Abstract
Background
Avian haemosporidian parasites can cause severe disease in their hosts due to excessive exo-erythrocytic merogony and anaemia caused by blood stages. Notably, the development of megalomeronts by species of Haemoproteus and Leucocytozoon has been associated with mortalities in birds. Diagnosis of lethal infections is currently accomplished by the detection of parasites’ tissue stages in histological sections combined with PCR and sequencing. However, sequences frequently are not reliably obtained and the generic discrimination of exo-erythrocytic tissue stages based on morphological characters is challenging. Therefore, the present study aimed at developing specific molecular probes for the identification of Haemoproteus spp. and Leucocytozoon spp. in histological sections using chromogenic in situ hybridization.
Methods
Parasite subgenus-specific oligonucleotide probes were designed to target the 18S ribosomal RNA of Haemoproteus species (subgenus Parahaemoproteus) and Leucocytozoon spp. (subgenus Leucocytozoon) and were in situ hybridized to sections from formalin-fixed, paraffin-embedded tissue samples determined positive for these parasites by PCR and histopathology. To confirm the presence of parasites at sites of probe hybridization, consecutive sections were stained with haematoxylin–eosin and examined.
Results
Parahaemoproteus- and Leucocytozoon-specific probes labelled erythrocytic and exo-erythrocytic stages of Haemoproteus spp. and Leucocytozoon spp., respectively. Binding of probes to parasites was consistent with detection of the same exo-erythrocytic meronts in consecutive haematoxylin–eosin-stained sections. Cross-reactivity of the probes was ruled out by negative chromogenic in situ hybridization when applied to samples positive for a parasite of a genus different from the probes’ target.
Conclusions
Chromogenic in situ hybridization using 18S ribosomal RNA-specific oligonucleotide probes reliably identifies and discriminates Haemoproteus and Leucocytozoon parasites in tissue sections and enables unequivocal diagnosis of haemosporidioses.
Electronic supplementary material
The online version of this article (10.1186/s13071-019-3536-2) contains supplementary material, which is available to authorized users.
Keywords: In situ hybridization, Haemoproteus, Leucocytozoon, Birds, Pathology, Exo-erythrocytic meronts, 18S ribosomal RNA, Haemosporidians
Background
Avian haemosporidian parasites (Haemosporida) are widespread almost all over the world and infect birds of diverse families and orders. The majority of described parasites belong to the genera Plasmodium, Haemoproteus and Leucocytozoon, comprising more than 200 morphologically distinct species [1]. The life-cycle of haemosporidians in the avian host includes development both in tissues (exo-erythrocytic merogony) and blood cells. Haemosporidians always undergo exo-erythrocytic merogony producing meronts in tissue cells before they infect blood cells and form gametocytes, the parasite stage infective for vectors [2].
The pathogenic impact of haemosporidians on the host can be variable at different developmental stages, and severity of infection in avian hosts depends on many factors [3]. Generally, haemosporidian infection is subclinical in birds; however, it is well established that parasites not only cause haematological disorders but can also elicit severe damage to organs due to marked merogony in tissues [2]. For example, Plasmodium infections can result in excessive multiplication of parasites in various organs including the brain, where meronts cause blockage of capillaries, ultimately leading to death of the host [4]. Such lethal infections were frequently reported in immunologically naive hosts, which are highly susceptible to infection, e.g. penguins [5–7]. However, there are also cases, albeit less numerous, which demonstrate mortalities linked to malaria in wild birds inhabiting regions with haemosporidian transmission [8–10]. Exo-erythrocytic merogony of Haemoproteus and Leucocytozoon, the most diverse avian haemosporidian genera besides Plasmodium, has been investigated only fragmentarily because of methodological difficulties to access these life-cycle stages, particularly in wild animals. Data on complete tissue merogony are available only for a few of these blood parasite species [2]. Experimental research might provide convincing data [1], but is challenging to design with Haemoproteus and Leucocytozoon parasites, mainly because of difficulties to obtain sporozoites, the only infective stage that can initiate exo-erythrocytic development of these haemosporidians in vertebrate hosts. Cultivation and experimental infection of vectors (species of Ceratopogonidae, Hippoboscidae and Simuliidae) are needed for such experiments. With rare exceptions, Haemoproteus and Leucocytozoon parasites were usually considered to be relatively benign in avian hosts, particularly in wildlife [11]. That hampered research in this field of parasitology and veterinary medicine for better understanding virulence of haemosporidian infections. Numerous veterinary observations and field case reports contradicted this opinion, but they did not receive due attention [12–16]. Haemoproteus and Leucocytozoon parasites have been identified to cause morbidity and mortality in several bird species. Severe haemosporidioses caused by these parasites have been repeatedly reported in both captive [12–15, 17–25] and wild birds [9, 10, 16, 18, 26] and were commonly associated with the development of megalomeronts in diverse organs of their hosts.
Unequivocal determination of parasitic tissue stages in haemosporidian infections is difficult. Diagnosis of haemosporidian infections in lethal cases is primarily achieved by necropsy followed by histopathological examination and PCR-based testing. Parasites can be detected in routinely stained histological sections; however, microscopic examination is time-consuming and parasites can be overlooked or confounded with nuclear fragments in necrotic tissue or cell debris. Although morphological features can indicate their generic identity, the correct assignment of parasites is often challenging, especially in cases of aberrant infections due to unusual morphology and location in atypical hosts [2]. There are several reports presenting severe cases of haemosporidioses, in which conclusive determination of the parasites based on tissue stages was not achieved, particularly in cases of Haemoproteus and Leucocytozoon infections [10, 26–28]. In many cases, identification of involved parasites is accomplished by histological analysis in combination with PCR and sequencing. However, even sensitive PCR does not always reliably detect parasites, especially in co-infections, which are common in birds [29] hampering correct attribution of detected genotypes to morphological stages observed in histology. This is a particularly sensitive issue in distinguishing growing (young) exo-erythrocytic meronts of Plasmodium and Haemoproteus parasites, which usually are morphologically identical at this stage of development [1].
The aim of the present study was to develop molecular probes for the specific detection of Haemoproteus (subgenus Parahaemoproteus) and Leucocytozoon (subgenus Leucocytozoon) parasites in formalin-fixed, paraffin wax-embedded (FFPE) tissue samples by using chromogenic in situ hybridization (CISH). This method has already been established for the detection of Plasmodium species by using probes which target the 18S ribosomal RNA (rRNA) of parasites [7]. Based on these convincing results, molecular probes targeting the 18S rRNA of Haemoproteus species and Leucocytozoon spp. were designed.
Methods
Probe design
Oligonucleotide probes were designed to target the 18S rRNA of parasites from the avian haemosporidian genera Haemoproteus (subgenus Parahaemoproteus) and Leucocytozoon (subgenus Leucocytozoon). Haemosporidian 18S ribosomal DNA (rDNA) sequences were isolated from genomes published in NCBI GenBank [30] (accession numbers X13706, AY625607, PRJEB9073, PRJEB9074) and aligned with sequences obtained from an analysis of nuclear 18S rDNA of a selection of Haemoproteus spp., Leucocytozoon spp. and Plasmodium spp. (JH, unpublished data) using MAFFT v. 7 [31]. Alignments were checked in Bioedit [32] and regions of sequence homology suitable for parasite subgenus-specific probes, i.e. conserved within one target group but with differences (mismatches) to other groups, were determined. Probe design was conceived to address species of the subgenera Parahaemoproteus (Haemoproteus) and Leucocytozoon (Leucocytozoon), as they comprise the majority of avian Haemoproteus and Leucocytozoon species.
For the detection of Haemoproteus (Parahaemoproteus) species, the probe P-Haemo18S_1 (5′-CTA ACC GTA GTT ATA GTC GCC ATC TC-3′) was designed. This probe is 100% complementary to the 18S rRNA of Haemoproteus minutus (TURDUS2), H. syrnii (CULKIB01), H. lanii (RBS06), H. balmorali (ROBIN01), H. tartakovskyi (SISKIN1), and the Haemoproteus sp. lineages STAL2, LK03 and EMCIR01. Because of substantial 18S rDNA sequence differences between Leucocytozoon species belonging to the Leucocytozoon toddi-species complex and other Leucocytozoon spp. (JH, unpublished data), two distinct probes were designed to target parasites of the subgenus Leucocytozoon: the probe Leuco18S_1 (5′-TAG GAC TCC CCA CTT GTC TTT TTC TTG A-3′), which is 100% complementary to the 18S rRNA of Leucocytozoon sp. lineages CIAE02, SYCON05, TUPHI06, PARUS20, COCOR18, STAL05, BUBO01, ASOT06, MILVUS02, COCOR9, BT1, COCOR13 and STAL3, and the probe Ltod18S_1 (5′-GCT AAC CGT AGT TAT AGT CGC CAT CTC-3′), which is 100% complementary to Leucocytozoon sp. lineages ACNI03, CIAE03 and BUTBUT03, and targets members of the L. toddi-species complex. To ensure specificity of probes, they were designed to include at least six nucleotide mismatches to 18S rRNA sequences of other target groups. Quality, i.e. GC-content, formation of self-dimers, and hybridization temperature of probes was checked in silico using AmplifX v. 1.7 [33], and a BLAST search against bird genomes was performed in NCBI GenBank to exclude unintentional hybridization to bird DNA. Probes were commercially synthesized by Microsynth Austria (Vienna, Austria) including 3′ ends labelled with digoxigenin.
Tissue samples
Formalin-fixed, paraffin wax-embedded (FFPE) tissue samples including various organs from infected wild birds were obtained from the archive of the Institute of Pathology and the Research Institute of Wildlife Ecology at the University of Veterinary Medicine Vienna (Austria). For testing the new probes, only samples determined as monoinfections by PCR were used. Tissue samples were sectioned at 1 µm, mounted on SuperFrost Plus Slides (Thermo Fisher Scientific, Waltham, Massachusetts, USA) and air-dried before being subjected to haematoxylin–eosin (HE) staining or CISH. To be able to precisely correlate histological findings with CISH results, serial sections were prepared, one of which was stained with HE and the others used for CISH with each single probe. Probes were tested on a selection of samples positive for different lineages in order to evaluate applicability of probes on a subgenus level (Table 1).
Table 1.
Host species and identified lineages from samples used for testing subgenus-specific probes
| Host | Parasite | MalAvi lineage | GenBank ID |
|---|---|---|---|
| Asio otus | Haemoproteus sp. | CIRCUM06 | MK330165 |
| Strix uralensis | Haemoproteus sp. | STRURA03 | MK330140 |
| Strix uralensis | H. syrnii | CULKIB01 | MK330143, MK330144 |
| Tetrao urogallus | Haemoproteus sp. | TETURO01 | MK330141 |
| Tetrao urogallus | Haemoproteus sp. | TETURO02 | MK330139 |
| Cyanoramphus novaezelandiae | H. minutus | TUPHI01 | MK330147 |
| Cyanoramphus novaezelandiae | H. minutus | TURDUS2 | MK330158 |
| Turdus merula | H. minutus | TURDUS2 | MK330151 |
| Passer domesticus | H. lanii | RBS06 | MK330149 |
| Lanius collurio | H. lanii | RBS2 | MK330164 |
| Emberiza citrinella | Haemoproteus sp. | EMCIR01 | MK330150, MK330152 |
| Falco tinnunculus | Haemoproteus sp. | LK03 | MK330145, MK330146 |
| Sylvia atricapilla | H. parabelopolskyi | SYAT41 | MK330159 |
| Passer domesticus | Haemoproteus sp. | PADOM03 | MK330161, MK330162, MK330163 |
| Bubo bubo | Leucocytozoon danilewskyi | BUBO01 | MK330142 |
| Corvus corone cornix | Leucocytozoon sp. | COCOR13 | MK330138 |
| Turdus merula | Leucocytozoon sp. | ASOT06 | MK330148 |
| Garrulus glandarius | Leucocytozoon sp. | GAGLA06 | MK330153 |
| Turdus merula | Leucocytozoon sp. | TUMER10 | MK330154 |
| Brotogeris cyanoptera | Leucocytozoon sp. | CIAE02 | MK330160 |
| Buteo buteo | L. toddi | BUTBUT03 | MK330155 |
| Turdus merula | Plasmodium matutinum | LINN1 | MK330156, MK330157 |
Molecular analysis
Molecular analysis was performed for all samples that were used for probe design and CISH. Total DNA was extracted from frozen and FFPE tissue samples and, if available, also from dried blood spots. For frozen samples and dried blood spots the DNeasy Blood & Tissue Kit (Qiagen, Venlo, Netherlands), and for FFPE tissue samples the FFPE Kit (Qiagen) were used according to the manufacturer’s recommendations with the exception that two elutions with each 100 µl AE buffer were performed, the second of which was used as template for the PCR. Samples were screened for haemosporidians using a nested PCR protocol [34] targeting a section of the mitochondrial cytochrome b gene (cytb) of parasites. In the first PCR, the primers HaemNFI and HaemNR3 were used to amplify parasite mitochondrial DNA of Plasmodium spp., Haemoproteus spp. and Leucocytozoon spp. In the second PCR, the primer pairs HaemF/HaemR2 and HaemFL/HaemR2L were used to amplify mitochondrial DNA of Plasmodium spp./Haemoproteus spp. and Leucocytozoon spp., respectively. Each DNA extract was amplified once. The primers of Hellgren et al. [34] do not allow for amplification of cytb sequences in members of the L. toddi-species complex, which are primarily found in raptors belonging to the order Accipitriformes. Based on the alignment of cytb sequences from species of the L. toddi-species complex published on NCBI GenBank under the accession numbers DQ177235–DQ177273 [35] and HM142915–HM142923 [36], a nested PCR protocol was designed to specifically target this group: in the first PCR, the primers CytB_L2_F (5′-GAG AGT TAT GGG CTG GAT GGT-3′) and CytB_L2_R (5′-TAG AAA GCC AAG AAA TAC CAT TCT G-3′), and in the second PCR, the primers CytB_L2_nF (5′-GCT GGA TGG TGT TTT AGA TAY ATG C-3′) and CytB_L2_nR (5′-CCA TTC TGG AAC AAT ATG TAA AGG TG-3′) were used to amplify a 528 bp section of the cytb from respective parasites. The selected section covers the entire cytb barcoding region, which is amplified using the protocol of Hellgren et al. [34].
PCR was performed using the GoTaq G2 Flexi DNA Polymerase (Promega, Madison, Wisconsin, USA). The volume for each reaction mixture was 25 µl and contained 14.375 µl nuclease-free water, 5 µl 5× Green GoTaq Flexi Buffer, 2 µl MgCl2 solution (25 mM), 0.5 µl PCR nucleotide mix (10 mM, Promega), 0.125 µl GoTaq G2 Flexi DNA Polymerase (5 u/µl), each 1 µl forward and reverse primer (10 pmol/µl) and 1 µl of DNA template. For the second PCR, instead of a DNA template, 1 µl of the PCR product from the first PCR was used. Thermocycler conditions were the following: initial denaturation at 94 °C for 2 min, 35 cycles of denaturation at 94 °C for 30 s, primer annealing at 50 °C (Hellgren primers)/55 °C (L. toddi primers) for 30 s, and extension at 72 °C for 1 min, followed by a final extension at 72 °C for 10 min. PCR products were run on 1% agarose gels stained with Midori Green Advance (Nippon Genetics Europe, Dueren, Germany) and visualized with a BioSens SC-Series 710 gel documentation system (GenXpress, Wiener Neudorf, Austria). All DNA extracts were amplified once. Tissue samples confirmed positive in earlier screenings were used as positive controls. Negative controls contained nuclease free water instead of DNA templates. PCR amplicons from the second PCRs were sent to Microsynth Austria for bi-directional sequencing with forward and reverse primers. Obtained sequences were edited and aligned in Bioedit [32] and electropherograms were carefully checked for double base-calling to rule out mixed infections. Sequences were submitted to BLAST search in the avian malaria database MalAvi [37] and NCBI GenBank.
Chromogenic in situ hybridization
CISH was applied to tissue sections following a previously established protocol [7] with some modifications. Tissue sections were dewaxed 2 × 5 min in Neo-Clear solution (Merck Millipore, Burlington, Massachusetts, USA), rehydrated in a series of 100%, 90% and 70% ethanol and distilled water 5 min each and subjected to proteolytic treatment with proteinase K (Roche, Basel, Switzerland) 3 µg/ml in 0.5 M Tris-HCl buffered saline at 37 °C for 40 min. After proteolysis, sections were rinsed in distilled water, dehydrated in 90% and 100% ethanol for 5 min and air-dried. Tissue sections were covered with a hybridization mixture containing 11 µl distilled water, 20 µl 20× saline-sodium citrate (SSC) buffer, 50 µl formamide, 5 µl herring sperm, 2 µl 50× Denhardt’s solution, 10 µl dextran sulphate (50%, w/v) and 1 ng probe per 100 µl. Slides were incubated at 95 °C for 6 min and shock-frozen on crushed ice, followed by incubation overnight in a humid chamber at 40 °C. All following steps were performed in a humid chamber at room temperature. First, sections were washed 3 × 10 min in 2×, 1× and 0.1× SSC buffer and pre-incubated with Tris-HCL buffered saline, normal goat serum and 10% Triton X-100 for 30 min before incubation with anti-digoxigenin-AP Fab-fragments (Roche) at a concentration of 1:200 for 1 h. After washing 2 × 15 min in distilled water, the chromogenic substrates NBT (nitro-blue tetrazolium chloride, Roche) and BCIP (5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt, Roche) were mixed with levamisole in 0.1 M Tris-buffered saline (pH 9.5) and with applied to sections for a minimum of 40 min. Development of signal was examined by visual control and colour reaction was terminated by placing the sections in Tris-EDTA buffer (pH 8.0) for 10 min. Finally, sections were counterstained with haematoxylin and mounted using Aquatex (Merck Millipore) and coverslips.
In a first test, different concentrations of probes (0.25–1 ng/100 µl hybridization mixture) and colour reaction times (40–120 min) were applied to determine optimal working concentrations for each probe. After testing the two probes for Leucocytozoon species individually, they were combined in a hybridization-cocktail at optimal working concentration for each probe.
Subgenus-specificity of probes was tested by applying them on samples positive for parasites belonging to subgenera different from the probes’ targets, including a Plasmodium sp. positive sample. Conversely, a Plasmodium-specific probe [7] was applied to rule out co-infections which might have been missed by PCR screening. For negative controls, probes were omitted in the hybridization mixture. Furthermore, probes were tested for cross-hybridization with a range of other organisms including viral (West Nile virus, Usutu virus), fungal (Aspergillus sp., Encephalitozoon cuniculi) and protozoal (Eimeria sp., Cryptosporidium sp., C. tortoise, Toxoplasma sp., Babesia sp., Tetratrichomonas gallinae, Histomonas meleagridis, Entamoeba sp., Blastocystis sp.) pathogens.
Microscopy
Haematoxylin–eosin stained sections and CISH results were evaluated by bright field microscopy using 200×, 400× and 1000× magnifications. Images were acquired using an Olympus BX51 microscope (Olympus Europa, Hamburg, Germany) equipped with an Olympus DP71 camera. Images were adjusted for brightness and contrast and assembled in Adobe Photoshop CC 2015 (Adobe, San José, California, USA).
Results
Molecular analysis
Samples used in this study were determined as single infections by nested PCR. Sequence electropherograms did not show any double peaks indicating absence of co-infections in the samples. Detected cytb sequences were submitted to BLAST search in NCBI GenBank and MalAvi [37]. Most sequences were 100% identical with already published lineages (Table 1). Five new haplotypes were identified in samples that were used for testing the probes (STRURA03, TETURO01, TETURO02, TUMER10, BUTBUT03), and were consequently submitted to MalAvi [37]. Nucleotide sequences were deposited in NCBI GenBank under the accession numbers MK330138–MK330165 (Table 1).
Detection of erythrocytic and exo-erythrocytic parasite stages by CISH
Haemoproteus and Leucocytozoon parasites were successfully visualized by CISH using parasite subgenus-specific probes in positive tissue samples. Parasite stages were labelled by distinct dark purple signals of the chromogenic reaction products when subgenus-specific probes matching the parasites’ rRNA in the sample were applied (Fig. 1b, d). Signals were observed in different organs, including lungs, liver, spleen, brain, intestine, heart and skeletal muscle. The abundance and intensity of signals varied between samples and organs depending on the distribution of parasite stages. Purple signals were discernible in blood cells of capillaries and larger vessels indicating labelling of erythrocytic and leucocytic parasite stages of Haemoproteus spp. and Leucocytozoon spp. Signals attributed to exo-erythrocytic meronts perfectly matched the parasites’ shapes and locations as observed in corresponding HE-stained sections. Application of probes not matching the parasites’ subgenera remained negative demonstrating high subgenus-specificity of probes (Additional file 1: Figure S1c, d, f, h, j, k).
Fig. 1.
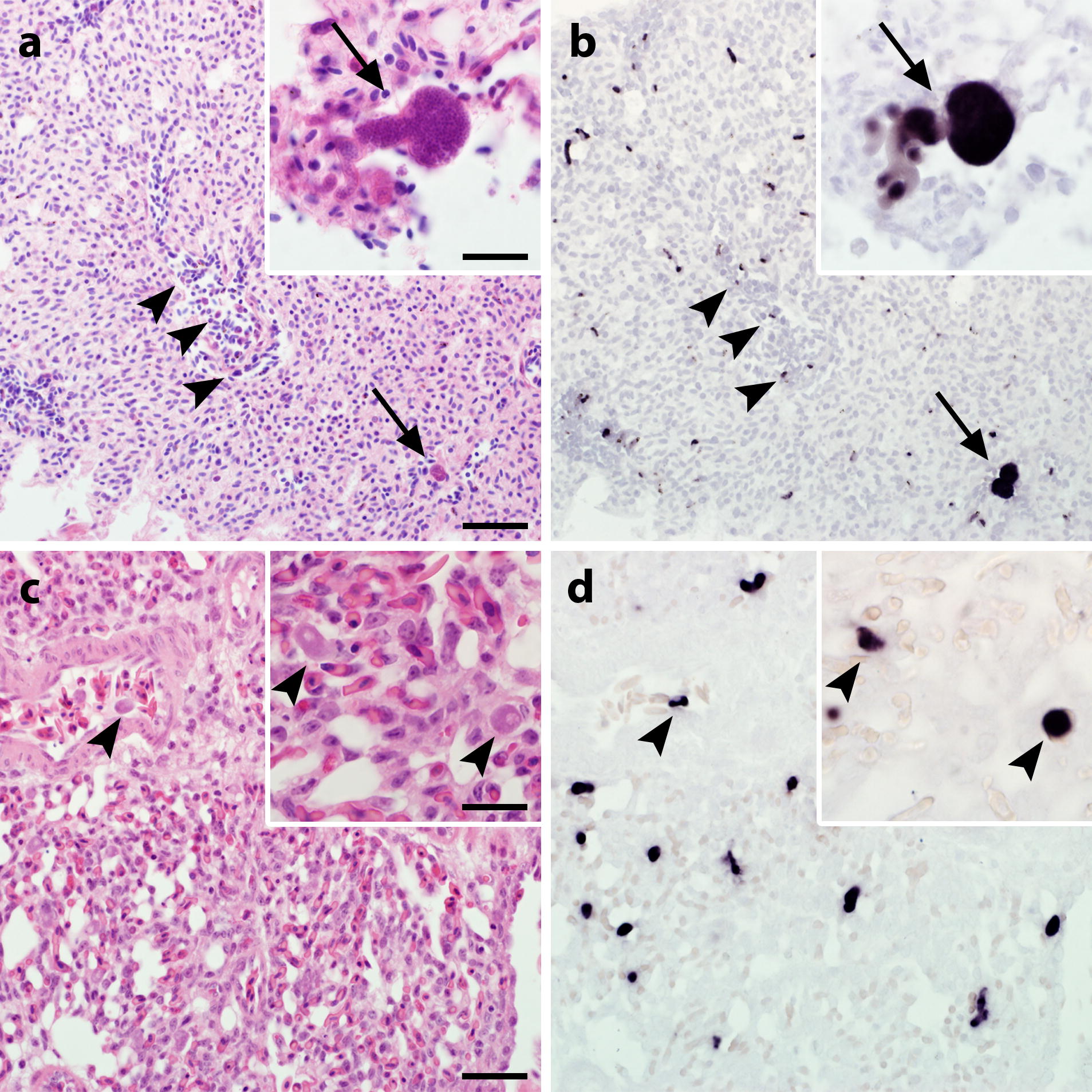
Erythrocytic and exo-erythrocytic parasite stages in lung sections from infected birds visualized by chromogenic in situ hybridization (CISH). Serial lung sections from a yellowhammer (Emberiza citrinella) infected with Haemoproteus sp. lineage EMCIR01 (a, b) and a crow (Corvus cornix) infected with Leucocytozoon sp., lineage COCOR13 (c, d) were stained with haematoxylin–eosin (HE; a, c) and subjected to CISH (b, d). Gametocytes (arrowheads) and exo-erythrocytic meronts (arrows) of parasites were observed in HE-stained sections (a, c) and labelled by subgenus-specific probes for Haemoproteus (Parahaemoproteus) spp. (b) and Leucocytozoon (Leucocytozoon) spp. (d) showing distinct purple signals in corresponding in situ-hybridized sections. Note that small parasites are difficult to see in HE-stained sections, but are readily visible in sections subjected to CISH. Scale-bars: 20 µm (inserts, 10 µm)
Labelling of megalomeronts of Haemoproteus and Leucocytozoon parasites in aberrant hosts
To investigate whether the probes also reliably bind to megalomeronts of Haemoproteus spp. and Leucocytozoon spp. in non-adapted hosts, three cases of aberrant infections were examined. Two of them were red-crowned parakeets (Cyanoramphus novaezelandiae) infected with H. minutus, lineages TUPHI01 and TURDUS2, and one was a cobalt-winged parakeet (Brotogeris cyanoptera) infected with Leucocytozoon cf. californicus, lineage CIAE02. In situ hybridization with subgenus-specific probes revealed robust labelling of megalomeronts in different tissues (Fig. 2b, d). Staining intensity varied between megalomeronts from different organs of the same sample, but also across megalomeronts found within the same organ (Fig. 2b). Similarly, colour variations in regard to the basophilic and eosinophilic nature of respective structures were observed in corresponding HE-stained sections (Fig. 2a). Hybridization of probes not complementary to the parasites’ genera remained negative (Additional file 2: Figure S2c, d, f, h).
Fig. 2.
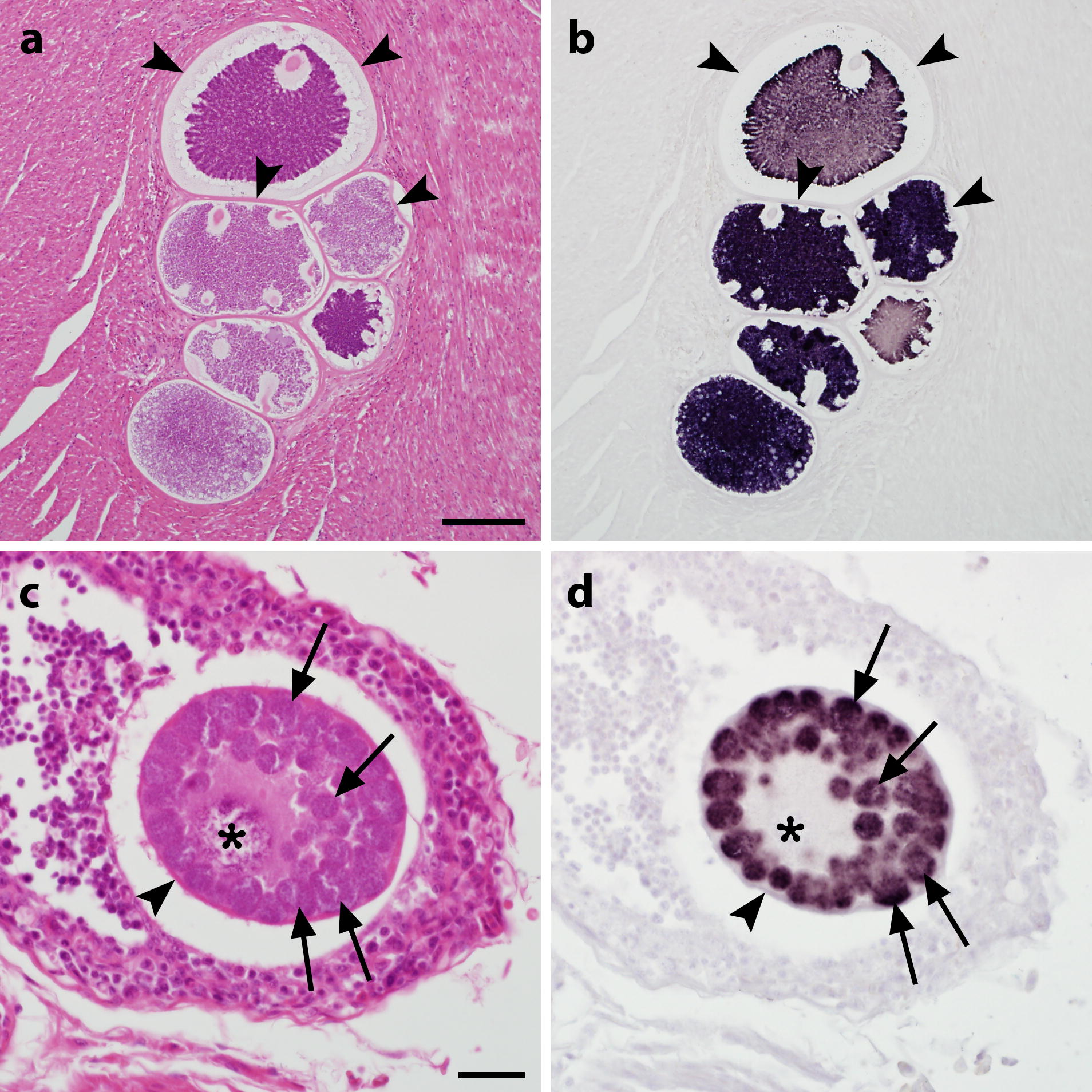
Megalomeronts of Haemoproteus minutus (a, b) and Leucocytocoon sp. (c, d) in histological sections from infected parakeets stained by haematoxylin and eosin (HE; a, c) and chromogenic in situ hybridization (CISH; b, d). Multiple megalomeronts of H. minutus, lineage TUPHI01, and Leucocytozoon cf. californicus, lineage CIAE02, were observed in HE-stained sections of the cardiac muscle in a red-crowned parakeet (Cyanoramphus novaezelandiae; a) and in the bursa of Fabricius in a blue-winged parakeet (Brotogeris cyanoptera; c), respectively. Application of subgenus-specific probes for Haemoproteus (Parahaemoproteus) spp. (b) and Leucocytozoon (Leucocytozoon) spp. (d) with CISH unequivocally indicated generic identity of parasites. Signals were confined to cytomeres (arrows) and merozoites of the parasites whereas structures not attributed to the parasite such as host cell nucleus (asterisk) and prominent capsule-like walls (arrowheads) around megalomeronts remained negative. Note numerous roundish cytomeres (arrows) and a readily visible host cell nucleus (or ‘central body’) present in the megalomeront of Leucocytozoon sp., but absent in megalomeronts of Haemoproteus parasites. Scale-bars: a, 100 µm; c, 20 µm
Cross-reactivity testing
In situ hybridization failed to produce signals in tissue samples positive for different viral, fungal and protozoal organisms ruling out undesirable binding of probes with pathogens which potentially might co-occur in infected birds.
Discussion
In the present study, oligonucleotide probes were developed for the identification of Haemoproteus (Parahaemoproteus) species and Leucocytozoon (Leucocytozoon) spp. in FFPE tissues samples from infected birds. The results demonstrate that the new probes reliably detect erythrocytic and exo-erythrocytic parasite stages and distinguish between parasites belonging to different genera using CISH, providing opportunities to gain new knowledge about exo-erythrocytic development of haemosporidians using samples collected in the wild.
A similar methodological approach has already been successfully applied for detection of Plasmodium parasites in histological sections of infected birds [4, 7, 8]. A different CISH protocol using a cytb gene probe was used for the detection of Haemoproteus parasites [23]. However, in the latter study, the probe was designed to target the so-called barcode region of the mitochondrial cytb of Haemoproteus species and Plasmodium spp. and does not allow for discrimination between parasites of these genera. In the present study, probes for Haemoproteus species and Leucocytozoon spp. were designed to target the 18S rRNA of parasites due to two major reasons. First, rRNA is highly abundant in the cell cytoplasm, improving detectability of parasites as compared to other target molecules. Secondly, the patchy conservation of the 18S rRNA gene allowed for the design of subgenus-specific probes, which are necessary for the discrimination of parasites belonging to the most important avian haemosporidian genera.
Because 18S rDNA sequences of Haemoproteus and Leucocytozoon parasites were not available in NCBI GenBank except for H. tartakovskyi [38], probe design was based on newly generated sequence data of Haemoproteus (Parahaemoproteus) spp. and Leucocytozoon (Leucocytozoon) spp. (JH, unpublished data). Acquired 18S rDNA sequences and hence probe design included a relatively small selection of Haemoproteus and Leucocytozoon lineages (8 and 16, respectively), taking into account the huge number of reported parasite lineages from these subgenera. However, selected lineages represented diverse clades within both subgenera and the results demonstrated successful hybridization of the probes to lineages not included for probe design. Therefore, it can be presumed that the specified probes also detect other Haemoproteus and Leucocytozoon lineages, as selected target regions of the 18S rRNA gene seem to be homologous across the majority of clades within the subgenus Parahaemoproteus and the two clades of the subgenus Leucocytozoon.
Despite evident advantages of targeting rRNA, some limitations may arise with respect to parasite detection by means of CISH when working with tissue samples that are not adequately fixed before being subjected to molecular analysis. Weak or false negative results may be obtained if tissues are poorly fixed, as this will result in RNA degradation. Conversely, extensive fixation over longer time periods (a month to years) leads to fragmentation or masking of nucleic acids and prevents hybridization of probes [39]. Ideally, tissue samples should be fresh and fixed immediately after necropsy to minimize autolytic degradation. Conventional fixation in 10% neutral buffered formalin for 12–72 hours should provide good morphology and detectability of rRNA by CISH.
Failure to detect parasites may also be the result of low cellular ribosome content, which could be the case in stages with reduced cellular activity, e.g. potential quiescent or dormant stages, similar to human malaria parasites [40]. Therefore, CISH results should always be carefully evaluated and compared to HE-stained sections to confirm absence of parasites.
The present study showed varying abundance and intensity of CISH signals between samples and different organs of individual samples, depending on parasite load and stage of infection. While some cases exhibited signals exclusively in blood cells, others presented both labelled erythrocytic and exo-erythrocytic stages. It might be the case that sparsely distributed meronts in the test samples were missed by investigation of only few sections during probe testing. The scope of the present study was to establish molecular probes rather than detailed investigation of the test samples; however, it should be stressed, that multiple sections must be examined before drawing conclusions on parasite load and presence of tissue meronts in general.
Megalomeronts detected in Haemoproteus- and Leucocytozoon-infected tissue samples showed different staining intensities in both in situ-hybridized and corresponding HE-stained sections. These variations possibly reflect different developmental stages of megalomeronts exhibiting variable levels of rRNA content; however, this assumption remains speculative.
This study proves that the clusters of megalomeronts (or so called multilocular megalomeronts) covered by thick walls (Fig. 2a, b) belong to Haemoproteus parasites, as suggested by Earlé et al. [14]. These parasites were formerly believed to be stages of development of Leucocytozoon species or even Besnoitia parasites [11]. However, it remains unclear whether multilocular megalomeronts develop only in non-adapted (aberrant) avian hosts or they are an essential stage of development of Haemoproteus species in natural hosts as well. During this study, only small meronts were reported in naturally infected yellowhammers, a natural host of Haemoproteus sp. lineage EMCIR01 (Fig. 1a, b). Application of CISH provides an opportunity to answer this question, which is important for better understanding mechanisms of virulence during haemoproteosis.
In addition to traditional histological methods for the diagnosis of haemosporidian infections, CISH can be used to localize and identify Haemoproteus spp. and Leucocytozoon spp. in tissue sections. A major benefit of CISH is that it enables specific parasite detection in morphologically preserved tissues and simultaneous evaluation of potentially associated lesions. This is particularly important considering numerous reports on the pathogenic effects that exo-erythrocytic parasite stages can have on their hosts [4, 7, 9, 10, 18, 22–26]. The correct determination of parasites associated with severe microscopic lesions is crucial for gaining a better understanding on the pathogenicity of diverse avian haemosporidian parasites. The methodology and probes presented here provide a suitable approach for the unequivocal generic assignment of Haemoproteus spp. and Leucocytozoon spp., facilitating the diagnosis of avian haemosporidioses in histological samples. Direction of future research will be the design of probes allowing identification of parasites at species level, an application which will enable new insights into tissue merogony of haemosporidians in mixed infections.
Conclusions
This study demonstrates that Haemoproteus and Leucocytozoon parasite tissue stages can be readily detected and discriminated by CISH using 18S rRNA-specific probes. In addition to the formerly established Plasmodium-specific probe, the new probes for Haemoproteus species and Leucocytozoon spp. represent powerful tools not only for diagnostic purposes but also for the study of exo-erythrocytic merogony of haemosporidian parasites in general. Further development of the in situ hybridization protocol towards the combined application of the probes will be useful to investigate co-infections, which predominate in birds, but have not been addressed from the histological point of view as far.
Additional files
Additional file 1: Figure S1. Erythrocytic and exo-erythrocytic parasite stages in lung sections from infected birds visualized by chromogenic in situ hybridization (CISH). Serial lung sections from Emberiza citrinella infected with Haemoproteus sp. (EMCIR01) (a–d), Corvus cornix infected with Leucocytozoon sp. (COCOR13) (e–h) and Turdus merula infected with Plasmodium matutinum (LINN1) (i–l) were stained with haematoxylin–eosin (HE; a, e, i) and subjected to CISH with probes for Haemoproteus (Parahaemoproteus) spp. (b, f, j), Leucocytozoon (Leucocytozoon) spp. (c, g, k) and Plasmodium spp. (d, h, l). Blood stages (arrowheads) and exo-erythrocytic meronts (arrows) of parasites were observed in HE-stained sections (a, e, i) and labelled in corresponding in situ-hybridized sections (b, g, l). Sections treated with probes not matching the parasites’ genera, remained negative (c, d, f, h, j, k). Scale-bars: 20 µm (inserts: 10 µm).
Additional file 2: Figure S2. Megalomeronts of Haemoproteus minutus (a–d) and Leucocytocoon sp. (e–h) in histological sections from infected parakeets stained by haematoxylin-eosin (HE; a, e) and chromogenic in situ hybridization (CISH; b–d, f–h). Multiple megalomeronts of Haemoproteus minutus (TUPHI01), and Leucocytozoon cf. californicus (CIAE02), were observed in HE-stained sections of the cardiac muscle of Cyanoramphus novaezelandiae (a) and in the bursa of Fabricius of Brotogeris cyanoptera (c). CISH with probes for Haemoproteus (Parahaemoproteus) spp. (b, f), Leucocytozoon (Leucocytozoon) spp. (c, g) and Plasmodium spp. (d, h) indicated generic identity of parasites. Signals were confined to cytomeres (arrows) and merozoites of the parasites whereas host tissue structures like nucleus (asterisk) and capsule-like walls (arrowheads) around megalomeronts remained negative. Note numerous cytomeres (arrows) and a host cell nucleus present in the megalomeront of Leucocytozoon sp., but absent in megalomeronts of Haemoproteus parasites. Sections treated with probes not matching the parasites’ genera were negative (c, d, f, h). Scale-bars: a, 100 µm; e, 20 µm.
Acknowledgements
We would like to thank Nora Nedorost and Karin Fragner from the Molecular Pathology Laboratory, Institute of Pathology, University of Veterinary Medicine, Vienna, Austria, for their great assistance and expertise with CISH and the staff from the Histology Laboratory, Institute of Pathology, University of Veterinary Medicine, Vienna, Austria, for their technical support. The present study was funded by the FWF Austrian Science Fund (grant P 29628).
Authors’ contributions
TH, JH and HW: study conception and design. TH, JH: molecular analysis. JH: probe design. TH: procedure, analysis of CISH experiments and drafting of manuscript. TH, HW: microscopy. CJS, AKH, CK, NF, MI, GV: sample collection and processing. All authors critically revised the manuscript. All authors read and approved the final manuscript.
Funding
The study was funded by the FWF Austrian Science Fund (grant P 29628).
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article and its additional files.
Ethics approval and consent to participate
The study was part of the FWF-project P 29628 and approved by the institutional ethics and animal welfare committee and the national authority according to §§ 26ff. of Animal Experiments Acts, Tierversuchsgesetz 2012–TVG 2012, Austria (BMWFW-68.205/0036-WF/V/3b/2017). Material collection was approved by the Environmental Protection Agency, Lithuania (permit 2018-04-13, no. 24).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Valkiūnas G. Avian malaria parasites and other haemosporidia. 1. Boca Raton: CRC Press; 2005. [Google Scholar]
- 2.Valkiūnas G, Iezhova TA. Exo-erythrocytic development of avian malaria and related haemosporidian parasites. Malar J. 2017;16:101. doi: 10.1186/s12936-017-1746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson CT, Thomas NJ, Hunter DB, editors. Parasitic diseases of wild birds. 1. Oxford: Wiley-Blackwell; 2008. [Google Scholar]
- 4.Ilgūnas M, Bukauskaitė D, Palinauskas V, Iezhova TA, Dinhopl N, Nedorost N, et al. Mortality and pathology in birds due to Plasmodium (Giovannolaia) homocircumflexum infection, with emphasis on the exoerythrocytic development of avian malaria parasites. Malar J. 2016;15:256. doi: 10.1186/s12936-016-1310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanstreels RET, Da Silva-Filho RP, Kolesnikovas CKM, Bhering RCC, Ruoppolo V, Epiphanio S, et al. Epidemiology and pathology of avian malaria in penguins undergoing rehabilitation in Brazil. Vet Res. 2015;46:30. doi: 10.1186/s13567-015-0160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taunde PA, Bianchi MV, Perles L, da Silva FS, Guim TN, Stadler RA, et al. Pathological and molecular characterization of avian malaria in captive Magellanic penguins (Spheniscus magellanicus) in South America. Parasitol Res. 2018;118:599–606. doi: 10.1007/s00436-018-6155-5. [DOI] [PubMed] [Google Scholar]
- 7.Dinhopl N, Mostegl MM, Richter B, Nedorost N, Maderner A, Fragner K, et al. Application of in-situ hybridization for the detection and identification of avian malaria parasites in paraffin wax-embedded tissues from captive penguins. Avian Pathol. 2011;40:315–320. doi: 10.1080/03079457.2011.569533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinhopl N, Nedorost N, Mostegl MM, Weissenbacher-Lang C, Weissenböck H. In situ hybridization and sequence analysis reveal an association of Plasmodium spp. with mortalities in wild passerine birds in Austria. Parasitol Res. 2015;114:1455–1462. doi: 10.1007/s00436-015-4328-z. [DOI] [PubMed] [Google Scholar]
- 9.Niedringhaus KD, Fenton HMA, Cleveland CA, Anderson AN, Schwartz D, Alex CE, et al. Case Series: virulent hemosporidiosis infections in juvenile Great horned owls (Bubo virginianus) from Louisiana and California, USA. Vet Parasitol Reg Stud Reports. 2018;12:49–54. doi: 10.1016/j.vprsr.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Lederer R, Adlard RD, O’Donoghue PJ. Severe pathology associated with protozoal schizonts in two pied currawongs (Strepera graculina) from Queensland. Vet Rec. 2002;150:520–522. doi: 10.1136/vr.150.16.520. [DOI] [PubMed] [Google Scholar]
- 11.Bennett GF, Peirce MA, Ashford RW. Avian Haematozoa: mortality and pathogenicity. J Nat Hist. 1993;27:993–1001. doi: 10.1080/00222939300770621. [DOI] [Google Scholar]
- 12.Borst GHA, Zwart P. An aberrant form of Leucocytozoon infection in two quaker parakeets (Myiopsitta monachus Boddaert, 1783) Zeitsch Parasitenkd. 1972;40:131–138. doi: 10.1007/BF00329148. [DOI] [PubMed] [Google Scholar]
- 13.Gardiner CH, Jenkins HJ, Mahoney KS. Myositis and death in bobwhites, Colinus virginianus (L.), due to hemorrhagic cysts of a haemosporozoan of undetermined taxonomic status. J Wildl Dis. 1984;20:308–318. doi: 10.7589/0090-3558-20.4.308. [DOI] [PubMed] [Google Scholar]
- 14.Earle RA, Bastianello SS, Bennett GF, Krecek RC. Histopathology and morphology of the tissue stages of Haemoproteus columbae causing mortality in Columbiformes. Avian Pathol. 1993;22:67–80. doi: 10.1080/03079459308418901. [DOI] [PubMed] [Google Scholar]
- 15.Commichau C, Jonas D. Eine durch Leukozytozoon simondi verursachte Erkrankung bei Entenküken unter besonderer Berücksichtigung des histologischen Nachweises. Zbl Vet Med B. 2010;24:662–667. doi: 10.1111/j.1439-0450.1977.tb01037.x. [DOI] [PubMed] [Google Scholar]
- 16.Farmer JN. Gizzard lesions associated with Haemoproteus sacharovi infections of pigeons. Proc Iowa Acad Sci. 1964;71:537–542. [Google Scholar]
- 17.Kelly EJ, Baldwin TJ, Frame DD, Childress AL, Wellehan JFX. Haemoproteus (Parahaemoproteus) spp. in captive-bred bobwhite quail (Colinus virginianus) in southern Utah, USA. J Wildl Dis. 2018;54:726–733. doi: 10.7589/2017-01-014. [DOI] [PubMed] [Google Scholar]
- 18.Baker KC, Rettenmund CL, Sander SJ, Rivas AE, Green KC, Mangus L, et al. Clinical effect of hemoparasite infections in snowy owls (Bubo scandicus) J Zoo Wildl Med. 2018;49:143–152. doi: 10.1638/2017-0042R.1. [DOI] [PubMed] [Google Scholar]
- 19.Ortiz-Catedral L, Brunton D, Stidworthy MF, Elsheikha HM, Pennycott T, Schulze C, et al. Haemoproteus minutus is highly virulent for Australasian and South American parrots. Parasit Vectors. 2019;12:40. doi: 10.1186/s13071-018-3255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardona CJ, Ihejirika A, McClellan L. Case report—Haemoproteus lophortyx infection in bobwhite quail. Avian Dis. 2002;46:249–255. doi: 10.1637/0005-2086(2002)046[0249:HLIIBQ]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.Tarello W. Fatal Haemoproteus psittaci infection in an African grey parrot. Vet Rec. 2005;157:32. doi: 10.1136/vr.157.1.32-b. [DOI] [PubMed] [Google Scholar]
- 22.Ferrell ST, Snowden K, Marlar AB, Garner M, Lung NP. Fatal hemoprotozoal infections in multiple avian species in a zoological park. J Zoo Wildl Med. 2007;38:309–316. doi: 10.1638/1042-7260(2007)038[0309:FHIIMA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Donovan TA, Schrenzel M, Tucker TA, Pessier AP, Stalis IH. Hepatic hemorrhage, hemocoelom, and sudden death due to Haemoproteus infection in passerine birds: eleven cases. J Vet Diagn Invest. 2008;20:304–313. doi: 10.1177/104063870802000307. [DOI] [PubMed] [Google Scholar]
- 24.Olias P, Wegelin M, Zenker W, Freter S, Gruber AD, Klopfleisch R. Avian malaria deaths in parrots. Europe. Emerg Infect Dis. 2011;17:950–952. doi: 10.3201/eid1705.101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pacheco MA, Escalante AA, Garner MM, Bradley GA, Aguilar RF. Haemosporidian infection in captive Masked bobwhite quail (Colinus virginianus ridgwayi), an endangered subspecies of the Northern bobwhite quail. Vet Parasitol. 2011;182:113–120. doi: 10.1016/j.vetpar.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peirce MA, Lederer R, Adlard RD, O’Donoghue PJ. Pathology associated with endogenous development of haematozoa in birds from southeast Queensland. Avian Pathol. 2004;33:445–450. doi: 10.1080/03079450410001724076. [DOI] [PubMed] [Google Scholar]
- 27.Stidworthy MF, Greenwood AG. Deaths in aviary birds associated with protozoal megaloschizonts. Vet Rec. 2006;159:606. doi: 10.1136/vr.159.18.606. [DOI] [PubMed] [Google Scholar]
- 28.Pennycott T, Wood A, MacIntyre C, MacIntyre D, Patterson T. Deaths in aviary birds associated with protozoal megaloschizonts. Vet Rec. 2006;159:499–500. doi: 10.1136/vr.159.15.499. [DOI] [PubMed] [Google Scholar]
- 29.Valkiūnas G, Bensch S, Iezhova TA, Krizanauskiene A, Hellgren O, Bolshakov CV. Nested cytochrome b polymerase chain reaction diagnostics underestimate mixed infections of avian blood haemosporidian parasites: microscopy is still essential. J Parasitol. 2006;92:418–422. doi: 10.1645/GE-3547RN.1. [DOI] [PubMed] [Google Scholar]
- 30.Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Res. 2016;44:D67–D72. doi: 10.1093/nar/gkv1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall TA. BioEdit: a user-friendly biological sequences alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 33.Jullien N. AmplifX v. 1.7. https://inp.univ-amu.fr/en/amplifx-manage-test-and-design-your-primers-for-pcr.
- 34.Hellgren O, Waldenström, Bensch S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol. 2004;90:797–802. doi: 10.1645/GE-184R1. [DOI] [PubMed] [Google Scholar]
- 35.Sehgal RNM, Hull AC, Anderson NL, Valkiūnas G, Markovets MJ, Kawamura S, et al. Evidence for cryptic speciation of Leucocytozoon spp. (Haemosporida, Leucocytozoidae) in diurnal raptors. J Parasitol. 2006;92:375–379. doi: 10.1645/GE-656R.1. [DOI] [PubMed] [Google Scholar]
- 36.Jasper MA, Hull JM, Hull AC, Sehgal RNM. Widespread lineage diversity of Leucocytozoon blood parasites in distinct populations of western red-tailed hawks. J Ornithol. 2014;155:767–775. doi: 10.1007/s10336-014-1064-6. [DOI] [Google Scholar]
- 37.Bensch S, Hellgren O, Pérez-Tris J. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour. 2009;9:1353–1358. doi: 10.1111/j.1755-0998.2009.02692.x. [DOI] [PubMed] [Google Scholar]
- 38.Bensch S, Canbäck B, DeBarry JD, Johansson T, Hellgren O, Kissinger JC, et al. The genome of Haemoproteus tartakovskyi and its relationship to human malaria parasites. Genome Biol Evol. 2016;8:1361–1373. doi: 10.1093/gbe/evw081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mostegl MM, Richter B, Dinhopl N, Weissenböck H. Influence of prolonged formalin fixation of tissue samples on the sensitivity of chromogenic in situ hybridization. J Vet Diagn Invest. 2011;23:1212–1216. doi: 10.1177/1040638711425584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voorberg-van der W, Annemarie Roma G, Bouwmeester T, Lee BH, Carbone W, Kinzel B, Pasini E, et al. A comparative transcriptomic analysis of replicating and dormant liver stages of the relapsing malaria parasite Plasmodium cynomolgi. Elife. 2017;6:1–22. doi: 10.7554/eLife.29605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Erythrocytic and exo-erythrocytic parasite stages in lung sections from infected birds visualized by chromogenic in situ hybridization (CISH). Serial lung sections from Emberiza citrinella infected with Haemoproteus sp. (EMCIR01) (a–d), Corvus cornix infected with Leucocytozoon sp. (COCOR13) (e–h) and Turdus merula infected with Plasmodium matutinum (LINN1) (i–l) were stained with haematoxylin–eosin (HE; a, e, i) and subjected to CISH with probes for Haemoproteus (Parahaemoproteus) spp. (b, f, j), Leucocytozoon (Leucocytozoon) spp. (c, g, k) and Plasmodium spp. (d, h, l). Blood stages (arrowheads) and exo-erythrocytic meronts (arrows) of parasites were observed in HE-stained sections (a, e, i) and labelled in corresponding in situ-hybridized sections (b, g, l). Sections treated with probes not matching the parasites’ genera, remained negative (c, d, f, h, j, k). Scale-bars: 20 µm (inserts: 10 µm).
Additional file 2: Figure S2. Megalomeronts of Haemoproteus minutus (a–d) and Leucocytocoon sp. (e–h) in histological sections from infected parakeets stained by haematoxylin-eosin (HE; a, e) and chromogenic in situ hybridization (CISH; b–d, f–h). Multiple megalomeronts of Haemoproteus minutus (TUPHI01), and Leucocytozoon cf. californicus (CIAE02), were observed in HE-stained sections of the cardiac muscle of Cyanoramphus novaezelandiae (a) and in the bursa of Fabricius of Brotogeris cyanoptera (c). CISH with probes for Haemoproteus (Parahaemoproteus) spp. (b, f), Leucocytozoon (Leucocytozoon) spp. (c, g) and Plasmodium spp. (d, h) indicated generic identity of parasites. Signals were confined to cytomeres (arrows) and merozoites of the parasites whereas host tissue structures like nucleus (asterisk) and capsule-like walls (arrowheads) around megalomeronts remained negative. Note numerous cytomeres (arrows) and a host cell nucleus present in the megalomeront of Leucocytozoon sp., but absent in megalomeronts of Haemoproteus parasites. Sections treated with probes not matching the parasites’ genera were negative (c, d, f, h). Scale-bars: a, 100 µm; e, 20 µm.
Data Availability Statement
The dataset supporting the conclusions of this article is included within the article and its additional files.


