Abstract
Background
Amyloid β (Aβ) and tau proteins are considered as critical factors that affect Alzheimer’s disease (AD) and mild cognitive impairment (MCI). Although many studies have conducted on these two proteins, little study has investigated the relationship between their spatial distributions. This study aims to explore the associations of spatial patterns between Aβ deposition and tau deposition in patients with MCI and normal control (NC).
Methods
We used multimodality positron emission tomography (PET) data from a clinically heterogeneous population of patients with MCI and NC. All data were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database containing information of 65 patients with MCI and 75 NC who both had undergone AV45 (Aβ) and AV1451 (tau) PET. To assess the spatial distribution of Aβ and tau deposition, we employed parallel independent component analysis (pICA), which enabled the joint analysis of multimodal imaging data. pICA was conducted to identify the significant difference and correlation relationship of brain networks between Aβ PET and tau PET in MCI and NC groups.
Results
Our results revealed the strongly correlated network between Aβ PET and tau PET were colocalized with the default-mode network (DMN). Simultaneously, in comparison of the spatial distribution between Aβ PET and tau PET, it was found that the significant differences between MCI and NC were mainly distributed in DMN, cognitive control network and visual networks. The altered brain networks obtained from pICA analysis are consistent with the abnormalities of brain network in MCI patients.
Conclusions
Findings suggested the abnormal spatial distribution regions of tau PET were correlated with the abnormal spatial distribution regions of Aβ PET, and both of which were located in DMN network. This study revealed that combining pICA with multimodal imaging data is an effective approach for distinguishing MCI patients from NC group.
Electronic supplementary material
The online version of this article (10.1186/s12888-019-2149-9) contains supplementary material, which is available to authorized users.
Keywords: Parallel independent component analysis, multivariate data analysis; Amyloid imaging; Tau imaging; Networks
Background
Amyloid β (Aβ) and tau proteins have been recognized as two important factors that cause Alzheimer’s disease (AD) and Mild cognitive impairment (MCI) [1, 2]. Many studies have focused on these two proteins separately [3, 4], and Brier MR et al. [5] have calculated the correlation between the deposition of the two proteins on the voxel-wise based on neuroimages. However, little imaging study has investigated the correlated brain networks of these two proteins thus far.
Several brain image studies have been carried out with different technologies [6, 7]. Multivariate statistical paradigms (such as principal component analysis (PCA) or independent component analysis (ICA)) assess distributed alterations and their interrelationships in multiple neuroimaging data. ICA is a data-driven analysis method to study brain networks conducted by neuroimaging. It was widely used in functional magnetic resonance imaging (fMRI) [8, 9], magnetoencephalography [10], electroencephalography [11], structural MRI [12], and PET imaging [13]. As a variation of ICA, parallel ICA (pICA) could estimate independent components in multimodal data [14]. A prior study reported that the multivariate techniques could be sensitive for early diagnosis of AD [15]. pICA was used to identify the mechanism of Aβ deposition that leads to neurodegeneration and cognitive decline in MCI and AD patients [16, 17]. Moreover, Fu L et al. [18] conducted on the spatial correlation network of Aβ protein and fluorodeoxyglucose (FDG).
Study has found the regions of interest (ROI) were correlated with the scale scores in MCI patients [19], while other paper examined whether ROI regions contribute to distinguish patients from normal people [20]. Tapan Gandhi et al. [21] utilized K-fold cross-validation method to validated the wavelet coefficients of EEG data and pointed out that K-fold cross-validation was a rigorous method.
The goal of our research includes two aspects. Firstly, we use pICA method to explore the significant difference and correlation of spatial distribution between AV1451 (tau PET) and AV45 (Aβ PET). Secondly, we combine pICA with multimodal imaging data to distinguish MCI patients from NC group.
Materials and methods
Subjects
Tau PET and Aβ PET images were downloaded from the Alzheimer’s Disease Neuroimaging Initiative(ADNI) website (http://adni.loni.usc.edu/), belonging to ADNI-3 phase [22]. The unified preprocessing description of the collected PET data in ADNI database was added in Additional file 1. A total of 140 individuals (65 patients with MCI and 75 NCs) who both had tau and Aβ PET images were included for pICA analysis.
We also recorded scores for the Mini–Mental State Examination (MMSE) [23], and Clinical Dementia Rating (CDR) [24] from the ADNI database as well as the CSF-Aβ value and CSF-Tau value.
Data acquisition and preprocessing
The acquisition parameters for all scanners have been described in the Additional file 1. PET images were coregistered, averaged, normalized (standardized image and voxel size), and smoothed to produce a uniform resolution (8 mm full-width at half-maximum). PET scans require dynamic 30-min six-frame (5-min each) acquisition beginning 30 min after the injection of 18F-labeled AV1451 and 18F-labeled AV45. We normalized all images spatially according to the PET Montreal Neurological Institute (MNI) brain space template; subsequently, we scaled and averaged the same images using Statistical Parametric Mapping 12 (SPM12: https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) by MATLAB 2014a on the Centos 6.5 operating system. The images adopted were acquired using Siemens, GE, and Philips PET scanners in a resting state. The spatial normalization included a 12-parameter affine transformation; this process was followed by a nonlinear iterative spatial transformation using SPM12.
pICA
More details about pICA were introduced in [25]. Utilizing multimodal imaging data, pICA identifies the independent components of each image modality. It also estimates the correlation between these components, as well as different image modality. Using Akaike Information Criterion (AIC) and Minimum Description Length Criterion, the number of independent components of each mode were identified [20]. In order to balance the fitting accuracy and complexity of the independent component model, we chose the lowest independent component set of AIC values. In each modality, the contribution of each independent component to the variance across all subjects is expressed by the loading parameters performed in pICA analysis. Making all the components more intuitive, we set the z-score to be |z| > 2.5. The number of independent estimated components is eight [18]. In this study, independent components of tau PET and Aβ PET were identified by pICA method, furthermore the most significantly different regions in tau PET and Aβ PET among patients with MCI were found. For tau PET spatial distribution, the voxel-wise two-sample t-test was used to found the significantly different components between MCI patients and NC. The ROI features for later analysis were identified by components with significant differences. Aβ PET carried on the same process.
Moreover, the Pearson’s correlation coefficients for all pairs of tau PET and Aβ PET independent components were calculated and the variations of age, sex, and all the statistical values were assessed. Significant relationships between Aβ and tau accumulation were measured by Pearson’s correlation coefficients, and these coefficients should be corrected for multiple comparisons. The false discovery rate (FDR) was performed (p < 0.05) on the results. Classification analysis separately assessed the contribution of each component to the classification of MCI and NC.
K-fold cross-validation
The process of cross-validation is to divide the data into subsets, and then select one subset for calculation, while other remaining subsets are used to verify the accuracy of the previous analysis. It considers the initial subset as the training set and the other subsets as the test set [26].
K-fold cross-validation is a common data analysis method. The advantage of K-fold cross-validation is that all samples are used for training and testing, and each sub-sample is treated as a test data only once. Based on our sample size, we set the K value as 5. Therefore, all data were randomly divided into five groups. Four groups were combined in the pICA, and the last group was used to detect the validity of ROI features extracted from the pICA results. The above process was repeated for five times. A single estimation was obtained from the averaged 5 times calculation values [27].
Statistical analysis
A two-sample t test was conducted to identify any significant differences in age or MMSE, CSF-tau and CSF-Aβ. The Mann-Whitney test was conducted to identify any significant difference in CDR score. A chi-square test was performed to identify significant differences with respect to sex or between patients who were APOE4 carriers and noncarriers.
Results
Patient characteristics
The characteristics of all 140 subjects are listed in Table 1. No significant difference was observed in sex, age, or APOE4 between the MCI and NC groups. Cognitive performance, estimated from CDR and MMSE results, was significantly worse in MCI group than in NC group.
Table 1.
Demographic data of all subjects
| MCI | NC | p | |
|---|---|---|---|
| N (total N = 80) | 65 | 75 | – |
| Age | 73.27 ± 5.75 | 76.27 ± 6.22 | 0.3721b |
| Genger (male:female) | 71:69 | 65:75 | 0.423a |
| APOE4 (carriers:noncarriers) | 32:33 | 33:42 | 0.348b |
| MMSE | 25.7 ± 2.3 | 27.9 ± 1.7 | < 0.001a |
| CDR | 0.5 | – | < 0.001c |
| CSF-Tau | 251.3 ± 104.9 | 254.6 ± 128.2 | 0.89a |
| CSF-Aβ | 1036 ± 358 | 875 ± 321 | 0.30a |
Data are presented as a mean ± standard deviations. p was obtained using athe two-sample t test, bthe chi-square test and c the Mann-Whitney test
Individual tau PET and Aβ PET components
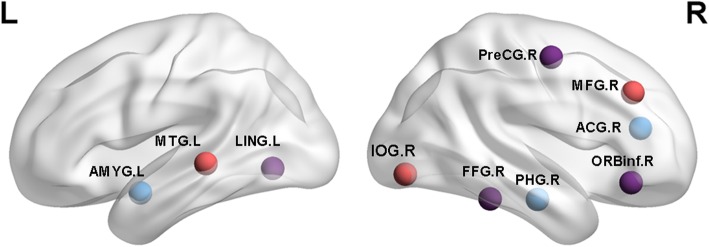
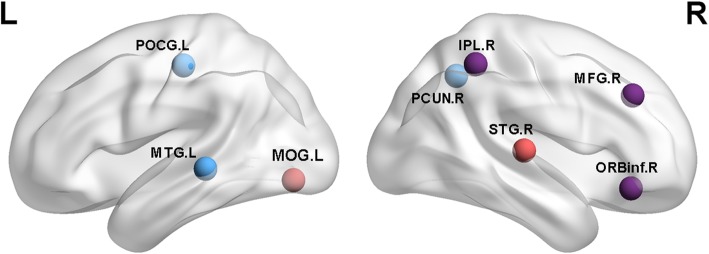
Each kind of data was found three components with significant differences between MCI and NC group. They were discovered to frequently occur (see Table 2 (tau), Table 3 (Aβ), Fig. 1 (tau), and Fig. 2 (Aβ)). We recorded the maximum |z| and P values in each respective region. We detected the networks with significant differences in tau PET group as follows: visual network (VN) including right fusiform gyrus; left lingual gyrus; left middle temporal gyrus; right inferior occipital gyrus. The cognitive control network (CCN) including right inferior frontal gyrus (opercular part); right precentral gyrus; right middle frontal gyrus; right parahippocampal gyrus. The default-mode network (DMN) including left amygdala; right anterior cingulate and paracingulate gyri. We detected significant differences in the following networks in Aβ PET group: The VN including right middle occipital gyrus. The CCN including the right middle frontal gyrus; right inferior frontal gyrus (opercular part); right inferior parietal but supramarginal and angular gyri; left postcentral gyrus; right superior temporal gyrus. The DMN including left middle temporal gyrus and right precuneus gyrus.
Table 2.
Components with significant differences in tau
| Brain regions | |z| | Networks | P value (components) | X | Y | Z |
|---|---|---|---|---|---|---|
| Fusiform_R | 3.523 | visual | 0.0362 | 38 | −11 | −30 |
| Lingual_L | 3.176 | visual | 0.0362 | −12 | −93 | −14 |
| Frontal_Inf_Orb_R | 3.298 | cognitive | 0.0362 | 48 | 33 | −11 |
| Precentral_R | 3.517 | cognitive | 0.0362 | 58 | 12 | 43 |
| Temporal_Mid_L | 3.81 | visual | 0.0210 | −43 | −57 | −7 |
| Occipital_Inf_R | 3.047 | visual | 0.0210 | 33 | −57 | −9 |
| Frontal_Mid_R | 3.425 | cognitive | 0.0210 | 30 | 40 | 28 |
| ParaHippocampal_R | 3.628 | cognitive | 0.0013 | 24 | −5 | 22 |
| Amygdala_L | 3.624 | subcortical | 0.0013 | −23 | −6 | −21 |
| Cingulum_Ant_R | 4.117 | DMN | 0.0013 | −2 | −18 | 22 |
Abbreviation: Frontal_Mid_R Right middle frontal gyrus, Frontal_Inf_Orb_R Right Inferior frontal gyrus(orbital part), Parietal_Inf_R Right Inferior parietal, but supramarginal and angular gyri, Occipital_Mid_R Right Middle occipital gyrus, Temporal_Sup_R Right Superior temporal gyrus, Temporal_Mid_L Left Middle temporal gyrus, Postcentral_L Left Postcentral gyrus, Precuneus_R Right Precuneus
Table 3.
Components with significant differences in Aβ
| Brain regions | |z| | Networks | P value (component) | X | Y | Z |
|---|---|---|---|---|---|---|
| Frontal_Mid_R | 2.81 | cognitive | 0.0358 | 24 | 35 | 33 |
| Frontal_Inf_Orb_R | 2.97 | cognitive | 0.0358 | 38 | 15 | 31 |
| Parietal_Inf_R | 3.027 | cognitive | 0.0358 | 36 | −41 | 42 |
| Occipital_Mid_R | 4.461 | Visual | 0.0117 | 20 | −86 | 19 |
| Temporal_Sup_R | 3.312 | cognitive | 0.0117 | 53 | −38 | 19 |
| Temporal_Mid_L | 2.91 | DMN | 0.0095 | 55 | −20 | −8 |
| Postcentral_L | 3.72 | cognitive | 0.0095 | −27 | −42 | 62 |
| Precuneus_R | 3.327 | DMN | 0.0095 | 0 | −53 | 25 |
Abbreviation: Frontal_Mid_R Right middle frontal gyrus, Frontal_Inf_Orb_R Right Inferior frontal gyrus(orbital part), Parietal_Inf_R Right Inferior parietal but supramarginal and angular gyri, Occipital_Mid_R, Right middle occipital gyrus, Temporal_Sup_R Right superior temporal gyrus, Temporal_Mid_L Left Middle temporal gyrus, Postcentral_L Left Postcentral gyrus, Precuneus_R Right Precuneus
Fig. 1.
Purple nodes: significant differences between the MCI and NC groups in AV1451 were observed in right fusiform gyrus (FFG.R), left lingual gyrus (LING.L), right inferior frontal gyrus(orbital part) (ORBinf.R), and right precentral gyrus (PreCG.R). Red nodes: significant differences were noted in the left middle temporal gyrus (MTG.L), right inferior occipital gyrus (IOG.R), and right middle frontal gyrus (MFG.R). Blue nodes: significant differences were observed in the right parahippocampal (PHG.R), left amygdala (AMYG.L), right anterior cingulate and paracingulate gyri (ACG.R)
Fig. 2.
Purple nodes: significant differences between the MCI and NC groups in AV45 were observed in the right middle frontal gyrus (MFG.R), right inferior frontal gyrus(opercular part) (ORBinf.R), right inferior parietal but supramarginal and angula gyri (IPL.R). Red nodes: significant differences were noted in the right middle occipital gyrus (MOG.R), right superior temporal gyrus (STG.R). Blue nodes: significant differences were observed in the left middle temporal gyrus (MTG.L), left postcentral gyrus (POCG.L), and right precuneus gyrus (PCUN.R)
Correlated tau PET and Aβ PET components
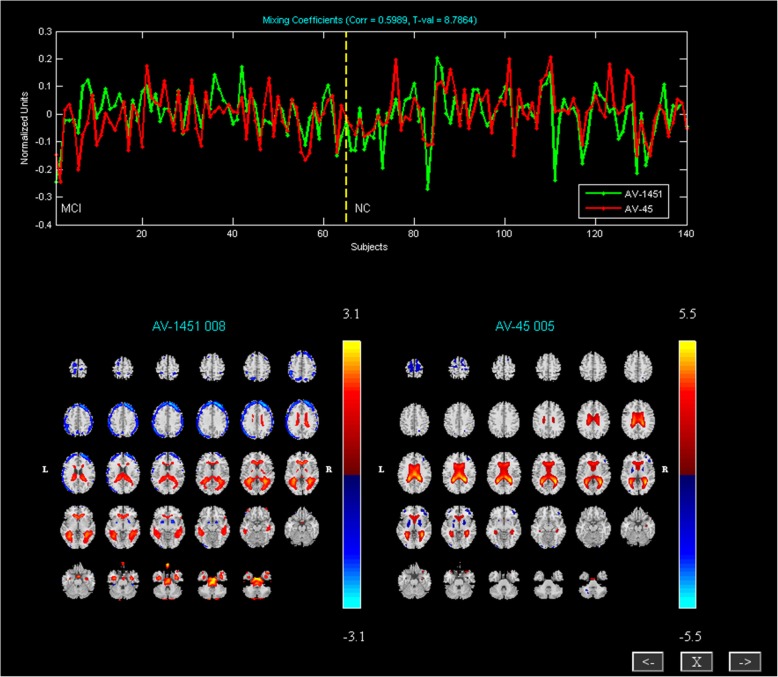
We also found the correlated networks. One pair of components with the highest correlation (R = 0.5989) was identified between the tau PET and Aβ PET. They were largely colocalized with the DMN. These components mainly contained bilateral precuneus, bilateral angular gyrus, left anterior cingulate cortex, left superior frontal gyrus, left middle temporal gyrus, left middle frontal gyrus, left inferior frontal gyrus (Fig. 3).
Fig. 3.
Correlated components of AV1451 and AV45. The top presents loading parameters with a significant correlation in all participants with AV1451 (green) and AV45 (red). Correlated components of AV1451 (left) and AV45 (right), including the medial frontal gyrus; anterior cingulate cortex; posterior cingulate cortex; precuneus; superior temporal gyrus
Feature test
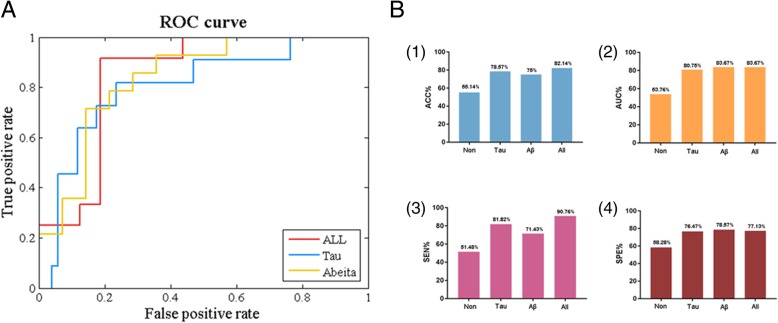
Using the method of five-fold cross validation, we detected the regions with significant differences extracted by pICA analysis in distinguishing MCI from NC group. Figure 4 and Table 4presented the contribution of these differential components in the two proteins to classification. The final improvements in the ACCs of all features and the AUCs were respectively 78.57 and 80.75% for tau protein, 75 and 83.67% for Aβ protein, and 82.14 and 84.38% after the fusion of the two proteins. In the original data, the accuracy rate was only slightly more than 50%. The accuracy has been improved and the area under the curve (AUC) has been increased, which suggested features obtained by pICA analysis were effective. The mean values of ACC, AUC, Sensitivity (SEN), and Specificity (SPE) were from five experiments (Table 4).
Fig. 4.
a Receiver operating characteristic (ROC) curves of different types of features. Different colors were used to represent the ROC curves of all four types of features. b Comparison of the classification accuracy (1); area under the curve (2); sensitivity (3); specificity (4)
Table 4.
Classification results
| ACC | AUC | SEN | SPE | |
|---|---|---|---|---|
| Non | 55.14 | 53.76 | 51.48 | 58.28 |
| Tau | 78.57 | 80.75 | 81.82 | 76.47 |
| Aβ | 75 | 83.67 | 71.43 | 78.57 |
| All | 82.14 | 83.67 | 90.76 | 77.13 |
Discussion
Group comparison of tau PET and Aβ PET in brain networks
MCI patients exhibited that components with significant differences for tau and Aβ protein were mainly in DMN, VN, CCN and subcortical networks (SN). These networks are parts of the intrinsic connected networks (ICNs) of human brain. ICNs exhibit a consistent distribution of activity during rest and tasks, which are associated with specific neurocognitive functions [28]. DMN has been considered as a critical role in supporting internal mentation and monitoring external environment [29]. Evidence suggested that Aβ deposition was most likely to occur in DMN, probably due to high synaptic activity [30]. Tau deposition is primarily targeted at high-level cognitive networks such as DMN [31]. Altered connections within DMN in AD and MCI have also been reported in the study [32]. Greicius MD et al. [33] demonstrated that there were close links between DMN and episodic memory processing. Visual function is a major complex sensory domain affected by mental diseases [34]. The abnormalities in VN are associated with the aberrant processing of visual information and visual hallucinations [35]. A prior study indicated that the impairment of ventral visual function, including wrong recognition of an object, face and color, were also well noted in MCI patients [36]. Therefore, our results might discover the abnormalities of the visual network in MCI patients may be affected by the presence of tau and Aβ proteins. Several studies suggested that emotion regulation involved increased activity in cortical regions was associated with CCN [37]. The impaired response of CCN to verbal memory is partly responsible for the decline of memory ability in AD/MCI patients [38]. In addition, the Aβ accumulation is a major trait of the pathogenesis of dementia. Recent studies has observed that there was considerable spatial overlap of Aβ accumulating regions with other ICNs, such as CCN [39]. Hansson O et al. [31] indicated that the regional deposition of hyperphosphorylated tau aggregates in AD generally affected CCN. It has been known that amygdala belonging to SN has a central role in emotional learning and memory [40]. Further, recent morphological analysis suggested there was substantial atrophy within the amygdala in AD/MCI [41]. According to aforementioned findings, it can preliminarily speculate that tau and Aβ proteins have an effect on the brain network of MCI patients. Impairments in brain network may lead to mental dysfunction in MCI patients.
Strongly correlated tau PET and Aβ PET networks
This study also elucidated spatially disparate relationships between the patterns of tau and Aβ deposition across a heterogenous MCI population. We discovered that a significant correlated pair of components between tau PET and Aβ PET were identified using pICA. Tau protein levels in the bilateral precuneus, right angular gyrus, left anterior cingulate cortex, left angular gyrus, left superior frontal gyrus, and left middle temporal gyrus were strongly correlated to Aβ protein levels in the precuneus, bilateral angular gyrus, left middle frontal gyrus, and left inferior frontal gyrus. They are largely colocalized with the DMN [42]. It was well-documented that the alterations in the brain structure, function, and cognition in MCI patients were related with alterations in brain networks [43]. Utilizing resting state functional connectivity MRI (rs-fMRI), networks correlations have been detected in patients with MCI. These networks mainly were involved in DMN and other networks [44]. Although MCI is related with widespread disruption of network connections, DMN is usually most affected. As a sensory-visceromotor link related to social behavior, emotional control and motivation drive, DMN played many potential roles and had a great relationship with personality composition [45]. Therefore, in MCI, the brain regions associated with DMN were damaged, resulting in phenomenon of metabolic reduction and amyloid abnormalities [46]. In the context of current models of the AD pathophysiological cascade [47], our findings might indicate that Aβ diffusion was similar to that of tau and that the spatial distribution of Aβ and tau may be strongly correlated. Several studies pointed out that Aβ aggregation may be driven by the total flow of neuronal activity, while tau aggregation may be driven by transneuronal spread, generating patterns of neurodegeneration that coincide with specific functional networks and ultimately lead to specific clinical phenotypes [48], which were similar to results in present results.
Role of multivariate analysis
Multivariate techniques are widely used in neuroimaging data analysis. Multivariate methods mainly focus on the level of brain regions to analyze the correlation and covariance of brain regions. The advantage of these methods is that different modal neuroimaging data can be combined to represent pathophysiology of a disease comprehensively [49]. Unlike the univariate method, multivariate analysis has obvious advantages in studying the mechanism of interregional brain cooperation [50]. Results from multivariate analysis can be seen as a feature of neural network, which is an important perspective to study the brain damage induced by mental illness [51, 52]. In order to guarantee the statistical results more accurate, conservative corrections for voxel wise multiple comparisons were added in the multivariate methods. All in all, the use of multivariate methods will help to obtain more discriminatory features in diagnostic classification. In this study, the combined analysis of tau PET and Aβ PET performed better than that of tau PET and Aβ PET alone in distinguishing MCI patients from NC group.
Limitations
This study has several limitations. Firstly, pICA assumes that measurements in each image voxel are independent and that the overall noise is uniformly distributed, and these assumptions may not be entirely accurate for PET data. Therefore, future research should focus more on the diversity of data. Secondly, the lack of AD subjects in our research is another limitation due to the incomplete data of ADNI database. After collecting enough AD data in the next step, we will plan to conduct a comparison and combination study of AD and MCI data separately.
Conclusions
In the present study, we explored the tau PET and Aβ PET spatial distribution pattern in MCI patients and NCs. The pICA results revealed that the abnormal pattern detected by tau PET was in agreement with the abnormal pattern detected by Aβ PET, both of which shared the location of the DMN. Moreover, these regions were helpful for distinguishing patients with MCI from those in the NC group. These results indicated that tau PET and Aβ PET are reliable biomarkers of neurological function and might be helpful for diagnosis.
Additional file
Acquisition parameters of PET data. We have revised in the manuscript. (DOCX 13 kb)
Acknowledgements
The authors would like to thank the contribution of Yongchao Li and Hongzhi Cui made to this research project.
Funding
This study was supported by the National Basic Research Program of China (973 Program) (No.2014CB744600), the National Natural Science Foundation of China (Grant, No.61632014), the Program of Beijing Municipal Science & Technology Commission (No.Z171100000117005), the National Key Research and Development Program of China (No.2016YFC1307203) and the Fundamental Research Funds for the Central Universities (lzujbky-2017-kb08).
Availability of data and materials
Not applicable.
Abbreviations
- ACC
Accuracy
- AD
Alzheimer’s disease
- ADNI
Alzheimer’s Disease Neuroimaging Initiative
- APOE4
Apolipoprotein E ε4 allele
- AUC
Area under the curve
- Aβ PET
AV45
- Aβ
Amyloid β
- CCN
Cognitive control network
- CDR
Clinical Dementia Rating
- DMN
Default-mode network
- FDG
Fluorodeoxyglucose
- fMRI
Functional magnetic resonance imaging
- ICNs
Intrinsic connected networks
- MCI
Mild cognitive impairment
- MMSE
Mini–Mental State Examination
- MNI
Montreal Neurological Institute
- NC
Normal control
- PET
Positron emission tomography
- pICA
Parallel independent component analysis
- ROI
Regions of interest
- rs-fMRI
Resting state functional connectivity MRI
- SEN
Sensitivity
- SN
Subcortical networks
- SPE
Specificity
- Tau PET
AV1451
- VN
Visual network
Authors’ contributions
Conceived and designed the experiments, YL BH ZJY. Analyzed the data, YL YZ. Contributed reagents/materials/analysis tools, YL ZJY. Wrote the paper, YL BH ZJY YZ YF YY. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study procedures were approved by the institutional review boards of all participating centres (https://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf), and written informed consent was obtained from all participants or their authorised representatives. The investigators within the ADNI contributed to the design and implementation of the ADNI and/or provided data but did not participate in analysis or writing of this report.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Laferla FM, Oddo S. Alzheimer's disease: Aβ, tau and synaptic dysfunction. Trends Mol Med. 2005;11(4):170–176. doi: 10.1016/j.molmed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Salvadó G, Molinuevo JL, Brugulat-Serrat A, Falcon C, Grau-Rivera O, Suárez-Calvet M, Pavia J, Niñerola-Baizán A, Perissinotti A, Lomeña F. Centiloid cut-off values for optimal agreement between PET and CSF core AD biomarkers. Alzheimers Res Ther. 2019;11(1):27. doi: 10.1186/s13195-019-0478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duan H, Jiang J, Xu J, Zhou H, Huang Z, Yu Z, Yan Z. Differences in Aβ brain networks in Alzheimer’s disease and healthy controls. Brain Res. 2017;1655:77–89. doi: 10.1016/j.brainres.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 4.M S SNL, DR S, JP ON, M J RO, SL B, JW V, J F HDS. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89(5):971–982. doi: 10.1016/j.neuron.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, Owen C, Aldea P, Su Y, Hassenstab J. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med. 2016;8(338):338ra366. doi: 10.1126/scitranslmed.aaf2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee W, Kim S, Kim D. Individual biometric identification using multi-cycle electrocardiographic waveform patterns. Sensors. 2018;18(4):1005. doi: 10.3390/s18041005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jang J, Bang K, Jang H, Hwang D. Quality evaluation of no-reference MR images using multidirectional filters and image statistics. Magn Reson Med. 2018;80(3):914. doi: 10.1002/mrm.27084. [DOI] [PubMed] [Google Scholar]
- 8.McKeown MJ, Jung T-P, Makeig S, Brown G, Kindermann SS, Lee T-W, Sejnowski TJ. Spatially independent activity patterns in functional MRI data during the Stroop color-naming task. Proc Natl Acad Sci. 1998;95(3):803–810. doi: 10.1073/pnas.95.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao W, Cao X, Hou C, Li T, Yan C, Jiang L, Cheng L, Li C, Yao D. Effects of cognitive training on resting-state functional connectivity of default mode, salience, and central executive networks. Front Aging Neurosci. 2016;8(152):1–11. [DOI] [PMC free article] [PubMed]
- 10.Vigário R, Särelä J, Jousmiki V, Hämäläinen M, Oja E. Independent component approach to the analysis of EEG and MEG recordings. IEEE Trans Biomed Eng. 2000;47(5):589–593. doi: 10.1109/10.841330. [DOI] [PubMed] [Google Scholar]
- 11.Grandchamp R, Braboszcz C, Makeig S, Delorme A. Stability of ICA decomposition across within-subject EEG datasets. Conf Proc IEEE Eng Med Biol Soc. 2012;2012(4):6735–6739. doi: 10.1109/EMBC.2012.6347540. [DOI] [PubMed] [Google Scholar]
- 12.Xu L, Groth KM, Pearlson G, Schretlen DJ, Calhoun VD. Source-based morphometry: the use of independent component analysis to identify gray matter differences with application to schizophrenia. Hum Brain Mapp. 2010;30(3):711–724. doi: 10.1002/hbm.20540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park HJ, Kim JJ, Youn T, Lee DS, Lee MC, Kwon JS. Independent component model for cognitive functions of multiple subjects using [15O] H2O PET images. Hum Brain Mapp. 2003;18(4):284–295. doi: 10.1002/hbm.10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calhoun VD, Adali T, Kiehl KA, Astur R, Pekar JJ, Pearlson GD. A method for multitask fMRI data fusion applied to schizophrenia. Hum Brain Mapp. 2010;27(7):598–610. doi: 10.1002/hbm.20204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brickman AM, Habeck C, Zarahn E, Flynn J, Stern Y. Structural MRI covariance patterns associated with normal aging and neuropsychological functioning. Neurobiol Aging. 2007;28(2):284–295. doi: 10.1016/j.neurobiolaging.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Duygu T, Norbert S, Mathis CA, William J, Weiner MW. Spatial patterns of brain amyloid-beta burden and atrophy rate associations in mild cognitive impairment. Brain. 2011;134(4):1077–1088. doi: 10.1093/brain/awr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shashwath AM, Balaji N, Jingyu L, Nora IP-B, Michael CS, Vince DC, David CG, Li S, Shannon LR, Andrew JS. A large scale multivariate parallel ICA method reveals novel imaging-genetic relationships for Alzheimer's disease in the ADNI cohort. NeuroImage. 2012;60(3):1608–1621. doi: 10.1016/j.neuroimage.2011.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu L, Liu L, Zhang J, Xu B, Fan Y, Tian J. Brain network alterations in Alzheimer's disease identified by early-phase PIB-PET. Contrast Media Mol Imaging. 2018;2018(8):6830105. doi: 10.1155/2018/6830105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leyton CE, Villemagne VL, Sharon S, Pike KE, Ballard KJ, Olivier P, Burrell JR, Rowe CC, Hodges JR. Subtypes of progressive aphasia: application of the international consensus criteria and validation using β-amyloid imaging. Brain. 2011;134(10):3030–3043. doi: 10.1093/brain/awr216. [DOI] [PubMed] [Google Scholar]
- 20.Jr RL, Tosun D, Ghosh P, Lehmann M, Madison CM, Weiner MW, Miller BL, Jagust WJ, Rabinovici GD. Parallel ICA of FDG-PET and PiB-PET in three conditions with underlying Alzheimer's pathology. Neuroimage Clinical. 2014;4:508–516. doi: 10.1016/j.nicl.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandhi T, Panigrahi BK, Anand S. A comparative study of wavelet families for EEG signal classification. Neurocomputing. 2011;74(17):3051–3057. [Google Scholar]
- 22.Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack CR, Jagust W, Trojanowski JQ, Toga AW, Beckett L. Ways toward an early diagnosis in Alzheimer’s disease: the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005;1(1):55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, Mchugh PR. “Mini-mental state” : a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.JC M. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Pearlson G, Windemuth A, Ruano G, Perronebizzozero NI, Calhoun V. Combining fMRI and SNP data to investigate connections between brain function and genetics using parallel ICA. Hum Brain Mapp. 2010;30(1):241–255. doi: 10.1002/hbm.20508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohavi R. A study of cross-validation and bootstrap for accuracy estimation and model selection. Proceedings of the 14th International Joint Conference on Artificial Intelligence (Morgan Kaufmann Publishers Inc San Francisco). 1995;2:1137–45.
- 27.Gandhi T, Panigrahi BK, Anand S. A comparative study of wavelet families for EEG signal classification. 2011. [Google Scholar]
- 28.Laird AR, P Mickle F, Eickhoff SB, Turner JA, Ray KL, D Reese M, Glahn DC, Beckmann CF, Smith SM, Fox PT. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci. 2014;23(12):4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong Y, Huang L, Cai S, Zhang Y, Deneen KMV, Ren A, Ren J. Altered effective connectivity patterns of the default mode network in Alzheimer's disease: an fMRI study. Neurosci Lett. 2014;578:171–175. doi: 10.1016/j.neulet.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Haan W, Mott K, van Straaten EC, Scheltens P, Stam CJ. Activity dependent degeneration explains hub vulnerability in Alzheimer's disease. PLoS Comput Biol. 2012;8(8):e1002582. doi: 10.1371/journal.pcbi.1002582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansson O, Grothe MJ, Strandberg TO, Ohlsson T, Hägerström D, Jögi J, Smith R, Schöll M. Tau pathology distribution in Alzheimer's disease corresponds differentially to cognition-relevant functional brain networks. Front Neurosci. 2017;11:167. [DOI] [PMC free article] [PubMed]
- 32.Li X, Li TQ, Andreasen N, Wiberg MK, Westman E, Wahlund LO. Ratio of Aβ42/P-tau181p in CSF is associated with aberrant default mode network in AD. Sci Rep. 2013;3:1339. doi: 10.1038/srep01339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.BODIS-WOLLNER I, MARX MS, MITRA S, BOBAK P, MYLIN L, YAHR M. Visual dysfunction in Parkinson's disease: loss in spatiotemporal contrast sensitivity. Brain. 1987;110(6):1675–1698. doi: 10.1093/brain/110.6.1675. [DOI] [PubMed] [Google Scholar]
- 35.Sang SC, Strafella AP, Duff-Canning S, Zurowski M, Vijverman AC, Bruno V, Aquino CC, Criaud M, Rusjan PM, Houle S. The relationship between serotonin-2A receptor and cognitive functions in nondemented Parkinson's disease patients with visual hallucinations. Mov Disord Clin Pract. 2017;4:n/a. doi: 10.1002/mdc3.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng Y, Shi L, Lei Y, Wang D. Altered topological organization of high-level visual networks in Alzheimer’s disease and mild cognitive impairment patients. Neurosci Lett. 2016;630:147–153. doi: 10.1016/j.neulet.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 37.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Dhanjal NS, Wise RJ. Frontoparietal cognitive control of verbal memory recall in Alzheimer's disease. Ann Neurol. 2014;76(2):241–251. doi: 10.1002/ana.24199. [DOI] [PubMed] [Google Scholar]
- 39.Grothe MJ, Teipel SJ. Spatial patterns of atrophy, hypometabolism, and amyloid deposition in Alzheimer's disease correspond to dissociable functional brain networks. Hum Brain Mapp. 2016;37(1):35–53. doi: 10.1002/hbm.23018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29(5):272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Qiu A, Fennema-Notestine C, Dale AM, Miller MI. Regional shape abnormalities in mild cognitive impairment and Alzheimer's disease. Neuroimage. 2009;45(3):656–661. doi: 10.1016/j.neuroimage.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grabner RH, Ansari D, Koschutnig K, Reishofer G, Ebner F. The function of the left angular gyrus in mental arithmetic: evidence from the associative confusion effect. Hum Brain Mapp. 2013;34(5):1013–1024. doi: 10.1002/hbm.21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickerson BC, Salat DH, Bates JF, Monika A, Killiany RJ, Greve DN, Dale AM, Stern CE, Deborah B, Albert MS. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2010;56(1):27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrella JR, Sheldon FC, Prince SE, Calhoun VD, Doraiswamy PM. Default mode network connectivity in stable vs progressive mild cognitive impairment. Neurology. 2011;76(6):511–517. doi: 10.1212/WNL.0b013e31820af94e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raichle ME. The brain's default mode network. Annu Rev Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 46.Buckner RL, Snyder AZ, Shannon BJ, Gina LR, Rimmon S, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neurology TL. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seeley WW, Crawford RK, Juan Z, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tosun D, Schuff N, Mathis CA, Jagust W, Weiner MW. Spatial patterns of brain amyloid-β burden and atrophy rate associations in mild cognitive impairment. Brain. 2011;134(4):1077–1088. doi: 10.1093/brain/awr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Devanand DP, Habeck CG, Tabert MH, Scarmeas N, Pelton GH, Moeller JR, Mensh BD, Tarabula T, Van Heertum RL, Stern Y. PET network abnormalities and cognitive decline in patients with mild cognitive impairment. Neuropsychopharmacology. 2006;31(6):1327. doi: 10.1038/sj.npp.1300942. [DOI] [PubMed] [Google Scholar]
- 51.Lehmann M, Ghosh PM, Madison C, Laforce R, Corbettarastelli C, Weiner MW, Greicius MD, Seeley WW, Gornotempini ML, Rosen HJ. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain. 2013;136(3):844–858. doi: 10.1093/brain/aws327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang S, Li CS. Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage. 2012;59(4):3548–3562. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Acquisition parameters of PET data. We have revised in the manuscript. (DOCX 13 kb)
Data Availability Statement
Not applicable.