Abstract
Background
Both-bone forearm fractures are a common fracture, accounting for 3.4% of all paediatric fractures. For now, elastic stable intramedullary nailing (ESIN) and open reduction and internal fixation (ORIF) are the common surgical procedures for paediatric both-bone forearm fractures. Both ORIF and ESIN have their shortcomings. Therefore, we need to find another surgical treatment which can decrease the rate of complications and improve the clinical efficacy. Our study plans to test hybrid fixation, using an ESIN fixation for the radius and an ORIF for the ulna. Our study will conduct a randomised controlled trial (RCT) comparing double plate fixation with hybrid fixation for treatment of both-bone forearm fractures in older children between 10 and 16 years of age. The objectives of this trial are to compare the effectiveness between double plate fixation and hybrid fixation for treatment of both-bone forearm fractures in older children.
Methods
An RCT will be conducted, and the participants included will be randomly divided into either the hybrid fixation group or the double plate fixation group, at a ratio of 1:1. The primary clinical outcome measures are the Disabilities of the Arm, Shoulder and Hand score and radiological evaluation. Secondary clinical outcome measures are intraoperative blood loss, surgical duration, visual analogue scale score after surgery, hospital duration after surgery and complications. Follow-up will be conducted at 2 weeks and 1, 3, 6 and 12 months postoperatively.
Discussion
The trial will provide a new surgical treatment for forearm fractures in older children. Our hypothesis is that there is no clinically relevant difference in the primary outcome measures between the two treatment groups.
Trial registration
Chinese Clinical Trial Registry, ChiCTR1800018060. Registered on 26 August 2018.
Electronic supplementary material
The online version of this article (10.1186/s13063-019-3458-5) contains supplementary material, which is available to authorized users.
Keywords: Forearm fractures, Elastic stable intramedullary nailing, Hybrid fixation, Randomised controlled trial
Background
Both-bone forearm fractures are a common fracture, accounting for 3.4% of all paediatric fractures and 26% of paediatric upper extremity fractures [1, 2]. For children under 10 years old, most both-bone forearm fractures can be treated successfully with casting due to the considerable bone remodelling potential [3–5]. Although closed reduction and casting is a feasible choice for children older than 10 years old [6], there are adverse outcomes of conservative treatment, such as nonunion of the fracture, malunion and secondary surgery [7]. Also, the standard of amount of angulation or malrotation is not clear in this age group. In addition, for open fractures, fractures associated with compartment syndromes, elbow injuries, combined injuries such as Monteggia fractures and Monteggia equivalents, significant comminution or further displacement with nonoperative treatment, operative treatment is required [8–10]. For now, elastic stable intramedullary nailing (ESIN) and plate screw fixation are the common surgical procedures for paediatric both-bone forearm fractures [11]. The advantages of ESIN fixation for paediatric both-bone forearm fractures compared with plate fixation include less wound infection, shorter operative time, smaller edge, less soft tissue dissection, ease of implant removal and early return to activity after implant removal [12–16]. However, ESIN fixation also has its own shortcomings, including delayed union and nonunion, refracture, implant migration or failure and compartment syndrome [17–21]. Compared with younger children, the rate of complications is obviously increased in those over 10 years old [22–24]. Open reduction and internal fixation (ORIF) is a surgical alternative in this age group and offers some potential benefits, including immediate fracture stabilisation and anatomic reduction, which are important for restoring forearm rotation [25, 26]. However, ORIF has been criticised because of its longer operation time, the amount of soft tissue dissection and periosteal stripping, the increased amount of bleeding and the increased risk of wound infection [27, 28].
Both ORIF and ESIN fixation have their shortcomings. Therefore, we need to find another surgical treatment which can decrease the rate of complications and improve the clinical efficacy. Our study plans to test hybrid fixation, using an ESIN fixation for the radius, and open reduction and plate screw fixation for the ulna. Compared with double plate fixation, hybrid fixation not only reduces soft tissue dissection and potentially refracture rates after implant removal, but it also incorporates some advantages of ESIN fixation. Feng et al. conducted a case-control study and found that mixed fixation was a feasible method [29]. Our study will conduct a randomised controlled trial (RCT) comparing double plate fixation with hybrid fixation for treatment of both-bone forearm fractures in older children between 10 and 16 years of age.
The objectives of this trial are to compare the effectiveness between double plate fixation and hybrid fixation for treatment of both-bone forearm fractures in older children. Our hypothesis is that there is no clinically relevant difference in the primary outcome measures between the two treatment groups.
Methods
Study setting
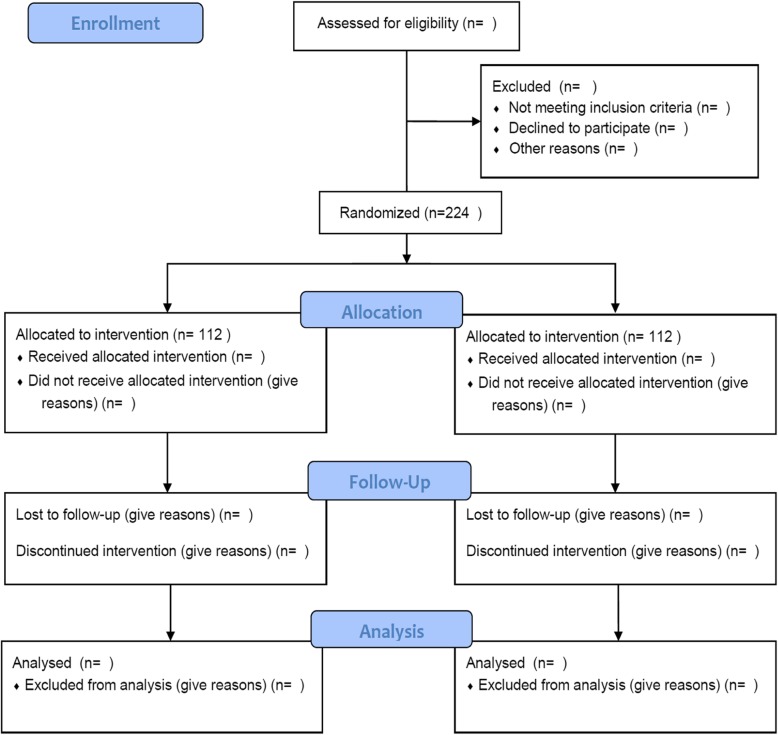
The study, based on an RCT, will be conducted in our hospital. The trial was approved and monitored by the Ethics Research Committee of our hospital, and it conforms to the Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. This trial has been registered at the Chinese Clinical Trial Registry (ChiCTR1800018060). The protocol conforms to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines (see Additional file 1). Figure 1 shows a flow chart of the trial design.
Fig. 1.
Flow chart of the trial
Consent
Informed consent takes place in a face-to-face setting at the research site. Patients’ parents will have at least 24 h to consider participation and will be encouraged to discuss the study with their family and other healthcare professionals. A full verbal explanation of the study, a written Patient Information Sheet (detailing rationale, design and personal implications of trial entry) and informed consent form will be provided. Participants may withdraw at any stage of the trial. Consent will be obtained prior to collection of baseline assessment data and subsequent randomisation.
Participants
Some members of our group will assess patients with both-bone forearm fractures for eligibility. The diagnosis will be verified using anteroposterior and laterolateral radiographs. All eligible patients’ parents will be introduced to the study, given detailed written information about it and then asked to participate and to sign the written informed consent form. Inclusion and exclusion criteria are listed as follows.
Inclusion criteria
The inclusion criteria are as follows:
Boys or girls aged 10–16 years old
Only unilateral displaced closed both-bone forearm fractures
AO fracture classification types 22-A3 and 22-B3
Fracture has been present for less than 10 days
The time from injury to operation is less than 14 days
Parents have signed informed consent form and are willing to participate in all follow-up visits.
Exclusion criteria
The exclusion criteria are:
Bilateral fracture, open fractures, complex forearm fractures (Monteggia fractures, Galeazzi fractures, intra-articular elbow or wrist fractures) and pathologic fractures
History of trauma of the same upper extremity causing functional deficit
Disease that significantly affects the general condition of the patient
Significantly impaired ability to cooperate for any reason (substance abuse, mental disorder, dementia)
Unwilling to accept both treatment methods.
Participant withdrawal criteria
Patients will be withdrawn for the trial for the following reasons:
Patients request to withdraw from the trial
Occurrence of a serious adverse event
Occurrence of factors making it difficult to sustain the process or investigator’s decision to terminate because of clinical trial results affected by some factors
Patient death or patient lost to follow-up.
Participant timeline
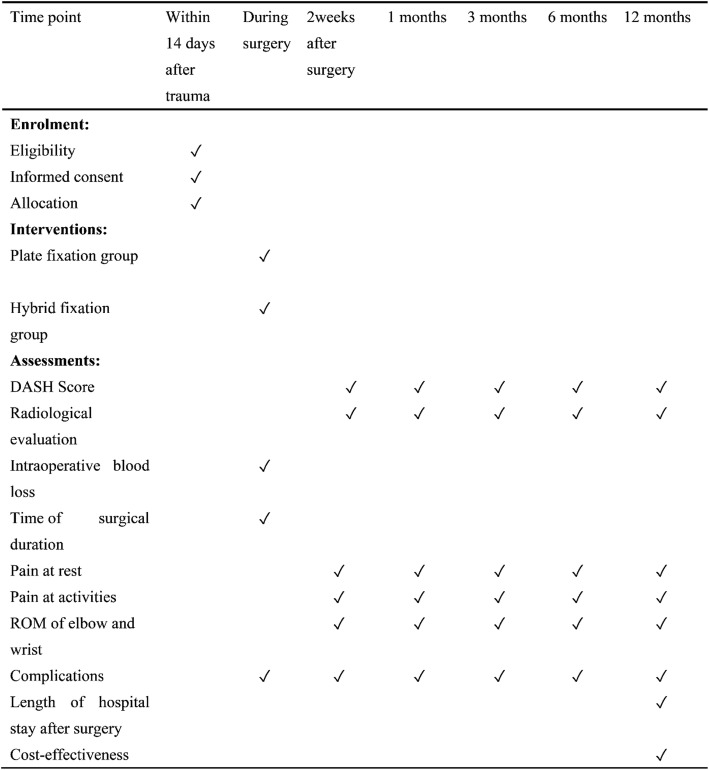
A flow chart of the trial is presented in Fig. 1. The time schedule of enrolment, interventions, assessments and visits is shown in Fig. 2.
Fig. 2.
Schedule of enrolment, interventions and assessments
Sample size
The sample size calculation is performed using G*Power 3.1 [30] and is based on Disabilities of the Arm, Shoulder and Hand (DASH) score as the primary outcome measure in this trial. For the sample size calculation, we used an α level of 0.05 and a β level of 0.1. We assumed the minimal clinically important difference (MCID) of the DASH to be 5 points, with the standard deviation (SD) being 14.7 [31]. Using these assumptions, the required sample size is 98 per group with 90% power to show a clinically important difference between the treatment methods with a two-sided type I error rate of 5%. With an assumption of 12.5% lost to follow-up, we decided to include 112 participants per group.
Allocation and randomisation
Prerandomisation eligibility checks will be carried out to ensure that participants are eligible for inclusion in the study. Patients will be randomly assigned to one of two groups (experimental or control) using a computer-generated random assignment in a 1:1 ratio. The computer randomly extracts 112 numbers from the 1–224 number as the hybrid fixation group, and the remaining numbers as the plate fixation group. According to the order of inclusion, patients will be numbered 1–224. Patients, researchers performing the follow-up measurements and the trial statistician will be blinded to the group allocations.
Blinding
Blinding in this study is almost impossible. The surgical approach differs considerably between these two methods. All patients, researchers and surgeons can also see the differences in the skin incisions between the ESIN and ORIF treatments.
Interventions
Surgical treatment will be performed either by or under the supervision of an experienced orthopaedic surgeon within 2 weeks after initial trauma.
Plate fixation group
A 3.5-mm narrow locking compression plate (DePuy Synthes, Raynham, MA, USA) will be the preferred choice of the treating surgeon. The length of the plate will be at least 7 holes to ensure stability of osteosynthesis, and at least three bicortical screws will be used on both sides of the fracture line. For dual plating fixation constructs, ORIF of the ulna will be performed using the direct approach to the subcutaneous ulnar shaft. The radius will be exposed with a standard anterior (Henry) approach. The dynamic compression plates will be used for both the radius and ulna. Reduction will be obtained and provisionally fixated; fluoroscopic image intensification will be used to verify rotational alignment and reduction of both fractures. Fixation of the implants will then be definitively secured.
Hybrid fixation group
The Titanium Elastic Nail System from Synthes (DePuy Synthes) with ESIN nails of 2.0–3.0 mm diameter, appearing to be one third of the diameter of the central bony canal, are usually used in our Department of Trauma Surgery. For hybrid constructs, the patient will be placed on the table and an image intensifier will be used to localise the placement of skin incisions. The radial ESIN nail is inserted through a 1- to 2-cm mini incision, to protect the superficial radial nerve, at the distal lateral radius. When the nail reaches the fracture site, the fracture is reduced by manipulation and traction under image intensifier control. Once reduction, alignment and provisional fixation of both fractures is satisfactory, the straight rod will be removed and an appropriately sized elastic intramedullary nail will be inserted into the radius. The plate on the ulnar will then be definitively affixed to the bone. An above-elbow plaster cast will be applied and maintained for 2 weeks.
Outcomes
Clinical radiological evaluation of union, functional evaluation of outcome and rate of complications will be performed immediately, and at 2 weeks and 1, 3, 6 and 12 months.
Primary outcome measures
Primary outcome measures are DASH score and radiological evaluation:
The primary outcome measure of this study is the DASH score, which will be recorded at 1, 3, 6 and 12 months postoperatively. The primary time point is at 12 months. DASH is a widely used and validated tool assessing upper extremity-related deficits and symptoms in daily life reported by the patient. It has been shown to be a valid instrument to monitor changes in symptoms and function over time [32–34].
Radiological evaluation based on postoperative X-ray, including nonunion of the fracture and malunion of the fracture, will be performed at 2 weeks and 1, 3, 6 and 12 months postoperatively.
Secondary outcome measures
Measurements are recorded at 2 weeks and 1, 3, 6 and 12 months postoperatively.
Intraoperative blood loss will be recorded in the anaesthesia records and will include the blood in suction bottles (after subtracting the lavage fluid used during the surgery) and that in the weighed sponges used during the operation.
Time of surgical duration.
Pain at rest (0–10 score with visual analogue scale).
Pain at activities (0–10 score with visual analogue scale).
Range of motion (ROM) of elbow and wrist.
Complications: wound infection, reoperations and nerve injury.
Length of hospital stay after surgery.
Cost-effectiveness.
Data collection and management
Questionnaire forms on paper will be the primary data collection tools for the study. The questionnaires will be completed at the outpatient clinic during the baseline and control visits. On receipt of the questionnaire forms, the researcher will make a visual check of the responses and will query missing data when possible. The paper forms will be securely stored at both study sites. Double data entry will be used to minimise typing errors. A research nurse and a research assistant will enter the data independently into two separate electronic databases. First, the research nurse enters the data into an electronic database, which is located in a secure network drive and protected with access codes known only by the research nurse. Missing, implausible and inconsistent data in the electronic database will be checked by the research nurse at the coordinating centre. If a missing or implausible item is noticed, the patient will be contacted and asked about the item. The answer will be corrected on the original paper form with a note that the answer was retrieved by a phone call and the corrected data had been entered to the database.
Patient records in the participating hospitals will also be used when collecting missing data or interpreting inconsistent or implausible data. After 12 months follow-up visits are completed and all data stored, a research assistant, not involved in the trial, will enter all the data from the paper forms into a separate database. The two databases will be compared for consistency. Discrepancies will be checked from the original paper forms by a research nurse at the coordinating centre. Final interpretation of the data will be corrected into the master database, which will be the source for the final data analysis.
Monitoring
Data monitoring
We will conduct the study without a data monitoring committee (DMC). Both treatment methods are widely used in daily practice and have been proven to provide acceptable results. Since there is no DMC, we will not conduct an interim analysis during the trial.
Harms auditing
All the medical records of the participating patients will be carefully assessed, and all harms and complications of the treatment will be reported when reporting the results of this trial. The harms will be categorised as serious and minor adverse events as described in the section.
Auditing
We will not conduct auditing between the participant centres during the trial.
Follow-up
Follow-up will be conducted at 2 weeks and 1, 3, 6 and 12 months postoperatively.
Statistical analysis
The trial data will be analysed using SPSS for Windows software (V.19.0; SPSS, Chicago, IL, USA). For continuous variables, the Shapiro-Wilk test will be applied to determine if they follow a normal distribution. For normally distributed variables, the means will be calculated and compared using the independent samples t test (Student’s t test) or analysis of variance (ANOVA); otherwise, the Mann-Whitney U test will be used for group comparisons. The χ2 test will be used to analyse qualitative variables. In all analyses, p < 0.05 will be taken to indicate statistical significance.
Ethics and dissemination
Research ethics approval
The trial was approved and monitored by the Ethics Research Committee of our hospital, which conforms to the Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. This trial has been registered at the Chinese Clinical Trial Registry (ChiCTR1800018060). The protocol conforms to the Standard Protocol Items: Recommendations for Interventional Trials.
Protocol amendments
All modifications of the study protocol will be communicated by updating the trial registry (Chinese Clinical Trial Registry.gov).
Dissemination policy
The findings of this study will be disseminated through peer-reviewed publications and conference presentations. Patients participating in the trial will be sent a letter with information on the results after the primary outcome results are published.
Discussion
Potential impact and significance of the study
ORIF treatment of forearm double fracture requires two longer surgical incisions to achieve satisfactory fracture reduction and fixation. To obtain adequate exposure, a significant amount of soft tissue dissection and periosteal stripping may be necessary, which has been regarded as a risk factor for nonunions [35, 36]. Compared with ORIF, ESIN has the advantage of smaller incisions, shorter operative time and less bleeding in the treatment of forearm fractures. However, this method may lead to increased redisplacement and reduced clinical results, which may fail to provide rotational stability [16]. Both ORIF and ESIN fixation have their shortcomings. Therefore, we need to find another surgical treatment which can decrease the rate of complications and improve the clinical efficacy.
The purpose of our study is to evaluate hybrid fixation construct feasibility, using an ESIN fixation for the radius and open reduction and plate screw fixation for the ulna.
The titanium elastic nail is known for its good flexibility with antirotation performance to some extent, which can be remodelled according to the curvature of the radius. It can also be easily prebent to make a fixation with two or more points in accordance with the fracture characteristics and location. Plate fixation of the ulna makes the forearm more stable, further enhancing the antirotation performance. Thus, patients do not need a long-time plaster cast applied, which is conducive to early exercise. Salvi [37] considered that the radius and ulna meet different functions; the radius had more complex functions, such as pronation and supination, whereas the ulna played a greater role in maintaining the stability of the forearm with respect to the radius, especially when subjected to buckling and torsional stress. So, when the ulna was intact, the radius fractures treated using intramedullary fixation would achieve a greater antirotation performance [38]. Therefore, restoring the original function of the ulna was necessary to rebuild the forearm stability, and ulnar plate fixation achieved this goal precisely.
In this protocol paper we describe an RCT comparing plate fixation with hybrid fixation for treatment of both-bone forearm fractures in older children between 10 and 16 years of age. After completion, this trial will provide valuable evidence on the treatment of both-bone forearm fractures in older children.
Strengths and limitations of the study
This study has potential limitations. Participants are under 16 years old, and their responses might not really reflect their subjective feelings. Thus, certain external validity problems will remain as a significant proportion of these patients are noncooperative. In addition, our follow-up period is only 1 year, because of limited funds and researchers.
Expectations
Our expectation is that there will be no clinically relevant difference in the primary outcome measure between the two treatment groups. Hybrid fixation will be another effective option for treatment of both-bone forearm fractures in older children.
Trial status
This trial is ongoing, and patient recruitment began in October 2018. Recruitment is expected to be completed in October 2019.
Additional file
SPIRIT 2013 checklist: recommended items to address in a clinical trial protocol and related documents. (DOC 124 kb)
Acknowledgements
We sincerely thank our schools and hospitals for their help and the patients who participated in the experiment. The authors thank all the staff at the university.
Abbreviations
- ChiCTR
Chinese Clinical Trial Registry
- DASH
Disabilities of the Arm, Shoulder and Hand
- ESIN
Elastic stable intramedullary nailing
- MCID
Minimal clinically important difference
- ORIF
Open reduction and plate screw fixation
- RCT
Randomised controlled trial
- ROM
Range of motion
Authors’ contributions
LYC is the guarantor. LYC and CHC were involved in the conception of the project. CHC, LZX and WHZ drafted the manuscript. CHC and WHZ will oversee enrolment and data collection. HC provided methodological expertise. All authors read, provided feedback and approved the final manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Availability of data and materials
The data that support the findings of this study will be available from Wenzhou Medical University; however, restrictions apply to the availability of these data. The data will be used under a license published for the current study and will not be publicly available. However, the data will be available from the corresponding author upon reasonable request and with permission from the institutions.
Ethics approval and consent to participate
The trial was approved and monitored by the Ethics Research Committee of our hospital, which conforms to the Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. This trial has been registered at the Chinese Clinical Trial Registry (ChiCTR1800018060). Written informed consent will be obtained from all patients prior to trial participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chunhui Chen and Linzhen Xie are co-first authors.
References
- 1.Mann DC, Rajmaira S. Distribution of physeal and nonphyseal fractures in 2,650 long-bone fractures in children aged 0–16 years. J Pediatr Orthop. 1990;10:713–716. doi: 10.1097/01241398-199011000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Landin LA. Fracture patterns in children. Analysis of 8,682 fractures with special reference to incidence, etiology and secular changes in a Swedish urban population 1950–1979. Acta Orthop Scand Suppl. 1983;202:1–109. [PubMed] [Google Scholar]
- 3.Price CT, Scott DS, Kurzner ME, Flynn JC. Malunited forearm fractures in children. J Pediatr Orthop. 1990;10:705–712. doi: 10.1097/01241398-199011000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Noonan KJ, Price CT. Forearm and distal radius fractures in children. J Am Acad Orthop Surg. 1998;6:146–156. doi: 10.5435/00124635-199805000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Jones K, Weiner DS. The management of forearm fractures in children: a plea for conservatism. J Pediatr Orthop. 1999;19:811–815. [PubMed] [Google Scholar]
- 6.Zionts LE, Zalavras CG, Gerhardt MB. Closed treatment of displaced diaphyseal both-bone forearm fractures in older children and adolescents. J Pediatr Orthop. 2005;25:507–512. doi: 10.1097/01.bpo.0000158005.53671.c4. [DOI] [PubMed] [Google Scholar]
- 7.Kay S, Smith C, Oppenheim WL. Both-bone midshaft forearm fractures in children. J Pediatr Orthop. 1986;6:306–310. doi: 10.1097/01241398-198605000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Ortega R, Loder RT, Louis DS. Open reduction and internal fixation of forearm fractures in children. J Pediatr Orthop. 1996;16:651–654. doi: 10.1097/01241398-199609000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Nicol RO, Stott NS. Intramedullary fixation for pediatric unstable forearm fractures. Clin Orthop Relat Res. 2002;402:245–250. doi: 10.1097/00003086-200209000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Wyrsch B, Mencio GA, Green NE. Open reduction and internal fixation of pediatric forearm fractures. J Pediatr Orthop. 1996;16:644–650. doi: 10.1097/01241398-199609000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Kapila R, Sharma R, Chugh A, Goyal M. Evaluation of clinical outcomes of management of paediatric bone forearm fractures using titanium elastic nailing system: a prospective study of 50 cases. J Clin Diagn Res. 2016;10:RC12–RC15. doi: 10.7860/JCDR/2016/22040.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang SN, Mangwani J, Ramachandran M, Paterson JM, Barry M. Elastic intramedullary nailing of paediatric fractures of the forearm: a decade of experience in a teaching hospital in the United Kingdom. J Bone Joint Surg Br. 2011;93:262–265. doi: 10.1302/0301-620X.93B2.24882. [DOI] [PubMed] [Google Scholar]
- 13.Richter D, Ostermann PA, Ekkernkamp A, Muhr G, Hahn MP. Elastic intramedullary nailing: a minimally invasive concept in the treatment of unstable forearm fractures in children. J Pediatr Orthop. 1998;18:457–461. [PubMed] [Google Scholar]
- 14.Luhmann SJ, Gordon JE, Schoenecker PL. Intramedullary fixation of unstable both-bone forearm fractures in children. J Pediatr Orthop. 1998;18:451–456. [PubMed] [Google Scholar]
- 15.Reinhardt KR, et al. Comparison of intramedullary nailing to plating for both-bone forearm fractures in older children. J Pediatr Orthop. 2008;28:403–409. doi: 10.1097/BPO.0b013e31816d71f2. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez FF, et al. Unstable diaphyseal fractures of both bones of the forearm in children: plate fixation versus intramedullary nailing. Injury. 2005;36:1210–1216. doi: 10.1016/j.injury.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Hayasaka S, Mizuno K, Yabata K, Saito T, Tada K. Atypical gyrate atrophy of the choroid and retina associated with iminoglycinuria. Arch Ophthalmol. 1982;100:423–425. doi: 10.1001/archopht.1982.01030030425007. [DOI] [PubMed] [Google Scholar]
- 18.Jubel A, et al. Outcomes and complications of elastic stable intramedullary nailing for forearm fractures in children. J Pediatr Orthop B. 2005;14:375–380. doi: 10.1097/01202412-200509000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Yuan PS, Pring ME, Gaynor TP, Mubarak SJ, Newton PO. Compartment syndrome following intramedullary fixation of pediatric forearm fractures. J Pediatr Orthop. 2004;24:370–375. doi: 10.1097/01241398-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Ogonda L, Wong-Chung J, Wray R, Canavan B. Delayed union and non-union of the ulna following intramedullary nailing in children. J Pediatr Orthop B. 2004;13:330–333. doi: 10.1097/01202412-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Cullen MC, Roy DR, Giza E, Crawford AH. Complications of intramedullary fixation of pediatric forearm fractures. J Pediatr Orthop. 1998;18:14–21. [PubMed] [Google Scholar]
- 22.Ho CA, Jarvis DL, Phelps JR, Wilson PL. Delayed union in internal fixation of pediatric both-bone forearm fractures. J Pediatr Orthop B. 2013;22:383–387. doi: 10.1097/BPB.0b013e328361c7ea. [DOI] [PubMed] [Google Scholar]
- 23.Martus JE, et al. Complications and outcomes of diaphyseal forearm fracture intramedullary nailing: a comparison of pediatric and adolescent age groups. J Pediatr Orthop. 2013;33:598–607. doi: 10.1097/BPO.0b013e3182a11d3b. [DOI] [PubMed] [Google Scholar]
- 24.Flynn JM, Jones KJ, Garner MR, Goebel J. Eleven years experience in the operative management of pediatric forearm fractures. J Pediatr Orthop. 2010;30:313–319. doi: 10.1097/BPO.0b013e3181d98f2c. [DOI] [PubMed] [Google Scholar]
- 25.Shah AS, et al. Stabilization of adolescent both-bone forearm fractures: a comparison of intramedullary nailing versus open reduction and internal fixation. J Orthop Trauma. 2010;24:440–447. doi: 10.1097/BOT.0b013e3181ca343b. [DOI] [PubMed] [Google Scholar]
- 26.Schemitsch EH, Richards RR. The effect of malunion on functional outcome after plate fixation of fractures of both bones of the forearm in adults. J Bone Joint Surg Am. 1992;74:1068–1078. doi: 10.2106/00004623-199274070-00014. [DOI] [PubMed] [Google Scholar]
- 27.Beaupre GS, Csongradi JJ. Refracture risk after plate removal in the forearm. J Orthop Trauma. 1996;10:87–92. doi: 10.1097/00005131-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Rosson JW, Shearer JR. Refracture after the removal of plates from the forearm. An avoidable complication. J Bone Joint Surg Br. 1991;73:415–417. doi: 10.1302/0301-620X.73B3.1670441. [DOI] [PubMed] [Google Scholar]
- 29.Feng Y, Shui X, Wang J, Cai L, Wang G, Hong J. Comparison of hybrid fixation versus dual intramedullary nailing fixation for forearm fractures in older children: case-control study. Int J Surg. 2016;30:7–12. doi: 10.1016/j.ijsu.2016.03.070. [DOI] [PubMed] [Google Scholar]
- 30.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 31.Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American academy of orthopaedic surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84-A:208–215. doi: 10.2106/00004623-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Beaton DE, et al. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001;14:128–146. doi: 10.1016/S0894-1130(01)80043-0. [DOI] [PubMed] [Google Scholar]
- 33.Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG) Am J Ind Med. 1996;29:602–608. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 34.Gummesson C, Atroshi I, Ekdahl C. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord. 2003;4:11. doi: 10.1186/1471-2474-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones DJ, Henley MB, Schemitsch EH, Tencer AF. A biomechanical comparison of two methods of fixation of fractures of the forearm. J Orthop Trauma. 1995;9(3):198–206. doi: 10.1097/00005131-199506000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Wei SY, Born CT, Abene A, Ong A, Hayda R, WG DL., Jr Diaphyseal forearm fractures treated with and without bone graft. J Trauma. 1999;46(6):1045–1048. doi: 10.1097/00005373-199906000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Salvi AE. Forearm diaphyseal fractures: which bone to synthesize first? Orthopedics. 2006;29(8):669–671. doi: 10.3928/01477447-20060801-19. [DOI] [PubMed] [Google Scholar]
- 38.Wright RR, Schmeling GJ, Schwab JP. The necessity of acute bone grafting in diaphyseal forearm fractures: a retrospective review. J Orthop Trauma. 1997;11(4):288–294. doi: 10.1097/00005131-199705000-00012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SPIRIT 2013 checklist: recommended items to address in a clinical trial protocol and related documents. (DOC 124 kb)
Data Availability Statement
The data that support the findings of this study will be available from Wenzhou Medical University; however, restrictions apply to the availability of these data. The data will be used under a license published for the current study and will not be publicly available. However, the data will be available from the corresponding author upon reasonable request and with permission from the institutions.