Abstract
Background
Cervical cancer is the fourth most common cancer affecting women worldwide. Paclitaxel/Carboplatin is one of the most commonly prescribed regimens in cervical cancer treatment. Although chemotherapeutic drugs are very effective, severe side effects and development of drug resistance limits the use of these drugs. The use of natural products with anticancer activity may help to partially overcome these issues.
Methods
In the present study, we investigated the ability of Gallic acid, to potentiate the anti-cancer effects of Paclitaxel, Carboplatin and Paclitaxel/Carboplatin combination in human HeLa cells by performing MTT assay, cell cycle analysis and RT-PCR assay and Western blotting for some apoptotic markers.
Results
Our results revealed that the highest cytotoxic effect, the highest induction of apoptosis and significant elevation in P53 and Caspase 3 levels was seen in Paclitaxel/Gallic acid combination.
Conclusion
These results indicate that Gallic acid potentiates Paclitaxel effect and that Paclitaxel/Gallic acid combination could represent a promising alternative with lower side effects-for Paclitaxel/Carboplatin combination in treatment of cervical cancer treatment.
Keywords: HeLa cells, Gallic acid, Paclitaxel, P53, Caspase 3
Background
Cancer is the second leading cause of death globally, and is responsible for an estimated 9.6 million deaths in 2018 [1]. Lung cancer is the most frequent cancer and the leading cause of cancer death among males, followed by prostate and colorectal cancer (for incidence) and liver and stomach cancer (for mortality). Among females, breast cancer is the most commonly diagnosed cancer and the leading cause of cancer death, followed by colorectal and lung cancer (for incidence), and vice versa (for mortality). Cervical cancer ranks fourth cancer type, for both incidence and mortality, affecting women worldwide [2]. Treatment of cancer with a single therapy is rarely effective as cancer development involves many features of the cell [3]. Combination therapy is a prominent approach in cancer chemotherapy. The toxicity is significantly less because different pathways will be targeted and lower dosage of each individual drug is required [4, 5].
Taxane and platinum-based combination chemotherapy is the treatment of choice for ovarian cancer [6]. Platinum-based drugs exerts cytotoxicity mainly through the formation of DNA adducts leading to hindering of DNA replication and induction of apoptosis, while Taxanes target microtubules leading to suppression of spindle-microtubule dynamics, which results in cell cycle arrest and eventually, apoptosis [7, 8]. Upon diagnosis, most ovarian cancer patients are administered combination therapy containing dose-dense Taxanes, usually Paclitaxel, and a platinum agent, usually Carboplatin, as it causes much less cytotoxicity and nephrotoxicity compared with Cisplatin as the platinum agent [9].
It has been proposed that cell cycle arrest induced by Paclitaxel possibly inhibits DNA repair leading to accumulation of Carboplatin-DNA adducts and enhances the antitumor activity [10].
However, the use of many chemically synthesized anticancer drugs has caused considerable side effects to patients. Therefore, the discovery and development of new drugs based on natural products have been the focus of much research [11, 12].
Some reports have shown to indicate the use of natural products in suppressing cancer proliferation via different mechanisms in the treatment of different types of cancer as breast cancer, head and neck cancer cell [13–15].
Gallic acid (GA) is a polyhydroxyphenolic compound which can be found in various natural products, such as green tea, grapes, strawberries, bananas and many other fruits [16]. A number of studies have demonstrated the potential anticancer activity of Gallic acid and its derivatives, both in vivo and in vitro [17, 18]. In fact, anti-cancer activity of Gallic acid has been reported in various cancer cells, including human ovarian cancer cells [19–22]. It was proven that the anticancer effect of Gallic acid is due to its ability to inhibit cell proliferation and to induce apoptosis [19, 23, 24].
In this study, we aimed to investigate the possible synergistic or antagonistic effect of Gallic acid with Paclitaxel, Carboplatin, Paclitaxel/Carboplatin combination therapy.
Materials and methods
To compare the anticancer effects of Paclitaxel, Carboplatin and Gallic acid individually and in-combination on growth and apoptosis in HeLa cells, Growth inhibition assay, cell cycle analysis, RT-PCR assay and Western blot analysis for some apoptotic markers were performed.
Reagents
Paclitaxel (Taxol®, 6 mg/ml, Bristol-Myers Squibb, Egypt) and Carboplatin (Carboplatin®10 mg/ml, MYLAN, France) were purchased from Egyptian market.
Gallic acid was purchased from Sigma Aldrich, USA. 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide dye was purchased from BIO BASIC, Canada. An Annexin V-fluorescein isothiocyanate (FITC)/Propidium iodide (PI) double staining kit was purchased from BioVision Research Products, USA. A RNeasy Mini Kit was purchased from QIAGEN, Venlo, Netherlands and a One-Step RT-PCR Kit was purchased from Bio-Rad, California, USA. Primary and secondary antibodies for western blotting were purchased from Cell Signaling Technology, Massachusetts, USA and Danko, California, USA, respectively. Materials and chemicals for electrophoresis were obtained from Bio-Rad (California, USA), Sigma Aldrich, USA and Roche, Basel, Switzerland.
Cell culture
The HeLa cells, a human cervical cancer HeLa cell line, were supplied by VACSERA (Giza, Egypt). The cells were cultured in RPMI-1640 medium (Lonza, Switzerland) supplemented with 10% fetal bovine serum (Gibco), 1% penicillin and 1% streptomycin (Sigma Aldrich, USA) at 37 °C under an atmosphere of 5% CO2 and 95% air.
Growth inhibition assay
The cell growth inhibition effects of serial dilutions of Paclitaxel (600, 300, 150, 75, 37.5, 18.75, 9.37, 4.68, 2.34, 1.17 µg/ml), Carboplatin (1000, 500, 250, 125, 62.5, 31.25, 15.62, 7.81 µg/ml), Gallic acid (10,000, 5000, 2500, 1250, 625, 312.51 µg/ml) and combinations of various treatment were detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide dye (MTT) assay [25]. Drug combinations and concentrations tested are shown in Table 1.
Table 1.
The tested drug combinations
| Mixture | Drugs and its concentration |
|---|---|
| Mix 1 | IC50 of Paclitaxel (112.5 µg/ml) + IC50 of Carboplatin (323.5 µg/ml) |
| Mix 2 | IC50 of Paclitaxel (112.5 µg/ml) + MNTC of Gallic acid (312.5 µg/ml) |
| Mix 3 | IC50 of Carboplatin (323.5 µg/ml) + MNTC of Gallic acid (312.5 µg/ml) |
| Mix 4 | IC50 of Paclitaxel (112.5 µg/ml) + IC50 of Carboplatin (323.5 µg/ml) + MNTC of Gallic acid (312.5 µg/ml) |
| Mix 5 | 1/10 from IC50 of Paclitaxel (11.2 µg/ml) + 1/10 from IC50 of Carboplatin (32.3 µg/ml) |
| Mix 6 | 1/10 from IC50 of Paclitaxel (11.2 µg/ml) + MNTC of Gallic acid (312.5 µg/ml) |
| Mix 7 | 1/10 from IC50 of Carboplatin (32.3 µg/ml) + MNTC of Gallic acid (312.5 µg/ml) |
| Mix 8 | 1/10 from IC50 of Paclitaxel (11.2 µg/ml) + 1/10 from IC50 of Carboplatin (32.3 µg/ml) + MNTC of Gallic acid (312.5 µg/ml) |
Briefly, 1 × 105 cells per well were seeded in 96-well microtiter plate and incubated at 37 °C for 24 h. to develop a complete monolayer sheet. After exposure to the indicated amounts of drugs for 24 h, medium was discarded and twenty microliters of MTT (BIO BASIC CANADA INC) solution (5 mg/ml in PBS) were added to each well of 96-well plate. The plate was placed on a shaking table, 150 rpm for 5 min, to thoroughly mix the MTT into the media then incubated for four additional hours at 37 °C and 5% CO2. Medium in plates was removed and 200 μl DMSO was added to each well to solubilize the formazan crystals (MTT metabolic product). Optical density was measured at 560 nm using a microplate reader (Mindray Mr-96A, China). Influences of tested compounds on HeLa cells proliferation were evaluated by the calculation of cell viability (%) using the following equation:
In vitro cytotoxic activity was evaluated by the calculation of the concentration of the drug required to kill 50% of cells relative to the untreated cultures known as the half maximal inhibitory concentration (IC50). For Gallic acid the minimum dilution of Gallic acid with no toxic effect on the cells which corresponds to maximum non-toxic concentration (MNTC) was also detected.
Cell cycle analysis and apoptosis assay
Annexin V-fluorescein isothiocyanate (FITC)/Propidium iodide (PI) double staining kit (BioVision Research Products, USA) was used to identify the cells in different phases. HeLa cells were treated with Paclitaxel (112.5 µg/ml), Carboplatin (323.5 µg/ml), Gallic acid (312.5 µg/ml), Mix. 1 (Paclitaxel (112.5 µg/ml) + Carboplatin (323.5 µg/ml), Mix. 2 (Paclitaxel (112.5 µg/ml) + Gallic acid (312.5 µg/ml), Mix 3 (Carboplatin (323.5 µg/ml) + Gallic acid (312.5 µg/ml), Mix 4 (Paclitaxel (112.5 µg/ml) + Carboplatin (323.5 µg/ml) + Gallic acid (312.5 µg/ml) for 24 h. After incubation, cells were harvested from the treated and normal samples. The cells were then resuspended in 500 μl of binding buffer, and then 5 μl of annexin V-FITC and 5 μl of PI were added in the dark for 5 min. The cell cycle distribution was then detected by flow cytometry (BD FACSCalibur, India).
Real time quantitative PCR (RT-qPCR) assay for some apoptotic markers
HeLa cells were treated with selected doses of individual and combination drugs as mentioned previously in cell cycle analysis and incubated for 24 h. to perform mRNA expression analysis. Total RNA was then isolated from control HeLa cell and treated HeLa cells. RNA was extracted using RNeasy Mini Kits (QIAGEN, Venlo, Netherlands) according to the manufacturer’s protocol. RT-qPCR was performed by use of SYBR® Green dye (iScriptTM One-Step RT-PCR Kit, Bio-Rad, California, United States) on Rotor-Gene Q real-time PCR cycler (QIAGEN, Venlo, Netherlands). The forward and reverse primers used for amplification of P53, Bcl-2, and Caspase 3 are listed in Table 2. Quantitative analysis was performed by the measurement of threshold cycle (CT) values during the exponential phase of amplification. ΔCT was calculated by the difference between the CT values of the P53, Bcl-2, Caspase-3 and the CT value of β-actin gene. Finally, fold changes of treated cells compared with untreated cells were calculated using the following equation: 2^ΔΔCT.
Table 2.
Forward and reverse primer PCR sequences for real-time PCR
| Primer | Sequence of nucleotides (nt) | Size (nt) |
|---|---|---|
| P53 | ||
| Forward | 5′-CCCCTCCTGGCCCCTGTCATCTTC-3′ | 24 |
| Reverse | 5′-GCAGCGCCTCACAACCTCCGTCAT-3′ | 24 |
| Bcl-2 | ||
| Forward | 5′-CCTGTG GAT GAC TGA GTA CC-3′ | 20 |
| Reverse | 5′-GAGACA GCC AGG AGA AAT CA-3′ | 20 |
| Caspase 3 | ||
| Forward | 5′-TTC ATT ATT CAG GCC TGC CGA GG-3′ | 23 |
| Reverse | 5′-TTC TGA CAG GCC ATG TCA TCC TCA-3′ | 24 |
ΔCTE: the difference between the CT values of the P53, BCL-2, Caspase-3 and the CT value of B-actin gene in treated cells.
ΔCTC: the difference between the CT values of the P53, BCL-2, Caspase-3 and the CT value of B-actin gene in control HeLa cells.
Western blot analysis
The expression of P53, Procaspase 3, Cleaved Caspase 3 proteins were evaluated using western plot analysis according to [26, 27]. In brief, HeLa cells were treated with DMSO (control), individual and combination drugs (mentioned previously in cell cycle analysis section) and incubated for 24 h. Then cells were then lysed in cold lysis buffer (100 mM NaCl, 10 mM Tris, 25 mM ethylenediaminetetraacetic acid (EDTA), 25 mM Ethylene glycol bis (2-aminoethyl) tetraacetic acid (EGTA), 1% (v/v) Triton X-100, 1% (v/v) NP-40 (pH 7.4), with 1:300 protease inhibitor cocktail (Sigma) and Phosphatase inhibitor cocktail Tablet (Roche). The cells were then immediately frozen at − 20 °C for 1 h for further lysis, and collected by cell scraper and sonicated 2 × 10 s. Protein concentration in the supernatant was measured with Bradford method. Equal amounts (20 µg) of total protein from control and treated cell extracts samples were mixed and boiled with SDS Loading buffer (750 mM Tris–HCl (pH 6.8); 600 mM dithiothreitol (DTT); 12% SDS; 0.012% Bromophenol blue; 60% glycerol) for 10 min, allowed to cool on ice and then loaded into SDS-polyacrylamide gel and separated by Cleaver electrophoresis unit (Cleaver, UK), transferred onto polyvinylidene fluoride (PVDF) membranes (BioRad) for 30 min using a Semi-dry Electroblotter (Biorad, USA) at 2.5 A and 25 V for 30 min. Membranes were blocked with 5% nonfat milk in Tris Buffered Saline Tween (TBS-T) for 2 h. at 37 °C and then incubated overnight at 4 °C with each primary antibody at indicated dilution (The primary antibodies against Caspase-3 antibody (1:1000, Cell Signaling Technology), Cleaved Caspase-3 antibody (1:750, Cell Signaling Technology), p53 (1:1000, Abcam), and β-actin (1:2000, Sigma). The blots were washed three times with TBS-T and incubated with secondary antibody (horse radish peroxidase-linked secondary antibodies (Dako) for another hour at room temperature, followed by washing for three times with TBS-T. Substrates were visualized by using chemiluminescent Western ECL substrate (Perkin Elmer, Waltham, MA) and analyzed using a CCD camera-based imager (Chemi Doc imager, Biorad, USA), and the bands intensities were then measured by ImageLab (Biorad). β-actin was used as the loading control.
Statistical analysis
Data were analyzed using the GraphPad Prism 6.0 statistical program (GraphPad Software, Inc.), and statistical differences between groups were evaluated using two -way analysis of variance. P < 0.05 was considered to indicate a statistically significant difference.
Results
Growth inhibition assay
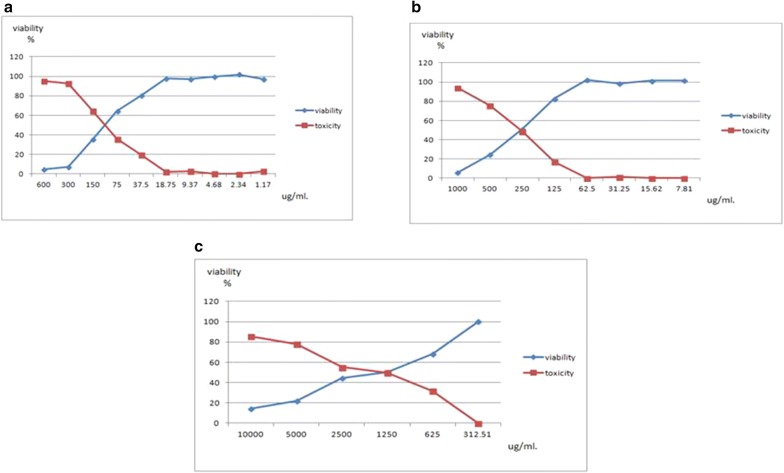
The cytotoxic activities of Paclitaxel, Carboplatin, and Gallic acid were first assessed individually on HeLa cells via MTT assay (Table 3, Fig. 1). The three compounds showed significant inhibition of cell proliferation in a dose dependent manner. HeLa cells were most sensitive to Paclitaxel showing the IC50 of 112.53 µg/ml followed by Carboplatin that exhibited an IC50 of 323.57 µg/ml. The highest IC50 (1948.95 µg/ml) was recorded for Gallic acid. Also the MNTC for Gallic acid (312.51 µg/ml). These results reflect the lower cytotoxicity of Gallic acid when used on its own.
Table 3.
Effect of individual drugs and combinations on cell viability of HeLa cells after 24 h
| ID | Conc. µg/ml | Mean O.D | ST.E | Viability % | Toxicity % | IC50 |
|---|---|---|---|---|---|---|
| HeLa | – | 0.183 | 0.006351 | 100 | 0 | µg/ml |
| Paclitaxel | 600 | 0.009 | 0.000577 | 4.918033 | 95.08196721 | 112.53 |
| 300 | 0.013333 | 0.000882 | 7.285974 | 92.7140255 | ||
| 150 | 0.064667 | 0.004485 | 35.33698 | 64.66302368 | ||
| 75 | 0.117333 | 0.002186 | 64.11658 | 35.88342441 | ||
| 37.5 | 0.147333 | 0.00318 | 80.51002 | 19.48998179 | ||
| 18.75 | 0.179 | 0.001528 | 97.81421 | 2.18579235 | ||
| 9.37 | 0.177667 | 0.002963 | 97.08561 | 2.9143898 | ||
| 4.68 | 0.182 | 0.003055 | 99.45355 | 0.546448087 | ||
| 2.34 | 0.186333 | 0.003844 | 101.8215 | 0 | ||
| 1.17 | 0.178 | 0.002646 | 97.26776 | 2.732240437 | ||
| Carboplatin | 1000 | 0.011333 | 0.000333 | 6.193078 | 93.80692168 | 323.57 |
| 500 | 0.045333 | 0.006333 | 24.77231 | 75.2276867 | ||
| 250 | 0.094 | 0.002517 | 51.36612 | 48.63387978 | ||
| 125 | 0.151667 | 0.005044 | 82.87796 | 17.12204007 | ||
| 62.5 | 0.187 | 0.003 | 102.1858 | 0 | ||
| 31.25 | 0.180667 | 0.000882 | 98.72495 | 1.275045537 | ||
| 15.62 | 0.185667 | 0.002186 | 101.4572 | 0 | ||
| 7.81 | 0.186 | 0.007506 | 101.6393 | 0 | ||
| Gallic acid | 10,000 | 0.026667 | 0.00786 | 14.57195 | 85.428051 | 1948.95 |
| 5000 | 0.04 | 0.006658 | 21.85792 | 78.1420765 | ||
| 2500 | 0.082 | 0.002309 | 44.80874 | 55.19125683 | ||
| 1250 | 0.092 | 0.001528 | 50.27322 | 49.72677596 | ||
| 625 | 0.125 | 0.005508 | 68.30601 | 31.69398907 | ||
| 312.51 | 0.183 | 0.003786 | 100 | 0 | ||
| Drugs combinations | HeLa | 0.192667 | 0.003756 | 100 | 0 | |
| Mix 1 | 0.033 | 0.005196 | 17.12773 | 82.87226865 | ||
| Mix 2 | 0.030333 | 0.003383 | 15.74367 | 84.25632775 | ||
| Mix 3 | 0.051333 | 0.00348 | 26.64314 | 73.35686234 | ||
| Mix 4 | 0.030333 | 0.003844 | 15.74367 | 84.25632775 | ||
| Mix 5 | 0.056 | 0.001155 | 29.06524 | 70.93475891 | ||
| Mix 6 | 0.051 | 0.005686 | 26.47013 | 73.52986973 | ||
| Mix 7 | 0.037333 | 0.003528 | 19.37683 | 80.62317261 | ||
| Mix 8 | 0.035333 | 0.004177 | 18.33878 | 81.66121693 |
Fig. 1.
Effect of Paclitaxel (a), Carboplatin (b), and Gallic acid (c) on cell viability of HeLa cells at 24 h
Regarding the combination treatment, the highest cytotoxicity where seen in Mix. 2 (IC50 of Paclitaxel (112.5 µg/ml) + MNTC of Gallic acid (312.5 µg/ml) and Mix 4 (IC50 of Paclitaxel (112.5 µg/ml) + IC50 of Carboplatin (323.5 µg/ml) + MNTC of Gallic acid (312.5 µg/ml) which showed an 84.26% inhibition of cell viability. Interestingly, 81.66%, 80.62% and 73.53% inhibition of cell viability were noted, respectively in Mix. 7 (1/10 from IC50 of Carboplatin (32.3 µg/ml) + MNTC of Gallic acid (312.5 µg/ml) and Mix. 8 (1/10 from IC50 of Paclitaxel (11.2 µg/ml) + 1/10 from IC50 of Carboplatin (32.3 µg/ml) + MNTC of Gallic acid (312.5 µg/ml) and Mix 6 (1/10 from IC50 of Paclitaxel (11.2 µg/ml) + MNTC of Gallic acid (312.5 µg/ml).
Mix 6, 7 and 8, the concentration of treatments used was 1/10 of IC50 of Paclitaxel, Carboplatin and MNTC of Gallic acid; these combinations showed high cell cytotoxicity claiming for the potentiation of Gallic acid with Paclitaxel and Carboplatin even in low doses.
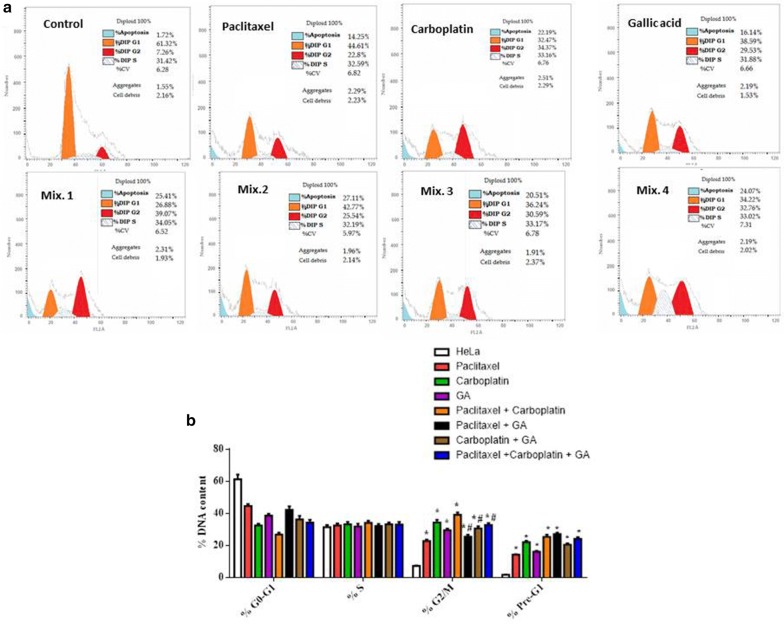
Cell cycle progression
The cell-cycle distribution and cell death, including apoptosis was analyzed using Annexin V-FITC/PI staining and flow cytometry. It was determined how each individual drug and the mentioned drug combinations changed cell cycle distribution and affected the anti-tumour activity (Table 4, Fig. 2).
Table 4.
Cell cycle analysis of HeLa cells following treatment with different drugs
| Sample data | Results | |||
|---|---|---|---|---|
| ID | %G0–G1 | %S | %G2/M | %Pre-G1 |
| Cont. (HeLa) | 61.32 ± 2.95 | 31.42 ± 1.34 | 7.26 ± 0.23 | 1.72 ± 0.02 |
| Paclitaxel | 44.61 ± 1.29 | 32.59 ± 1.23 | 22.8 ± 0.84 | 14.25 ± 0.32 |
| Carboplatin | 32.47 ± 1.09 | 33.16 ± 1.52 | 34.37 ± 1.72 | 22.19 ± 0.77 |
| Gallic acid | 38.59 ± 1.28 | 31.88 ± 1.76 | 29.53 ± 0.92 | 16.14 ± 0.63 |
| Mix. 1 | 26.88 ± 1.22 | 34.05 ± 1.39 | 39.07 ± 1.49 | 25.41 ± 1.27 |
| Mix. 2 | 42.27 ± 2.17 | 32.19 ± 1.31 | 25.54 ± 1.12 | 27.11 ± 0.92 |
| Mix. 3 | 36.24 ± 2.19 | 33.17 ± 1.27 | 30.59 ± 1.38 | 20.51 ± 0.78 |
| Mix. 4 | 34.22 ± 1.76 | 33.02 ± 1.61 | 32.76 ± 1.17 | 24.07 ± 1.13 |
Fig. 2.
Cell cycle analysis of HeLa cells following treatment with different drugs. a Histogram showing the percentages of cells in different cell cycle phases. b Bar chart showing the percentages of cells in different cell cycle phases. *Significant from control group at P < 0.0001, #significant from Paclitaxel + Carboplatin group at P < 0.0001; GA Gallic acid
Flow cytometry analysis showing induction of apoptosis by Gallic acid
Cell cycle analysis showed that treatment of HeLa cells with Paclitaxel, Carboplatin, Gallic acid and the mentioned drug combinations resulted in growth arrest at the G2/M phase and it further showed an increase in the cell population in pre G1 population, which could be indicative of apoptotic cells. The highest induction of apoptosis was seen in the doublet combination of Paclitaxel/Gallic acid (27.11%) which showed significant increase than single treatment with Paclitaxel and non-significant increase than the doublet combination Paclitaxel/Carboplatin. On the contrary, the doublet combination of Carboplatin/Gallic acid showed non-significant decrease in apoptotic population compared to single treatment with Carboplatin and the doublet combination Paclitaxel/Carboplatin.
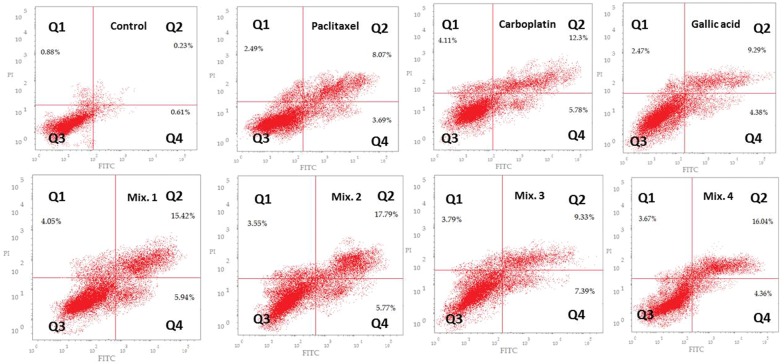
Evaluation of apoptosis by annexin V-FITC staining
Since accumulation of cells at G2/M phase during cell cycle analysis was observed following treatment with Paclitaxel, Carboplatin, Gallic acid and the mentioned drug combinations, we were interested in quantifying the different types of apoptotic cells. In order to detect and quantify the apoptosis, Annexin V-FITC/PI double staining was used. Staining with Annexin V is typically used in conjunction with a vital dye such as PI for identification of early and late apoptotic cells. Viable cells with intact membranes exclude PI, whereas the membranes of dead and damaged cells are permeable to PI. Annexin V is capable of staining apoptotic cells as soon after initiating apoptosis; cells translocate the membrane phosphatidylserine (PS) from the inner face of the plasma membrane to the cell surface. Once on the cell surface, PS can be easily detected by staining with a fluorescent conjugate of Annexin Vas it has a high affinity for PS. Therefore, cells that are considered viable are both Annexin V and PI negative, while cells that are in early apoptosis are Annexin V positive and PI negative, and cells that are in late apoptosis or already dead are both Annexin V and PI positive. After HeLa cells were treated with selected doses of individual and combination drugs and stained with annexin V/PI, the cell cycle distribution was then detected by flow cytometry and results were recorded (Table 5) and represented as Dot plot graph representing four quadrant images (Fig. 3). Our results showed that both the doublet combination of Paclitaxel/Gallic acid (Mix. 2) and triplet combination of Paclitaxel/Carboplatin/Gallic acid (Mix. 4) showed the highest proportion of cells in late apoptotic stage (stained by Annexin V-FITC and PI). These results suggested synergistic or additive effect of Gallic acid with Paclitaxel and Paclitaxel/Carboplatin combination.
Table 5.
Detection of different types of apoptotic cells induced in HeLa cells following treatment with different drugs using annexin VFITC/PI staining
| ID | Total | Early apoptosis | Late apoptosis | Necrosis |
|---|---|---|---|---|
| Cont. (HeLa) | 1.72 | 0.61 | 0.23 | 0.88 |
| Paclitaxel | 14.25 | 3.69 | 8.07 | 2.49 |
| Carboplatin | 22.19 | 5.78 | 12.3 | 4.11 |
| Gallic acid | 16.14 | 4.38 | 9.29 | 2.47 |
| Mix. 1 | 25.41 | 5.94 | 15.42 | 4.05 |
| Mix. 2 | 27.11 | 5.77 | 17.79 | 3.55 |
| Mix. 3 | 20.51 | 7.39 | 9.33 | 3.79 |
| Mix. 4 | 24.07 | 4.36 | 16.04 | 3.67 |
Fig. 3.
Dot plot representing four quadrant images observed by flow cytometric analysis. Q1: shows necrotic cells, Q2: shows later period apoptotic cells, Q3: shows normal cells and the Q4: shows early apoptotic cells
Real time quantitative PCR (RT-qPCR) assay for some apoptotic markers
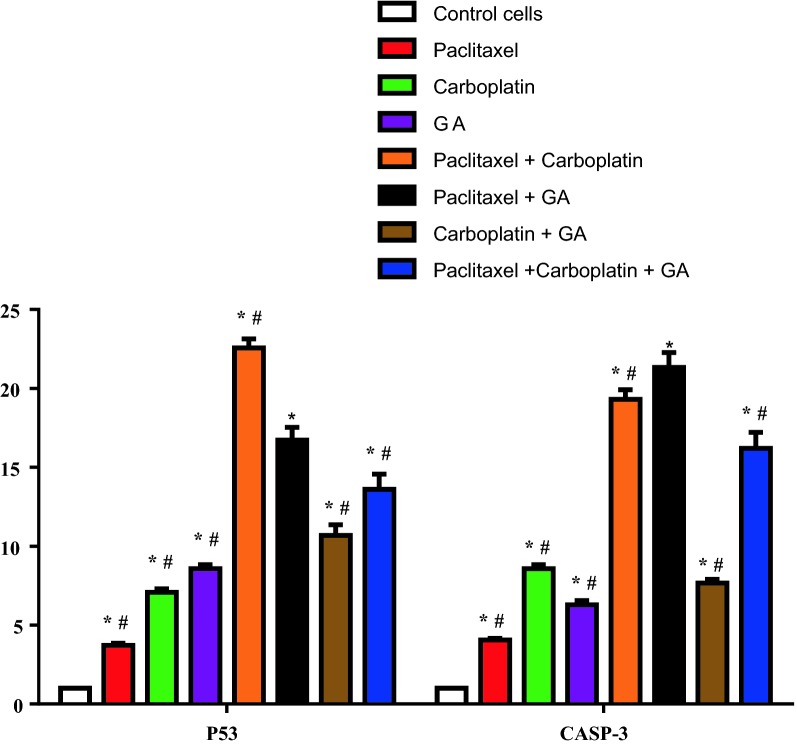
In order to analyze whether treatment with individual drugs or in combination for 24 h. affected genes controlling apoptosis including P53, Bcl-2, and Caspase 3, changes in the expression of these genes using quantitative real-time PCR were performed. The results are shown in Table 6 and Fig. 4. All cells treated with Paclitaxel, Carboplatin, Gallic acid and the mentioned drug combinations, showed significant increase in mRNA expression of P53 and Caspase 3 as compared to control HeLa cells, while BCl2 levels showed insignificant decrease than control HeLa cells. It was noticed that treatment of HeLa cell with Paclitaxel alone showed the least level of P53 (3.73 ± 0.14) and Caspase 3 (4.06 ± 0.1) among all treated group but upon addition of Gallic acid to Paclitaxel (Mix. 2), this combination therapy showed the highest level of Caspase3 (21.33 ± 0.95) and the second highest level of P53 (16.73 ± 0.81) preceded only by Paclitaxel/Carboplatin combination (Mix. 1) (22.56 ± 1.58). Paclitaxel/Gallic acid combination (Mix. 2) showed significant increase in P53 level as compared to all treated group except of Paclitaxel/Carboplatin combination (Mix. 1) at P < 0.0001. Paclitaxel/Gallic acid combination (Mix. 2) also showed significant increase in Caspase 3 level as compared to all treated group at P < 0.0001. These results suggested strong synergistic effect of Gallic acid with Paclitaxel.
Table 6.
The expression of P53, Bcl-2, and Caspase 3 by RT-qPCR
| Sample data | Results | ||
|---|---|---|---|
| Fold change | |||
| ID | P53 |
bcl2 |
Casp3 |
| Cont. HeLa | 1 | 1 | 1 |
| Paclitaxel | 3.73 ± 0.14 | 0.38 ± 0.14 | 4.06 ± 0.1 |
| Carboplatin | 7.08 ± 0.22 | 0.24 ± .005 | 8.59 ± 0.25 |
| Gallic acid | 8.59 ± 0.25 | 0.28 ± 0.07 | 6.29 ± 0.27 |
| Mix. 1 | 22.56 ± 1.58 | 0.28 ± 0.06 | 19.31 ± 0.61 |
| Mix. 2 | 16.73 ± 0.81 | 0.28 ± 0.02 | 21.33 ± 0.95 |
| Mix. 3 | 10.68 ± 68 | 0.22 ± 0.01 | 7.68 ± 0.23 |
| Mix. 4 | 13.62 ± 0.96 | 0.31 ± 0.02 | 16.22 ± 1.01 |
The results were represented as mean of fold changes (2^ΔΔCT) ± SD. β-actin gene was used as a control gene
Fig. 4.
The expression of P53 and Caspase 3 by RT-qPCR. The results were represented as mean of fold changes ± SD (P < 0.0001). β-actin gene was used as a control gene. *Significant from HeLa group at P < 0.0001, #significant from Paclitaxel + GA group at P < 0.0001
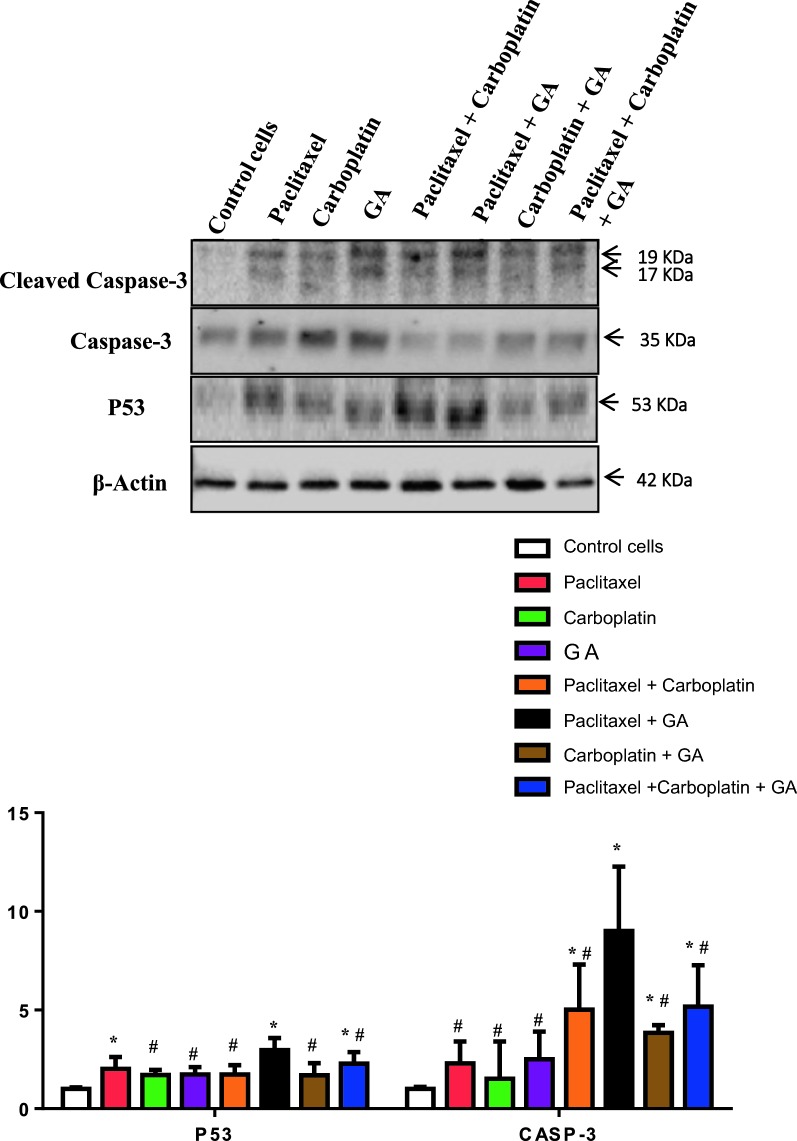
Western blotting for some apoptotic markers
In an attempt to investigate the effect of different tested drugs on apoptotic pathway, level of P53 protein and ratio of Cleaved Caspase-3/Caspase 3 were assessed by western blotting (Fig. 5). The protein levels of both P53 and Caspase 3 in all tested drugs were significantly increased as compared to control HeLa cells. Paclitaxel/Gallic acid combination (Mix. 2) showed significant increase in P53 and Caspase 3 levels as compared to all groups. These results suggests that Paclitaxel/Gallic acid combination has the highest apoptotic effect as evidenced by its induction of both p53, a tumor suppressor protein that regulates apoptosis, and Caspase 3, a key enzyme in the execution of apoptosis, (Fig. 6). Taken together, Paclitaxel/Gallic acid combination could be an effective and safer alternative for Paclitaxel/Carboplatin combination.
Fig. 5.
The immunoblotting of P53 and cleaved Caspase 3. Control group for both P53 and cleaved caspase-3/Caspase ratio and values were represented as mean ± SD of treated cells compared with Control cells (untreated cells) (P < 0.0001). The detected proteins were quantified and normalized to β-actin. *Significant from HeLa group at P < 0.01, #significant from Paclitaxel + GA group at P < 0.05. The upper part of the figure illustrates immunoblot of P53, Cleaved Caspase-3/Caspase and β-actin from three separate experiments
Fig. 6.
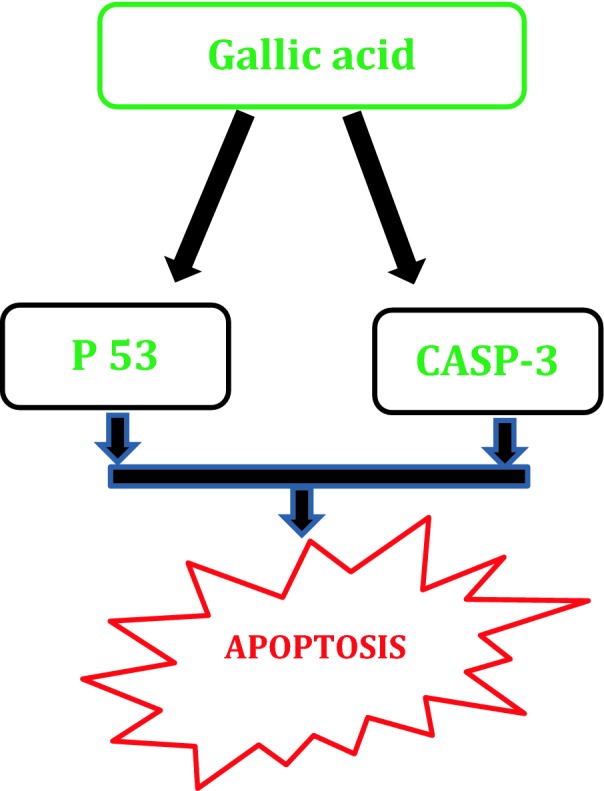
Schematic diagram showing the mechanism of action of Gallic acid in the induction of apoptosis in Hela cells
Discussion
Although chemotherapeutic drugs are very effective, severe side effects and development of drug resistance limits the use of these drugs. The use of natural products with anticancer activity may help to partially overcome these issues. In the present study, we investigated the ability of Gallic acid, to potentiate the anti-cancer effects of Paclitaxel, Carboplatin and Paclitaxel/Carboplatin combination in human HeLa cells by performing MTT assay, cell cycle analysis, RT-PCR assay for P53, Bcl-2, and Caspase 3 genes and Western blot analysis for P53 and Caspase 3. Our results revealed that the most promising combination was Gallic acid with Paclitaxel as it showed the highest cytotoxic effect by MTT assay, the highest induction of apoptosis by cell cycle analysis and significant elevation in P53 and Caspase 3 levels.
A number of previous studies investigated the cytotoxic effect of GA in various cell lines and showed that the IC50 for Calu-6 (human, Caucasian, lung, adenocarcinoma), A 549 (adenocarcinomic human alveolar basal epithelial cells), HeLa (human epithelial carcinoma), and (HUVECs) Human umbilical vein endothelial cells were 10–50, 100–200, 80, 400 µM, respectively [28–31]. These results suggest that Gallic acid induced cell death in tumour cells with a relatively high selectivity. In the present study, Gallic acid treatment decreased the growth of HeLa cells in a dose-dependent manner, with the IC50 value of ~ 11.5 µM at 24 h. The cytotoxic effect of Gallic acid may be explained by its pro-oxidant property which has been recognized as apoptosis inducer in different cancer cell lines including HeLa cells [32]. The difference in IC50 of Gallic acid in HeLa cell seen in our study can be explained by the fact that although MTT assay has been used for 30 years in cancer research [33–35], it rarely yielded a consistent IC50 value for a given chemical compound against a given cancer cell line. According to He et al. [36], this issue was attributed to differences among the manufacturers and formulae used by different laboratories, even within the same laboratory, the MTT assay results gives variable IC50 values among different staff researchers and between different experimental repeats performed by the same researcher. This inconsistency can be explained by variation in control wells, which are used as a basis for the calculations of IC50 values, these variations depend on the initial cell seeding density and the proliferation potential of the cell line [36].
In the present study, flow cytometric analysis showed that Gallic acid caused G2/M phase cell cycle arrest. Subsequently, the apoptotic sub-G1 phase increased obviously, suggesting the occurrence of sequential events of cell cycle arrest followed by apoptosis. Gallic acid was found to induce apoptosis in different in vitro [30] and in vivo studies [37–39].
The ability of Gallic acid to induce G2/M arrest was also reported by Ou et al. [40] who explained this effect by alterations in cell cycle regulators in a human bladder transitional carcinoma cell line. The regulation of cell cycle is controlled by a family of cyclin/cyclin-dependent kinase (CDK) complexes and the CDK inhibitors (CDKIs) [41]. G2/M transition is regulated mainly by the sequential activation and deactivation of CDK-regulatory proteins and cyclin complexes. Cdc2 is also known as Cdk1, which initially forms a complex with Cyclin B1 to drive the cell from G2 to M phase [42]. Ou et al. [40] suggested that the anticancer effect of Gallic acid seems to be based on activation of ataxia telangiectasia-mutated (ATM)/checkpoint kinase 2 (Chk2)-which cause phosphorylation and activation of cdc25c, a phosphatase that activates the Cdk1/cyclin B1 kinase complex, leading to accumulation of cdc2 in Tyr-15 phosphorylated inactive form and consequently cell cycle arrest and apoptosis occur.
Apoptosis is a complex process that proceeds through at least two main pathways (extrinsic and intrinsic), each of which can be regulated at multiple levels. The intrinsic pathway centers on the mitochondria, the release of proapoptotic factors from the mitochondria, such as cytochrome c, into the cytoplasm promotes the activation of initiator caspase-9, which in turn activates effector caspases such as caspase-3 or -7. The extrinsic pathway relies on the activation of death receptors from the tumour necrosis factor (TNF) receptor family that promote the recruitment and activation of initiator caspase-8 via adaptor proteins such as Fas-associated death domain (FADD) or TNFRSF1A-associated via death domain (TRADD) [43]. Major regulators of the intrinsic pathway are the pro- and anti-death members of the Bcl-2 family [44]. These proteins reside at, or translocate to the mitochondria, controlling the release of p53, a major tumour suppressor, serves as a regulator of the apoptotic process that can modulate key control points in both the extrinsic and intrinsic pathways [45].
In our study, Gallic acid induced apoptosis as manifested by the results of both MTT assay and flow cytometric analysis. In order to understand the role of Gallic acid in induction of apoptosis, P53, Bcl-2, and Caspase 3 expression was detected by RT-PCR assay and Western blot analysis.
In our study, Paclitaxel/Gallic acid combination showed the second highest level of P53 preceded only by Paclitaxel/Carboplatin combination. P53 is necessary to maintain a G2 arrest following DNA damage, since tumor cells lacking this proteins enter into mitosis with accelerated kinetics. The mechanism of p53-dependent G2 arrest involves an initial inhibition of cyclin B1/Cdc2 activity by p21 and a subsequent reduction of cyclin B1 and Cdc2 protein levels [46]. The ability of Gallic acid to up regulate p53 seen in our study is in agreement with Wang et al. [47] who revealed that Gallic acid exhibited its anticancer effect on human SCLC H446 cells via the ROS-dependent mitochondrial apoptotic pathway.
Caspase 3 is one of key enzymes to play a pivotal role in the terminal execution phase of apoptosis [48]. In Our study, Paclitaxel/Gallic acid combination showed the highest level of Caspase 3. The involvement of different Caspases in GA-induced apoptosis in HeLa cells, including Caspase 3, was reported by You et al. [28] who found that treatment of GA-treated HeLa cells with various Caspase inhibitors resulted in significantly but not completely prevention of apoptosis and decreased dead cell percentage.
The potentiating effect of co-treatment of Gallic acid with Paclitaxel seen in our study, is in agreement with Sánchez-Carranza et al. [49] who reported that the cytostatic action of Paclitaxel is increased in the human ovarian carcinoma A2780 cells (drug sensitive) and A2780AD cells (multi-drug-resistant ovarian cancer) models via co-treatment with Gallic acid.
Sánchez-Carranza et al. explained this result by the ability of Gallic acid to potentiate the Paclitaxel-induced G2/M phase arrest. Using the drug-resistant cell line, they also demonstrated that proliferation inhibition and G2/M phase arrest are mediated by Gallic acid-provoked reactive oxygen species overproduction, and by reactive oxygen species-mediated inhibition of Paclitaxel-provoked extracellular signal-regulated kinases (ERKs) activation [50]. ERK is a subfamily of mitogen-activated protein kinases (MAPKs) that control a vast array of physiological processes. The extracellular signal-regulated kinases (ERKs) function in the control of cell division, and inhibitors of these enzymes are being explored as anticancer agents [40].
Conclusion
The present study suggests that the combination of Paclitaxel and Gallic acid may be a promising protocol for treatment of cervical cancer and it could be a possible replacement of the current commonly used regimen Paclitaxel/Carboplatin. The Paclitaxel/Gallic acid combination offers a number of advantages over existing protocols such as higher efficacy, lower dose and less side effects. More detailed mechanistic and efficacy studies are warranted in cell culture, animal models and finally clinical trials to evaluate Paclitaxel/Gallic acid combination therapy.
Acknowledgements
The authors would like to acknowledge Dr. Mohamed Ali Abd El-Baseer, Director of science way for scientific research.
Authors’ contributions
NMA and NO contributed in this work. NMA suggested the research point. Both authors read and approved the final manuscript.
Funding
This research is not supported by any funding agency.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent of publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nora M. Aborehab, Email: naborehab@msa.eun.eg
Nada Osama, Email: Nada_dardeer@yahoo.com.
References
- 1.Cancer. https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed 1 May 2019.
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Adhami VM, Malik A, Zaman N, Sarfaraz S, Siddiqui IA, Syed DN, Afaq F, Pasha FS, Saleem M, Mukhtar H. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clin Cancer Res. 2007;13(5):1611–1619. doi: 10.1158/1078-0432.CCR-06-2269. [DOI] [PubMed] [Google Scholar]
- 4.Albain KS, Nag SM, Calderillo-Ruiz G, Jordaan JP, Llombart AC, Pluzanska A, Rolski J, Melemed AS, Reyes-Vidal JM, Sekhon JS, et al. Gemcitabine plus Paclitaxel versus Paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J Clin Oncol. 2008;26(24):3950–3957. doi: 10.1200/JCO.2007.11.9362. [DOI] [PubMed] [Google Scholar]
- 5.Mokhtari RB, Kumar S, Islam SS, Yazdanpanah M, Adeli K, Cutz E, Yeger H. Combination of carbonic anhydrase inhibitor, acetazolamide, and sulforaphane, reduces the viability and growth of bronchial carcinoid cell lines. BMC Cancer. 2013;13:378. doi: 10.1186/1471-2407-13-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guidance on the use of paclitaxel in the treatment of ovarian cancer. https://www.nice.org.uk/guidance/ta55. Accessed 1 May 2019.
- 7.Stordal B, Davey M. Understanding cisplatin resistance using cellular models. IUBMB Life. 2007;59(11):696–699. doi: 10.1080/15216540701636287. [DOI] [PubMed] [Google Scholar]
- 8.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4(4):253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 9.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 10.Stewart DJ. Mechanisms of resistance to cisplatin and carboplatin. Crit Rev Oncol/Hematol. 2007;63(1):12–31. doi: 10.1016/j.critrevonc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Frazier AL, Stoneham S, Rodriguez-Galindo C, Dang H, Xia C, Olson TA, Murray MJ, Amatruda JF, Shaikh F, Pashankar F, et al. Comparison of carboplatin versus cisplatin in the treatment of paediatric extracranial malignant germ cell tumours: a report of the Malignant Germ Cell International Consortium. Eur J Cancer. 2018;98:30–37. doi: 10.1016/j.ejca.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Jiang S, Pan AW, Lin TY, Zhang H, Malfatti M, Turteltaub K, Henderson PT, Pan CX. Paclitaxel enhances carboplatin-dna adduct formation and cytotoxicity. Chem Res Toxicol. 2015;28(12):2250–2252. doi: 10.1021/acs.chemrestox.5b00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharya S, Muhammad N, Steele R, Kornbluth J, Ray RB. Bitter Melon enhances natural killer-mediated toxicity against head and neck cancer cells. Cancer Prev Res. 2017;10(6):337–344. doi: 10.1158/1940-6207.CAPR-17-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright GD. Opportunities for natural products in 21(st) century antibiotic discovery. Nat Prod Rep. 2017;34(7):694–701. doi: 10.1039/C7NP00019G. [DOI] [PubMed] [Google Scholar]
- 15.Yao H, Liu J, Xu S, Zhu Z, Xu J. The structural modification of natural products for novel drug discovery. Expert Opin Drug Discov. 2017;12(2):121–140. doi: 10.1080/17460441.2016.1272757. [DOI] [PubMed] [Google Scholar]
- 16.Muhammad N, Steele R, Isbell TS, Philips N, Ray RB. Bitter melon extract inhibits breast cancer growth in preclinical model by inducing autophagic cell death. Oncotarget. 2017;8(39):66226–66236. doi: 10.18632/oncotarget.19887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhattacharya S, Muhammad N, Steele R, Peng G, Ray RB. Immunomodulatory role of bitter melon extract in inhibition of head and neck squamous cell carcinoma growth. Oncotarget. 2016;7(22):33202–33209. doi: 10.18632/oncotarget.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun J, Chu YF, Wu X, Liu RH. Antioxidant and antiproliferative activities of common fruits. J Agric Food Chem. 2002;50(25):7449–7454. doi: 10.1021/jf0207530. [DOI] [PubMed] [Google Scholar]
- 19.Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15(10):7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De A, De A, Papasian C, Hentges S, Banerjee S, Haque I, Banerjee SK. Emblica officinalis extract induces autophagy and inhibits human ovarian cancer cell proliferation, angiogenesis, growth of mouse xenograft tumors. PLoS ONE. 2013;8(8):e72748. doi: 10.1371/journal.pone.0072748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sourani ZM, Pourgheysari BP, Beshkar PM, Shirzad HP, Shirzad MM. Gallic Acid inhibits proliferation and induces apoptosis in lymphoblastic leukemia cell line (C121) Iran J Med Sci. 2016;41(6):525–530. [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshino M, Haneda M, Naruse M, Htay HH, Iwata S, Tsubouchi R, Murakami K. Prooxidant action of gallic acid compounds: copper-dependent strand breaks and the formation of 8-hydroxy-2′-deoxyguanosine in DNA. Toxicol in Vitro. 2002;16(6):705–709. doi: 10.1016/S0887-2333(02)00061-9. [DOI] [PubMed] [Google Scholar]
- 23.de Mejia EG, Ramirez-Mares MV, Puangpraphant S. Bioactive components of tea: cancer, inflammation and behavior. Brain Behav Immun. 2009;23(6):721–731. doi: 10.1016/j.bbi.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 24.He Z, Li B, Rankin GO, Rojanasakul Y, Chen YC. Selecting bioactive phenolic compounds as potential agents to inhibit proliferation and VEGF expression in human ovarian cancer cells. Oncol Lett. 2015;9(3):1444–1450. doi: 10.3892/ol.2014.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han YH, Park WH. Growth inhibition in antimycin A treated-lung cancer Calu-6 cells via inducing a G1 phase arrest and apoptosis. Lung Cancer. 2009;65(2):150–160. doi: 10.1016/j.lungcan.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Burnette W. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory: Cold Spring Harbor; 1989. [Google Scholar]
- 28.You BR, Moon HJ, Han YH, Park WH. Gallic acid inhibits the growth of HeLa cervical cancer cells via apoptosis and/or necrosis. Food Chem Toxicol. 2010;48(5):1334–1340. doi: 10.1016/j.fct.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 29.You BR, Kim SZ, Kim SH, Park WH. Gallic acid-induced lung cancer cell death is accompanied by ROS increase and glutathione depletion. Mol Cell Biochem. 2011;357(1–2):295–303. doi: 10.1007/s11010-011-0900-8. [DOI] [PubMed] [Google Scholar]
- 30.You BR, Park WH. Gallic acid-induced lung cancer cell death is related to glutathione depletion as well as reactive oxygen species increase. Toxicol in Vitro. 2010;24(5):1356–1362. doi: 10.1016/j.tiv.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Park WH. Gallic acid induces HeLa cell death via increasing GSH depletion rather than ROS levels. Oncol Rep. 2017;37(2):1277–1283. doi: 10.3892/or.2016.5335. [DOI] [PubMed] [Google Scholar]
- 32.Dorniani D, Saifullah B, Barahuie F, Arulselvan P, Hussein MZ, Fakurazi S, Twyman LJ. Graphene oxide-Gallic Acid nanodelivery system for cancer therapy. Nanoscale Res Lett. 2016;11(1):491. doi: 10.1186/s11671-016-1712-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 34.Barltrop JA, Owen TC, Cory AH, Cory JG. 5-(3-Carboxymethoxyphenyl)-2-(4,5-dimethylthiazolyl)-3-(4-sulfophenyl) tetrazolium, inner salt (MTS) and related analogs of 3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT) reducing to purple water-soluble formazans as cell-viability indicators. Bioorg Med Chem Lett. 1991;1:611–614. doi: 10.1016/S0960-894X(01)81162-8. [DOI] [Google Scholar]
- 35.Ishiyama M, Miyazono Y, Sasamoto K, Ohkura Y, Ueno K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta. 1997;44(7):1299–1305. doi: 10.1016/S0039-9140(97)00017-9. [DOI] [PubMed] [Google Scholar]
- 36.He Y, Zhu Q, Chen M, Huang Q, Wang W, Li Q, Huang Y, Di W. The changing 50% inhibitory concentration (IC50) of cisplatin: a pilot study on the artifacts of the MTT assay and the precise measurement of density-dependent chemoresistance in ovarian cancer. Oncotarget. 2016;7(43):70803–70821. doi: 10.18632/oncotarget.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen YJ, Lin KN, Jhang LM, Huang CH, Lee YC, Chang LS. Gallic acid abolishes the EGFR/Src/Akt/Erk-mediated expression of matrix metalloproteinase-9 in MCF-7 breast cancer cells. Chem Biol Interact. 2016;252:131–140. doi: 10.1016/j.cbi.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 38.Zhao B, Hu M. Gallic acid reduces cell viability, proliferation, invasion and angiogenesis in human cervical cancer cells. Oncol Lett. 2013;6(6):1749–1755. doi: 10.3892/ol.2013.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verma S, Singh A, Mishra A. Gallic acid: molecular rival of cancer. Environ Toxicol Pharmacol. 2013;35(3):473–485. doi: 10.1016/j.etap.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 40.Ou TT, Wang CJ, Lee YS, Wu CH, Lee HJ. Gallic acid induces G2/M phase cell cycle arrest via regulating 14-3-3beta release from Cdc25C and Chk2 activation in human bladder transitional carcinoma cells. Mol Nutr Food Res. 2010;54(12):1781–1790. doi: 10.1002/mnfr.201000096. [DOI] [PubMed] [Google Scholar]
- 41.Agarwal C, Singh RP, Dhanalakshmi S, Tyagi AK, Tecklenburg M, Sclafani RA, Agarwal R. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene. 2003;22(51):8271–8282. doi: 10.1038/sj.onc.1207158. [DOI] [PubMed] [Google Scholar]
- 42.Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20(15):1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- 43.Martin P, Pognonec P. ERK and cell death: cadmium toxicity, sustained ERK activation and cell death. FEBS J. 2010;277(1):39–46. doi: 10.1111/j.1742-4658.2009.07369.x. [DOI] [PubMed] [Google Scholar]
- 44.Tsujimoto Y. Cell death regulation by the Bcl-2 protein family in the mitochondria. J Cell Physiol. 2003;195(2):158–167. doi: 10.1002/jcp.10254. [DOI] [PubMed] [Google Scholar]
- 45.Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22(56):9030–9040. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- 46.Flatt PM, Tang LJ, Scatena CD, Szak ST, Pietenpol JA. p53 regulation of G(2) checkpoint is retinoblastoma protein dependent. Mol Cell Biol. 2000;20(12):4210–4223. doi: 10.1128/MCB.20.12.4210-4223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang R, Ma L, Weng D, Yao J, Liu X, Jin F. Gallic acid induces apoptosis and enhances the anticancer effects of cisplatin in human small cell lung cancer H446 cell line via the ROS-dependent mitochondrial apoptotic pathway. Oncol Rep. 2016;35(5):3075–3083. doi: 10.3892/or.2016.4690. [DOI] [PubMed] [Google Scholar]
- 48.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281(5381):1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez-Carranza JN, Diaz JF, Redondo-Horcajo M, Barasoain I, Alvarez L, Lastres P, Romero-Estrada A, Aller P, Gonzalez-Maya L. Gallic acid sensitizes paclitaxel-resistant human ovarian carcinoma cells through an increase in reactive oxygen species and subsequent downregulation of ERK activation. Oncol Rep. 2018;39(6):3007–3014. doi: 10.3892/or.2018.6382. [DOI] [PubMed] [Google Scholar]
- 50.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.