Abstract
Since the intentional release of Bacillus anthracis spores through the U.S. Postal Service in the fall of 2001, research and development related to decontamination for this biological agent have increased substantially. This review synthesizes the advances made relative to B. anthracis spore decontamination science and technology since approximately 2002, referencing the open scientific literature and publicly available, well-documented scientific reports. In the process of conducting this review, scientific knowledge gaps have also been identified. This review focuses primarily on techniques that are commercially available and that could potentially be used in the large-scale decontamination of buildings and other structures, as well as outdoor environments. Since 2002, the body of scientific data related to decontamination and microbial sterilization has grown substantially, especially in terms of quantifying decontamination effcacy as a function of several factors. Specifically, progress has been made in understanding how decontaminant chemistry, the materials the microorganisms are associated with, environmental factors, and microbiological methods quantitatively impact spore inactivation. While advancement has been made in the past 15 years to further the state of the science in the inactivation of bacterial spores in a decontamination scenario, further research is warranted to close the scientific gaps that remain.
Graphical Abstract

INTRODUCTION
Although the military has been developing decontamination methods for their purposes for decades,1 research conducted or funded by nondefense government agencies with a focus on the decontamination of civilian facilities had been minimal prior to 2001. That changed following the intentional release of Bacillus anthracis spores through the U.S. Postal Service in the fall of 20012 (referred to as the Amerithrax attack), with research and development (R&D) related to decontamination for this bioterrorism agent increasing significantly since then.3 (B. anthracis is the bacterium causing anthrax disease, and can infect livestock, wildlife, and humans.4) One driver for this new research was the overall cost of the remediation efforts across the United States, which was estimated to have been approximately $320 million.5 It required the use of sporicidal chemicals to essentially sterilize large buildings, which was unprecedented.6 This is because bacterial spores are one of the most resistant microbial forms to inactivate with biocides,7 and may survive for centuries if left undisturbed.8 When aerosolized, bacterial spores such as those of B. anthracis can remain aloft for hours and thus have the potential to widely disperse,9 greatly expanding the extent of contamination, and further exacerbating recovery efforts.
Other more recent, unintentional incidents of B. anthracis contamination, whether naturally occurring10 or manmade,11 continue to demonstrate the need to advance decontamination science and technology. Examples include several incidents of contamination and numerous fatalities resulting from drumming-associated activities or heroin use, both traced to B. anthracis-contaminated goat skins.12 The recently developed National Biodefense Strategy further attests to the need to develop decontamination approaches for all biological threats, regard-less of their origin.13
A few years following the Amerithrax incident, a review of B. anthracis spore inactivation techniques2 and a compilation of building decontamination alternatives14 were published. Two other articles have been published since then that provide overviews of decontamination approaches for B. anthracis.3,15 While these previous review articles provide helpful, qualitative summaries of decontamination approaches and operational aspects that could be employed after a release of B. anthracis spores, they generally lack detailed data to document the conditions in which the decontaminants are effective.
This current review highlights the scientific and technological advances made relative to B. anthracis spore decontamination technologies since these initial reviews, that is, from approximately 2002, and identifies knowledge gaps. The scientific advances gained over the past 15 years or so primarily include the development of a large amount of data and information related to the chemistry and environmental conditions in which these decontaminants are effective. Thus, we have taken a more quantitative approach, vis-à-vis the synopsis of effcacy data for numerous decontamination techniques. The data are presented as a function of the materials the spores are deposited on, and also other important factors such as the chemistry (e.g., active ingredient concentration, contact time, dosage) and environ-mental (e.g., materials, temperature, humidity) conditions in which they are effective. Lastly, while the primary focus of our review is on B. anthracis, we present data for other spore-forming microorganisms as well. Many of the techniques discussed in this review for the inactivation of B. anthracis spores can also be effectively used for other virulent spore-producing bacteria that present public health concerns.
MATERIALS AND METHODS
There are numerous bacterial spore inactivation techniques of various scale, readiness, and application. Therefore, to provide a more succinct review, we have limited the scope to techniques that can be used at a relatively larger scale (such as for a building) and are commercially viable. Further, our review focuses on both liquid- and gaseous-based chemistries, as well as a few physical-based techniques. Liquid decontaminants are primarily used for surfaces, whereas gases are employed for volumetric decontamination, that is, inactivation of spores on surfaces as well as aerosolized spores, in large enclosed areas. Additionally, the following is a review of the scientific literature, limited to peer-reviewed journals and government reports, if the reports are publicly available and the methods are suffciently documented.
Scope of Technologies Under Review.
While there are well-established techniques for sterilization of materials such as foodstuffs, medical instruments, and pharmaceuticals, these techniques are typically confined for use on a small scale or may not be easily transportable (e.g., because of the use of hazardous or radioactive materials). Examples include ethylene oxide (which is flammable and carcinogenic),16,17 as well as ionizing radiation in the forms of gamma irradiation,18,19 X-rays,20 and electron beam.21 Although excluded from the scope of this review paper, some of the above techniques were used to decontaminate certain items following the Amerithrax attacks,3 and thus would be considered as decontamination tools for small, valuable, or personal items that would be sensitive to chemical exposure (e.g., cash, mail, jewelry, artwork, mechanical devices, electronics).
There are several emerging decontamination and sterilization techniques undergoing extensive R&D, such as the use of cold atmospheric plasma.22,23 For these newer techniques that have undergone significant development but are not quite commercialized, we have provided a brief synopsis in the Supporting Information (SI). While physical removal of bacterial spores from a substrate (e.g., via washing or vacuuming24,25) may be considered “decontamination”, and may play a role in an overall remediation plan for a wide area contamination event,3 for brevity we have focused this review only on techniques that inactivate spores (e.g., chemical, irradiative). Prophylactic approaches that would inactivate spores that come in contact with a material embedded with an antimicrobial26,27 are also excluded from this review.
Since the focus of this review is on decontamination, which implies the presence of a surface, material, fomite, or reusable object, our review generally excludes spore inactivation techniques or studies that do not involve the use of substrates, such as those involving liquid suspensions, water treatment,28 water infrastructure,29 and wastewater treatment.30 Spores are much more difficult to inactivate when they are deposited on a surface compared to being suspended in a liquid.31 Similarly, spores suspended in air are more readily inactivated (at least by gaseous or physical-based decontaminants) compared to spores associated with a surface, since there are no chemical and/or physical interactions between the microorganism, the material, and the decontaminant to diminish effcacy. For this reason, we have this excluded aerosol studies (e.g., see Grinshpun et al.32). Nevertheless, we acknowledge that the health effects associated with inhalation of aerosolized B. anthracis spores are more severe (higher morbidity and mortality) than with dermal contact of a contaminated surface.33
As we emphasize throughout this review, the contaminated material is a predominant factor in the effcacy of the technology and has often been overlooked in previous reviews for spore inactivation or decontamination techniques. For example, hard, nonporous, and inorganic materials (e.g., glass, stainless steel) are typically easier to effectively decontaminate than porous or organic materials (e.g., ceiling tile, soil). From a mechanistic standpoint, many decontaminants rely on oxidation chemistry, and so spores associated with organic materials tend to be inactivated less effectively. Porous materials may provide micro-locations for spores to escape contact with decontaminants. These material interactions and chemical mechanisms affecting effcacy are discussed throughout this review. Thus, we synthesize in this review the chemistry of the decontaminants as well as the complexity of surfaces and materials found outside a hospital, clean room, or laboratory that make the task of decontamination following a B. anthracis spore release highly challenging, and for which rigorous effcacy testing using realistic materials and applications is critical.34
Focus on Decontamination Effcacy.
We emphasize in this review decontamination effcacy, which is typically reported in terms of log10 reduction (LR), in which LR = log10 (mean CFU (colony forming units) recovered from positive control carriers) − log10 (mean CFU recovered from decontaminated carriers).35 (Positive controls are the carriers or coupons inoculated with bacterial spores but not exposed to the decontaminant.) Effcacy is a function of the myriad experimental variables that may be investigated, and as discussed above, the contaminated material is a major factor. (The presence of soil or organic loading on a material may also diminish the decontaminant’s effcacy, especially if the spore inactivation mechanism for the decontaminant is oxidation. Therefore, most registered antimicrobials require a clean surface before use.) Other factors affecting effcacy that we discuss in this review include decontaminant chemistry and related characteristics (e.g., chemical concentration, mass applied to surface, contact time (CT)), environmental conditions (e.g., temperature, relative humidity (RH)), and microbiological factors (species, strain, spore loading,36 spore preparation, inoculation method,37 extraction method, active ingredient neutralization method, etc.). This multitude of factors that may affect effcacy has typically been poorly elucidated in previous reviews. Most of the techniques we review here utilize chemical-based sporicides, but we also discuss two physical-based sterilization techniques (e.g., heat treatment38 and ultraviolet light39). Nearly all the tests described in this review were conducted at laboratory ambient temperature (20−25 °C); decontamination effcacy data for lower temperatures (pertinent to outdoor decontamination) is a research gap.
In this review, we refer to a decontamination technique as “effective” against bacterial spores on a material if effcacy is ≥6 LR. Note, however, that a decontamination test result may also be considered effective if the population of spores on the material is completely inactivated, even when the recovery of spores from the positive control is less than 6 log CFU and hence resulting in <6 LR. The 6 LR criterion originates from guidance established for effcacy testing of antimicrobial products with claims to inactivate B. anthracis spores on inanimate surfaces,40 using sporicidal test methods such as AOAC International Method 2008.0541 with virulent B. anthracis spores or a surrogate. Additionally, refer to Ryan et al.37 and other references7,42 for further information related to the use of effcacy test methods for antimicrobial pesticides and sporicidal decontaminants, how test methods and carrier material can affect effcacy results, and related policy implications thereof. Larger-scale decontamination tests utilizing an aerosol release of spores may use surface sampling followed by analysis using culture or PCR to characterize effcacy.43
To allow for reporting additional granularity of data, in this review we refer to a decontamination test result as “moderately effective” if LR is 3.00−5.99, and “ineffective” if LR is <3.00. We acknowledge that some sporicidal effcacy test methods and associated policy result in only a “pass/fail” determination, and do not allow for use of a “moderately effective” nomenclature. Lastly, in an actual contamination incident with B. anthracis spores, offcials may require no detectable spores as a remediation goal, and thus the required LR in an actual incident would depend on the contamination level. Alternatively, at some point it may become too expensive or disruptive to require decontamination in a building with very low, but detectable, levels of B. anthracis spores.44
Although we focus primarily on decontamination effcacy in this review, there are other criteria for selecting a decontamination method. These may include whether the technology has been demonstrated at full-scale, its cost, availability (technology, chemicals, expertise, personnel), material compatibility,45 health and safety (most techniques are hazardous) issues, and environmental impacts.
B. anthracis Strains and Other Spore-Forming Bacteria Included in Review.
While the primary focus of our review is for B. anthracis, we have also included data from other Bacillus species. Additionally, most of the decontamination data in the literature for spores of virulent B. anthracis are from tests using the Ames strain. Very few effcacy data for other virulent strains such as Vollum31 are reported (this is a gap). As such, unless otherwise noted, “B. anthracis” in this review refers to the Ames strain. Occasionally, the avirulent vaccine strain “Sterne” is used in tests.
This review also includes decontamination effcacy data for other spore-producing Bacillus species, which may be tested as surrogates for B. anthracis or other microbial contaminants. While Bacillus atrophaeus has long been used by the biodefense community as a simulant,46 we have included surrogate data only if previous experimentation included both B. anthracis and the surrogate organism, and that the surrogate demonstrated similar or greater resistance to inactivation compared to B. anthracis. The data we have included in this review do confirm the historical use of B. atrophaeus (aka Bacillus globigii aka BG) and its phenotypic relative Bacillus subtilis as appropriate surrogates for B. anthracis when decontaminating with chlorine dioxide gas,47 hydrogen peroxide vapor,48 formaldehyde gas,49 ozone gas,50 and several liquid sporicides such as peracetic acid (PAA), aqueous hydrogen peroxide, hypochlorite, and aqueous chlorine dioxide.31,51,52 In the following sections, other Bacillus data may also be presented for a decontaminant according to the criteria noted above. Interestingly, whereas B. anthracis, Bacillus thuringiensis, and Bacillus cereus may be considered the same species based on genetic evidence,53 B. thuringiensis or B. cereus have not often been used31 in decontamination studies, although their use is gaining traction; see for example Sagripanti et al.31 and Buhr et al.54−56
This review of bacterial spore inactivation techniques may be applicable for other virulent spore-producing bacteria, such as B. cereus, Clostridium botulinum, and Clostridium difficile. Because C. difficile is a major public health concern and a source of nosocomial infections,57 we have presented further discussion of inactivation techniques and data for this spore-former in the SI.
Physiology of Bacterial Spores Relative to Inactiva-tion Mechanisms.
While the primary focus of this review is to elucidate the chemical or physical treatment conditions con-ducive to effective inactivation of bacterial spores as a function of substrate (i.e., at the macro scale), we also briefly present here and throughout the review the microbiological mechanisms thought to be responsible for inactivation of bacterial spores. Some good overviews related to spore physiology and sporicidal mechanisms may be found elsewhere.7,8,58,59 Briefly, spore killing mechanisms may involve damage to one or more of the following cellular components or metabolic activities, and depend on the decontaminant chemistry: damage to DNA, the inner membrane, the germination apparatus, or inactivation of core enzymes. The structure of bacterial spores, including the presence of an intact spore coat, plays a major role in their resistance to inactivation.59
LIQUID-BASED SPORICIDES
The application of liquid-based decontaminants is a simpler approach than the use of gaseous decontaminants (here occasionally referred to as fumigants) and may be more amenable for use by a facility owner/occupant in the event of an intentional wide-area B. anthracis spore release.60 In addition to chemical decontaminant parameters such as active ingredient concentration and CT that affect effcacy, the pH of the liquid and the mass of active ingredient applied to a surface are other operational parameters that may affect effcacy and are reported here if available and relevant.
One advantage of using liquid decontaminants is that they may be applied to surfaces or materials using a variety of different techniques. These application methods include spraying (usually low pressure is preferred to minimize reaerosolization of spores), immersion (may be useful for materials that would be discarded as waste), fogging (generation of microscopic droplets; useful for volumetric or surfaces), gels (use of a binding agent such as fumed silica; may be useful for vertical surfaces or ceilings), foams (entrained air; vertical surfaces, ceilings), prewetted wipes (useful for small items with complex surfaces), and simply using a mop or sponge. With regard to spraying or fogging, an electrostatic charge may be applied to the droplets generated to improve their adherence to complex surfaces (as is done routinely in other applications, such as in agriculture61), although the documentation of improvement in effcacy is lacking. In addition, although foams and gels may allow for longer CT of the liquid decontaminant on a surface due to their ability to adhere (important for a ceiling or a vertical surface), the mass of active ingredient in actual contact with the surface may be less due to the thickness of the foam or gel; research to clarify whether foam or gelling agents improve decontamination effcacy of liquid sporicides is also needed. One drawback with the use of liquid sporicidal chemicals is that they’re primarily used for surface decontamination (with fogging an exception) and not for killing aerosolized spores. To what extent surface decontamination using liquid sporicides affects a reduction in the number of aerosolized spores in an enclosed volume is a research gap.
Hypochlorous Acid.
In aqueous solution, hypochlorous acid (HOCl) exists in equilibrium with its conjugate base, hypochlorite. Together these two species are referred to as free available chlorine (FAC). Chlorine bleach, an aqueous solution of sodium hypochlorite, is the most common source of HOCl in studies of B. anthracis inactivation on building and environmental surfaces. The protonated HOCl is the more effective sporicide; for surfaces treated with a constant FAC, acidifying to pH≤ 6 increases sporicidal effcacy.62 As with other oxidizing compounds (e.g., ClO2, peroxides, ozone; discussed below) the spore killing mechanism of hypochlorite is thought to involve spore inner membrane damage, with spore coat protein offering resistance.58,59
pH-Adjusted Bleach.
As summarized in Table 1, spray-applied solutions of acidified bleach (pH-adjusted bleach or pAB) with FAC levels ranging from 5000 to 7000 ppm (ppm) were effective (>6 LR) against B. anthracis on nonporous building surfaces, although effcacy on porous surfaces varied by material.34,51,52,63 Effcacy on porous surfaces may be limited by consumption of FAC through oxidation of organic materials (which often comprise porous surfaces) or by low surface wettability.
Table 1.
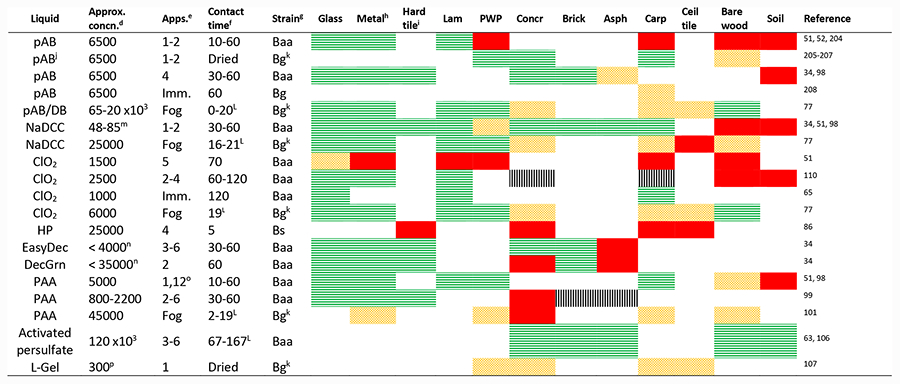
|
Effcacy: Horizontal green lines LR ≥ 6 or complete inactivation; Orange trellis, 6 > LR ≥ 3; complete red fill, LR < 3; no fill, not tested; vertical, mixed results.
DB, dilute bleach; EasyDec, EasyDecon; DecGrn, DeconGreen, other sporicide abbreviations defined in abbreviations section.
Lam, decorative laminate; PWP, painted wallboard paper; Concr, concrete; Asph, asphalt; Carp, carpet; Ceil tile, ceiling tile.
Concentration in mg/L unless otherwise noted; for pAB/DB and NaDCC, concentration is ppm FAC.
Number of spray applications or Imm., surface was immersed in liquid; fog, surface was fogged with liquid.
Contact time in minutes (unless otherwise noted) until surface was neutralized or sampled; dried, surface dried overnight before sampling;for fog applications, contact time is the time elapsed after fogging of liquid completed (dwell time).
Baa, B. anthracis ames; Bg, B. globigii/atrophaeus; Bs, B. subtilis.
Stainless steel, aluminum or galvanized metal.
Granite, porcelain, or ceramic.
Efficacy reported as surface log reduction (chemical inactivation and physical removal) with viable spores in neutralized rinsate/runoff and carpet vacuumed before treatment.
Surface inoculated with aerosolized spores,
hours,
wt % concentration NaDCC in formulation.
ppm of HP in formulation.
Soil received 12 applications of PAA.
g/L of Oxone in formulation.
A large field study effectively used spray-applied pAB against B. atrophaeus on a variety of nonporous building surfaces,64 although the porous materials including ceiling tiles, furniture, and carpet were bagged and removed from the building and treated as waste. Some of these porous items are good candidates for immersion in pAB. Low effcacy against B. anthracis on carpet, for example, can be overcome through immersion of the carpet in pAB for at least 30 min.65
Although chlorine bleach is corrosive, no damage was observed to building material coupons assessed in a laboratory study,34 but during a field-scale application, wood-laminate floor was damaged.64 While the aforementioned studies typically used acetic acid (such as vinegar) to lower the pH of the bleach solutions to a range of 6−7, Frazer et al.66 demonstrated that acidified bleach was equally effective when using HCl for acidification.
Hypochlorite Solutions Diluted with Water, no pH Adjustment.
These solutions were effective against B. anthracis (Ames and other strains such as Vollum and Albia) on nonporous surfaces immersed in the liquid (3000−5000 ppm FAC),31,67 but effcacy was reduced or required substantially higher concentrations in the presence of organic burden.68–70 Several spray-applied commercial off-the-shelf cleaning products containing bleach (≥20 000 ppm FAC or 2% hypochlorite) and surfactants, with pH of approximately 12.5, were effective against spores of B. atrophaeus on porous and nonporous building surfaces71 The use of carbon nanotubes improved effcacy of hypochlorite solutions as well.72 Diluted bleach and consumer products may be less hazardous to work with (compared to pAB) because less chlorine gas is released from the liquid at higher pH, potentially reducing the level of respiratory protection needed. Chlorine gas workplace exposure limits range from 0.1 to 1.0 ppm by volume (ppmv).73
Sodium Dichloroisocyanurate.
An alternative method of producing HOCl in solution is the dissolution of commercially available tablets or powders of sodium dichloroisocyanurate (NaDCC; e.g., for swimming pool disinfection). NaDCC hydrolyzes to form FAC, monochloroisocyanurate, and isocyanurate in equilibrium. At near neutral pH, approximately half the available chlorine exists as mono- or dichloroisocyanurate.74 This equilibrium between chloroisocyanurates and FAC is the suggested cause of the enhanced microbiocidal effcacy of NaDCC under organic burden compared to the effcacy of bleach.75 As FAC is consumed by reaction with organic material, it is slowly replenished by hydrolysis of the chloroisocyanurates. The continued release of FAC provides a low concentration of FAC over an extended period. In the case of bleach, all the available chlorine present in solution is FAC that may be consumed immediately by reaction with organic material. Examples of where NaDCC offers increased effcacy on organic materials compared to pAB or dilute bleach are found here.34,51,76 Despite these few studies, the FAC levels and CTs needed for effective use of spray-applied NaDCC against B. anthracis on porous and nonporous surfaces is a research gap.
Application Methods.
In addition to application as a spray, many of the above-mentioned HOCl-based decontaminants are also effective as fogs, wetted wipes, or gels. Fogging with pAB, diluted bleach, or NaDCC was effective (>6 LR) against spores of B. atrophaeus on nonporous surfaces but only moderately effective or ineffective on most porous materials (Table 1).77 Several commercial off the shelf bleach-wetted wipes were effective (>6 LR) against B. atrophaeus on nonporous surfaces. Acidified bleach mixed into a polymeric gel was effective against B. anthracis Vollum on painted steel.78 This gel was able to adhere to vertical surfaces, potentially providing greater wetted CT per application than a spray-applied liquid.
Electrochemical Generation.
Hypochlorous acid can also be generated electrochemically from an aqueous solution of NaCl.79–81 In an electrolytic cell, chloride ions are oxidized at the anode to form Cl2 gas, which reacts to form hydrochloric acid and HOCl (a solution referred to as anolyte water).82 The primary advantage of this system is the generation of HOCl on site without the need to transport or store large volumes of bleach. The effcacy of electrochemically generated HOCl is similar to that of pAB: effective against B. anthracis on tested nonporous surfaces (>6 LR) but only moderately effective or ineffective on tested porous surfaces.83
Calcium Hypochlorite.
Refer to the SI for further information and data on this source of HOCl sporicidal chemistry.
Peroxide Compounds.
Although there are several commercial decontaminants containing hydrogen peroxide (HP) combined with additional ingredients (e.g., surfactants, chelators, activators, and catalysts), aqueous HP alone may be effective on materials only when used at relatively high concentrations. From the United States Environmental Protection Agency’s (USEPA) list of registered antimicrobial products used as sterilizers, the minimum concentration of aqueous HP when used without additional ingredients is 35%.84 Other studies also confirm the need for relatively high concentrations when using HP by itself.70,85,86
H2O2 with Activators or Catalysts.
The effectiveness of aqueous HP can be improved by adding bleaching activators such as glycerol diacetate (diacetin) or catalysts such as bicarbonate and molybdate ions. Bleaching activators are a class of compounds with O- or N-bound acetyl groups that react with HP to form PAA.87 For example, tetraacetylethylenediamine combined with sodium perborate (both compounds may be found in laundry detergents; the latter dissociates to HP in aqueous solutions87) was shown to have antimicrobial activity.88 Further, diacetin is cited as the activator in a patent89 for a two-component product currently sold as Easy Decon 200. Triacetin and potassium carbonate were added to HP solution in a decontamination study using B. atrophaeus spores, to produce an activated HP solution that was shown to be effective on a number of mostly nonporous materials when applied as a spray, but was ineffective on wood and concrete.90 Bicarbonate and molybdate ions catalyze the oxidative reactions involving HP by forming peroxymonocarbonate (HCO −4) and peroxomolybdate intermediates, respectively, which are attributed to increased oxidation rates of organic compounds.91–94 Decon Green, a formulation developed by the military, contains both bicarbonate and molybdate catalysts.95 These amended or activated formulations of HP can be effective at lower concentrations than the concentrations required in unamended solutions. One study found that spray-applied EasyDecon 200 (<4 wt % HP) and Decon Green (<35 wt % HP) were effective against B. anthracis (>7 LR) on a variety of outdoor building materials (Table 1), but not on the organic materials treated wood or asphalt.34
Peracetic Acid.
Products utilizing PAA chemistry are generally some of the most effective liquid sporicides that are commercially available (hospital/medical instrument applications), and are produced in equilibrium with acetic acid and HP. Against a suspension of B. atrophaeus spores, PAA was substantially more effective (several orders of magnitude) than HP.96 Leggett et al.97 confirmed the synergistic activity of the combination of PAA and HP via suspension tests using B. subtilis spores, and hypothesized that aqueous HP weakens the spore coat, but that inactivation is primarily due to PAA. As noted in Table 1, spray-applied formulations of PAA (0.08−0.5 wt %) were effective against B. anthrac is on several indoor and outdoor building materials, but notably ineffective on unpainted concrete.34,51,98,99 PAA has also been evaluated via application with wipes100 and fog.101 In a comprehensive study, fogging of PAA (and also aqueous HP) was found to be effective on most of the subway railcar materials that were tested, in at least one of the conditions evaluated.102 Like the spray-applied tests, however, fogging of PAA was ineffective on unpainted concrete. The reduced effcacy of HP-based sporicides on unpainted concrete is thought to be related to the decomposition of HP caused by chemical interactions with this material (e.g., material demand).
Solid powders of peracetyl borate that dissolve in water to form PAA have been suggested as less hazardous and more economical to transport than PAA. A commercial formulation of peracetyl borate was effective (>6 LR) against B. anthracis spores on a variety of naval equipment surfaces, using an immersion test approach.54 Further evaluation of peracetyl borate and development of other solid precursor materials for PAA is recommended.
Other Noteworthy Peroxides.
Sodium persulfate, also referred to as sodium peroxodisulfate, when activated produces highly reactive but persistent sulfate radicals, and is used com-mercially as an oxidant to treat organic contaminants in soil103 and groundwater104 and has been tested for inactivation of microbes.105 In a systematic study involving several test materials and other parameters, sodium persulfate activated with 8% aqueous HP was effective in inactivating spores of B. anthracis on variety of difficult-to-treat porous and organic outdoor sur-faces including asphalt, brick, soil, and concrete (Table 1).63,106 There are several activators other than HP that can be used with sodium persulfate105 to produce sulfate radicals, and this area is suggested for further R&D.
A few studies evaluated aqueous solutions or gels of potassium peroxymonosulfate (KHSO5) by dissolving the commercially available salt 2KHSO5·KHSO4·K2SO4 (Oxone, DuPont, Wilmington, DE).107 Addition of sodium chloride to buffered solutions of peroxymonosulfate results in the formation of HOCl, which greatly increased the effcacy of 100-g/L Oxone against a suspension of B. atrophaeus.108
Aqueous Chlorine Dioxide.
Due to its volatility and potential to degrade during storage,109 most aqueous chlorine dioxide (ClO2) solutions tested for effcacy against B. anthracis spores on building or environmental surfaces are prepared at the point of use. Methods of generating ClO2 include mixing solutions of sodium chlorite and bleach under acidic conditions, the use of commercial products containing sodium chlorite and activating compounds that react when mixed in solution, and electrochemical generation from sodium chlorite.
Spray-applied solutions of ClO2 at measured concentrations of 3000−4000 ppm were effective against B. anthracis on several nonporous building surfaces but ineffective or not consistently effective on porous surfaces and soils (Table 1).110 Spray-applied commercial products with lower ClO2 concentrations ranging from 200 to 1500 ppm (as reported by their vendors) have been mostly ineffective (<2 LR) on porous and nonporous surfaces.51,52
A separate study found that if porous materials (carpet and particle board) were immersed in a 1000 ppm solution of ClO2 at >6 LR of B. anthracis was achieved.65 Improved effcacy through immersion at a lower concentration suggests that the limited effcacy of spray-applied liquids could be due to spray-application parameters (e.g., droplet size or insuffcient number of applications). Although not yet substantiated, authors have also suggested that ClO2 in the aqueous phase could be lost through volatilization from spray droplets or from the wetted surface during the application.52,111
Several aqueous solutions of ClO2 in the range of 5000− 6000 mg/L were produced via an easy to use commercially available product that utilizes sodium chlorite and sodium bisulfate. When applied as a fog, the ClO2 solutions were effective on a number of materials.77 Further investigation of this simple ClO2 generation technology and application approach is warranted. In addition to chemical methods, aqueous ClO2 can be generated electrochemically using solutions of sodium chlorite and sodium bromide.112
Aldehydes.
The mechanism of spore killing by this chemistry is thought to be DNA and germination apparatus damage.59 While aqueous solutions of formaldehyde (e.g., formalin) are sporicidal, Spotts Whitney et al.2 suggest that the classification of formaldehyde as a possible carcinogen has limited its use. Further, the European Union classifies formaldehyde as a Category 1B carcinogen, and has approved its use as an antimicrobial only under certain conditions.113 Liberal applications of 5−38 wt % solutions were used to decontaminate Gruinard Island, a former site of B. anthracis (unspecified strain) weapons testing in Scotland, resulting in no detectable spores.114 No controlled laboratory studies reporting the effectiveness of aqueous formaldehyde against B. anthracis on surfaces or soil could be located.
Table 1, below, is a synthesis of the more pertinent test conditions and results for liquid-based decontaminants.
Other liquid sporicides of note, including glutaraldehyde and aqueous solutions of ozone, may be found in the SI.
GASEOUS DECONTAMINANTS
Gaseous decontaminants are generally used for enclosed volumes such as buildings, although tarpaulins may be used to contain the gas during decontamination of soil or other complex surfaces. With adequate air mixing, sporicidal chemicals in the gas-phase have the advantages (over liquid decontaminants) of inactivating aerosolized spores, can be widely dispersed, and can penetrate through cracks and crevices to decontaminate hard to reach surfaces. As with liquid decontaminants, gaseous decontaminants are generally more effective at higher concentrations, temperatures, and CTs. In addition to these parameters, relative humidity (RH) is also an important environmental factor in gas-based decontamination, with effcacy generally improving with increasing RH. Generating, achieving, and maintaining suffciently high concentrations of the sporicidal gas needed for effective decontamination inside a building or volume to be decontaminated tends to be a technical challenge. R&D is therefore moving toward finding effcacious conditions at relatively low concentrations coupled with longer CTs. The use of lower concentrations may also have the added benefit of improved compatibility of materials (research is needed to confirm this benefit), less hazardous operating conditions, and allowing more vendors to provide decontamination services in the event of wide area B. anthracis spore release. Lastly, all sporicidal gases are typically hazardous themselves, so there may be a need to minimize the release of the gas during or after decontamination via containment or capture techniques.115 Table 2, below, provides a synopsis of conditions and results for gaseous decontaminants. As with Table 1 for liquids, this table is not intended to be inclusive of all data, but rather is intended to provide a synopsis of some of the more relevant effcacy data.
Table 2.
Decontamination Effcacya Synopsis for Sporicidal Gasesb Tested under Select Conditions and Materialsc
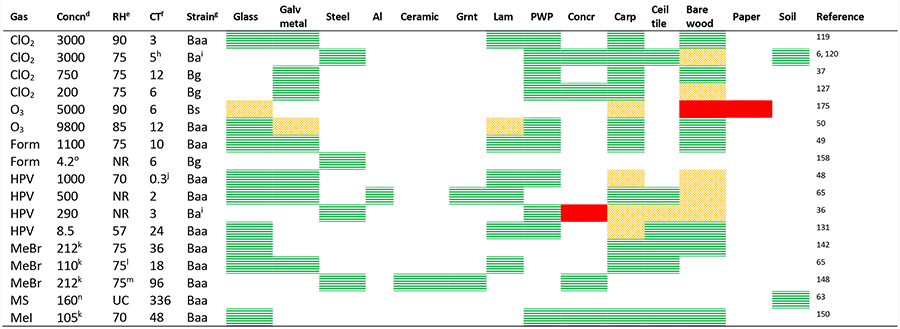
|
Effcacy: Green horizontal lines, LR ≥ 6 or complete inactivation; Orange trellis, 6 > LR ≥ 3; Complete red fill, LR < 3; no fill, not tested; vertical, mixed results.
Form, Formaldehyde; MS, metam sodium; other sporicide abbreviations defined in abbreviations section.
Galv metal, galvanized metal; Grnt, granite; Al, aluminum; others as in Table 1.
Concentration in ppmv unless otherwise noted.
RH at ambient temperature (20−25 °C) unless otherwise noted; NR, RH and temp not reported but believed to be variable; UC, uncontrolled RH at ambient temp.
Contact time in hours unless otherwise noted.
Baa, B. anthracis ames; Bg, B. globigii/atrophaeus; Bs, B. subtilis.
Topsoil tested at 2−4 h, 1 cm depth, using sterilized soil− see 2nd reference listed.
Soil is Baa, others are Ba NNR1d1.
Dwell time of 20 min with total cycle time (conditioning, gassing, dwell) of ~2 h.
Concentration in mg/L air.
Temp of 37 °C.
Temp of 10 °C.
160 μL of 42.5 wt % metam sodium applied to small amount of soil in Petri dish.
ClO2 Gas.
Following the Amerithrax incident, four buildings were decontaminated using gaseous ClO2. 116 Chlorine dioxide gas has also been used in several other B. anthrac is contamination incidents, such as the decontamination of a village hall in Scotland.10 Since these incidents, a substantial body of R&D in the use of this gas as a sterilant has been produced, and most of these studies show the gas to be a highly effective decontaminant. However, because of stability issues (i.e., unable to be compressed and stored), ClO2 gas must be generated at the point of use.117 Other issues with ClO2 gas, related to its detrimental interactions with materials (e.g., material demand, corrosivity, and formation of unwanted byproducts) have also been raised.118
ClO2 gas can be generated via a wet or dry chemical process, although no difference in decontamination effcacy was observed as a function of the generation technique.6 Over the past 15 years, decontamination effcacy testing with ClO2 gas has been conducted with numerous types of materials under a variety of operational and environmental conditions. Earlier tests with ClO2 gas were conducted mostly at relatively higher levels of the gas (i.e., >1000 ppm), and have demonstrated its generally high effcacy on numerous materials,6,119,47 including more difficult materials such as soils120,121 and grimyconcrete material obtained from a subway tunnel.122
In transitioning the research to tests with relatively lower levels of ClO2, associated with longer CTs, a 200 ppm level (75% RH) for 4 h was shown to be effective in inactivating spore populations of B. subtilis in galvanized metal ductwork from a mock heating, ventilation, and air conditioning system.123 Other studies have demonstrated the conditions required for effective decontamination using ClO2 gas levels ranging from 350 to 750 ppm.124–126 In a series of six small-chamber experiments conducted at either 100 or 200 ppm of ClO2 (75% RH, 24 °C; CTs ranging from 2 to 12 h), several building materials were effectively decontaminated in every test except for moderate decontamination effcacy of the wood coupons at 200 ppm.127
With respect to the effect of temperature and RH, in a study simulating the relatively cooler temperature that may be encountered in a subway system (11 °C), the lower temperature greatly diminished the decontamination effcacy128 In this same study, lowering RH from 75 to 50% (at 24 °C) also greatly reduced effcacy. Wang et al.129 confirmed that increasing concentration and RH significantly improved inactivation of B. atrophaeus spores on paper, with rapid improvement occurring when RH > 70%.
Hydrogen Peroxide Vapor.
Hydrogen peroxide vapor (HPV) is a well-established antimicrobial technology that has been described in patents since 1934130 and has been extensively investigated for its ability to inactivate all classes of microorganisms,131 Because of its benign decomposition products (O2 and H2O), HPV is increasingly being used in place of other gas-phase sterilants such as ethylene oxide and formal-dehyde.132 HPV has also been shown to be more compatible with electronics and other materials compared to sporicidal gases such as ClO2.
Since 2002, the use of HPV as a sporicide, and for B anthracis inactivation in particular, has evolved from use as a small chamber sterilant for medical devices to its application as a volumetric or building decontaminant. HPV was used to decontaminate two large buildings contaminated with B. anthracis spores following Amerithrax.116 In the fumigation of one of the contaminated buildings (Department of State SA-32), special efforts were required and difficulties were encountered to reach and maintain the target HPV concentration.14 In the full-scale field evaluation of HPV as part of the Bio-Response Operational Testing and Evaluation study,64 roughly one-third of the postdecontamination samples were positive for spores of the target organism Bacillus globigii. The poor results were similarly attributed to not being able to meet the target concentration of 250 ppm throughout the building.
Recent research on HPV has sought to elicit the effect of several variables on the effcacy of HPV, including material. Such studies48,65 have demonstrated that building materials such as wood, unpainted concrete, and carpet are more difficult to decontaminate with HPV compared to nonporous materials such as glass, laminate, and galvanized metal. Wood et al.131 confirmed that unpainted concrete was decontaminated ineffectively but that decontamination was effective for the majority of indoor materials and conditions that were tested. As with HP-based liquid sporicides, HPV’s poor decontamination performance on unpainted concrete is likely due to this material’s high demand for the HPV.133
The use of a proper surrogate for B. anthracis in HPV decontamination studies has also been investigated.48,134 For example, while G. stearothermophilus may be the internationally recognized biological indicator organism for HPV,134 Rogers et al.48 have shown that B. subtilis may be a more representative organism to model B. anthracis resistance to inactivation by HPV. Other studies have investigated the effect of spore loading36 and the inactivation of different spore producing species such as C. difficile57 and C. botulinum.135
As summarized by Unger-Bimczok et al.,132 disagreement remains in the literature as to the effect of RH on decontamination effcacy and whether condensation on surfaces should occur or be avoided with the use of HPV. They report that it is common procedure to dehumidify the volume to be decontaminated to avoid condensation of HPV, while others claim that condensation is critical to effective decontamination with HPV. For example, Pruss et al.136 claim that a lower surface temperature enhances condensation, and that this improves effcacy, although only for certain species of bacteria. UngerBimczok et al.132 showed that increasing RH improves decontamination effcacy for G. stearothermophilus spores (due to increased condensation), but this effect is more pronounced when decontaminating with relatively lower concentrations. In addition, they conclude that the molecular deposition of water and HP on surfaces is more important than the HPV concentration for effcacious microbial inactivation.
Because of the large volume of literature on the use of HPV as a sterilant, along with a myriad of experimental conditions, there is considerable variability in the concentrations and CTs (and thus dose, i.e., concentration × CT) that have been studied and recommended for effective inactivation of bacterial spores. Most of the studies have demonstrated the use of HPV at concentrations over 200 ppm, with associated CT on the order of minutes to a few hours. One vendor requires their HPV technology to achieve concentrations between 250 ppm to 930 ppm.137 More recently, however, R&D is moving toward simplifying the fumigation process via using lower HPV concentrations, coupled with longer CTs, than are typically used or suggested by vendors of HPV generating technology.138–140 Wood et al.131 showed that relatively low HPV concentrations (average of 5−10 ppm), produced simply via the use of inexpensive, commercially available humidifiers and 3−8% aqueous HP solutions, create sporicidal conditions on many different materials after a few days’ CT. Concrete was the exception, that is, it has been shown in other studies to be poorly decontaminated using HPV.36,131
Methyl Bromide, Methyl Iodide, and Metam Sodium.
Methyl bromide (MeBr), methyl iodide (MeI), and metam sodium are all fumigants that are currently used or have been used as pesticides for soils (agriculture), food commodities, and/or structures. MeBr and MeI are alkylating agents (like EtO), which kill spores via DNA damage.59
Methyl Bromide.
MeBr was recognized in 1950 as sporicidal for B. anthracis spores.141 In the event of a wide-area release of B. anthracis spores, MeBr has several advantages as a decontaminant including an existing industry with personnel experienced in its use; MeBr easily penetrates materials and is relatively compatible with materials. While it is being phased out under the Montreal Protocol on Substances That Deplete the Ozone Layer, several million kg of MeBr are still being used in the U.S. annually under quarantine, preshipment, and critical use exemptions.142 Global quarantine and preshipment use of MeBr in 2013 is estimated to be 10,000 t.143 To mitigate impacts to the stratospheric ozone layer, MeBr can be captured with activated carbon following its use as a decontaminant.144
Since Amerithrax, several laboratory and field studies have been undertaken to further understand the sterilant properties of MeBr. Juergensmeyer et al.145 conducted a study using spores of several microorganisms (B. anthracis ANR-I, G. stearothermophilus, B. atrophaeus, and B. thuringiensis) inoculated onto glass slides, with experiments conducted at 37 °C and varying MeBr concentrations. No B. anthracis spores were recovered (>7 LR) after a 48 h exposure to 80 mg/L. In another laboratory study with MeBr tests at 37 °C and 75% RH, greater than 6 LR was observed for B. anthracis spores on all materials tested except cellulose.65 In these tests, B. subtilis was much more resistant to MeBr than B. anthracis, a surprising finding.
From a comprehensive parametric study, Wood et al.142 reported on the required CT needed to achieve >6 LR of B. anthracis spores on the six building materials tested in their study, as a function of multiple fumigation conditions (varying RH, temperature, and concentration). As an example, 18 h CT was required with a MeBr concentration at 300 mg/L, 27 °C, 75% RH. MeBr was also found to be effective against B. anthracis spores in 1 cm depth topsoil at 25 °C (180 mg/L; CT of 36 h; RH > 75%).63
MeBr has been demonstrated in field-scale tests, such as with a 1,444 m3 building in which no damage to the building or its contents was observed.146 A full-scale field demonstration for decontaminating a subway railcar using MeBr was also successfully conducted.147 With the thought that MeBr could be used for decontamination of underground transportation systems (e.g., subway tunnels), tests were conducted at lower temperatures to assess the potential.148 MeBr was found to be effective in inactivating spores of B. anthracis Sterne on several tunnel materials at 10 and 4.5 °C, with extended CTs of 4 and 7 days, respectively.
Methyl Iodide.
Although no longer used or manufactured in the U.S., the pesticide MeI is used in several other countries as an alternative to MeBr.149 In one laboratory study with MeI, several conditions (100−400 mg/L) were found to be effective in inactivating B. anthracis Ames spores on all of the materials tested.150
Metam Sodium.
Lastly, metam sodium is the most widely used soil fumigant in the US and was effective in decontaminating topsoil (B. anthracis) and a test dust under a number of test conditions.63 Metam sodium reacts with moisture in the soil to produce methyl isothiocyanate gas, the chemical responsible for biocidal activity. Indeed, adding moisture to the soil was found to improve sporicidal activity against B. anthracis spores.
Formaldehyde Gas.
Formaldehyde gas has been used as a decontaminant and sterilant for over 100 years.151 It has been commonly used for decontamination of high efficiency particulate air filters, biological containment laboratories and safety cabinets, and animal housings, due to its effcacy, low cost, and material compatibility.152 Formaldehyde gas was used to decontaminate mail sorting equipment from a postal facility in Landover, Maryland.153 However, due to concerns over its toxicity, alternatives for the above-listed applications are being explored.154
Gaseous formaldehyde can be generated via the heating of paraformaldehyde (PF), a mixture of formaldehyde-based polymers that is solid at room temperature.155 The heating of PF can occur simply through the use of a hot plate156 or through a controlled process using commercially available equipment.49,152 Gaseous formaldehyde may also be generated via the heating of formalin, an aqueous mixture of formaldehyde (typically 37% by weight) and methanol. As with PF, formalin can be heated by simple means such as a wok154 or via more sophisticated and controllable equipment.157 Ngabo et al.158 recently investigated the effcacy of formaldehyde vapor generated using formalin, at several concentrations and exposure times, and reported that 4.2 mL formalin/m3 with a six h CT, was effective in inactivating spores of B. atrophaeus on stainless steel.
With respect to an effective or optimal formaldehyde gas concentration, 10.5 g solid PF/m3 of volume to be decontaminated has been recommended by various organizations159,160 and has been used in various studies.49,152,153 This level was originally recommended by Taylor et al.161 as a result of their investigations. Note that this concentration is the original amount of solid PF to begin with prior to sublimation and is not the actual concentration of formaldehyde in air. In fact, very few decontamination studies have actually measured and reported levels of formaldehyde gas in the air, with the exception of Rogers et al.49 and Ackland et al.162 The latter reported that the vapor phase equilibrium concentration of gaseous formaldehyde at 20−21 °C is 2 g/m3, and when exceeding this concentration, condensation of formaldehyde occurs. Indeed, in the study by Rogers et al.,49 the original amount of solid PF was equivalent to 10.5 g/m3, but actually measured only 1100 ppm formaldehyde (= 1.36 mg/L at 23 °C) in the air.
With formaldehyde condensation, it is common practice to employ ammonia gas to react with the formaldehyde to produce the relatively benign byproduct methenamine, a solid residue that forms on surfaces and is typically removed for practical reasons. Ammonia gas can be generated by heating ammonium carbonate or ammonium bicarbonate.156 Further research is needed to determine effective gas-phase formaldehyde levels that minimize methenamine production.
Spiner163 originally demonstrated that decontamination effcacy with formaldehyde gas is improved at elevated RH levels, and consistent with this finding, more recent studies have employed RH levels in the range of 70−90%.49,152 Rogers et al.49 demonstrated effective decontamination against B. anthracis on numerous building materials. We were unable to locate other formaldehyde gas decontamination data for B. anthracis nor with its use on realistic building materials.
Ozone.
The use of ozone gas as a biodecontaminant has been explored since 1982.164 While the use of ozone gas holds promise, it has not been demonstrated full-scale.14 Nevertheless, gaseous ozone can be generated in large quantities (up to 300 kg/h by one vendor) for use in water utilities and other industrial water and wastewater applications,165 and approximately 10% of U.S. water treatment plants use gaseous ozone.166 is gaining traction for use in healthcare environments for disinfection and sterilization,167–170 and has been approved for reprocessing (sterilization) of medical equipment that cannot be heat-treated.171
Mahfoudh et al.172 discuss the role that elevated RH plays in fumigation with ozone and other gaseous decontaminants, via the swelling of spores and creating “channels” for gas to diffuse into the spore. Other research groups50,173–175 have corroborated the finding that increasing RH levels (>80%) improves effcacy with ozone gas.
Using ozone gas at relatively low levels (1−25 ppm), Akbas et al.176 and Sharma et al.169 demonstrated moderate effcacy (2−4 LR) against spores of B. cereus and C. difficile on a number of different materials. With higher ozone concentrations, Aydogan et al.175 reported 2−4 LR (depending on material) in B. subtilis spore populations when decontaminating with ozone at approximately 5000 ppm, 90% RH for 4 h. In a large comprehensive study with numerous experiments utilizing spores of both B. anthracis and B. subtilis, effective decontamination with ozone gas was achieved at 85% RH, with required ozone levels ranging from 9800−12 000 ppm, depending on the material.50
PHYSICAL-BASED DECONTAMINANTS
Thermal Treatment.
Thermal treatment techniques for the inactivation of microorganisms have been used for millennia,55 and may be categorized as either wet or dry heat. Wet heat includes environments with air at elevated temperatures and saturated with moisture (100% RH) or boiling water.177 Autoclaves are an example of “wet heat”, where steam is used at elevated pressures and temperatures.178 While sterilization via autoclave is a ubiquitous technique except where there are material compatibility issues,177 studies have shown that under certain circumstances, typical autoclave operation for waste treatment may not completely inactivate spore populations.178,179 Protein damage is the likely spore inactivation mechanism for wet heat, while DNA damage is the major mechanism with dry heat.8,59
A dry heat environment is characterized with an RH level <100%, such as the environment found in an incinerator,180 an ampule in an oil bath, or infrared heating.181 Spores are generally more resistant to dry heat than wet heat.177 Peeler et al.182 showed that with dry heat (113 and 125 °C), inactivation of B. atrophaeus spores improved with increasing RH levels, except at RH levels less than 10%. More recently, Buhr et al.55,183 showed that in dry heat environments, increasing RH may be accompanied by an increase in effcacy, but their results were somewhat confounded by other test variables.
While research continues to further elucidate the mechanisms associated with wet184–186 and dry heat8,181,187 spore inactivation, thermal treatment applied on a large scale may be impractical.14 Nonetheless, Buhr et al.188 demonstrated decontamination of a C-130 aircraft using dry heat (75−80 °C, 70−90% RH, 7-day CT). While 89% of surface samples were negative for the test organism B. thuringiensis, overall effcacy was estimated to be >7 LR; all BIs and inoculated coupons were completely inactivated. No information was provided relative to impact of the dry heat treatment on aircraft materials.
UVC-254.
The germicidal effects of ultraviolet radiation (UV) have been known for 150 years, and the majority of papers published on the effects of UV on bacteria have focused on UV with a wavelength of 254 nm, which is produced via low pressure mercury vapor lamps.189 We refer to this wellestablished sterlization technique as UVC-254. The wavelength range for UVC is 190−290 nm; germicidal UVC produced from sources other than mercury vapor bulbs (e.g., light emitting diodes) is discussed in the SI section on emerging decontamination techniques. Medium pressure mercury lamps produce UV light over a broader range of wavelengths (200− 400 nm).190 UVC inactivates bacterial spores via damage to DNA.8,59,189
In a review of UVC-254,189 the authors conclude that “the literature revealed that many studies lack information on dosimetry, microbial quality, or experimental details that would allow comparative analysis of UVC data”, and we would agree with this assessment. In the cases where dosimetry data are reported, experimental procedures range widely in terms of the fluence rate, dosage, exposure time, the distance between UVC source and microbial population, and techniques for measuring UVC. For example, at the low end of the dosage range, Menetrez et al.191 report that 17.5 mJ/cm2 achieved a 59% reduction of Bacillus anthracis Sterne spores on Agar plates. At the upper end of the range, Kesavan et al.192 reported that a dose of 2300 mJ/cm2 provided a 2−3 LR.
While several studies report bacterial spore population inactivation kinetics, none of the literature reports UVC-254 dosages required to achieve effcacious decontamination, that is, ≥ 6 LR. That UVC-254 may be unable to achieve greater than 4 LR on materials could be due to a shielding or shading effect at the microscopic level.189 This shielding or shading may be due to effects of materials189 or from agglomeration of spores.192 Further, several studies have reported a tailing effect,189,192–194 whereby an initial 1−2 LR of spores occurs rapidly at low UVC-254 dosages, but with minimal additional decay after prolonged exposure. For example, Owens et al.193 showed that ~4 LR of B. anthracis Sterne was achieved with 1000 mJ/cm2 using a medium pressure Hg lamp, but that no further reduction occurred thereafter, up to a dose of 4000 mJ/cm2.
Most of the studies on UVC-254 for bacterial spore inactivation conducted over the past decade focused on development of additional dosimetry data for different materials. As with many other inactivation studies, the scientific literature for UVC-254 is typically lacking in decontamination effcacy data for materials other than rudimentary laboratory substrates or environmental matrices such as glass slides,195 filter paper,196 water or liquid suspension,194,197 air,198,199 or agar plates.200 Further, many of the UVC-254 data in the literature are for vegetative bacteria or spores of B. subtilis,201,202 B. atrophaeus,199 B. cereus,203 or B. anthracis Sterne;191,193,203 we were unable to find any UVC-254 dosage/effcacy data for virulent forms of B. anthracis spores. Other gaps in the literature include the effect of RH and fluence rate on effcacy with UVC-254, as well as effcacy for other UVC sources.
CONCLUSIONS AND RESEARCH RECOMMENDATIONS
Substantial progress has been made in the past 15 years in the science and technology of inactivating spores of B. anthracis and other bacteria. One of the more significant advancements has been the development of a large body of data which demonstrates decontaminant operational (e.g., chemical concentration, CT, dosage requirements) and environmental conditions (temperature, RH) required for effective killing of spores. Further, we have elucidated the chemical mechanisms of decontaminants and (related to this) learned that the building material or environmental media that the spore population is associated with has a significant effect on decontamination effcacy. While most of this research was conducted at bench scale, transitioning decontamination technology to increasingly larger scale is a current trend that will need to continue, to uncover issues of a more operational nature. For example, several technologies that were suggested years ago as having potential2,14 have since been demonstrated to be effective at larger scales (one case in point being MeBr).
For B. anthracis in particular, research has been focused on developing the capacity to respond to an intentional, wide area release of such spores. Since the availability of chemicals, personnel, and expertise will likely impact the decontamination effort after a wide-area incident, having numerous techniques available to decontaminate buildings and other environments would be advantageous. This implies developing more simple, readily available but effective methods, such as disseminating HPV through a humidifier,131 using off-the-shelf cleaning products found to be sporicidal;71 or using agricultural pesticides found to be sporicidal (such as MeBr), in which there is an existing industry. Research and development is expected to continue in this vein. Similarly, transfer of sterilization techniques used in the medical and food industries to biodefense applications is a current trend that will continue. Many of the techniques discussed in this review for killing B. anthracis can also be effectively used for C. difficile, and vice versa.
Additional research is needed to investigate technologies that are effective against spores of B. anthracis or other Bacilli in suspension or as an aerosol but have not been fully evaluated on relevant building or environmental surfaces. Emerging decontamination techniques (e.g., atmospheric cold plasma) need to be further developed to make them commercially viable and feasible for use at a larger scale. More research is also needed to identify effective techniques for challenging conditions such as low temperatures, and complex, outdoor materials such as soil and vegetation. Most laboratory studies are conducted at relatively high spore loadings to demonstrate a >6-LR; identification of less aggressive methods that achieve complete inactivation but at lower spore loadings will be useful. Lastly, additional efficacy data are needed for strains of B. anthracis that have been often overlooked in research.
In closing, please refer to Table 3 which provides a qualitative summary of the techniques discussed in this article, in terms of their advantages, disadvantages (e.g., material compatibility issues), and other noteworthy items (e.g., full-scale usage or demonstration).
Table 3.
Summary of Demonstrated Decontamination Techniques for the Inactivation of B. anthracis Sporesa
| technology | advantages | disadvantages | other notes |
|---|---|---|---|
| Liquid Sporicides | |||
| acidified chlorine bleach (pAB) | readily available (chlorine bleach, vinegar), effective on many materials | less effective on organic materials, expected material compatibility issues, more prone to producing chlorine gas than diluted bleach without pH adjustment77 | used in actual B. anthracis incidents116 and in two field-scale demonstrations64,209 |
| dilute chlorine bleach | readily available, COTS bleach cleaners with at least 2% hypochlorite effective, effective on many materials | less effective on organic materials, expected material compatibility issues | used as a fog in field test209 |
| NaDCC (dichlor) | readily available as swimming pool chemical; just add to | may leave residue,77 gaps remain for determining efficacy on materials, | may be more tolerant of organic burden compared to other HOCl-based |
| water, effective on many materials as CAS CAD | required concentrations—using swimming pool chemicals | decontaminants | |
| electrolyzed water | generates HO Cl in situ, by passing electrical current through salt water | expect similar issues as PAB | reduces need to transport large volumes of bleach |
| aqueous hydrogen peroxide | benign decomposition products (H20 and 02) | generally effective only at concentrations >35%, ineffective on unpainted concrete, less effective on wood | used in food industry |
| peracetic acid and related compounds | readily available, COTS, used in health-care and medical settings, effective on many materials | efficacy issues on unpainted concrete | most registered liquid sterilants use PAA or related chemistry as active ingredient,84 can also be generated in situ with peracetyl borate and water |
| activated hydrogen peroxide | formulated on-site by mixing HP with activator; removes transport issues, would expect similar efficacy and issues as PAA, some COTS formulations available | efficacy issues on unpainted concrete | activators such as diacetin react with HP to produce PAA or related per oxygen compounds, military formulation uses bicarbonate molybdate catalysts |
| activated persulfate | effective on high organic materials, such as soil and asphalt, produces sulfate radicals which have capacity to overcome organic burden | strong oxidant, expected material compatibility issues; may be best suited for outdoor materials and soil | used full-scale for soil remediation with organic chemical contaminants, persulfate may be activated to produce sulfate radicals via HP, iron, and high temperatures |
| aqueous C102 | mixed efficacy results; may require high concentration (>3000—4000 ppm) to achieve efficacy on some materials | typically generated at point of use, some COTS products available to ease generation of solution | |
| Gases | |||
| cio2 | generally effective on most materials | strong oxidant; material compatibility issues45 | has been used full-scale in several actual B. anthracis incidents10,116 and field tests,64 fumigation at relatively higher concentrations requires higher level of expertise and generation technology, which are lacking |
| HPV | generally effective on many materials, compatible with most materials45 | efficacy issues on unpainted concrete and possibly some organic materials such as carpet and wood | tests have shown that relatively lower concentrations coupled with longer contact times are effective, and may allow avoidance of expensive generation equipment,131 has been used full-scale in several actual B. anthracis incidents116 and a field test64 |
| MeBr | generally effective on most materials, highly penetrative of materials, compatible with most materials45 | issues with supply of the gas, due to international treaty limiting production | demonstrated at several full-scale field tests146,147 |
| Mel | effective on most materials tested | limited test data and information related to material compatibility | used as a structural fumigant in some countries, although not available in some countries |
| Metam sodium | useful/efficacious for soil decontamination | not tested against B. anthracis on materials other than soil | used widely full-scale as soil fumigant |
| formaldehyde | effective on most materials, compatible with most | typically neutralized with ammonia gas, which produces a residue that | has been widely used for BSL3 laboratory decontamination, but this is being phased out |
| materials, inexpensive, easy to generate gas | must be removed | due to suspected carcinogenicity, used full-scale at postal facility153 | |
| ozone | efficacious on many materials at high concentrations and high RH | strong oxidant, expected material compatibility issues | used full-scale at many water treatment facilities, but not tested at full-scale as a high concentration fumigant |
| ethylene oxide | widely used in small-scale chambers for medical instrument and related sterilization | flammable, highly toxic, not available for use at large scale | |
| Physical | |||
| wet heat | ubiquitous sterilization technique (e.g., autoclave), typically used for small items for medical/health fields, laboratories | some material compatibility issues, limited for use in relatively small chambers capable of withstanding pressure, although some commercial scale autoclaves available | |
| dry heat | may be more compatible to materials compared to wet heat | time and temperature requirements for efficacious conditions may be incompatible for several materials | tested full-scale on a military aircraft188 |
| UVC-254 | well-demonstrated technology, used commercially in several types of applications for room, surface, and air disinfection | no literature reports efficacy greater than 4 LR, which may be due to shielding/shading of materials and/or agglomeration of spores, may be better suited for nonporous materials | |
| Physical | |||
| ionizing radiation | highly penetrative, efficacious on most materials, compatible with most materials, useful for decontamination of small, valuable personal items | like ethylene oxide and autoclaves, this technology confined to use in chambers, i.e., not available for large-scale, uses ionizing radiation source, which is hazardous and requires licensing requirements for use | fairly widely available technology, dosage requirements for effective decontamination vary by material; data gaps remain |
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge other investigators at USEPA who have contributed to many of the publications referenced in this review, including Drs. Shawn Ryan, Worth Calfee, Lukas Oudejans, Paul Lemieux, Sang Don Lee, Shannon Serre, and Leroy Mickelsen. Alden Adrion was supported by an appointment to the Research Participation Program for the USEPA, Office of Research and Development, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and USEPA. This article has been subjected to the USEPA’s internal review and has been approved for publication. Note that approval does not signify that the contents necessarily reflect the views of the Agency. Mention of trade names, products, or services does not convey official USEPA approval, endorsement, or recommendation.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.8b05274.
Synopsis and table of emerging decontamination techniques; techniques for the inactivation of C. difficile spores; review of other sporicidal techniques such as calcium hypochlorite, ozonated water, and glutaraldehyde (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).U.S. Department of the Army. NBC Decontamination; FM 3-5, MCWP 3-–37.3; Washington, DC, July 28, 2000. [Google Scholar]
- (2).Spotts Whitney EA; Beatty ME; Taylor TH; Weyant R; Sobel J; Arduino MJ; Ashford DA Inactivation of Bacillus anthracis spores. Emerging Infect. Dis 2003, 9 (6), 623–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Campbell CG; Kirvel RD; Love AH; Bailey CG; Miles R; Schweickert J; Sutton M; Raber E Decontamination after a release of B. anthracis spores. Biosecur Bioterror 2012, 10 (1), 108–22. [DOI] [PubMed] [Google Scholar]
- (4).Hoffmann C; Zimmermann F; Biek R; Kuehl H; Nowak K; Mundry R; Agbor A; Angedakin S; Arandjelovic M; Blankenburg A Persistent anthrax as a major driver of wildlife mortality in a tropical rainforest. Nature 2017, 548 (7665), 82. [DOI] [PubMed] [Google Scholar]
- (5).Schmitt K; Zacchia NA Total decontamination cost of the anthrax letter attacks. Biosecurity and bioterrorism: biodefense strategy, practice, and science 2012, 10 (1), 98–107. [DOI] [PubMed] [Google Scholar]
- (6).Rastogi VK; Ryan SP; Wallace L; Smith LS; Shah SS; Martin GB Systematic evaluation of the efficacy of chlorine dioxide in decontamination of building interior surfaces contaminated with anthrax spores. Appl. Environ. Microbiol 2010, 76 (10), 3343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Leggett MJ; Setlow P; Sattar SA; Maillard JY Assessing the activity of microbicides against bacterial spores: knowledge and pitfalls. J. Appl. Microbiol 2016, 120 (5), 1174–80. [DOI] [PubMed] [Google Scholar]
- (8).Setlow P Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol 2006, 101 (3), 514–525. [DOI] [PubMed] [Google Scholar]
- (9).Stuart AL; Wilkening DA Degradation of Biological Weapons Agents in the Environment: Implications for Terrorism Response. Environ. Sci. Technol 2005, 39 (8), 2736–2743. [DOI] [PubMed] [Google Scholar]
- (10).Andrew Riley Report on the Management of an Anthrax Incident in the Scottish Borders http://news.bbc.co.uk/2/shared/bsp/hi/pdfs/13_12_07_anthrax.pdf (accessed June 18, 2018).
- (11).Cote CK; Buhr T; Bernhards CB; Bohmke MD; Calm AM; Esteban-Trexler JS; Hunter MC; Katoski SE; Kennihan N; Klimko CP, A Standard Method to Inactivate Bacillus anthracis Spores to Sterility Using γ-Irradiation. Appl. Environ. Microbiol 2018, AEM. 00106−18.84e00106–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Bennett E; Hall I; Pottage T; Silman N; Bennett A Drumming-associated anthrax incidents: exposures to low levels of indoor environmental contamination. Epidemiol. Infect 2018, 146 (12), 1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).U.S. Department of Defense, U.S. Department of Homeland Security, and U.S. Department of Agriculture. National Biodefense Strategy, 2018.
- (14).Science Applications International Corp. Compilation of Available Data on Building Decontamination Alternatives, EPA/600/R-05/036; U.S. Environmental Protection Agency: Washington, DC, 2005. [Google Scholar]
- (15).Price PN; Hamachi K; McWilliams J; Sohn MD Anthrax Sampling and Decontamination: Technology Trade-Offs, 2009.
- (16).U.S. Environmental Protection Agency. Evaluation of Ethylene Oxide for the Inactivation of Bacillus anthracis, EPA/600/R-13/220; U.S. Environmental Protection Agency: Washington, DC, 2013. [Google Scholar]
- (17).Dias FN; Ishii M; Nogaroto SL; Piccini B; Penna TCV Sterilization of medical devices by ethylene oxide, determination of the dissipation of residues, and use of green fluorescent protein as an indicator of process control. J. Biomed. Mater. Res., Part B 2009, 91B (2), 626–630. [DOI] [PubMed] [Google Scholar]
- (18).Dauphin LA; Newton BR; Rasmussen MV; Meyer RF; Bowen MD Gamma irradiation can be used to inactivate Bacillus anthracis spores without compromising the sensitivity of diagnostic assay. Appl. Environ. Microbiol 2008, 74 (14), 4427–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Broomall SM; Ichou MA; Krepps MD; Johnsky LA; Karavis MA; Hubbard KS; Insalaco JM; Betters JL; Redmond BW; Rivers BA Whole-genome sequencing in microbial forensic analysis of gamma-irradiated microbial materials. Appl. Environ. Microbiol 2016, 82 (2), 596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Moeller R; Setlow P; Horneck G; Berger T; Reitz G; Rettberg P; Doherty AJ; Okayasu R; Nicholson WL Roles of the major, small, acid-soluble spore proteins and spore-specific and universal DNA repair mechanisms in resistance of Bacillus subtilis spores to ionizing radiation from X rays and high-energy chargedparticle bombardment. J. Bacteriol 2008, 190 (3), 1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Helfinstine SL; Vargas-Aburto C; Uribe RM; Woolverton CJ Inactivation of Bacillus endospores in envelopes by electron beam irradiation. Appl. Environ. Microbiol 2005, 71 (11), 7029–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Liang Y; Wu Y; Sun K; Chen Q; Shen F; Zhang J; Yao M; Zhu T; Fang J Rapid Inactivation of Biological Species in the Air using Atmospheric Pressure Nonthermal Plasma. Environ. Sci. Technol 2012, 46 (6), 3360–3368. [DOI] [PubMed] [Google Scholar]
- (23).Sharma A; Pruden A; Yu Z; Collins GJ Bacterial Inactivation in Open Air by the Afterglow Plume Emitted from a Grounded Hollow Slot Electrode. Environ. Sci. Technol 2005, 39 (1), 339–344. [PubMed] [Google Scholar]
- (24).U.S. Environmental Protection Agency. Determination of the Effcacy of Spore Removal from Carpets Using Commercially-Available Wet/Vacuum Carpet Cleaning Systems, EPA/600/R-13/217; U.S. Environmental Protection Agency: Washington, DC, 2013. [Google Scholar]
- (25).Lutz EA; Sharma S; Casto B; Needham G; Buckley TJ Effectiveness of UV−C Equipped Vacuum at Reducing Culturable Surface-Bound Microorganisms on Carpets. Environ. Sci. Technol 2010, 44 (24), 9451–9455. [DOI] [PubMed] [Google Scholar]
- (26).Thornburg CC; Calomiris JJ Comparison of Bacillus anthracis to the Surrogate Bacillus atrophaeus for Spore Inactivation on a Novel Antimicrobial Fabric; AFRL-HE-WP-TP-2006-0061; Air Force Research Laboratory: Aberdeen Proving Ground, MD, 2006. [Google Scholar]
- (27).Fulmer PA; Wynne JH Coatings Capable of Germinating and Neutralizing Bacillus anthracis Endospores. ACS Appl. Mater. Interfaces 2012, 4 (2), 738–743. [DOI] [PubMed] [Google Scholar]
- (28).Forsyth JE; Zhou PR; Mao QX; Asato SS; Meschke JS; Dodd MC Enhanced Inactivation of Bacillus subtilis Spores during Solar Photolysis of Free Available Chlorine. Environ. Sci. Technol 2013, 47 (22), 12976–12984. [DOI] [PubMed] [Google Scholar]
- (29).Szabo JG; Muhammad N; Heckman L; Rice EW; Hall J Germinant-enhanced decontamination of Bacillus spores adhered to iron and cement-mortar drinking water infrastructures. Appl. Environ. Microbiol 2012, 78 (7), 2449–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Chen Q; Gao M; Li J; Shen F; Wu Y; Xu Z; Yao M Inactivation and magnetic separation of bacteria from liquid suspensions using electrosprayed and nonelectrosprayed nZVI particles: observations and mechanisms. Environ. Sci. Technol 2012, 46 (4), 2360–7. [DOI] [PubMed] [Google Scholar]
- (31).Sagripanti JL; Carrera M; Insalaco J; Ziemski M; Rogers J; Zandomeni R Virulent spores of Bacillus anthracis and other Bacillus species deposited on solid surfaces have similar sensitivity to chemical decontaminants. J. Appl. Microbiol 2007, 102 (1), 11–21. [DOI] [PubMed] [Google Scholar]
- (32).Grinshpun SA; Adhikari A; Yermakov M; Reponen T; Dreizin E; Schoenitz M; Hoffmann V; Zhang S Inactivation of aerosolized Bacillus atrophaeus (BG) endospores and MS2 viruses by combustion of reactive materials. Environ. Sci. Technol 2012, 46 (13), 7334–41. [DOI] [PubMed] [Google Scholar]
- (33).Inglesby TV; Henderson DA; Bartlett JG; Ascher MS; Eitzen E; Friedlander AM; Hauer J; McDade J; Osterholm MT; O’toole T Anthrax as a biological weapon: medical and public health management. JAMA 1999, 281 (18), 1735–1745. [DOI] [PubMed] [Google Scholar]
- (34).Calfee MW; Choi Y; Rogers J; Kelly T; Willenberg Z; Riggs K, Lab-scale assessment to support remediation of outdoor surfaces contaminated with Bacillus anthracis spores. J. Bioterrorism Biodef 2011, 2, (3). DOI: 10.4172/2157-2526.1000110 [DOI] [Google Scholar]
- (35).Tomasino SF; Rastogi VK; Wallace L; Smith LS; Hamilton MA; Pines RM Use of alternative carrier materials in AOAC official method SM 2008.05, efficacy of liquid sporicides against spores of Bacillus subtilis on a hard, nonporous surface, quantitative three-step method. J. AOAC Int 2010, 93 (1), 259–276. [PubMed] [Google Scholar]
- (36).Rastogi VK; Wallace L; Smith LS; Ryan SP; Martin B Quantitative method to determine sporicidal decontamination of building surfaces by gaseous fumigants, and issues related to laboratory-scale studies. Appl. Environ. Microbiol 2009, 75 (11), 3688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Ryan SP; Lee SD; Calfee MW; Wood JP; McDonald S; Clayton M; Griffin-Gatchalian N; Touati A; Smith L; Nysewander M Effect of inoculation method on the determination of decontamination efficacy against Bacillus spores. World J. Microbiol. Biotechnol 2014, 30 (10), 2609–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Wood JP; Lemieux P; Betancourt D; Kariher P; Griffin N Pilot-scale experimental and theoretical investigations into the thermal destruction of a Bacillus anthracis surrogate embedded in building decontamination residue bundles. Environ. Sci. Technol 2008, 42 (15), 5712–5717. [DOI] [PubMed] [Google Scholar]
- (39).Cates EL; Cho M; Kim JH Converting visible light into UVC: microbial inactivation by Pr(3+)-activated upconversion materials. Environ. Sci. Technol 2011, 45 (8), 3680–6. [DOI] [PubMed] [Google Scholar]
- (40).U.S. Environmental Protection Agency. Product Performance Test Guidelines OCSPP 810.2100: Sterilants, Sporicides, and Decontaminants, Guidance for Effcacy esting, EPA 712-C-17-003; Washington, DC, 2018. [Google Scholar]
- (41).AOAC International. Effcacy of Liquid Sporicides Against Spores of Bacillus subtilis on Nonporous and Porous Surfaces, AOAC 2008.05- 2008; AOAC International: Rockville, MD, 2008. [Google Scholar]
- (42).U.S. Environmental Protection Agency. Determining the Effcacy of Liquids and Fumigants in Systematic Decontamination Studies for Bacillus anthracis Using Multiple Test Methods, EPA/600/R-10/088; U.S. Environmental Protection Agency: Washington DC, 2010. [Google Scholar]
- (43).Buttner MP; Cruz P; Stetzenbach LD; Klima-Comba AK; Stevens VL; Cronin TD Determination of the efficacy of two building decontamination strategies by surface sampling with culture and quantitative PCR analysis. Appl. Environ. Microbiol 2004, 70 (8), 4740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Price PN; Sohn MD; LaCommare KS; McWilliams JA Framework for evaluating anthrax risk in buildings. Environ. Sci. Technol 2009, 43 (6), 1783–1787. [DOI] [PubMed] [Google Scholar]
- (45).U.S. Environmental Protection Agency. Technical Brief: Assessment of the Impact of Decontamination Fumigants on Electronic Equipment, EPA/600/R-14/316; U.S. Environmental Protection Agency: Washington, DC, September 2014, 2014. [Google Scholar]
- (46).Gibbons HS; Broomall SM; McNew LA; Daligault H; Chapman C; Bruce D; Karavis M; Krepps M; McGregor PA; Hong C Genomic signatures of strain selection and enhancement in Bacillus atrophaeus var. globigii, a historical biowarfare simulant. PLoS One 2011, 6 (3), No. e17836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).U.S. Environmental Protection Agency. CDG Research Corp. Bench-Scale Chlorine Dioxide Gas: Solid Generator, EPA/600/R-11/199; Washington, DC, 2004. [Google Scholar]
- (48).Rogers JV; Sabourin CL; Choi YW; Richter WR; Rudnicki DC; Riggs KB; Taylor ML; Chang J Decontamination assessment of Bacillus anthracis, Bacillus subtilis, and Geobacillus stearothermophilus spores on indoor surfaces using a hydrogen peroxide gas generator. J. Appl. Microbiol 2005, 99 (4), 739–48. [DOI] [PubMed] [Google Scholar]
- (49).Rogers JV; Choi YW; Richter WR; Rudnicki DC; Joseph DW; Sabourin CL; Taylor ML; Chang JC Formaldehyde gas inactivation of Bacillus anthracis, Bacillus subtilis, and Geobacillus stearothermophilus spores on indoor surface materials. J. Appl. Microbiol 2007, 103 (4), 1104–12. [DOI] [PubMed] [Google Scholar]
- (50).U.S. Environmental Protection Agency. Ozone Gas Decontamination of Materials Contaminated with Bacillus Anthracis Spores, EPA 600/R-11/142; United States Environmental Protection Agency: Washington, DC, November 2011, 2011. [Google Scholar]
- (51).Wood JP; Choi YW; Rogers JV; Kelly TJ; Riggs KB; Willenberg ZJ Efficacy of liquid spray decontaminants for inactivation of Bacillus anthracis spores on building and outdoor materials. J. Appl. Microbiol 2011, 110 (5), 1262–73. [DOI] [PubMed] [Google Scholar]
- (52).U.S. Environmental Protection Agency. Technology Evaluation Report: Evaluation of Spray-Applied Sporicidal Decontamination Technologies, EPA 600/R-06/146; Environmental Protection Agency: Washington, DC, 2006. [Google Scholar]
- (53).Helgason E; Økstad OA; Caugant DA; Johansen HA; Fouet A; Mock M; Hegna I; Kolstø A-B Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis–one species on the basis of genetic evidence. Appl. Environ. Microbiol 2000, 66 (6), 2627–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Buhr TL; Wells CM; Young AA; Minter ZA; Johnson CA; Payne AN; McPherson DC Decontamination of materials contaminated with Bacillus anthracis and Bacillus thuringiensis Al Hakam spores using PES-Solid, a solid source of peracetic acid. J. Appl. Microbiol 2013, 115 (2), 398–408. [DOI] [PubMed] [Google Scholar]
- (55).Buhr TL; Young AA; Barnette HK; Minter ZA; Kennihan NL; Johnson CA; Bohmke MD; DePaola M; CoraLao M; Page MA Test methods and response surface models for hot, humid air decontamination of materials contaminated with dirty spores of Bacillus anthracis Sterne and Bacillus thuringiensis Al Hakam. J. Appl. Microbiol 2015, 119 (5), 1263–77. [DOI] [PubMed] [Google Scholar]
- (56).Omotade TO; Bernhards RC; Klimko CP; Matthews ME; Hill AJ; Hunter MS; Webster WM; Bozue JA; Welkos SL; Cote CK The impact of inducing germination of Bacillus anthracis and Bacillus thuringiensis spores on potential secondary decontamination strategies. J. Appl. Microbiol 2014, 117 (6), 1614–1633. [DOI] [PubMed] [Google Scholar]
- (57).Lawley TD; Clare S; Deakin LJ; Goulding D; Yen JL; Raisen C; Brandt C; Lovell J; Cooke F; Clark TG Use of purified Clostridium difficile spores to facilitate evaluation of health care disinfection regimens. Appl. Environ. Microbiol 2010, 76 (20), 6895–6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Setlow B; Loshon C; Genest P; Cowan A; Setlow C; Setlow P Mechanisms of killing spores of Bacillus subtilis by acid, alkali and ethanol. J. Appl. Microbiol 2002, 92 (2), 362–375. [DOI] [PubMed] [Google Scholar]
- (59).Setlow P, Spore resistance properties In The Bacterial Spore: From Molecules to Systems; American Society of Microbiology, 2016; pp 201–215. [Google Scholar]
- (60).Krauter P; Tucker M A biological decontamination process for small, privately owned buildings. Biosecurity and bioterrorism: biodefense strategy, practice, and science 2011, 9 (3), 301–309. [DOI] [PubMed] [Google Scholar]
- (61).Law SE Agricultural electrostatic spray application: a review of significant research and development during the 20th century. J. Electrost 2001, 51, 25–42. [Google Scholar]
- (62).Wood JP; Calfee MW; Clayton M; Griffin-Gatchalian N; Touati A Optimizing acidified bleach solutions to improve sporicidal efficacy on building materials. Lett. Appl. Microbiol 2011, 53 (6), 668–72. [DOI] [PubMed] [Google Scholar]
- (63).U.S. Environmental Protection Agency. Decontamination of Soil Contaminated with Bacillus Anthracis Spores: Technology Evaluation Report, EPA/600/R-13/110; United States Environmental Protection Agency: U.S. Environmental Protection Agency: Washington, DC, 2013. [Google Scholar]
- (64).U.S. Environmental Protection Agency. Bio-Response Operational Testing and Evaluation (BOTE) Project - Phase 1: Decontamination Assessment, EPA/600/R-13/168; U.S. Environmental Protection Agency: Washington, DC, 2013. [Google Scholar]
- (65).U.S. Environmental Protection Agency Systematic Investigation of Liquid and Fumigant Decontamination Effcacy against Biological Agents Deposited on Test Coupons of Common Indoor Materials, EPA/600/R-11/076; U.S. Environmental Protection Agency: Washington, DC, 2011. [Google Scholar]
- (66).Frazer AC; Smyth JN; Bhupathiraju VK Sporicidal efficacy of pH-adjusted bleach for control of bioburden on production facility surfaces. J. Ind. Microbiol. Biotechnol 2013, 40 (6), 601–611. [DOI] [PubMed] [Google Scholar]
- (67).Majcher MR; Bernard KA; Sattar SA Identification by quantitative carrier test of surrogate spore-forming bacteria to assess sporicidal chemicals for use against Bacillus anthracis. Appl. Environ. Microbiol 2008, 74 (3), 676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Hilgren J; Swanson KMJ; Diez-Gonzalez F; Cords B Susceptibilities of Bacillus subtilis Bacillus cereus and Avirulent Bacillus anthracis Spores to Liquid Biocides. J. Food Prot 2009, 72 (2), 360–364. [DOI] [PubMed] [Google Scholar]
- (69).Amoako KK; Santiago-Mateo K; Shields MJ; Rohonczy E Bacillus anthracis spore decontamination in food grease. J. Food Prot 2013, 76 (4), 699–701. [DOI] [PubMed] [Google Scholar]
- (70).Hilgren J; Swanson KM; Diez-Gonzalez F; Cords B Inactivation of Bacillus anthracis spores by liquid biocides in the presence of food residue. Appl. Environ. Microbiol 2007, 73 (20), 6370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).U.S. Environmental Protection Agency. Evaluation of Bioagent Decontamination Options for Owner/Occupants, EPA/600/R-15/228; U.S. Environmental Protection Agency: Washington, DC, 2015. [Google Scholar]
- (72).Lilly M; Dong X; McCoy E; Yang L Inactivation of Bacillus anthracis Spores by Single-Walled Carbon Nanotubes Coupled with Oxidizing Antimicrobial Chemicals. Environ. Sci. Technol 2012, 46 (24), 13417. [DOI] [PubMed] [Google Scholar]
- (73).U.S. Occupational Safety and Health Administration OSHA Occupational Chemical Database, Report for Chlorine https://www.osha.gov/chemicaldata/chemResult.html?RecNo=650 (accessed June 5).
- (74).Bloomfield SF; Miles GA The antibacterial properties of sodium dichloroisocyanurate and sodium hypochlorite formulations. J. Appl. Bacteriol 1979, 46 (1), 65–73. [DOI] [PubMed] [Google Scholar]
- (75).Coates D Comparison of sodium hypochlorite and sodium dichloroisocyanurate disinfectants: neutralization by serum. J. Hosp. Infect 1988, 11 (1), 60–7. [DOI] [PubMed] [Google Scholar]
- (76).Guan J; Chan M; Brooks BW; Rohonczy L Influence of temperature and organic load on chemical disinfection of Geobacillus steareothermophilus spores, a surrogate for Bacillus anthracis. Can. J. Vet. Res 2013, 77 (2), 100–4. [PMC free article] [PubMed] [Google Scholar]
- (77).U.S. Environmental Protection Agency. Fogging of Chlorine-Based Sporicidal Liquids for the Inactivation of Bacillus anthracis Surrogate Spores, EPA/600/R-17/134; U.S. Environmental Protection Agency: Washington, DC, 2017. [Google Scholar]
- (78).Rogers JV; Richter WR; Choi YW; Judd AK Use of superabsorbent polymer gels for surface decontamination of Bacillus anthracis spores. Lett. Appl. Microbiol 2009, 48 (2), 180–6. [DOI] [PubMed] [Google Scholar]
- (79).Rogers JV; Ducatte GR; Choi YW; Early PC A preliminary assessment of Bacillus anthracis spore inactivation using an electrochemically activated solution (ECASOL (TM)). Lett. Appl. Microbiol 2006, 43 (5), 482–488. [DOI] [PubMed] [Google Scholar]
- (80).Zhang C; Li B; Jadeja R; Hung YC, Effects of Electrolyzed Oxidizing Water on Inactivation of Bacillus subtilis and Bacillus cereus Spores in Suspension and on Carriers. J. Food Sci 201681 M144. [DOI] [PubMed] [Google Scholar]
- (81).Robinson GM; Lee SH; Greenman J; Salisbury V; Reynolds DM Evaluation of the efficacy of electrochemically activated solutions against nosocomial pathogens and bacterial endospores. Lett. Appl. Microbiol 2010, 50 (3), 289–294. [DOI] [PubMed] [Google Scholar]
- (82).Huang YR; Hung YC; Hsu SY; Huang YW; Hwang DF Application of electrolyzed water in the food industry. Food Control 2008, 19 (4), 329–345. [Google Scholar]
- (83).U.S. Environmental Protection Agency. Evaluating a Decontamination Technology Based on the Electrochemical Generation of Anolyte Solution against B. anthracis Spores, EPA/600/R-11/124; U.S. Environmental Protection Agency: Washington, DC, 2011. [Google Scholar]
- (84).U.S. Environmental Protection Agency. List A: Antimicrobial Products Registered with the EPA as Sterilizers https://www.epa.gov/pesticide-registration/list-antimicrobial-products-registered-epa-sterilizers (accessed August 22, 2016).
- (85).Sagripanti JL; Bonifacino A Comparative sporicidal effect of liquid chemical germicides on three medical devices contaminated with spores of Bacillus subtilis. Am. J. Infect. Control 1996, 24 (5), 364–371. [DOI] [PubMed] [Google Scholar]
- (86).DeQueiroz GA; Day DF Disinfection of Bacillus subtilis spore-contaminated surface materials with a sodium hypochlorite and a hydrogen peroxide-based sanitizer. Lett. Appl. Microbiol 2008, 46 (2), 176–180. [DOI] [PubMed] [Google Scholar]
- (87).Hofmann J; Just G; Pritzkow W; Schmidt H Bleaching Activators and the Mechanism of Bleaching Activation. J. Prakt. Chem./Chem.-Ztg 1992, 334 (4), 293–297. [Google Scholar]
- (88).Shakouie S; Salem Milani A; Eskandarnejad M; Rahimi S; Froughreyhani M; Galedar S; Ranjbar E Antimicrobial activity of tetraacetylethylenediamine-sodium perborate versus sodium hypochlorite against Enterococcus faecalis. Journal of Dental Research, Dental Clinics, Dental Prospects 2016, 10 (1), 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Tucker MD; Engler DE Decontamination formulations for disinfection and sterilization 2007.
- (90).U.S. Environmental Protection Agency. Evaluation of Expedient Decontamination Options with Activated Peroxide-based Liquid Sporicides, EPA/600/R-13/009; U.S. Environmental Protection Agency: Washington, DC, 2013. [Google Scholar]
- (91).Richardson DE; Yao HR; Frank KM; Bennett DA Equilibria, kinetics, and mechanism in the bicarbonate activation of hydrogen peroxide: Oxidation of sulfides by peroxymonocarbonate. J. Am. Chem. Soc 2000, 122 (8), 1729–1739. [Google Scholar]
- (92).Richardson DE; Regino CA; Yao H; Johnson JV Methionine oxidation by peroxymonocarbonate, a reactive oxygen species formed from CO2/bicarbonate and hydrogen peroxide. Free Radical Biol. Med 2003, 35 (12), 1538–50. [DOI] [PubMed] [Google Scholar]
- (93).Yao HR; Richardson DE Epoxidation of alkenes with bicarbonate-activated hydrogen peroxide. J. Am. Chem. Soc 2000, 122 (13), 3220–3221. [Google Scholar]
- (94).Wagner GW; Procell LR; Yang YC; Bunton CA Molybdate/peroxide oxidation of mustard in microemulsions. Langmuir 2001, 17 (16), 4809–4811. [Google Scholar]
- (95).Wagner GW; Procell LR; Sorrick DC; Lawson GE; Wells CM; Reynolds CM; Ringelberg DB; Foley KL; Lumetta GJ; Blanchard DL All-Weather Hydrogen Peroxide Based Decontamination of CBRN Contaminants. Ind. Eng. Chem. Res 2010, 49 (7), 3099–3105. [Google Scholar]
- (96).Sagripanti JL; Bonifacino A Comparative sporicidal effects of liquid chemical agents. Appl. Environ. Microbiol 1996, 62 (2), 545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Leggett MJ; Schwarz JS; Burke PA; McDonnell G; Denyer SP; Maillard JY Mechanism of Sporicidal Activity for the Synergistic Combination of Peracetic Acid and Hydrogen Peroxide. Appl. Environ. Microbiol 2016, 82 (4), 1035–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).U.S. Environmental Protection Agency Evaluation Of Liquid And Foam Technologies For The Inactivation Of Bacillus Anthracis Spores On Topsoil, EPA/600/R-10/080; U.S. Environmental Protection Agency: Washington, DC, 2010. [Google Scholar]
- (99).U.S. Environmental Protection Agency Biological Agent Decontamination Technology Testing, EPA/600/R-10/087; U.S. Environmental Protection Agency: Washington, DC, 2010. [Google Scholar]
- (100).Meyer KM; Tufts JA; Calfee MW; Oudejans L Efficacy of sporicidal wipes for inactivation of a Bacillus anthracis surrogate. J. Appl. Microbiol 2014, 117 (6), 1634–44. [DOI] [PubMed] [Google Scholar]
- (101).Wood JP; Calfee MW; Clayton M; Griffin-Gatchalian N; Touati A; Egler K Evaluation of peracetic acid fog for the inactivation of Bacillus anthracis spore surrogates in a large decontamination chamber. J. Hazard. Mater 2013, 250–251, 61–67. [DOI] [PubMed] [Google Scholar]
- (102).Richter WR; Wood JP; Wendling MQ; Rogers JV Inactivation of Bacillus anthracis spores to decontaminate subway railcar and related materials via the fogging of peracetic acid and hydrogen peroxide sporicidal liquids. J. Environ. Manage 2018, 206, 800–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Wang L; Peng L; Xie L; Deng P; Deng D, Compatibility of surfactants and thermally activated persulfate for enhanced subsurface remediation. Environ. Sci. Technol 201751 7055. [DOI] [PubMed] [Google Scholar]
- (104).Li W; Orozco R; Camargos N; Liu H Mechanisms on the Impacts of Alkalinity, pH, and Chloride on Persulfate-Based Groundwater Remediation. Environ. Sci. Technol 2017, 51 (7), 3948–3959. [DOI] [PubMed] [Google Scholar]
- (105).Wordofa DN; Walker SL; Liu H Sulfate Radical-Induced Disinfection of Pathogenic Escherichia coli O157: H7 via IronActivated Persulfate. Environ. Sci. Technol. Lett 2017, 4 (4), 154–160. [Google Scholar]
- (106).U.S. Environmental Protection Agency. Decontamination of Outdoor Materials Contaminated with Anthrax Using Sodium Persulfate or Chloropicrin, EPA/600/R-15/101; U.S. Environmental Protection Agency: Washington, DC, 2015. [Google Scholar]
- (107).Raber E; McGuire R Oxidative decontamination of chemical and biological warfare agents using L-Gel. J. Hazard. Mater 2002, 93 (3), 339–352. [DOI] [PubMed] [Google Scholar]
- (108).Delcomyn CA; Bushway KE; Henley MV Inactivation of biological agents using neutral oxone-chloride solutions. Environ. Sci. Technol 2005, 39 (16), 2759–2764. [DOI] [PubMed] [Google Scholar]
- (109).Vogt H; Balej J; Bennett JE; Wintzer P; Sheikh SA; Gallone P; Vasudevan S; Pelin K, Chlorine Oxides and Chlorine Oxygen Acids In Ullmann’s Encyclopedia of Industrial Chemistry: Wiley-VCH Verlag GmbH & Co. KGaA, 2000. [Google Scholar]
- (110).U.S. Environmental Protection Agency Decontamination of Indoor and Outdoor Materials with Aqueous Chlorine Dioxide Solutions, EPA 600/R-12/516; U.S. Environmental Protection Agency: Washington, DC, 2012. [Google Scholar]
- (111).Chatuev BM; Peterson JW Analysis of the sporicidal activity of chlorine dioxide disinfectant against Bacillus anthracis (Sterne strain). J. Hosp. Infect 2010, 74 (2), 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Buhr TL; Young AA; Minter ZA; Wells CM; Shegogue DA Decontamination of a hard surface contaminated with Bacillus anthracis DeltaSterne and B. anthracis Ames spores using electrochemically generated liquid-phase chlorine dioxide (eClO2). J. Appl. Microbiol 2011, 111 (5), 1057–64. [DOI] [PubMed] [Google Scholar]
- (113).European Chemicals Agency Biocidal Products Committee Opinion on the Application for Approval of the Active Substance Formaldehyde, ECHA/BPC/181/2017; Helsinki: Finland, 2017. [Google Scholar]
- (114).Manchee RJ; Broster MG; Stagg AJ; Hibbs SE Formaldehyde Solution Effectively Inactivates Spores of BacillusAnthracis on the Scottish Island of Gruinard. Appl. Environ. Microbiol 1994, 60 (11), 4167–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Wood JP; Ryan SP; Snyder EG; Serre SD; Touati A; Clayton MJ Adsorption of chlorine dioxide gas on activated carbons. J. Air Waste Manage. Assoc 2010, 60 (8), 898–906. [DOI] [PubMed] [Google Scholar]
- (116).Canter DA Remediating anthrax-contaminated sites: Learning from the past to protect the future. Chem. Health Saf 2005, 12, 13–19. [Google Scholar]
- (117).Wood JP; Blair Martin G Development and field testing of a mobile chlorine dioxide generation system for the decontamination of buildings contaminated with Bacillus anthracis. J. Hazard. Mater 2009, 164 (2−3), 1460–7. [DOI] [PubMed] [Google Scholar]
- (118).Hubbard H; Poppendieck D; Corsi RL Chlorine dioxide reactions with indoor materials during building disinfection: surface uptake. Environ. Sci. Technol 2009, 43 (5), 1329–1335. [DOI] [PubMed] [Google Scholar]
- (119).U.S. Environmental Protection Agency. Evaluation of Sporicidal Decontamination Technology: Sabre Technical Services Chlorine Dioxide Gas Generator, EPA/600/R-06/048; U.S. Environmental Protection Agency: Washington, DC, 2006. [Google Scholar]
- (120).U.S. Environmental Protection Agency. Inactivation of Bacillus anthracis Spores in Soil Matrices with Chlorine Dioxide Gas, EPA/600/R-12/517; U.S. Environmental Protection Agency: Washington, DC, 2012. [Google Scholar]
- (121).U.S. Environmental Protection Agency. Assessment of the Decontamination of Soil Contaminated with Bacillus anthracis Spores Using Chlorine Dioxide Gas, Methyl Bromide, or Activated Sodium Persulfate, EPA/600/R-17/343; Washington, DC, 2017. [Google Scholar]
- (122).U.S. Environmental Protection Agency. Interactions of ClO2 and H2O2 Fumigants with Dirt and Grime on Subway Concrete, EPA/600/R-14/226; U.S. Environmental Protection Agency: Washington, DC, 2014. [Google Scholar]
- (123).U.S. Environmental Protection Agency. Evaluation of Chlorine Dioxide Gas and Peracetic Acid Fog for the Decontamination of a Mock Heating, Ventilation, and Air Conditioning Duct System, EPA/600/R14/014; U.S. Environmental Protection Agency: Washington, DC, 2014. [Google Scholar]
- (124).Kane SR; Létant SE; Murphy GA; Alfaro TM; Krauter PW; Mahnke R; Legler TC; Raber E Rapid, high-throughput, culture-based PCR methods to analyze samples for viable spores of Bacillus anthracis and its surrogates. J. Microbiol. Methods 2009, 76 (3), 278–284. [DOI] [PubMed] [Google Scholar]
- (125).Lowe JJ; Gibbs SG; Iwen PC; Smith PW; Hewlett AL Decontamination of a hospital room using gaseous chlorine dioxide: Bacillus anthracis, Francisella tularensis, and Yersinia pestis. J. Occup. Environ. Hyg 2013, 10 (10), 533–9. [DOI] [PubMed] [Google Scholar]
- (126).Pottage T; Macken S; Giri K; Walker JT; Bennett AM Low-temperature decontamination with hydrogen peroxide or chlorine dioxide for space applications. Appl. Environ. Microbiol 2012, 78 (12), 4169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).U.S. Environmental Protection Agency. Decontamination of a Mock Offce Using Chlorine Dioxide Gas, EPA/600/R-14/208; U.S. Environmental Protection Agency: Washington, DC, 2014. [Google Scholar]
- (128).U.S. Environmental Protection Agency Chlorine Dioxide Fumigation of Subway Materials Contaminated with B. anthracis Surrogate Spores; EPA/600/R-16/038; U.S. Environmental Protection Agency, Washington DC. [Google Scholar]
- (129).Wang T; Wu J; Qi J; Hao L; Yi Y; Zhang Z Kinetics of Inactivation of Bacillus subtilis subsp. niger Spores and Staph-ylococcus albus on Paper by Chlorine Dioxide Gas in an Enclosed Space. Appl. Environ. Microbiol 2016, 82 (10), 3061–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Muller J Treatment of Seeds for Sowing; United States Patent Offce, 1934; 1,962,996. [Google Scholar]
- (131).Wood JP; Calfee MW; Clayton M; Griffin-Gatchalian N; Touati A; Ryan S; Mickelsen L; Smith L; Rastogi V A simple decontamination approach using hydrogen peroxide vapour for Bacillus anthracis spore inactivation. J. Appl. Microbiol 2016, 121, n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Unger-Bimczok B; Kottke V; Hertel C; Rauschnabel J The Influence of Humidity, Hydrogen Peroxide Concentration, and Condensation on the Inactivation of Geobacillus stearothermophilus Spores with Hydrogen Peroxide Vapor. J. Pharm. Innov 2008, 3 (2), 123–133. [Google Scholar]
- (133).U.S. Environmental Protection Agency. Material Demand Studies: Materials Sorption of Vaporized Hydrogen Peroxide, EPA/600/R-10/002; U.S. Environmental Protection Agency: Washington, DC, 2010. [Google Scholar]
- (134).Kaspari O; Lemmer K; Becker S; Lochau P; Howaldt S; Nattermann H; Grunow R Decontamination of a BSL3 laboratory by hydrogen peroxide fumigation using three different surrogates for Bacillus anthracis spores. J. Appl. Microbiol 2014, 117 (4), 1095–103. [DOI] [PubMed] [Google Scholar]
- (135).Johnston MD; Lawson S; Otter JA Evaluation of hydrogen peroxide vapour as a method for the decontamination of surfaces contaminated with Clostridium botulinum spores. J. Microbiol. Methods 2005, 60 (3), 403–411. [DOI] [PubMed] [Google Scholar]
- (136).Pruss K; Stirtzel S; Kulozik U Influence of the surface temperature of packaging specimens on the inactivation of Bacillus spores by means of gaseous H2O2. J. Appl. Microbiol 2012, 112 (3), 493–501. [DOI] [PubMed] [Google Scholar]
- (137).U.S. Environmental Protection Agency. Vaprox Hydrogen Peroxide Sterilant - Amendment to Add Aseptic Food Processing Use EPA Pesticide; Label Reg. No. 58779–4, 2012. [Google Scholar]
- (138).Baron PA; Estill CF; Beard JK; Hein MJ; Larsen L Bacterial endospore inactivation caused by outgassing of vapourous hydrogen peroxide from polymethyl methacrylate (Plexiglas). Lett. Appl. Microbiol 2007, 45 (5), 485–90. [DOI] [PubMed] [Google Scholar]
- (139).Meyer KM; Calfee MW; Wood JP; Mickelsen L; Attwood B; Clayton M; Touati A; Delafield R Fumigation of a laboratory-scale HVAC system with hydrogen peroxide for decontamination following a biological contamination incident. J. Appl. Microbiol 2014, 116 (3), 533–41. [DOI] [PubMed] [Google Scholar]
- (140).Malik DJ; Shaw CM; Rielly CD; Shama G The inactivation of Bacillus subtilis spores at low concentrations of hydrogen peroxide vapour. J. Food Eng 2013, 114 (3), 391–396. [Google Scholar]
- (141).Kolb RW; Schneiter R The germicidal and sporicidal efficacy of methyl bromide for Bacillus anthracis. J. Bacteriol 1950, 59 (3), 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Wood JP; Wendling M; Richter W; Lastivka A; Mickelsen L Evaluation of the Efficacy of Methyl Bromide in the Decontamination of Building and Interior Materials Contaminated with Bacillus anthracis Spores. Appl. Environ. Microbiol 2016, 82 (7), 2003–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (143).United Nations Environment Program OzonAction Fact Sheet: QPS Uses of Methyl bromide and Their Alternatives http://www.unep.fr/ozonaction/information/mmcfiles/7766-esFactsheetQPSusesofMB.pdf (accessed June 18, 2018),
- (144).Wood JP; Clayton MJ; McArthur T; Serre SD; Mickelsen L; Touati A Capture of methyl bromide emissions with activated carbon following the fumigation of a small building contaminated with a Bacillus anthracis spore simulant. J. Air Waste Manage. Assoc 2015, 65 (2), 145–153. [DOI] [PubMed] [Google Scholar]
- (145).Juergensmeyer MA; Gingras BA; Scheffrahn RH; Weinberg MJ Methyl bromide fumigant lethal to Bacillus anthracis spores. J. Environ. Health 2007, 69 (6), 24–26. [PubMed] [Google Scholar]
- (146).Serre S; Mickelsen L; Calfee MW; Wood JP; Gray MS Jr.; Scheffrahn RH; Perez R; Kern WH Jr.; Daniell N, Whole-building decontamination of Bacillus anthracis Sterne spores by methyl bromide fumigation. J. Appl. Microbiol 2016120 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).U.S. Environmental Protection Agency. Subway Railcar Decontamination with Methyl Bromide; U.S. Environmental Protection Agency: Washington, DC, 2017. [Google Scholar]
- (148).U.S. Environmental Protection Agency. Decontamination of Subway Materials Contaminated with a Biological Spore using Methyl Bromide, EPA/600/R-17/187; U.S. Environmental Protection Agency: Washington, DC, 2017. [Google Scholar]
- (149).Sutton M; Kane SR; Wollard JR Methyl Iodide Fumigation of Bacillus anthracis Spores. J. Environ. Health 2015, 78 (2), 14–9. [PubMed] [Google Scholar]
- (150).U.S. Environmental Protection Agency. Evaluation of Methyl Iodide for the Inactivation of Bacillus anthracis, EPA/600/R-14/229; U.S. Environmental Protection Agency: Washington, DC, 2014. [Google Scholar]
- (151).Munro K; Lanser J; Flower R A comparative study of methods to validate formaldehyde decontamination of biological safety cabinets. Appl. Environ. Microbiol 1999, 65 (2), 873–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Gordon D; Madden B; Krishnan J; Klassen S; Dalmasso J; Theriault S Implications of paper vs stainless steel biological indicator substrates for formaldehyde gas decontamination. J. Appl. Microbiol 2011, 110 (2), 455–462. [DOI] [PubMed] [Google Scholar]
- (153).Canter DA; Gunning D; Rodgers P; O’connor L; Traunero C; Kempter CJ Remediation of Bacillus anthracis contamination in the US Department of Justice mail facility. Biosecurity and bioterrorism: biodefense strategy, practice, and science 2005, 3 (2), 119–127. [DOI] [PubMed] [Google Scholar]
- (154).Beswick AJ; Farrant J; Makison C; Gawn J; Frost G; Crook B; Pride J Comparison of multiple systems for laboratory whole room fumigation. Appl. Biosaf 2011, 16 (3), 139–157. [Google Scholar]
- (155).Gerberich HR; Seaman GC Formaldehyde In KirkOthmer Encyclopedia of Chemical Technology, 1994. [Google Scholar]
- (156).Luftman HS Neutralization of formaldehyde gas by ammonium bicarbonate and ammonium carbonate. Appl. Biosaf 2005, 10 (2), 101–106. [Google Scholar]
- (157).Macellaro A; Karlsson L; Emmoth E; Dergel I; Metreveli G; Bengtsson UA; Byström M; Hultén C; Johansson A-L Evaluation of Biological Indicator Spores as Tools for Assessment of Fumigation Decontamination Effectiveness. Appl. Biosaf 2015, 20 (4), 183–191. [Google Scholar]
- (158).Ngabo D; Pottage T; Bennett A; Parks S Cabinet Decontamination Using Formaldehyde. Appl. Biosaf 2017, 22 (2), 60–67. [Google Scholar]
- (159).NSF International; American National Standards Institute, Biosafety Cabinetry: Design, Construction, Performance, and Field Certification In Annex G, 2011. [Google Scholar]
- (160).Biosafety in Microbiological and Biomedical Laboratories, 5th ed.; U.S. Department of Health and Human Services, 2009; CDC21− 1112. [Google Scholar]
- (161).Taylor LA; Barbeito MS; Gremillion GG Paraformaldehyde for surface sterilization and detoxification. Appl. Microbiol 1969, 17 (4), 614–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Ackland N; Hinton M; Denmeade K Controlled formaldehyde fumigation system. Appl. Environ. Microbiol 1980, 39(3), 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Spiner DR; Hoffman RK Effect of relative humidity on formaldehyde decontamination. Appl. Microbiol 1971, 22 (6), 1138–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (164).Masaoka T; Kubota Y; Namiuchi S; Takubo T; Ueda T; Shibata H; Nakamura H; Yoshitake J; Yamayoshi T; Doi H Ozone decontamination of Bioclean rooms. Appl. Environ. Microbiol 1982, 43 (3), 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (165).Xylem Inc. PDOevo ozone system https://www.xylem.com/en-US/products-services/treatment-products-systems/disinfectionand-oxidation/ozone-systems/pdoevo-ozone-system (accessed May 9, 2017).
- (166).Rose L; Rice E Inactivation of bacterial biothreat agents in water, a review. J. Water Health 2014, 12 (4), 618–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (167).Sousa CS; Torres LM; Azevedo MP; de Camargo TC; Graziano KU; Lacerda RA; Turrini RN [Sterilization with ozone in health care: an integrative literature review]. Rev. Esc. Enferm. USP 2011, 45 (5), 1243–9. [DOI] [PubMed] [Google Scholar]
- (168).Davies A; Pottage T; Bennett A; Walker J Gaseous and air decontamination technologies for Clostridium difficile in the healthcare environment. J. Hosp. Infect 2011, 77 (3), 199–203. [DOI] [PubMed] [Google Scholar]
- (169).Sharma M; Hudson JB Ozone gas is an effective and practical antibacterial agent. Am. J. Infect. Control 2008, 36 (8), 559–563. [DOI] [PubMed] [Google Scholar]
- (170).Hudson J; Sharma M; Petric M Inactivation of Norovirus by ozone gas in conditions relevant to healthcare. J. Hosp. Infect 2007, 66 (1), 40–45. [DOI] [PubMed] [Google Scholar]
- (171).U.S. Food and Drug Administration. Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling Guidance for Industry and Food and Drug Administration Staff; Rockville, MD, 2015. [Google Scholar]
- (172).Mahfoudh A; Moisan M; Seguin J; Barbeau J; Kabouzi Y; Keroack D Inactivation of vegetative and sporulated bacteria by dry gaseous ozone. Ozone: Sci. Eng 2010, 32 (3), 180–198. [Google Scholar]
- (173).Menetrez M; Foarde K; Schwartz T; Dean T; Betancourt D An Evaluation of the Antimicrobial Effects of Gas-Phase Ozone. Ozone: Sci. Eng 2009, 31 (4), 316–325. [Google Scholar]
- (174).Currier RP; Torraco DJ; Cross JB; Wagner GL; Gladden PD; Vanderberg LA Deactivation of Clumped and Dirty Spores of Bacillus globigii. Ozone: Sci. Eng 2001, 23 (4), 285. [Google Scholar]
- (175).Aydogan A; Gurol MD Application of gaseous ozone for inactivation of Bacillus subtilis spores. J. Air Waste Manage. Assoc 2006, 56 (2), 179–185. [DOI] [PubMed] [Google Scholar]
- (176).Akbas MY; Ozdemir M Application of gaseous ozone to control populations of Escherichia coli, Bacillus cereus and Bacillus cereus spores in dried figs. Food Microbiol 2008, 25 (2), 386–391. [DOI] [PubMed] [Google Scholar]
- (177).Joslyn LJ Sterilization by Heat In Disinfection, Sterlization, and Preservation, 5th ed.; Block SS, Ed.; Lippincott Willimas & Wilkins, 2001; Vol.. [Google Scholar]
- (178).Lemieux P; Sieber R; Osborne A; Woodard A Destruction of spores on building decontamination residue in a commercial autoclave. Appl. Environ. Microbiol 2006, 72 (12), 7687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (179).Galvao MA; da Silva JC; Teixeira MC Efficacy of the decontamination of biological infectious waste after thermal treatment by autoclaving. Engenharia Sanitaria E Ambiental 2013, 18 (4), 323–331. [Google Scholar]
- (180).Wood JP; Lemieux P; Betancourt D; Kariher P; Gatchalian NG Dry thermal resistance of Bacillus anthracis (Sterne) spores and spores of other Bacillus species: implications for biological agent destruction via waste incineration. J. Appl. Microbiol 2009, 109 (1), 99–106. [DOI] [PubMed] [Google Scholar]
- (181).Xing Y; Li A; Felker DL; Burggraf LW Nanoscale structural and mechanical analysis of Bacillus anthracis spores inactivated with rapid dry heating. Appl. Environ. Microbiol 2014, 80 (5), 1739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Peeler J; Reyes A; Crawford R; Wehby A; Campbell J Thermal resistance of Bacillus subtilis var. niger in a closed system. Appl. Environ. Microbiol 1977, 33 (1), 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (183).Buhr TL; Young AA; Minter ZA; Wells CM; McPherson DC; Hooban CL; Johnson CA; Prokop EJ; Crigler JR Test method development to evaluate hot, humid air decontamination of materials contaminated with Bacillus anthracis Sterne and B. thuringiensis. Al Hakam spores. J. Appl. Microbiol 2012, 113 (5), 1037–51. [DOI] [PubMed] [Google Scholar]
- (184).Coleman WH; Zhang P; Li YQ; Setlow P Mechanism of killing of spores of Bacillus cereus and Bacillus megaterium by wet heat. Lett. Appl. Microbiol 2010, 50 (5), 507–14. [DOI] [PubMed] [Google Scholar]
- (185).Zhang PF; Kong LB; Setlow P; Li YQ Characterization of Wet-Heat Inactivation of Single Spores of Bacillus Species by Dual-Trap Raman Spectroscopy and Elastic Light Scattering. Appl. Environ. Microbiol 2010, 76 (6), 1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (186).Penna TCV; Ishii M; Machoshvili IA; Marques M The effect of bioindicator preparation and storage on thermal resistance of Bacillus stearothermophilus spores. Appl. Biochem. Biotechnol 2002, 98, 525–538. [DOI] [PubMed] [Google Scholar]
- (187).Setlow B; Parish S; Zhang P; Li YQ; Neely WC; Setlow P Mechanism of killing of spores of Bacillus anthracis in a high-temperature gas environment, and analysis of DNA damage generated by various decontamination treatments of spores of Bacillus anthracis, Bacillus subtilis and Bacillus thuringiensis. J. Appl. Microbiol 2014, 116 (4), 805–814. [DOI] [PubMed] [Google Scholar]
- (188).Buhr TL; Young AA; Bensman M; Minter ZA; Kennihan NL; Johnson CA; Bohmke MD; Borgers Klonkowski E; Osborn EB; Avila SD Hot, humid air decontamination of a C 130 aircraft contaminated with spores of two acrystalliferous Bacillus thuringiensis strains, surrogates for Bacillus anthracis. J. Appl. Microbiol 2016, 120 (4), 1074–84. [DOI] [PubMed] [Google Scholar]
- (189).Coohill TP; Sagripanti J-L Overview of the Inactivation by 254 nm Ultraviolet Radiation of Bacteria with Particular Relevance to Biodefense. Photochem. Photobiol 2008, 84 (5), 1084–1090. [DOI] [PubMed] [Google Scholar]
- (190).Kowalski W Ultraviolet Germicidal Irradiation Handbook; Springer: New York, 2009. [Google Scholar]
- (191).Menetrez MY; Foarde KK; Webber TD; Dean TR; Betancourt DA Efficacy of UV irradiation on eight species of Bacillus. J. Environ. Eng. Sci 2006, 5 (4), 329–334. [Google Scholar]
- (192).Kesavan J; Schepers D; Bottiger J; Edmonds J UV-C Decontamination of Aerosolized and Surface-Bound Single Spores and Bioclusters. Aerosol Sci. Technol 2014, 48 (4), 450–457. [Google Scholar]
- (193).Owens MU; Deal DR; Shoemaker MO; Knudson GB; Meszaros JE; Deal JL High-Dose Ultraviolet C Light Inactivates Spores of Bacillus Atrophaeus and Bacillus Anthracis Sterne on Nonreflective Surfaces. Appl. Biosaf 2005, 10 (4), 240. [Google Scholar]
- (194).Tran T; Racz L; Grimaila MR; Miller M; Harper WF Jr. Comparison of continuous versus pulsed ultraviolet light emitting diode use for the inactivation of Bacillus globigii spores. Water Sci. Technol 2014, 70 (9), 1473–80. [DOI] [PubMed] [Google Scholar]
- (195).Xue Y; Nicholson WL The Two Major Spore DNA Repair Pathways, Nucleotide Excision Repair and Spore Photoproduct Lyase, Are Sufficient for the Resistance of Bacillus subtilis Spores to Artificial UV-C and UV-B but Not to Solar Radiation. Appl. Environ. Microbiol 1996, 62 (7), 2221–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (196).Raguse M; Fiebrandt M; Stapelmann K; Madela K; Laue M; Lackmann J-W; Thwaite JE; Setlow P; Awakowicz P; Moeller R Improvement of biological indicators by uniformLy distributing Bacillus subtilis spores in monolayers to evaluate enhanced spore decontamination technologies. Appl. Environ. Microbiol 2016, 82 (7), 2031–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Würtele MA; Kolbe T; Lipsz M; Külberg A; Weyers M; Kneissl M; Jekel M Application of GaN-based ultraviolet-C light emitting diodes − UV LEDs − for water disinfection. Water Res 2011, 45 (3), 1481–1489. [DOI] [PubMed] [Google Scholar]
- (198).King B; Kesavan J; Sagripanti JL Germicidal UV Sensitivity of Bacteria in Aerosols and on Contaminated Surfaces. Aerosol Sci. Technol 2011, 45 (5), 645–653. [Google Scholar]
- (199).U.S. Environmental Protection Agency. Biological Inactivation Effsciency of HVAC In-Duct Ultraviolet Light Devices, EPA/600/S-11/002; U.S. Environmental Protection Agency: Wasington, DC, 2006. [Google Scholar]
- (200).Umezawa K; Asai S; Inokuchi S; Miyachi H A comparative study of the bactericidal activity and daily disinfection housekeeping surfaces by a new portable pulsed UV radiation device. Curr. Microbiol 2012, 64 (6), 581–7. [DOI] [PubMed] [Google Scholar]
- (201).Bruscolini F; Paolucci D; Rosini V; Sabatini L; Andreozzi E; Pianetti A Evaluation of ultraviolet irradiation efficacy in an automated system for the aseptic compounding using challenge test. Int. J. Qual. Health Care 2015, 27 (5), 412–7. [DOI] [PubMed] [Google Scholar]
- (202).Gardner D; Shama G The kinetics of Bacillus subtilis spore inactivation on filter paper by uv light and uv light in combination with hydrogen peroxide. J. Appl. Microbiol 1998, 84 (4), 633–641. [Google Scholar]
- (203).Blatchley ER; Meeusen A; Aronson AI; Brewster L Inactivation of Bacillus spores by ultraviolet or gamma radiation. J. Environ. Eng 2005, 131 (9), 1245–1252. [Google Scholar]
- (204).U.S. Environmental Protection Agency. Evaluation of Liquid and Foam Technologies for the Decontamination of B. anthracis and B. subtilis on Building and Outdoor Materials: Technology Evaluation Report, EPA/600/R 09/150; U.S. Environmental Protection Agency: Washington, DC, 2009 [Google Scholar]
- (205).U.S. Environmental Protection Agency. Effectiveness of Physical and Chemical Cleaning and Disinfection Methods for Removing, Reducing or Inactivating Agricultural Biological Threat Agents, EPA/600/R-11/092; U.S. Environmental Protection Agency: Washington, DC, 2011; p 124. [Google Scholar]
- (206).U.S. Environmental Protection Agency. Assessment Of Liquid And Physical Decontamination Methods For Environmental Surfaces Contaminated Withbacterial Spores: Evaluation Of Spray Method Parameters And Impact Of Surface Grime, EPA/600/R-12/591; U.S. Environmental Protection Agency: Washington, DC, 2012. [Google Scholar]
- (207).U.S. Environmental Protection Agency. Assessment of Liquid and Physical Decontamination Methods for Environmental Surfaces Contaminated with Bacterial Spores: Development and Evaluation of the Decontamination Procedural Steps, EPA/600/R-12/025; U.S. Environ-mental Protection Agency: Washington, DC, 2012. [Google Scholar]
- (208).U.S. Environmental Protection Agency. Expedient Approaches for the Management of Wastes Generated from Biological Decontamination Operations in an Indoor Environment-Evaluation of Waste Sampling and Decontamination Procedures, EPA 600/R-14/262; U.S. Environ-mental Protection Agency: Washington, DC, 2014. [Google Scholar]
- (209).U.S. Environmental Protection Agency. Underground Tran-port Restoration (UTR) Operational Technology Demonstration (OTD), EPA/600/R-17/272; U.S. Environmental Protection Agency: Washington, DC, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


