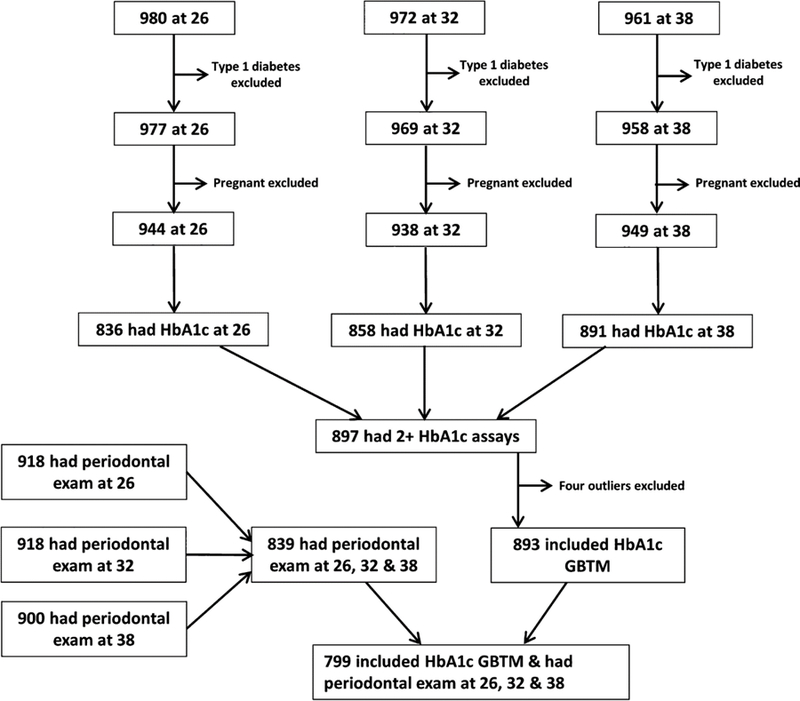
Flow chart for participation rates and sample sizes.
Table 1.
HbA1c trajectory group by demographic, HbA1c and periodontal variables. Percentages or standard deviation in parentheses. N = 799
| HbA1c trajectory group |
|||
|---|---|---|---|
| Low (N=85) | Medium (N=436) | High (N=278) | |
| Demographic characteristics | |||
| Male (N=408, 51.1%) | 40 (47.1) | 202 (46.3) | 166 (59.7)a |
| Low SES at 26 (N=196, 24.5%)1 | 17 (20.2) | 93 (22.7) | 86 (32.0)b |
| Low SES at 32 (N=230, 28.8%) | 18 (21.2) | 130 (29.8) | 92 (29.5) |
| Low SES at 38 (N=146, 18.3%)2 | 5 (5.9) | 72 (16.6) | 69 (24.9)c |
| HbA1c measures | |||
| Mean HbA1c at 26 IFCC (SD)3 [NGSP % units]* | 25.9 (1.9) [4.5%] | 29.9 (2.1) [4.9%] | 33.4 (2.2) [5.2%]d |
| Mean HbA1c at 32 IFCC (SD)4 [NGSP % units]* | 28.5 (2.2) [4.8%] | 33.2 (2.1) [5.2%] | 36.6 (2.2) [5.5%]d |
| Mean HbA1c at 38 IFCC (SD)5 [NGSP % units]* | 29.6 (2.1) [4.9%] | 34.1 (2.3) [5.3%] | 38.8 (2.7) [5.7%]d |
| Periodontitis prevalence | |||
| 1+ sites with 4+mm AL at 26 (N=141, 17.6%) | 8 (9.4) | 73 (16.7) | 60 (21.6)b |
| 1+ sites with 4+mm AL at 32 (N=165, 20.7%) | 10 (11.8) | 84 (19.3) | 71 (25.5)b |
| 1+ sites with 4+mm AL at 38 (N=271, 33.9%) | 23 (27.1) | 148 (33.9) | 100 (36.0) |
| 2+ sites with 4+mm AL at 26 (N=57, 7.1%) | 1 (1.2) | 29 (6.7) | 27 (9.7)b |
| 2+ sites with 4+mm AL at 32 (N=98, 12.3%) | 5 (5.9) | 50 (11.5) | 43 (15.5)b |
| 2+ sites with 4+mm AL at 38 (N=177, 22.2%) | 13 (15.3) | 92 (21.1) | 72 (25.9) |
| Anthropometric characteristics | |||
| Mean waist-height ratio at 26 (SD)6 | 0.46 (0.04) | 0.46 (0.05) | 0.47 (0.06) |
| Mean waist-height ratio at 32 (SD)7 | 0.48 (0.05) | 0.48 (0.06) | 0.50 (0.07)e |
| Mean waist-height ratio at 38 (SD)8 | 0.49 (0.05) | 0.50 (0.07) | 0.51 (0.08)e |
| High waist-height group at 26 (N=167, 21.7%)6 | 11 (13.4) | 79 (18.7) | 77 (28.8)a |
| High waist-height group at 32 (N=291, 37.5%)7 | 26 (32.1) | 150 (35.3) | 115 (42.6) |
| High waist-height group at 38 (N=354, 44.8%)8 | 34 (40.0) | 179 (41.6) | 141 (51.1)b |
| Smoking | |||
| Smoker at 26 (N=304, 38.0%) | 17 (20.0) | 162 (37.2) | 125 (45.0)a |
| Smoker at 32 (N=252, 31.5%) | 15 (17.6) | 133 (30.5) | 104 (37.4)a |
| Smoker at 38 (N=200, 25.1%)9 | 7 (8.2) | 102 (23.4) | 91 (32.9)a |
p<0.005; chi-square test.
p<0.05; chi-square test.
p<0.001; chi-square test
p<0.001; Kruskal–Wallis test.
p<0.05; Kruskal–Wallis test.
It was not possible to convert very small HbA1c values (such as standard deviations) from IFCC to valid NGSP values.
N=762.
N=797.
N=732.
N=759.
N=779.
N=771.
N=776.
N=791.
N=798
The first generalisation of the HbA1c GBTM model (N = 784) linked baseline (age 26) covariates sex, SES, smoking, periodontitis (as measured by the prevalence of 1+ sites with 4+mm AL), and high waist-hip group membership to the probability of group membership. Being male, being a smoker and being in the high waist-height ratio group increased the odds of being in the “High” trajectory; being a smoker also increased the odds of being in the “Medium” trajectory (Table 2).
Table 2.
Adjusted odds ratio (OR) for HbA1c trajectory group membership. N = 784.
| HbA1c trajectory group membership OR (CI) |
|||
|---|---|---|---|
| Low | Medium | High | |
| Male | 1.00 | 0.88 (0.44, 1.75) | 1.69 (0.87, 3.28) |
| Low SES at 26 | 1.00 | 1.22 (0.56, 2.65) | 1.41 (0.67, 2.95) |
| Smoker at 26 | 1.00 | 1.98 (0.95, 4.13) | 3.61 (1.72, 7.58) |
| Periodontitis at 261 | 1.00 | 1.41 (0.56, 3.54) | 1.77 (0.69, 4.52) |
| High waist-height group at 262 | 1.00 | 1.79 (0.71, 4.51) | 3.24 (1.35, 7.77) |
Reference: Low trajectory group. CI; confidence intervals.
Statistically significant associations in bold type.
One or more sites with 4+mm attachment loss.
Waist-height ratio ≥0.50.
For the second generalisation of the HbA1c GBTM model (N = 784), the time-varying covariate periodontitis (as measured by prevalence of 1+ sites with 4+ AL) was added to the trajectories themselves to examine whether periodontitis at 32 and 38 altered the course of the “Low”, “Medium” and “High” HbA1c trajectories. An attrition analysis was conducted for this sample found that there were proportionately fewer individuals of low SES and smokers at 26, 32 and 38 included in this analysis (data not shown).
All of the coefficients were positive, indicating that having periodontitis (as defined by having 1+ sites with 4+mm AL) at 32 and 38 was associated with an upward shift in trajectory (Table 3). Only the “High” trajectory coefficients had confidence intervals that did not contain the value zero. The addition of periodontitis case status to the model raised the BIC to −5628.83. These values represented an improvement of 12.2% over the completely unadjusted (no time-invariant or time-varying covariates) model.
Table 3.
Coefficients for shift in HbA1c trajectory at ages 32 and 38 per unit change for prevalence of 1+ sites with 4+mm AL at 32 and 38. N = 784.
| HbA1c trajectory group |
||||
|---|---|---|---|---|
| Low | Medium | High | BIC | |
| Shift in trajectory | ||||
| Unadjusted model1 | 0.787 (−0.253, 1.827) | 0.245 (−0.146, 0.636) | 0.596 (0.211, 0.982) | −6055.00 |
| Fully adjusted model2 | 0.495 (−0.577, 1.568) | 0.238 (−0.178, 0.653) | 0.562 (0.152, 0.971) | −5628.83 |
Unadjusted model includes time-varying covariate (periodontitis) only.
The fully adjusted model includes both time-invariant covariates (sex, SES at 26, smoking at 26 and membership of the high waist-height group at 26) and the time-varying covariate (periodontitis).
Statistically significant coefficients shown in bold type.
A detailed examination of the predicted values for each of the trajectories confirmed this upward shift associated with periodontitis for the “Low”, “Medium” and “High” trajectories at ages 32 and 38 (Table 4). This shows the predicted values for HbA1c for two scenarios: (1) no periodontitis at any age; and (2) no periodontitis at 26, and periodontitis at 32 and 38. Neither scenario has periodontitis at age 26 so the values are the same at this age. At ages 32 and 38, the differences between the two scenarios can be seen. However, overlapping confidence intervals were seen for all of the parameters. While the upward shift in the trajectories can be seen graphically, the overlapping confidence intervals are also evident (Fig 3). A more detailed explanation of these findings is in the online supplement.
Table 4.
Predicted values for HbA1c GBTM groups (both IFCC mmol/mol and NGSP %) for two scenarios. Scenario 1 has no periodontitis at any age. Scenario 2 has no periodontitis at age 26 and periodontitis at ages 32 and 38. Confidence intervals in parentheses. N = 784.
| HbA1c trajectory group |
|||
|---|---|---|---|
| Low | Medium | High | |
| IFCC (mmol/mol) | |||
| Age 26 | |||
| Scenario 1- No periodontitis at 26, 32 and 38 | 25.6 (24.6, 26.6) | 29.6 (29.1, 30.1) | 32.8 (32.4, 33.2) |
| Scenario 2 - Periodontitis at 32 and 38 | 25.6 (24.6, 26.6) | 29.6 (29.1, 30.1) | 32.8 (32.4, 33.2) |
| Age 32 | |||
| Scenario 1- No periodontitis at 26, 32 and 38 | 28.3 (27.1, 29.4) | 32.9 (32.3, 33.4) | 36.2 (35.8, 36.6) |
| Scenario 2 - Periodontitis at 32 and 38 | 28.7 (27.3, 30.2) | 33.1 (32.5, 33.7) | 36.8 (36.2, 37.2) |
| Age 38 | |||
| Scenario 1- No periodontitis at 26, 32 and 38 | 29.5 (28.5, 30.5) | 33.7 (33.0, 34.2) | 38.2 (37.7, 38.7) |
| Scenario 2 - Periodontitis at 32 and 38 | 30.0 (28.8, 31.2) | 33.9 (33.2, 34.5) | 38.7 (38.2, 39.3) |
| NGSP (%) | |||
| Age 26 | |||
| Scenario 1- No periodontitis at 26, 32 and 38 | 4.5 (4.4, 4.6) | 4.9 (4.8, 4.9) | 5.2 (5.1, 5.2) |
| Scenario 2 - Periodontitis at 32 and 38 | 4.5 (4.4, 4.6) | 4.9 (4.8, 4.9) | 5.2 (5.1, 5.2) |
| Age 32 | |||
| Scenario 1- No periodontitis at 26, 32 and 38 | 4.7 (4.6, 4.8) | 5.2 (5.1, 5.2) | 5.4 (5.4, 5.5) |
| Scenario 2 - Periodontitis at 32 and 38 | 4.8 (4.6, 4.9) | 5.2 (5.1, 5.3) | 5.5 (5.4, 5.5) |
| Age 38 | |||
| Scenario 1- No periodontitis at 26, 32 and 38 | 4.9 (4.8, 5.0) | 5.3 (5.2, 5.3) | 5.6 (5.6, 5.7) |
| Scenario 2 - Periodontitis at 32 and 38 | 4.9 (4.8, 5.0) | 5.3 (5.2, 5.3) | 5.7 (5.6, 5.7) |
Fully adjusted model includes time-invariant covariates sex, SES at 26, smoking at 26 and membership of the high waist-height group at 26, and time-varying covariate prevalence of periodontitis (as measured by prevalence of 1+ sites with 4+mm AL) at 32 and 38.
Fig 3.
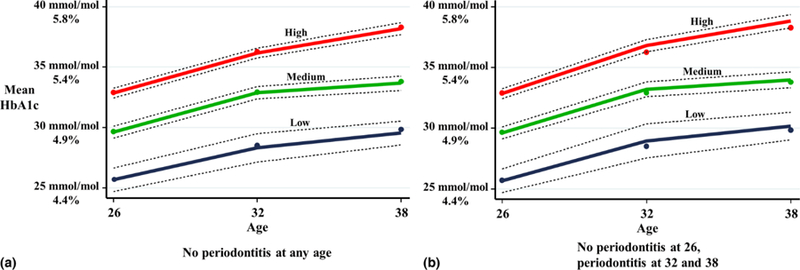
GBTM HbA1c trajectories (mmol/mol and %) for (a) no periodontitis at any age and (b) no periodontitis at age 26, and periodontitis at 32 and 38.
As a sensitivity analysis, this generalisation was carried out for the more rigorous periodontitis case definition of 2+ sites with 4+mm AL. The findings were similar to those for 1+ sites with 4+mm AL analysis (see online supplement). In addition, the generalisation was also carried out using body mass index (BMI) as the obesity measure, and the findings were almost identical to those in which waist-height ratio was used (see online supplement). Finally, a second analysis of the data was carried out using linear mixed effect modeling which integrates both fixed and random effects, and this found no associations between mean AL and HbA1c (see online supplement).
Discussion
The DMHDS data have provided a unique opportunity to explore the associations between periodontitis and dysglycaemia in an initially healthy population over 12 years from early adulthood into early middle age. This study is the first to examine the longitudinal associations between periodontitis and dysglycaemia, as measured by HbA1c, at an early stage in the glycaemia continuum. GBTM was used to track developmental trajectories of HbA1c over 12 years (Shearer et al., 2016). This paper used a GBTM generalisation to identify effect modifiers associated with deviations from the group trajectory. In this way, associations of periodontitis with the HbA1c trajectories in the fourth decade of life were explored. A minimal difference in mean HbA1c was seen between those with periodontitis, and those without periodontitis. However, overlapping confidence intervals between those with and those without periodontitis meant that an effect by periodontitis on HbA1c trajectory groups could not be established.
The strengths of this study include the use of a birth cohort with a very high retention rate; standardised age; comprehensive objective data on both periodontitis and glycaemia at three points over 12 years; and the use of GBTM to explore the influence of periodontitis on the HbA1c trajectories in the fourth decade of life. The former means that sample is representative of its source population (the South Island of New Zealand). The issue of whether the findings can be generalised to the whole New Zealand population, and to other populations (particularly the U.S.) must be considered. A 2006 paper provided broad support for the generalisability of the Dunedin Study findings to the total New Zealand population (Poulton et al., 2006). Another paper concluded that the periodontal findings from the DMHDS can be generalised to the New Zealand and U.S. populations (Thomson et al., 2006). The prevalence of dysglycaemia in the cohort was consistent with data from the U.S. (Marcinkevage et al., 2013) and the U.K. (Wilmot et al., 2013), and it is therefore likely that the findings are generalisable to these populations. The use of GBTM to identify latent HbA1c trajectory groups is a further strength of this study. The main advantage of GBTM over other trajectory modelling techniques is that it does not determine a priori the existence of trajectories of a specific form; rather, it allows distinctive latent developmental trajectories to emerge from the data (Nagin, 2005). It facilitates the examination of factors that may determine trajectory group membership or trajectory course, and it enables the dissemination and communication of findings in a form which is readily understood by non-technical audiences. In addition, while any attempt to categorise risk must be regarded as somewhat arbitrary, GBTM differs from the practice of defining thresholds for normoglycemia, prediabetes and diabetes; essentially, those are stages in the natural history of dysglycemia rather than separate diseases. GBTM instead characterizes risk in terms of a subpopulation’s developmental trajectory, and it provides a different perspective on identifying those most at risk. Generalisations of the model allowed the statistical linking of baseline characteristics to group membership probability, and identified effect modifiers associated with deviations from the group trajectory.
Some limitations must be acknowledged. Although the proportion (7.5%) who self-identify as Māori at age 26 in the cohort does match the proportion of Māori in the South Island, Māori are under-represented with respect to the total New Zealand Maori (14.9% in the 2013 Census) population. The DMHDS data are right-censored. Data to age 38 have been gathered, but we have no information on what will happen beyond this age. No appropriate data on diet were available. The ideal protocol for periodontal studies is the full-mouth examination of six sites per tooth (Papapanou, 2012; Savage et al., 2009). Although full-mouth periodontal examinations were carried out at ages 32 and 38, it was necessary to convert these to half-mouth data to allow longitudinal comparisons with the age 26 data. In addition, recordings were made from three sites only per tooth. This has likely led to underestimation of the prevalence of periodontitis, and possibly an attenuation of the strength of the associations between periodontitis and dysglycaemia. However, research has shown that, of the three random half-mouth protocols in general use, the one used in the DMHDS (three-site mesio-buccal, mid-buccal, and disto-lingual) produced less bias than the others (Susin et al., 2005). Finally, the power of the study may have been a limitation with a small number of participants with periodontitis in the “Low” trajectory group.
There is substantial evidence that periodontitis is associated with type 2 diabetes; it is plausible that the chronic inflammation of periodontitis may influence glycaemic control in susceptible individuals. However, there have been conflicting findings, with some studies suggesting that periodontitis may influence HbA1c levels, IGT and glycaemic control in those with diabetes or diabetes risk (Arora et al., 2014; Borgnakke et al., 2013; Choi et al., 2011; Demmer et al., 2010; Lamster et al., 2014; Morita et al., 2012; Saito et al., 2004; Saito et al., 2006), and others finding no associations (Kowall et al., 2015; Saito et al., 2005). The three studies by Saito and colleagues on Japanese samples had inconsistent findings: a greater risk of progression to IGT with greater periodontal probing depth (Saito et al., 2004); no associations between IGT and either deep pocketing or severe attachment loss in a female-only sub-sample of the previous study (Saito et al., 2005); and associations between alveolar bone loss and IGT in a different sample (Saito et al., 2006). A 2010 German paper found periodontitis at baseline in non-diabetics to be associated with HbA1c progression five years later (Demmer et al., 2010), while a biological gradient was observed between periodontitis and IFG in a US sample (Choi et al., 2011). Another Japanese study found associations between periodontitis at baseline and the risk of higher HbA1c five years later (Morita et al., 2012). Lamster et al. found that participants with previously unidentified pre-diabetes had periodontitis experience at a level between those observed for normoglycaemic participants and those with diabetes (Lamster et al., 2014). By contrast, a recent SHIP-Trend study found no association between pre-diabetes and the extent of teeth with 4mm+ AL or mean probing depth (Kowall et al., 2015). Meanwhile, the Continuous NHANES 2009–2010 survey found associations between severe periodontitis and IGT, but not IFG, and no associations between moderate periodontitis and either IGT or IFG (Arora et al., 2014). Of these studies, one was retrospective (Saito et al., 2004) and two were prospective (Demmer et al., 2010; Morita et al., 2012). However, participation/retention rates were low, so the findings were not generalisable to the source populations.
Comparisons between the present study and others are not straightforward. First, the age groups in the other studies were both older and (with the exception of the 2006 Saito et al paper) had a wider range of ages. The Saito studies had participants aged 40 years and older (Saito et al., 2004; Saito et al., 2005) and 50–55 years (Saito et al., 2006). Both the SHIP and the NHANES III samples were 20 years and older (Choi et al., 2011; Demmer et al., 2010). The Morita et al sample was aged 30–69 years; the Columbia University study sample was age 30 and over; the Continuous NHANES 2009–2010 sample was ages 30–80; and the SHIP-Trend sample was ages 20–82 (Arora et al., 2014; Kowall et al., 2015; Lamster et al., 2014; Morita et al., 2012).
Second, studies have used different definitions for dysglycaemia. Most papers used IGT as an indicator of dysglycaemia (Choi et al., 2011; Kowall et al., 2015; Saito et al., 2004; Saito et al., 2005; Saito et al., 2006); changes in mean HbA1c were used in two studies (Demmer et al., 2010) (Morita et al., 2012); and HbA1c levels recorded by a point-of-care device were used in one (Lamster et al., 2014). Another study found that whether there were associations or not depended on how dysglycaemia was assessed: either by IGT or IFG (Arora et al., 2014). The present study used mean HbA1c to track glycaemic levels over the 12 years. While no method of measurement is perfect, research indicates (and many organisations are currently recommending) that HbA1c be used for screening and diagnosis of diabetes (American Diabetes Association, 2014; The International Expert Committee, 2009; World Health Organization, 2011).
Third, there was even more variation among studies in how periodontitis experience was defined. Globally, there is a lack of standardisation and agreement in establishing criteria for the diagnosis of the condition, so comparisons among studies are problematic (Hugoson and Norderyd, 2008; Leroy et al., 2010; Page and Eke, 2007). Within the studies examining the associations between periodontitis and diabetes, periodontitis was variously assessed by the Community Periodontal Index of Treatment Need (Morita et al., 2012), clinical attachment loss (AL) and pocket depth (Arora et al., 2014; Choi et al., 2011; Demmer et al., 2010; Kowall et al., 2015; Saito et al., 2004; Saito et al., 2005), pocket depth only (Lamster et al., 2014), and by alveolar bone loss determined radiographically (Saito et al., 2006). Most periodontal examinations (including the age-26 data in the present study) were half-mouth only (Choi et al., 2011; Demmer et al., 2010; Kowall et al., 2015; Saito et al., 2004; Saito et al., 2005); full-mouth recordings were made in only two studies, and those were both cross-sectional (Arora et al., 2014; Lamster et al., 2014). For the current study, it was necessary to convert the age 32 and 38 measures to half-mouth data, and data were gathered from only three sites per tooth.
The current study focused on the 12 years between ages 26 and 38; this is a time of life when the foundations of poor health are being laid down, but it may also be a developmental epoch in which it is just too early to see clear associations between periodontitis and dysglycaemia. While the prevalence of both conditions was relatively high in the Dunedin cohort by age 38, the experience of neither was very severe. We could not demonstrate periodontitis to be a predictor of dysglycaemia at this early stage in the life course in this initially healthy cohort. Since both periodontitis and dysglycaemia generally worsen with time, it is possible that such associations may develop as the cohort ages (Kassebaum et al., 2014; Pani et al., 2008).
Conclusions
In this initially healthy population, periodontitis was not found to be associated with dysglycaemia over 12 years from early adulthood into early middle age. This suggests that any influence periodontitis may have on glycaemic health develops later in life. This finding adds to the theoretical understanding of how these two conditions interrelate, and it gives rise to new hypotheses. It is possible that dysglycaemia may need to reach a particular threshold of severity to be influenced by periodontitis, or periodontitis may need to reach a particular threshold of severity to exert an influence on dysglycaemia, or a combination of the two. It is essential to continue to study the complex links and temporal relationship between the two conditions in order to understand how they develop and interact over the next decades of life.
Supplementary Material
Clinical Relevance.
Scientific rationale for study:
Research conducted to date on the associations between periodontitis and early dysglycaemia/prediabetes has all been cross-sectional, and none has specifically examined associations in an initially healthy population from early adulthood into early middle age.
Principal findings:
Periodontitis could not be demonstrated to be a predictor of dysglycaemia at this early stage in the life course.
Practical implications:
This suggests that any influence periodontitis may have on glycaemic health develops later in life. It is possible that periodontitis may need to reach a particular threshold of severity to exert an influence on dysglycaemia.
Acknowledgements.
The authors thank the DMHDS Study members, and their families and friends, for their continuing participation in the Dunedin Study. We also thank Unit research staff and the Study founder, Dr Phil Silva. We are grateful to Professors Terrie Moffitt and Avshalom Caspi (Departments of Psychology and Neuroscience and Psychiatry and Behavioural Science, and Institute for Genome Sciences and Policy, Duke University, Durham, North Carolina) for their support and collection of the HbA1c data.
Funding. The Dunedin Multidisciplinary Health and Development Research Unit is supported by the Health Research Council of New Zealand (NZ HRC). The age-26 dental data collection was supported by the New Zealand Dental Association Research Foundation and the University of Otago. The age-32 dental data collection was supported by from the National Institute of Dental and Craniofacial Research, National Institutes of Health (grant R01 DE-015260-01A1), and a programme grant from the NZ HRC. The age-38 data collection was supported by a programme grant from the NZ HRC. This work was also supported by the following grants: the UK Medical Research Council (grants G0100527, G0601483 and MR/K00381X), the National Institute of Mental Health (grants MH45070 and MH49414), the US National Institute of Health/National Institute on Aging (grants AG032282, R01AG032282 and R01AG048895), and the UK Economic and Social Research Council grant ES/M010309/1.
Footnotes
Competing interests: The authors declare that there are no competing interests associated with this manuscript.
References
- American Diabetes Association (2014). Diagnosis and classification of diabetes mellitus. Diabetes Care 37, S81–90. [DOI] [PubMed] [Google Scholar]
- Arora N, Papapanou PN, Rosenbaum M, Jacobs DR Jr., Desvarieux M, Demmer RT (2014). Periodontal infection, impaired fasting glucose and impaired glucose tolerance: results from the Continuous National Health and Nutrition Examination Survey 2009–2010. Journal of Clinical Periodontology 41, 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwell M, Gunn P, Gibson S (2012). Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obesity reviews 13, 275–286. [DOI] [PubMed] [Google Scholar]
- Borgnakke WS, Ylöstalo PV, Taylor GW, Genco RJ (2013). Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. Journal of Clinical Periodontology 40, S135–S152. [DOI] [PubMed] [Google Scholar]
- Braatvedt GD, Cundy T, Crooke M, Florkowski C, Mann JI, Lunt H et al. (2012). Understanding the new HbA1c units for the diagnosis of Type 2 diabetes. New Zealand Medical Journal 125, 70–80. [PubMed] [Google Scholar]
- Browning LM, Hsieh SD, Ashwell M (2010). A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0.5 could be a suitable global boundary value. Nutrition Research Reviews 23, 247–269. [DOI] [PubMed] [Google Scholar]
- Choi YH, McKeown RE, Mayer-Davis EJ, Liese AD, Song KB, Merchant AT (2011). Association between periodontitis and impaired fasting glucose and diabetes. Diabetes Care 34, 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmer RT, Desvarieux M, Holtfreter B, Jacobs DR Jr., Wallaschofski H, Nauck M et al. (2010). Periodontal status and A1C change: longitudinal results from the study of health in Pomerania (SHIP). Diabetes Care 33, 1037–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugoson A, Norderyd O (2008). Has the prevalence of periodontitis changed during the last 30 years? Journal of Clinical Periodontology 35, S338–S345. [DOI] [PubMed] [Google Scholar]
- Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W (2014). Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. Journal of Dental Research 93, 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowall B, Holtfreter B, Volzke H, Schipf S, Mundt T, Rathmann W et al. (2015). Pre-diabetes and well-controlled diabetes are not associated with periodontal disease: the SHIP Trend Study. Journal of Clinical Periodontology 42, 422–430. [DOI] [PubMed] [Google Scholar]
- Lamster IB, Cheng B, Burkett S, Lalla E (2014). Periodontal findings in individuals with newly identified pre-diabetes or diabetes mellitus. Journal of Clinical Periodontology 41, 1055–1060. [DOI] [PubMed] [Google Scholar]
- Leroy R, Eaton KA, Savage A (2010). Methodological issues in epidemiological studies of periodontitis--how can it be improved? BMC oral health 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkevage JA, Alverson CJ, Narayan KM, Kahn HS, Ruben J, Correa A (2013). Race/ethnicity disparities in dysglycemia among U.S. women of childbearing age found mainly in the nonoverweight/nonobese. Diabetes Care 36, 3033–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne B, Byun U, Lee A (2013). New Zealand socio-economic index 2006 Wellington: Statistics New Zealand. [Google Scholar]
- Morita I, Inagaki K, Nakamura F, Noguchi T, Matsubara T, Yoshii S et al. (2012). Relationship between periodontal status and levels of glycated hemoglobin. Journal of Dental Research 91, 161–166. [DOI] [PubMed] [Google Scholar]
- Nagin DS (2005). Group-Based Modeling of Development Cambridge, MA: Harvard University Press. [Google Scholar]
- Nagin DS, Odgers CL (2010). Group-based trajectory modeling in clinical research. Annual Review of Clinical Psychology 6, 109–138. [DOI] [PubMed] [Google Scholar]
- Page RC, Eke PI (2007). Case definitions for use in population-based surveillance of periodontitis. Journal of Periodontology 78, S1387–S1399. [DOI] [PubMed] [Google Scholar]
- Pani LN, Korenda L, Meigs JB, Driver C, Chamany S, Fox CS et al. (2008). Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001–2004. Diabetes Care 31, 1991–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapanou PN (2012). The prevalence of periodontitis in the US: forget what you were told. Journal of Dental Research 91, 907–908. [DOI] [PubMed] [Google Scholar]
- Poulton R, Hancox R, Milne B, Baxter J, Scott K, Wilson N (2006). The Dunedin Multidisciplinary Health and Development Study: are its findings consistent with the overall New Zealand population? New Zealand Medical Journal 119, U2002. [PubMed] [Google Scholar]
- Poulton R, Moffitt TE, Silva PA (2015). The Dunedin Multidisciplinary Health and Development Study: overview of the first 40 years, with an eye to the future. Social Psychiatry and Psychiatric Epidemiology 50, 679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K et al. (2012). Periodontitis and diabetes: a two-way relationship. Diabetologia 55, 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Iida M et al. (2004). The Severity of Periodontal Disease is Associated with the Development of Glucose Intolerance in Non-diabetics: The Hisayama Study. Journal of Dental Research 83, 485–490. [DOI] [PubMed] [Google Scholar]
- Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Iida M et al. (2005). Relationship between obesity, glucose tolerance, and periodontal disease in Japanese women: the Hisayama study. Journal of Periodontal Research 40, 346–353. [DOI] [PubMed] [Google Scholar]
- Saito T, Murakami M, Shimazaki Y, Matsumoto S, Yamashita Y (2006). The extent of alveolar bone loss is associated with impaired glucose tolerance in Japanese men. Journal of Periodontology 77, 392–397. [DOI] [PubMed] [Google Scholar]
- Savage A, Eaton KA, Moles DR, Needleman I (2009). A systematic review of definitions of periodontitis and methods that have been used to identify this disease. Journal of Clinical Periodontology 36, 458–467. [DOI] [PubMed] [Google Scholar]
- Shearer DM, Thomson WM, Broadbent JM, McLean R, Poulton R, Mann J. (2016). High risk glycated hemoglobin trajectories established by mid-twenties: findings from a birth cohort study. BMJ Open Diabetes Res Care 4:1 e000243 doi: 10.1136/bmjdrc-2016-000243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susin C, Kingman A, Albandar JM (2005). Effect of partial recording protocols on estimates of prevalence of periodontal disease. Journal of Periodontology 76, 262–267. [DOI] [PubMed] [Google Scholar]
- Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC et al. (1996). Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. Journal of Periodontology 67, S1085–S1093. [DOI] [PubMed] [Google Scholar]
- The International Expert Committee (2009). International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32, 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson WM, Broadbent JM, Poulton R, Beck J (2006). Changes in periodontal disease experience from 26 to 32 years of age in a birth cohort. Journal of Periodontology 77, 947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson WM, Broadbent JM, Welch D, Beck J, Poulton R (2007). Cigarette smoking and periodontal disease among 32-year-olds: a prospective study of a representative birth cohort. Journal of Clinical Periodontology 34, 828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmot EG, Edwardson CL, Biddle SJ, Gorely T, Henson J, Khunti K et al. (2013). Prevalence of diabetes and impaired glucose metabolism in younger ‘at risk’ UK adults: insights from the STAND programme of research. Diabetic Medicine 30, 671–675. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2011). Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation Geneva. [PubMed] [Google Scholar]
- Zeng J, Williams SM, Fletcher DJ, Cameron CM, Broadbent JM, Shearer DM et al. (2014). Reexamining the association between smoking and periodontitis in the dunedin study with an enhanced analytical approach. Journal of Periodontology 85, 1390–1397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


