Abstract
Background
Early diagnosis of bacterial sepsis in neonates is hampered by non-specific symptoms and the lack of rapid responding laboratory measures. The biomarker soluble CD14 subtype (sCD14-ST) seems promising in the diagnostic process of neonatal sepsis. In order to evaluate the differences in diagnostic accuracy of sCD14-ST between early onset sepsis (EOS) and late onset sepsis (LOS) we assessed this systematic review and meta-analysis.
Results
Twelve articles were included in the systematic review and 10 in the meta-analysis. There was a high risk of bias on patient selection, index test and/or flow and timing. The overall quality of the included studies was moderate.
At sepsis onset a consequently higher level of sCD14-ST was found in septic neonates compared to healthy controls with significant higher levels in LOS compared to EOS. In the first 24 h after sepsis onset a significant increase in pooled means of plasma sCD14-ST levels was seen in EOS (t(71.6) = 7.3, p < .0001) while this was not seen in LOS or healthy controls. Optimal cut-off values ranged from 305 to 672 ng/l for EOS cases versus healthy controls. The pooled sensitivity was 81% (95%CI: 0.76–0.85), the pooled specificity was 86% (0.81–0.89) with an AUC of 0.9412 (SE 0.1178). In LOS optimal cut-off values ranged from 801 to 885 ng/l with a pooled sensitivity of 81% (0.74–0.86) and a pooled specificity of 100% (0.98–1.00). An AUC and SROC was not estimable in LOS because of the low number of studies.
Conclusions
sCD14-ST is a promising and rapid-responding diagnostic biomarker for EOS and LOS. The difference in pooled means between EOS and LOS underlines the importance to consider EOS and LOS as two different disease entities, requiring separate analysis in original articles and systematic reviews.
Electronic supplementary material
The online version of this article (10.1186/s12865-019-0298-8) contains supplementary material, which is available to authorized users.
Keywords: Neonatal sepsis, Early onset sepsis, Late onset sepsis, Soluble CD14 subtype, Presepsin
Background
Worldwide bacterial sepsis is one of the major causes of neonatal morbidity and mortality and claims the lives of more than 1000 neonates every day [1]. Neonatal sepsis is commonly divided into early-onset sepsis (EOS) and late-onset sepsis (LOS). EOS reflects transplacental and ascending infections from the maternal genital tract within 72 h after birth with an incidence estimated at 0.98 per 1000 live births and [2]. LOS (≥72 h after birth) is associated with the nosocomial environment with incidence inversely associated with birth weight and gestational age [3, 4]. Rapid diagnosis of both EOS and LOS is problematic because clinical signs start subtle and look like those caused by various non-infective conditions. The golden standard diagnostic test for bacterial sepsis is microbacterial confirmation. Unfortunately, bacterial culture is time-consuming and lacks sensitivity. Therefore, neonates with risk factors for infection or clinical signs and symptoms of infection are treated with antibiotics empirically.
To avoid unnecessary treatment of non-infected neonates, an early, sensitive and specific laboratory test would be helpful to guide clinicians to decide whether or not to start antibiotics. The upcoming biomarker soluble cluster of differentiation 14 subtype (sCD14-ST), also named presepsin, could be of diagnostic value for the detection of neonatal sepsis. sCD14-ST is a soluble and truncated form of CD14, a multifunctional cell surface glycoprotein expressed by various components of the innate immunity, like monocytic and dendritic cells and neutrophils. CD14 operates as a high-affinity receptor for e.g. lipopolysaccharides (LPS) and activates the toll-like receptor 4-specific proinflammatory signaling cascade [5, 6]. Furthermore CD14 is involved in the recognition of a wide variety of other bacterial products, like peptidoglycans of gram-positive bacteria [5]. During systemic inflammation and bacterial infections circulating plasma proteases cleave the soluble fraction of CD14 and sCD14-ST is released in the circulation. In an in vitro human experimental sepsis model (challenge with LPS in a human cell line of monocytic cells, and in peripheral mononuclear cells) sCD14-ST was detected 1 h after LPS exposure and peaked at 3 h [7]. The quick detection makes sCD14-ST a potential and promising candidate bedside biomarker for neonatal sepsis. Data about the diagnostic accuracy of plasma sCD14-ST in neonatal sepsis is limited, however recently the first systematic review and meta-analysis has been published [8]. Bellos et al. focussed on diagnostic accuracy and concluded that sCD14-ST has high sensitivity and specificity for the diagnosis of neonatal sepsis (EOS and LOS together) and is superior when compared to C-reactive protein (CRP) and procalcitonin (PCT). However, EOS and LOS were analysed together while the serum levels of sCD14-ST in both disease entities seem to differ as a result of differences in blood sampling time due to a different moment of clinical presentation. In EOS an increase in sCD14-ST plasma levels is seen in the first 24 h [9], in contrast to LOS [10]. After treatment initiation sCD14-ST levels decrease in the next few days in both EOS and LOS [9–13]. In order to evaluate the differences between EOS and LOS and its influence on the diagnostic accuracy of sCD14-ST we performed a new meta-analysis of the current literature, analyzing EOS and LOS separately.
Methods
The present study was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses Statement (PRISMA, see Additional file 1) [14]. The protocol of this review was published in advance on the PROSPERO database with reference number CRD42017061026.
Literature search
A systemic literature search was performed on March 16th 2017 using the following databases and other sources: PUBMED-Medline, EMBASE, The Cochrane Library, Web of Science, Clinicaltrials.gov and WHO ICTRP Clinical Trials in Children (CTC). There were no restrictions in language and publication period. Search terms or database specific medical subject headings (MeSH terms) used were “presepsin”, “sCD14”, “sCD14-ST”, “soluble CD14” and “P-SEP”. The following keywords and MeSH terms were used to identify only studies in neonates: “child”, “Infant, Newborn”, “newborn”, “prematur”, “low birth weight”, “VLBW”, “LBW”, “infant”, “neonat”, “postmatur”, “preterm”, “new-born” and “neo-nat”. Keywords were truncated if necessary by using “*” to broaden the search (for details and logbook of the literature search see Additional file 2). During the writing process updates of the initial search were performed with relevant articles included after the initial search (last time June 4th 2018).
Study selection
Eligibility assessment was performed in a blinded standardized manner by three reviewers. After the literature search was completed, all articles were uploaded in Rayyan and checked for duplication. Rayyan is a web and mobile app for systematic reviews which helps in the initial screening of articles based on titles and abstracts [15]. First, three independent researchers (IM, CN and DV) screened all retrieved articles on title and abstract using Rayyan application. Subsequently, all selected articles were read in full text by the three reviewers to receive the final inclusion. Any disagreement between the three reviewers regarding the eligibility of particular studies was resolved through discussion.
Quality assessment
Two independent reviewers (IM and CN) assessed the methodological quality and the risk of bias of the included studies using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool [16]. Disagreements between the two review authors about the risk of bias in particular studies were resolved through discussion and if needed involvement by the third review author (DV) was requested. Quality assessment according to the QUADAS-2 tool involved four domains; patient selection, index test, reference standard and flow and timing. In addition, applicability concerns of the first three domains was also assessed. Using the QUADAS-2 tool bias can be judged in terms of “low” or “high” risk of bias and in case there was insufficient data reported to make a judgement also as “unclear” risk.
Data extraction
Data extraction was performed by the primary and secondary reviewer (IM and CN). Qualitative data and information was extracted from each individual study on study characteristics and study results using a structured data table. Characteristics which were obtained included study design, inclusion and exclusion criteria, number of patients, gestational age and type of measurement (including measuring instruments and time of measurement). Extracted study outcomes were reference ranges and cut-off values of sCD14-ST in both healthy and septic neonates and descriptive measures, like median, interquartile range (IQR), mean and standard deviation (SD). Components of diagnostic accuracy were also obtained and included sensitivity, specificity, area under the curve (AUC), positive predicted value (PPV), negative predicted value (NPV). EOS and LOS were analysed separately.
Most articles reported the values of sCD14-ST in ng/l as unit of measurement, but some studies reported levels in pg/ml. Since the value of both units of measurement are the same, we choose to report all values and measurements for sCD14-St in ng/l, as this is the unit most used in recent literature. In this study t = 0 represents the moment (in days) when the infant newborn is suspected of having sepsis and blood culture is taken prior to start antibiotics.
From articles in which the necessary values of sCD14-ST were only reported in graphical form, the required data were interpolated from the figures by two independent researchers.
Data synthesis
MetaDiSc 1.4 software was used to perform the analyses of the meta-analysis [17]. When the mean and SD were reported in the article, these values were directly adapted to calculate the pooled mean and SD. When these data were not reported the estimated mean and SD were calculated based on studies of Hozo et al. and Wan et al. [18, 19], (for details see Appendix). An one-way ANOVA with post-hoc test was conducted to compare pooled means of EOS cases, LOS cases and healthy controls. An unpaired t test with Welch’s correction was performed to analyse the difference of sCD14-ST plasma levels between different time-points. The remainder of extracted data is presented in tabular or graphical form using MetaDiSc or GraphPad Prism version 7 [20]. Heterogeneity of the included studies was calculated by the inconsistency index (I-squared [21]) and is illustrated in a summary ROC plot (SROC plot) [22]. The potential presence of threshold effect was evaluated by calculating the Spearman rank correlation coefficient [17]. Publication bias was not expected to play a role since sCD14-ST is a relatively new subject of interest in scientific literature and only data for diagnostic accuracy were analysed. A funnel plot in meta-analysis of diagnostic test accuracy can be misleading and therefore was not determined [23]. In this article results of EOS, LOS and neonatal sepsis (EOS and LOS combined) will be reported separately because serum concentrations of sCD14-ST in these entities are expected to be different due to timing of blood sample collection based on clinical symptoms.
Results
Selection of articles
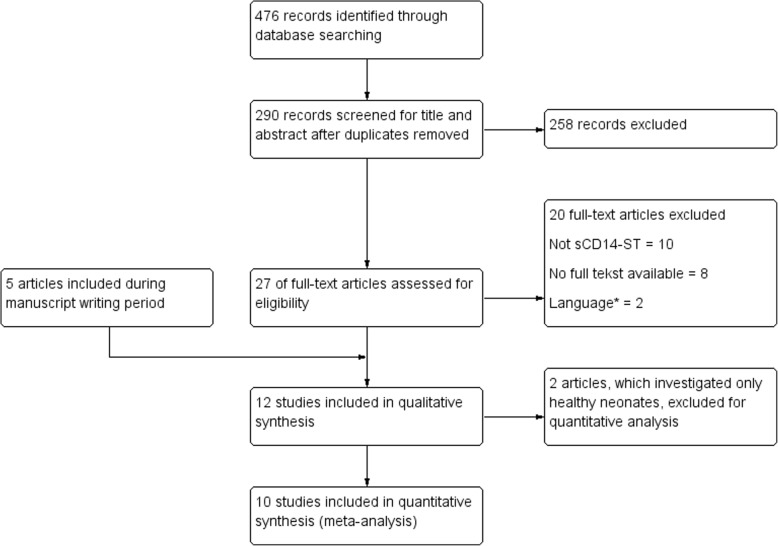
After duplicates were removed, 290 articles remained and were screened for eligibility on title and abstract. Secondly, 263 articles were excluded because they did not match the inclusion criteria (i.e. sepsis, neonates or presepsin/sCD14-ST). The remaining 27 articles were read in full text and in total, seven articles were initially selected to get used in this systematic review. Within the data extraction and manuscript writing period three relevant articles were published and included nonetheless afterwards. As mentioned before, another meta-analysis on sCD14-ST in neonatal sepsis was published in this period [8]. In this review five articles were not found in the databases we used for our literature search. Out of those five articles, one article was not available in full text [24] and two other articles were not eligible for our review (in one article it was unclear whether they investigated EOS or LOS [25] and in one article unusual ranges of sCD14-ST values were used [26]). Therefore out of this review, two articles [27, 28] were found to be appropriate to use in our review and were included afterwards. The literature search is summarized in Fig. 1.
Fig. 1.
Flow diagram literature search. * Two studies were excluded during full text reading based on language (Polish and Turkish) because none of the reviewers were competent in this languages
Study characteristics
In total 12 studies were included for qualitative synthesis in this systematic review. An overview of the characteristics of these studies is shown in Table 1 [9–13, 27–33]. All included studies were prospective observational studies and studied septic and healthy neonates. There were two studies which only investigated healthy neonates to asses reference ranges [29, 31]. Three studies investigated neonates with EOS only [9, 11, 28], two with LOS only [10, 12] and five studies investigated both EOS and LOS [13, 27, 30, 32, 33]. All sCD14-ST measurements were done on a PATHFAST analyser, which is a non-competitive chemiluminescent enzyme immunoassay (CLEIA). However, there were two producers; Mitsubishi Chemical Medience Corporation from Tokyo, Japan and Gepa Diagnostics from Milan, Italy. All studies which included both healthy and septic neonates measured sCD14-ST at t = 0. Most studies used bacterial culture as reference standard.
Table 1.
Overview and characteristics of included studies
| Sepsis type | Author | Case definition | Cases | GA | BW | Controls | GA | BW | PATHFASTb | Sampling timec |
|---|---|---|---|---|---|---|---|---|---|---|
| N | Weeks | Gram | N | Weeks | Gram | Days | ||||
| EOS | Montaldo [9] | Culture positive | 32 | 29.9 ± 1.1 | 1082 ± 205 | 38 | 30.2 ± 1.4 | 1102 ± 192 | M | T = 0, ½, 1 and 2 |
| Ozdemir [11] | Clinical + laboratory signs | 29 | 39.1 ± 0.9 | 3286 ± 461 | 40 | 39.0 ± 1.0 | 3379 ± 421 | M | T = 0, 3 and 7 | |
| Motalib [28] | Clinical signs | 28 | 37.6 ± 1.7 | 3242 ± 269 | 34 | 38.3 ± 1.3 | 3198 ± 235 | M | T = 0 and 7 | |
| LOS | Poggi [10] | Clinical + laboratory signs | 19 | 25.6 ± 2.0 | 684 ± 215 | 21 | 28.8 ± 2.0 | 1021 ± 233 | G | T = 0, 1, 3 and 5 |
| Topcuoglu [12] | Clinical signs | 42 | 28.4 ± 2.6 | 1084 ± 318 | 40 | 28.9 ± 2.8 | 1140 ± 338 | M | T = 0, 2 and 6 | |
| Controls | Mussap [29] | n/a | n/a | n/a | n/a | 26 | 26–36 | n/a | G | T = 1–7d |
| Pugni [31] | n/a | n/a | n/a | n/a | 684 | ≥ 24 | n/a | M | T = 3–7d | |
| Combineda | Miyosawa [32] | Culture positive + clinical signs | 13 | 30.3 ± 6.6 | 1644 ± 1202 | 18 | 31.2 ± 3.4 | 1520 ± 605 | M | T = 0, 1 and 2 |
| Mussap [30] | Culture positive/ clinical signs | 25 | 35.0 (31.0–41.0) | 2508 (1295–3160) | 25 | 34.0 (29.0–36.0) | 1750 (1027–3000) | G | T = 0 | |
| Osman [27] | Clinical + laboratory signs | 40 | 37.5 ± 1.2 | 2518 ± 532 | 15 | 37.8 ± 1.6 | 2581 ± 513 | M | T = 0 | |
| Iskandar [33] | Culture positive | 35 | n/a | n/a | 16 | n/a | n/a | M | T = 0 | |
| Xiao [13]e | Culture positive | 42 | 37.9 ± 2.4 | 2950 ± 639 | 53 | 38.8 ± 1.4 | 3094 ± 586 | M | T = 0, 3 and 5 | |
| Clinical signs | 54 | 37.9 ± 2.6 | 3054 ± 563 | |||||||
| Total | 359 | 1010 |
EOS early-onset sepsis, LOS late-onset sepsis, astudies that analyzed both EOS and LOS;
GA gestational age in mean ± SD or median (IQR), BW birth weight (mean ± SD); n/a not applicable
bM Mitsubishi Chemical Medience Corporation, Tokyo, Japan; G Gepa Diagnostics, Milan, Italy
cDays after admission/medical evaluation; ddays after birth; eOnly blood culture confirmed cases and healthy controls used
Quality of studies
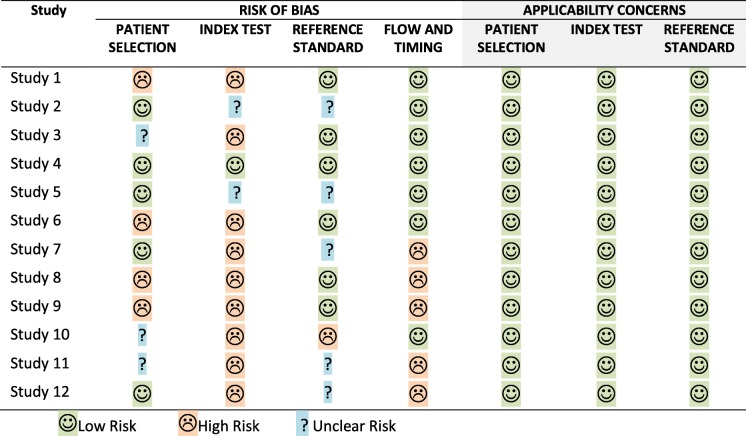
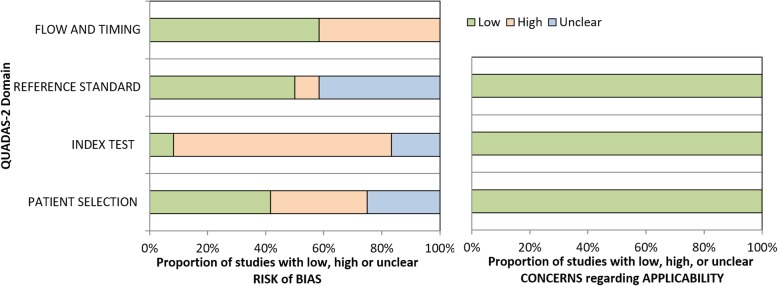
All selected studies where assessed on their methodological quality and the risk of bias using the QUADAS-2 tool. A summary of those results is shown in Table 2 and Fig. 2. There were no concerns regarding applicability in all selected studies. However, there was a high risk of bias in several studies on patient selection, index test and/or flow and timing. Some studies did not report sufficient data on patient selection, index test and/or reference standard and therefore these items were scored as an unclear risk of bias. The QUADAS-2 tool does not contain a total score of bias, but the overall quality of the included studies appears to be moderate.
Table 2.
QUADAS-2 quality assessment of selected studies tabular presentation
Fig. 2.
QUADAS-2 quality assessment of selected studies summary and graphical display
Heterogeneity
The part of heterogeneity which was due to the threshold effect was calculated with the Spearman rank correlation coefficient. The spearman correlation coefficient for the three studies on EOS was − 0.500 which indicates that there is a moderate possibility of threshold effect present. The Spearman correlation coefficient for (combined) neonatal sepsis (in total ten studies) was − 0.006, which indicates that there is a low possibility of threshold effect. Because of the low number of studies (two) it was not possible to calculate a spearman correlation coefficient for the LOS group. The inconsistency index of the included studies was between 94.5 and 96.0% for the sensitivity and 0.0 to 92.5% for specificity, which indicates that there was considerable heterogeneity among the included studies.
Findings
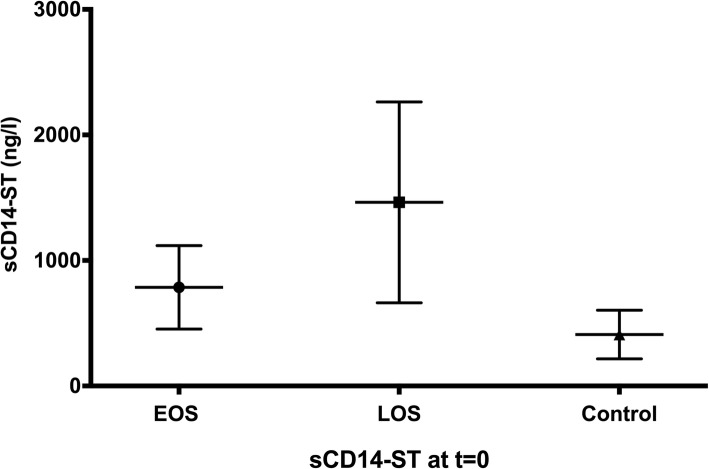
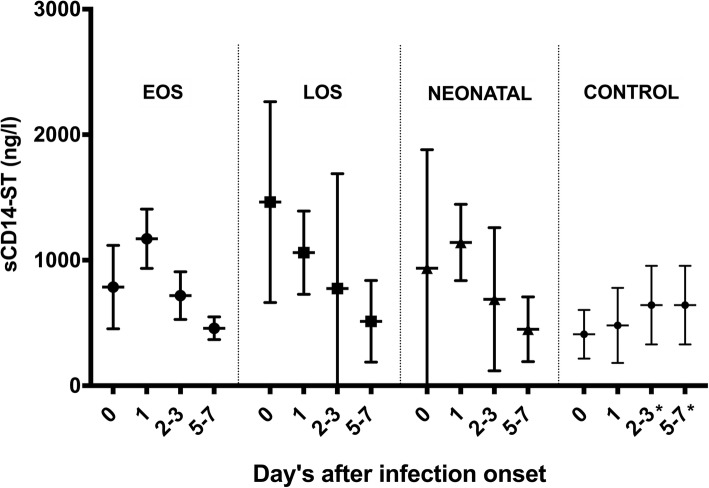
In total 12 studies were used for the qualitative analysis of neonatal sepsis and ten studies in the meta-analysis [9–13, 27, 28, 30, 32, 33]. The included studies analysed sCD14-ST at different time points after sepsis onset. If possible these time points were clustered together in 4 groups for the pooled analysis: t = 0, t = 1, t = 2–3, t = 5–7 (day’s after sepsis onset). The primary outcomes of the included studies are summarized in Additional file 3. The pooled means at t = 0 of EOS cases, LOS cases and healthy controls were unequal according to a one-way ANOVA, F(2, 453) = 219.7, p < .0001. Pairwise comparisons of the means using Tukey’s multiple comparisons test indicated a significant difference in all comparisons at t = 0 (Fig. 3 and Table 3). At the other time-points (t = 1, 2–3, 5–7) no significant differences were seen between EOS and LOS. The difference in serum level of sCD14-ST between EOS, LOS, neonatal sepsis and healthy controls is illustrated in Fig. 4. In the first 24 h after sepsis onset a significant increase in pooled means of plasma sCD14-ST levels was seen in EOS (t(71.6) = 7.3, p < .0001) while this was not seen in LOS, neonatal sepsis or healthy controls. The original data extracted from the studies on basis of which the calculation of the pool means is performed are reflected in Additional file 4.
Fig. 3.
Pooled mean and SD of sCD14-ST levels for EOS, LOS and healthy controls at t = 0. EOS = early-onset sepsis (n = 106), LOS = late-onset sepsis (n = 65), healthy controls (n = 285)
Table 3.
Pooled means and the differences per group at t = 0
| Mean 1(SD) | Mean 2 (SD) | Mean Diff | SE of diff | n1 | n2 | q | df | Adjusted p value | |
|---|---|---|---|---|---|---|---|---|---|
| EOS vs. LOS | 786 (332) | 1463 (800) | − 677 | 58.85 | 106 | 65 | 16.27 | 453 | <.0001 |
| EOS vs. Control | 786 (332) | 410 (194) | 376 | 42.5 | 106 | 285 | 12.51 | 453 | <.0001 |
| LOS vs. Control | 1463 (800) | 410 (194) | 1053 | 51.35 | 65 | 285 | 29 | 453 | <.0001 |
Fig. 4.
Pooled mean and SD of sCD14-ST levels of each subgroup at different timepoints. EOS = early onset sepsis, LOS = late onset sepsis, Neonatal = neonatal sepsis. * Time intervals 2–3 and 5–7 were taken together
Early-onset sepsis
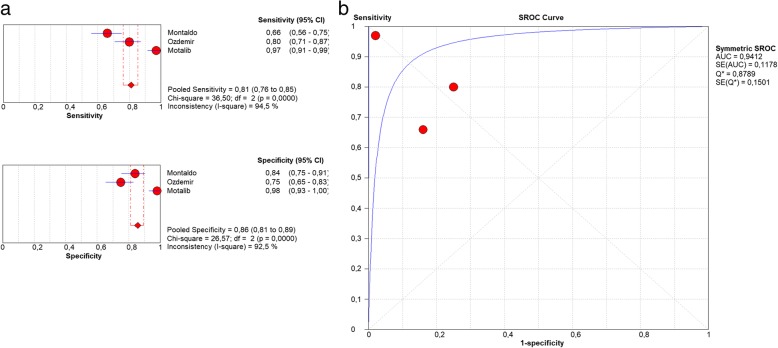
Optimal cut-off values at t = 0 ranged from 305 to 672 ng/l for EOS cases versus healthy controls. The pooled sensitivity was 81% (95%CI: 0.76–0.85), the pooled specificity was 86% (0.81–0.89) with an AUC of 0.9412 (SE 0.1178) (Fig. 5).
Fig. 5.
a Forest plot of sensitivity and specificity of sCD14-ST levels in EOS at t = 0. b Summary ROC plot of sensitivity and specificity of sCD14-ST for EOS at t = 0. EOS = early onset sepsis
Late-onset sepsis
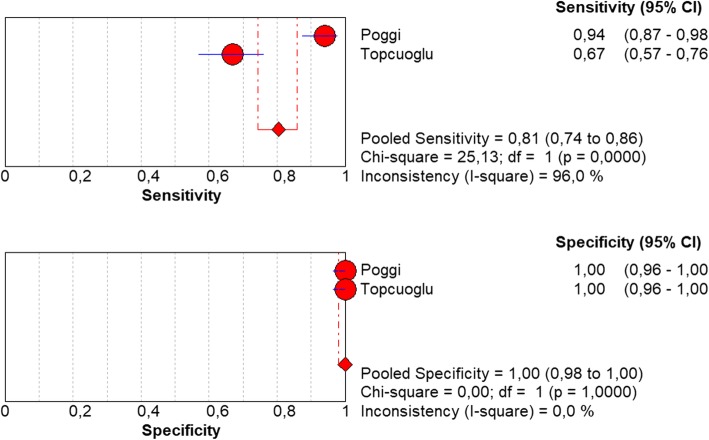
Optimal cut-off values at t = 0 ranged from 801 to 885 ng/l for LOS cases versus healthy controls. The pooled sensitivity was 81% (0.74–0.86) and the pooled specificity was 100% (0.98–1.00) (Fig. 6). An AUC and SROC was not estimable because of the low number of studies.
Fig. 6.
Forest plot of sensitivity and specificity of sCD14-ST levels in LOS at t = 0. LOS = late onset sepsis
Neonatal sepsis
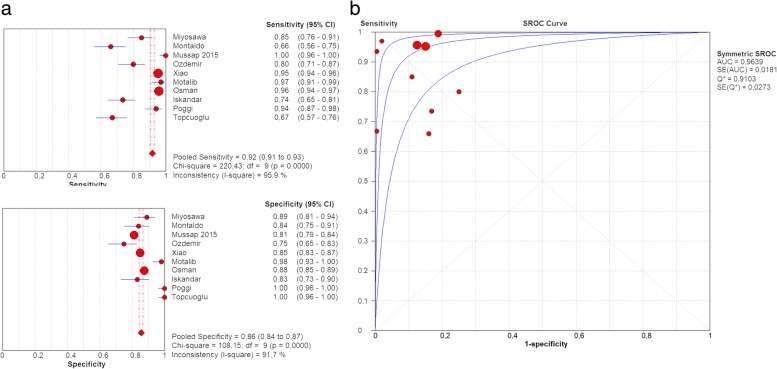
Taken all sepsis cases together (EOS, LOS and combined), cut-off values ranged from 305 to 885 ng/l. The pooled sensitivity was 92% (0.91–0.93) and the pooled specificity was 86% (0.84–0.87) with an AUC of 0.9639 (0.0181) (Fig. 7).
Fig. 7.
a Forest plot of sensitivity and specificity of sCD14-ST levels in neonatal sepsis at t = 0. b Summary ROC plot of sensitivity and specificity of sCD14-ST for neonatal sepsis at t = 0
Discussion
In this systematic review and meta-analysis, the current literature of sCD14-ST as biomarker for neonatal sepsis has been evaluated in order to facilitate rapid diagnosis and reduce the use of antibiotics in neonates. At sepsis onset a consequently higher level of sCD14-ST was found in septic neonates compared to healthy controls with higher levels in LOS compared to EOS. sCD14-ST levels increase in the first 24 h in EOS but not in LOS. Furthermore a decrease in sCD14-ST levels is seen in the days after treatment initiation for both EOS and LOS.
Our findings are in line with the promising results in adult research, were sCD14-ST has been proven to be a specific and quick-responding biomarker for bacterial infections [34]. In contrast to CRP and PCT, sCD14-ST is very specific for bacteria. Moreover sCD14-ST seems to be fast and more rapid-responding then CRP (increases after 12–24 h) and also PCT (increases after 3–4 h) with plasma levels already increased at sepsis onset (t = 0) [6, 35]. Although PCT seems promising in reducing the duration of antibiotic therapy after the initial start of the antibiotic treatment, there is no added value in the decision making before start of the antibiotics at t = 0 [36].
In the studies presented in this systematic review, differences in sCD14-ST levels were seen between EOS and LOS. This underlines the importance to consider EOS and LOS as two different disease entities, requiring separate analysis in original articles and systematic reviews. The hypothesis is that lower levels and increase in the first 24 h of sCD14-ST in EOS are caused by the fact that sepsis evaluation in EOS is determined in the early ‘pre-clinical’ stage of the infection, whereas sCD14-ST in LOS is analysed in the clinical stage with the first symptoms of infection. This finding, assuming a time-dependency of sCD14-ST, is in line with the decrease in sCD14-ST levels after initiation of therapy in both EOS and LOS. The change of sCD14-ST over time might be of future clinical value and can be used in both diagnosis and efficacy of treatment of neonatal sepsis.
The cut-off values, together with the specificity and sensitivity varied greatly between the studies and were depended on the type of infection (EOS vs LOS) and sampling time. In future practice, a cut-off value with a near 100% sensitivity or a determined amount of measurements is required to rule out the possibility of neonatal sepsis. Hence, a cut-off value of sCD14-ST for both EOS and LOS to rule out neonatal sepsis has yet to be determined, because most included studies have chosen a higher specificity over a higher sensitivity.
The literature search resulted in 12 articles covering the subject, indicating that this is a relatively new area of interest. Using the QUADAS-2 tool, the overall quality of the selected studies appears to be moderate. Importantly, with the QUADAS-2 tool we estimated there to be a high risk of bias on the thresholds of the index test in our included studies, because this was not pre-specified. However this result was distorted, as those studies were performed just to determine reference ranges.
This review has some limitations. First, the included studies used different reference standards to define neonatal sepsis, varying from blood culture to a combination of clinical signs. Although bacterial confirmation is the golden standard diagnostic test, it has a high-false negative rate in septic newborns [37]. Second, there were two PATHFAST measuring instruments from two different companies used in the included studies (Mitsubishi Chemical Medience Corporation from Tokyo, Japan & Gepa Diagnostics from Milan, Italy). A comparative study between the instruments produced by the different companies has not been performed, therefore the influence on the outcome of the sCD14-ST level is unclear. Third, most of the studies did not differentiate between EOS and LOS, making it difficult to analyse the two disease entities separately. Furthermore, the sample size in most of the included studies was small. Lastly, despite the possibility of a threshold effect in the LOS studies, we decided to perform a pooled accuracy analysis, therefore this should be interpreted with caution.
Conclusions
Regarding this meta-analysis we can conclude that sCD14-ST is a promising diagnostic biomarker for EOS and LOS. The difference in pooled means between EOS and LOS underlines the importance to consider EOS and LOS as two different disease entities, in which bloodsamples in EOS are measured in the early ‘pre-clinical’ stage of the infection and in LOS bloodsamples are measured in the clinical stage of infection. Therefore EOS and LOS requiring separate analysis in original articles and systematic reviews. In future daily practice sCD14-ST might be a valuable biomarker to rule out EOS prior to treatment initiation. Although a clear cut-off value with a high negative predictive value has still to be determined and evaluated in future prospective studies. Future studies should use a larger number of patients in their research populations. Lastly, more research on serial measurements of sCD14-ST in the first 24 h after birth is necessary for better understanding the role of sCD14-ST in EOS.
Additional files
Prisma 2009 checklist. (DOC 65 kb)
Logbook of literature search. (DOCX 36 kb)
Primary outcomes of the included studies. (DOCX 19 kb)
Original data of the included studies. (DOCX 21 kb)
Acknowledgements
Many thanks to A.H.L.C. van Kaam for his advice and contributions to the final version of the manuscript.
Abbreviations
- 95%CI
95% confidence interval
- ANOVA
Analysis of variance
- AUC
Area under the curve
- CLEIA
Chemiluminescent enzyme immunoassay
- CRP
C-reactive protein
- CTC
Clinical Trials in Children
- EOS
Early onset sepsis
- IQR
Interquartile range
- I-squared
Inconsistency index
- LBW
Low birth weight
- LOS
Late onset sepsis
- LPS
Lipopolysaccharides
- NPV
Negative predicted value
- PCT
procalcitonin
- PPV
Positive predicted value
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses Statement
- P-SEP
Presepsin
- QUADAS-2
Quality Assessment of Diagnostic Accuracy Studies 2
- sCD14-ST
Soluble cluster of differentiation 14 subtype
- SD
Standard deviation
- SE
Standard error
- SROC
Summary ROC
- VLBW
Very low birth weight
Appendix
Data synthesis
When median (md), minimum (a) and maximum (b) were reported, mean (m) and standard deviation (SD or S) were calculated based on the formulas of Hozo et al. [19] For samples sizes smaller than 25, the estimated mean was calculated with formula 1 = . For samples sizes larger than 25, the mean was estimated as the median (formula 2). The standard deviation for samples sizes smaller than 15 were calculated as based on formula 3 = . When sample sizes are between 16 and 70 formula 4 (= range/4) was used. When the median and interquartile range (q1-q3) were given calculations were based on a study of Wan and colleagues. [18] Formula 5 was applied to estimate the mean ((q1 + median + q3)/3). In this case the standard deviation was calculated using formula 6 = (q3-q1) / 1,35. The pooled mean was calculated by the formula and the pooled standard deviation was calculated by the formula , in which standard deviation equals the square root of the variance.
Authors’ contributions
DV started and monitored the project. DV, CN and IM were involved in the study selection. CN and IM contributed to quality assessment. IM did the data extraction and wrote the first draft of the manuscript. DV and CN also contributed to further versions of the manuscript. DV, CN and IM were involved in the data synthesis. All authors read and approved the final version of the manuscript.
Funding
Not applicable.
Availability of data and materials
All data analysed during this study are included in this published article (and its supplementary information files) and are available from the included studies, which are fully referenced.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Camacho-Gonzalez A, Spearman PW, Stoll BJ. Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatr Clin N Am. 2013;60(2):367–389. doi: 10.1016/j.pcl.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heron M. Deaths: leading causes for 2013. Natl Vital Stat Rep. 2016;65(2):1–95. [PubMed] [Google Scholar]
- 3.Boghossian NS, Page GP, Bell EF, et al. Late-onset sepsis in very low birth weight infants from singleton and multiple-gestation births. J Pediatr. 2013;162(6):1120–1124. doi: 10.1016/j.jpeds.2012.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballot DE, Chirwa T, Ramdin T, et al. Comparison of morbidity and mortality of very low birth weight infants in a central Hospital in Johannesburg between 2006/2007 and 2013. BMC Pediatr. 2015;15(1):20. [DOI] [PMC free article] [PubMed]
- 5.Mussap M, Noto A, Fravega M, Fanos V. Soluble CD14 subtype presepsin (sCD14-ST) and lipopolysaccharide binding protein (LBP) in neonatal sepsis: new clinical and analytical perspectives for two old biomarkers. J Matern Fetal Neonatal Med. 2011;24(2):12–14. doi: 10.3109/14767058.2011.601923. [DOI] [PubMed] [Google Scholar]
- 6.Delanghe JR, Speeckaert MM. Translational research and biomarkers in neonatal sepsis. Clin Chim Acta. 2015;451:46–64. doi: 10.1016/j.cca.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 7.Chenevier-Gobeaux C, Bardet V, Poupet H, Poyart H, Borderie D, Claessens YE, et al. Presepsin (sCD14-ST) secretion and kinetics by peripheral blood mononuclear cells and monocytic THP-1 cell line. Ann Biol Clin (Paris). 2016;74(1):93–7. [DOI] [PubMed]
- 8.Bellos I, Fitrou G, Pergialiotis V, Thomakos N, Perrea DN, Daskalakis G. The diagnostic accuracy of presepsin in neonatal sepsis: a meta-analysis. Eur J Pediatr. 2018;177(5):625–632. doi: 10.1007/s00431-018-3114-1. [DOI] [PubMed] [Google Scholar]
- 9.Montaldo P, Rosso R, Santantonio A, Chello G, Giliberti P. Presepsin for the detection of early-onset sepsis in preterm newborns. Pediatr Res. 2016;81(2):329–334. doi: 10.1038/pr.2016.217. [DOI] [PubMed] [Google Scholar]
- 10.Poggi C, Bianconi T, Gozzini E, Generoso M, Dani C. Presepsin for the detection of late-onset sepsis in preterm newborns. Pediatrics. 2015;135(1):68–75. doi: 10.1542/peds.2014-1755. [DOI] [PubMed] [Google Scholar]
- 11.Ozdemir AA, Elgormus Y. Diagnostic value of Presepsin in detection of early-onset neonatal Sepsis. Am J Perinatol. 2017;34(06):550–556. doi: 10.1055/s-0036-1593851. [DOI] [PubMed] [Google Scholar]
- 12.Topcuoglu S, Arslanbuga C, Gursoy T, et al. Role of presepsin in the diagnosis of late-onset neonatal sepsis in preterm infants. J Matern Fetal Neonatal Med. 2016;29(11):1834–1839. doi: 10.3109/14767058.2015.1064885. [DOI] [PubMed] [Google Scholar]
- 13.Xiao Ting, Chen Li-Ping, Zhang Li-hua, Lai Fu-Huang, Zhang Li, Qiu Qun-feng, Que Rong-Liang, Xie SiSi, Wu Ding-Chang. The clinical significance of sCD14-ST for blood biomarker in neonatal hematosepsis. Medicine. 2017;96(18):e6823. doi: 10.1097/MD.0000000000006823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberati Alessandro, Altman Douglas G., Tetzlaff Jennifer, Mulrow Cynthia, Gøtzsche Peter C., Ioannidis John P. A., Clarke Mike, Devereaux P. J., Kleijnen Jos, Moher David. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Medicine. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. [DOI] [PMC free article] [PubMed]
- 16.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 17.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6(31):31. [DOI] [PMC free article] [PubMed]
- 18.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed]
- 19.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed]
- 20.GraphPad Prism version 7.00 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com. Accessed 27 May 2019.
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and Presenting Results. In: Deeks JJ, Bossuyt PM, Gatsonis C, The Cochrane Collaboration, editors. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. 2010. [Google Scholar]
- 23.Van Enst WA, Ochodo E, Scholten RJPM, Hooft L, Leeflang MM. Investigation of publication bias in meta-analyses of diagnostic test accuracy: a meta-epidemiological study. BMC Med Res Methodol. 2014;14(1):70. [DOI] [PMC free article] [PubMed]
- 24.Tabl HA, Abed NT. Diagnostic value of presepsin in neonatal sepsis. Egypt J Immunol. 2016;23(2):29–37. [PubMed] [Google Scholar]
- 25.Sabry JH, Elfeky OA, Elsadek AE, Eldaly AA. Presepsin as an early reliable diagnostic and prognostic marker of neonatal sepsis. Int J Adv Res. 2016;4(6):1538–1549. doi: 10.21474/IJAR01/716. [DOI] [Google Scholar]
- 26.Mostafa RM, Kholouss SM, MZakaria N, Hafiz TR, Abdelaziz DM. Detection of presepsin and surface CD14 as a biomarker for early diagnosis of neonatal sepsis. J Am Sci. 2015;11(10):104–116.
- 27.Osman AS, Awadallah MG, Tabl HAEM, Abed NT, Goudah ESS. Presepsin as a novel diagnostic marker in neonatal septicemia. Egypt J Med Microbiol. 2015;24(3):21–26. doi: 10.12816/0024924. [DOI] [Google Scholar]
- 28.Ta M, Khalaf FA, El Hendawy G, Kotb SE, Ali AM, El Sharnoby A. Soluble CD14-subtype (Prespsin) and Hepcidin as diagnostic and prognostic markers in early onset neonatal sepsis. Egypt J Med Microbiol. 2015;24(3):45–52. doi: 10.12816/0024928. [DOI] [Google Scholar]
- 29.Mussap M, Puxeddu E, Burrai P, et al. Soluble CD14 subtype (sCD14-ST) presepsin in critically ill preterm newborns: preliminary reference ranges. J Matern Fetal Neonatal Med. 2012;25(sup5):51–53. doi: 10.3109/14767058.2012.717462. [DOI] [PubMed] [Google Scholar]
- 30.Mussap M, Puxeddu E, Puddu M, et al. Soluble CD14 subtype (sCD14-ST) presepsin in premature and full term critically ill newborns with sepsis and SIRS. Clin Chim Acta. 2015;451:65–70. doi: 10.1016/j.cca.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 31.Pugni Lorenza, Pietrasanta Carlo, Milani Silvano, Vener Claudia, Ronchi Andrea, Falbo Mariella, Arghittu Milena, Mosca Fabio. Presepsin (Soluble CD14 Subtype): Reference Ranges of a New Sepsis Marker in Term and Preterm Neonates. PLOS ONE. 2015;10(12):e0146020. doi: 10.1371/journal.pone.0146020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyosawa Yukihide, Akazawa Yohei, Kamiya Motoko, Nakamura Chizuko, Takeuchi Yusuke, Kusakari Mai, Nakamura Tomohiko. Presepsin as a predictor of positive blood culture in suspected neonatal sepsis. Pediatrics International. 2018;60(2):157–161. doi: 10.1111/ped.13469. [DOI] [PubMed] [Google Scholar]
- 33.Iskandar A, Arthamin MZ, Indriana K, et al. Comparison between presepsin and procalcitonin in early diagnosis of neonatal sepsis. J Matern Fetal Neonatal Med. 2018;1–6. Epub ahead of print [DOI] [PubMed]
- 34.Zheng Z, Jiang L, Ye L, Gao Y, Tang L, Zhang M. The accuracy of presepsin for the diagnosis of sepsis from SIRS: a systematic review and meta-analysis. Ann Intern Med. 2015;5(1):48. [DOI] [PMC free article] [PubMed]
- 35.Gilfillan M, Bhandari V. Biomarkers for the diagnosis of neonatal sepsis and necrotizing enterocolitis: clinical practice guidelines. Early Hum Dev. 2017;105:25–33. doi: 10.1016/j.earlhumdev.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Stocker M, van Herk W, El Helou S, et al. Procalcitonin-guided decision making for duration of antibiotic therapy in neonates with suspected early-onset sepsis: a multicentre, randomised controlled trial (NeoPIns) Lancet. 2017;390:871–881. doi: 10.1016/S0140-6736(17)31444-7. [DOI] [PubMed] [Google Scholar]
- 37.Sarkar S, Bhagat I, Wiswell TE, Spitzer AR. Role of multiple site blood cultures to document the clearance of bacteremia in neonates. J Perinatol. 2007;27:101–102. doi: 10.1038/sj.jp.7211636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Prisma 2009 checklist. (DOC 65 kb)
Logbook of literature search. (DOCX 36 kb)
Primary outcomes of the included studies. (DOCX 19 kb)
Original data of the included studies. (DOCX 21 kb)
Data Availability Statement
All data analysed during this study are included in this published article (and its supplementary information files) and are available from the included studies, which are fully referenced.