Abstract
Yes‐associated protein (YAP) and transcriptional co‐activator with PDZ‐binding motif (TAZ), the main effectors of the Hippo pathway, are emerging as important players in cancer biology and therapy response. The intracellular localization of YAP/TAZ is a key determinant in the regulation of their activity and their roles in signal transduction. This is particularly relevant for cancer: Aberrant nuclear localization of YAP and TAZ has been observed in numerous human cancers and may therefore represent an attractive target for cancer therapy. In this review, we describe the mechanisms that regulate the nucleo‐cytoplasmic shuttling of YAP/TAZ and their implications for cancer, and discuss how the new insights about this process may pave the way for novel therapeutic strategies.
Keywords: exportin 1, hippo pathway, nucleo‐cytoplasmic shuttling, TAZ, YAP
Abbreviations
- CAFs
cancer‐associated fibroblasts
- GPCR
G protein‐coupled receptors
- HCC
hepatocellular carcinoma
- LATS1 and LATS2
large tumor suppressor 1 and 2
- NES
nuclear export signal
- NLS
nuclear localization signal
- TAZ
transcriptional co‐activator with PDZ‐binding motif
- YAP
Yes‐associated protein
- Yki
Yorkie
1. Introduction
The transcriptional co‐factors Yes‐associated protein (YAP) and transcriptional co‐activator with PDZ‐binding motif (TAZ), the main effectors of the Hippo signal transduction pathway, are emerging as pivotal determinants of malignancy in human cancer (Harvey et al., 2013; Zanconato et al., 2016a 2016b). In addition, mounting evidence suggests that they play important roles in chemotherapeutic drug resistance and have significant impact on patient prognosis (Zhao and Yang, 2015). For instance, high levels of YAP and TAZ are observed in many human liver tumors (Han et al., 2014). Furthermore, liver‐specific YAP overexpression in transgenic mice leads to hepatocellular carcinoma (HCC) development, suggesting that YAP is a key driver of tumorigenesis in this type of cancer (Dong et al., 2007). Moreover, YAP was shown to mediate cisplatin resistance in HCC (Mao et al., 2014). Similarly, tumors with high TAZ levels are more invasive and metastatic and therefore difficult to treat. For example, TAZ contributes to Taxol resistance in breast cancer cells (Lai et al., 2011). Furthermore, it was shown that YAP/TAZ activity contributes to lung tumor progression and metastasis (Lau et al., 2014). More broadly, high YAP and/or TAZ expression and/or aberrant nuclear localization are correlated with poor prognosis in many cancers, such as pancreatic adenocarcinoma, endometrial carcinoma, melanoma, squamous cell carcinoma of the skin, Kaposi's sarcoma, colorectal cancer, gastric cancer, head and neck squamous cell carcinoma, ovarian cancer, urothelial carcinoma of the bladder, and esophageal squamous cell carcinoma (Zanconato et al., 2016a 2016b). All of these imply that overexpression and hyperactivation of YAP and TAZ favor tumorigenesis. Intriguingly, YAP may also act as a tumor suppressor in specific circumstances (Levy et al., 2007; Strano et al., 2005).
Despite the emerging importance of YAP and TAZ in cancer, the exact mechanisms driving their activation in human tumors still remain to be fully resolved. At the genomic level, Hippo pathway genes, including YAP/TAZ, are rarely mutated in cancer, with only a few exceptions in specific tumors (Chen et al., 2015; Wang et al., 2018). This suggests that additional and diverse mechanisms must control YAP/TAZ dysregulation in cancer; these might include epigenetic alterations, post‐translational modifications, crosstalk with various other pathways, and aberrant subcellular localization.
YAP/TAZ regulation is multilayered and involves numerous mechanisms and factors. Thus, a wide range of inputs including cell density, cell polarity, mechanical stress, ligands of G protein‐coupled receptors (GPCRs), and cellular energy status (Piccolo et al., 2014), have all been shown to regulate YAP/TAZ. In particular, the nucleo‐cytoplasmic distribution of YAP/TAZ is a key determinant of their activity and is a major target of their regulation by upstream components of the Hippo pathway. More specifically, the Hippo signaling pathway consists of a large network of proteins, the core of which is a conserved kinase cascade that limits tissue growth by promoting phosphorylation and inhibition of YAP and TAZ, or their orthologue Yorkie (Yki) in Drosophila (Huang et al., 2005; Lei et al., 2008; Zhao et al., 2007). Thus, when the Hippo pathway is inhibited, YAP/TAZ/Yki gain activity. Subsequently, the nuclear abundance of YAP/TAZ/Yki increases. Nuclear localization is crucial for the functionality of YAP/TAZ/Yki as transcriptional coactivators. The abundance of YAP/TAZ in the nucleus can be modulated by a variety of signaling pathways. For example, the mevalonate and the glucocorticoid receptor signaling pathways regulate YAP nuclear accumulation in breast cancer (Sorrentino et al., 2014, 2017). Indeed, YAP/TAZ nuclear localization can serve as a tool to screen for novel upstream modulators of the Hippo pathway (Sorrentino et al., 2014, 2017). As mentioned above, elevated nuclear presence of YAP and TAZ can often be observed in a variety of human malignancies, including liver, lung, breast, skin, colon, and ovarian cancer (Harvey et al., 2013; Johnson and Halder, 2014; Piccolo et al., 2013).
Surprisingly, YAP/TAZ/Yki lack a canonical nuclear localization signal (NLS), and therefore, the machinery responsible for their nuclear accumulation is not obvious (Wang et al., 2016b). Recently, Gao et al. (2017) added another layer of complexity to the picture. By using super‐resolution microcopy, they observed that, within several human cell lines, YAP is mainly distributed in nuclear clusters. Interestingly, cell contact and mechanical pressure weakened YAP clustering and transcriptional activity (Gao et al., 2017). Thus, not only subcellular, but also subnuclear distribution might be critical in the regulation of YAP transcriptional activity.
All in all, it is of great interest to understand how the nucleo‐cytoplasmic shuttling of these proteins is maintained and regulated under normal conditions, and how it becomes deregulated in cancer. Several recent studies (Ege et al., 2018; Elosegui‐Artola et al., 2017; Kofler et al., 2018; Manning et al., 2018) have begun to unravel this mystery. Here, we review the current understanding and discuss how these new insights may be exploited therapeutically.
2. Then and now: Regulation of YAP/TAZ/Yki nucleo‐cytoplasmic shuttling by the Hippo pathway
According to the canonical view of the mammalian Hippo pathway, mammalian STE20‐like protein kinase 1 and 2, together with the adaptor protein Salvador homologue 1, phosphorylate and activate large tumor suppressor 1 and 2 (LATS1 and LATS2). Activated LATS1/2, together with the adaptor proteins MOB kinase activator 1A and 1B, in turn phosphorylate YAP and TAZ. It is generally accepted that phosphorylation by LATS1/2 leads to YAP/TAZ nuclear exclusion, cytoplasmic sequestration by 14‐3‐3 anchoring factors, and/or proteasomal degradation. In Drosophila, the subcellular localization of Yki is regulated similarly by phosphorylation by the LATS orthologue Warts and subsequent 14‐3‐3 binding (Dong et al., 2007; Yu and Guan, 2013; Zhao et al., 2007). In the nucleus, Yki and YAP/TAZ exert their biological effects by regulating gene transcription (Dong et al., 2007; Yu and Guan, 2013; Zhao et al., 2007). Thus, through regulating the subcellular localization of YAP/TAZ/Yki, the Hippo kinase cascade maintains temporal control of their activity (Yu and Guan, 2013). Accordingly, prevention of YAP/Yki phosphorylation affects their biological functions and specifically increases their growth‐promoting activity (Dong et al., 2007; Zhao et al., 2007). Since YAP and TAZ do not have sequence‐specific DNA‐binding capability, they depend on interaction with sequence‐specific transcription factors for their recruitment to target sequences in the chromatin. In particular, TEAD family transcription factors serve as such chromatin anchors and therefore play an essential role in YAP/TAZ‐dependent gene expression and cell growth stimulation (Zhao et al., 2008).
Several recent studies have expanded this canonical model. First, contrary to the above dogma, it was shown that LATS‐dependent phosphorylated YAP can be retained in the nucleus and is not necessarily exported to the cytoplasm (Wada et al., 2011). Based on these observations and additional experiments, it was concluded that although phosphorylation of YAP is required for its exclusion from the nucleus, it not sufficient by itself (Wada et al., 2011), contrary to the canonical model. Second, in a study by Dupont et al. (2011) establishing the connection between YAP/TAZ and mechanotransduction, they showed that YAP/TAZ are entrapped in the cytoplasm upon treatment with cytoskeleton inhibitors. However, more in‐depth analysis revealed that YAP/TAZ actually shuttle between the cytoplasm and the nucleus, rather than being statically tethered within one subcellular compartment. This was shown by treating cells with a combination of both cytoskeletal inhibitors and nuclear export inhibitors; under such combined treatment, the cells exhibited increased nuclear localization of YAP/TAZ compared to treatment with cytoskeletal inhibitors alone, implying that they can shuttle into the nucleus and back even when the cytoskeleton is disrupted.
Challenging the existing model even further, Wang et al. (2016a) identified a biphasic YAP localization pattern in intestinal epithelial cells in response to mitogenic GPCR agonists. Specifically, they observed that YAP first translocates to the cytoplasm, and this is then followed by subsequent re‐entry into the nucleus, eventually driving changes in gene expression. The observations reported by these researchers are provocative for several reasons. First, whereas treatment with GPCR agonists or serum growth factors caused nuclear accumulation of YAP in some cell lines, it actually resulted in cytoplasmic sequestration in others. Thus, it seems that the same signal can either activate or inactivate YAP, depending on cellular context. Second, their findings imply that transient nuclear exit of YAP during the first phase is necessary for its nuclear re‐entry and activation. More specifically, exposure to leptomycin B, a nuclear export inhibitor, prevented the nuclear exit of YAP upon exposure to a GPCR agonist. Counterintuitively, it also blocked the expression of YAP‐regulated genes, despite the continued presence of YAP in the nucleus. Altogether, this suggests that dynamic nucleo‐cytoplasmic shuttling of YAP is important for its function, implying that YAP needs to be localized in the right place at the right time.
In addition, four new studies (Ege et al., 2018; Elosegui‐Artola et al., 2017; Kofler et al., 2018; Manning et al., 2018) thoroughly investigated mechanistic aspects of the regulation of YAP/TAZ/Yki nucleo‐cytoplasmic translocation. Three of these (Ege et al., 2018; Elosegui‐Artola et al., 2017; Manning et al., 2018) employed a similar approach, in which fluorescently labeled YAP/Yki was monitored by advanced microscopy tools and live imaging, to infer subcellular dynamics upon various treatments. Importantly, all three studies concluded that the majority of YAP/TAZ/Yki molecules rapidly and dynamically traffic between the cytoplasm and the nucleus.
Beyond the overall similar conclusions, each group also presented distinct mechanistic findings. Ege et al. (2018) compared YAP nuclear export and import rates in normal vs. cancer‐associated fibroblasts (CAFs) by using exogenously expressed YFP‐YAP protein and combining photobleaching with mathematical modeling. They concluded that nuclear export, accelerated by LATS‐mediated phosphorylation, is the key determinant of YAP localization. They identified exportin 1 (XPO1) as the exportin used by YAP to exit the nucleus. Surprisingly, dephosphorylation was not sufficient for YAP activation, and tyrosine phosphorylation by Src‐family kinases and cues from the cytoskeleton were necessary for effective YAP transcriptional activity.
Elosegui‐Artola et al. (2017) uncovered a novel mechanosensing mechanism directly converting force into nuclear import. They found that nuclear flattening, caused by mechanical force, leads to increased nuclear entry of YAP (and, potentially, other proteins) due to decreased mechanical restriction of molecular transport through nuclear pores. More specifically, fluorescence recovery after photobleaching and atomic force microscopy were used to follow the localization of overexpressed GFP‐YAP in mouse fibroblasts upon mechanical stress‐associated treatments. Interestingly, application of force to the nucleus was sufficient to translocate YAP into the nucleus independently of surface rigidity, focal adhesions, actin cytoskeleton, cell–cell adhesion, or LATS and MST overexpression. This suggests that nucleoskeletal changes override other signals to govern YAP subcellular localization. Interestingly, this study concluded that the nuclear import rate determines YAP location and activity.
Manning et al. (2018) explored Hippo pathway dynamics, focusing on the fly orthologue of YAP/TAZ, Yki. They endogenously tagged Yki with YFP and followed its dynamics in both larval wing and pupal notum. Intriguingly, they found that cell populations within the larval wing displayed different rates of Yki nucleo‐cytoplasmic shuttling. These differences suggest that regulation of YAP/TAZ/Yki nuclear localization is cell‐type‐specific even within the same tissue. Similar observations were also described in an earlier study on mammalian YAP localization in the developing lung (Mahoney et al., 2014). Furthermore, Yki localization was found to be cell cycle‐dependent, in that Yki was primarily cytoplasmic during interphase but chromatin‐bound in mitosis. By using Warts mutants, Manning et al. could show that Yki nuclear import rates were Hippo pathway dependent, similar to the findings of Elosegui‐Artola et al.
The aforementioned studies mainly focused on YAP/Yki localization. In parallel, a recent study by Kofler et al. (2018) elucidated the molecular requirements underlying TAZ nucleo‐cytoplasmic shuttling. By using diffusion‐limited TAZ constructs and inducible nuclear influx and efflux systems in pig cells, they demonstrated that TAZ localization is highly regulated. Specifically, they found that RhoA stimulation increased the nuclear import of TAZ. RhoA, a member of the Ras‐related family of GTPases and regulator of cytoskeleton dynamics (Hall, 1998; Ridley and Hall, 1992), has already been linked previously to TAZ/YAP activation (Ege et al., 2018; Elosegui‐Artola et al., 2017). Interestingly, at variance with the current dogma that YAP/TAZ lack nuclear import signals, Kofler et al. identified a noncanonical NLS in TAZ. In addition, they also identified a novel nuclear export signal (NES); both the NLS and the NES, identified in pig cells, are conserved also in human TAZ and YAP. The TAZ NLS represents a new class of import motifs, necessary and sufficient for efficient nuclear uptake, whereas the TAZ NES overlaps with the binding site for TEAD proteins. TEAD binding was previously shown to modulate YAP/TAZ nuclear localization (Chan et al., 2009; Ege et al., 2018; Lin et al., 2017); the recent findings provide an interesting explanation for this observation, namely that TEAD binding can mask the NES and consequently dampen nuclear export.
Together, these recent studies call for reevaluation of the canonical model of YAP/TAZ/Yki regulation. In agreement with previous models, YAP/TAZ/Yki transcriptional impact is primarily controlled by subcellular localization. However, a revised model would suggest that YAP/TAZ/Yki continually shuttle between the nucleus and the cytoplasm and are maintained at steady state by a balance of nuclear export and import rates. At the single molecule level, diverse modes of regulation, such as combinations of passive influx by controlling nuclear pore permeability with active regulation by protein modifications (like LATS/Warts serine phosphorylation or Src‐family kinases tyrosine phosphorylation) and interactions with specific binding partners (TEAD or 14‐3‐3), affect nuclear export and/or import rates (Fig. 1A). The outcome of this, at the cellular level, is that changes in the number of YAP/TAZ/Yki molecules in the nucleus lead to changes in transcriptional activation of their target genes (Fig. 1B). Thus, YAP/TAZ/Yki nucleo‐cytoplasmic shuttling is not a binary state, as suggested by classical models, but rather a snapshot of a range of continuous nuclear and cytoplasmic shuttling dynamics (Fig. 1C).
Figure 1.
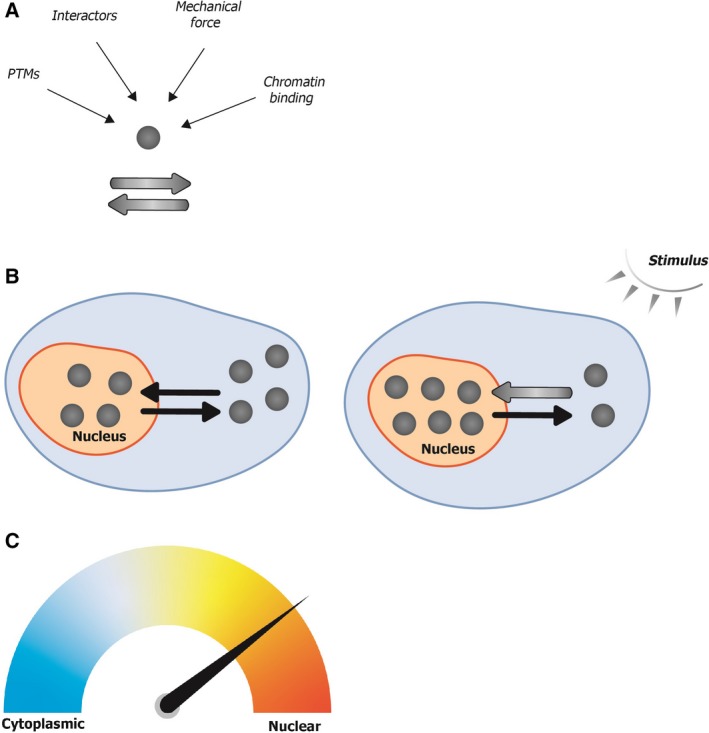
The balance between export and import rates dictates YAP/TAZ/Yki subcellular localization, which is a continuous and dynamic process. (A) At the single protein level, YAP/TAZ/Yki can be subject to several modes of regulation, such as post‐translational modifications (PTMs), interactions with specific proteins, binding to chromatin, and/or mechanical forces that alter nuclear pore permeability. The sum of these dictates the rates of YAP/TAZ/Yki nuclear export/import. Importantly, this nucleo‐cytoplasmic shuttling is continuously ongoing. (B) At the cellular level, the constant shuttling results in an equilibrium, making it appear as though some of the molecules reside stably in the nucleus while others remain cytoplasmic. When an activating stimulus is delivered to the cell, the balance between export and import is shifted, such that more molecules eventually end up in the nucleus. Gray circles represent single YAP/TAZ/Yki molecules. Black arrows represent the direction of translocation. A thicker gray arrow indicates a higher rate. (C) At the population level, YAP/TAZ/Yki nucleo‐cytoplasmic localization is not a binary state, but rather a range of states. The relative change between states is cell‐type‐ and/or stimulus‐dependent.
The complex subcellular regulation of YAP/TAZ/Yki underscores the need for their tight regulation in cell‐ and condition‐specific contexts. A combination of multiple points of regulation might allow a more refined response to different stimuli, which can result in a broad spectrum of nuclear to cytoplasmic YAP/TAZ/Yki ratios. Finally, the revised model suggests the existence of cell‐to‐cell variability with regard to YAP/TAZ/Yki subcellular localization, which might contribute to greater robustness at the population level.
3. Future perspectives
Several questions remain open. First, only a few exportins and importins have been shown so far to regulate directly YAP/TAZ/Yki nucleo‐cytoplasmic translocation (Ege et al., 2018; Wang et al., 2016b). It will thus be interesting to identify additional modulators of this process. Second, the apparent contradictions between some of the conclusions of the studies discussed above underline the importance of characterizing more comprehensively cell‐type‐ and condition‐specific YAP/TAZ/Yki subcellular dynamics. Furthermore, since YAP/TAZ display increased nuclear abundance in a variety of tumors (Zanconato et al., 2016b), it will be of great interest to elucidate the mechanisms responsible for their deregulated translocation in cancer cells.
Importantly, direct pharmacological inhibition of YAP/TAZ activity remains a clinical challenge. Current approaches toward meeting this challenge are reviewed in Guo and Teng (2015); Nakatani et al. (2016); Zanconato et al. (2016a, 2016b). For instance, a major effort is made for interfering with YAP/TAZ‐TEAD complexes. However, directly targeting YAP and TAZ may result in serious side effects, as YAP/TAZ are important for tissue homeostasis under physiological conditions. Thus, YAP/TAZ inhibition should preferably be targeted in a tissue‐specific and/or transient manner.
Inhibition of YAP/TAZ nuclear localization might serve as a potential therapeutic strategy, especially in view of the new insights gained from the aforementioned publications. One obvious approach might be to directly inhibit the nuclear export protein CRM1/XPO1. Indeed, this already represents a therapeutic promise in several types of cancer, and at least two such inhibitors are presently in clinical trials (Muqbil et al., 2018; Sun et al., 2016; Tai et al., 2014). Of course, these inhibitors will affect the localization of many additional proteins, but tumors with hyperactive nuclear YAP/TAZ may particularly be affected and may therefore be considered as prioritized candidates for such treatment. Furthermore, an interesting approach may be to target YAP/TAZ in the tumor microenvironment, especially in CAFs, in which YAP was shown to be activated and required for the tumor‐supportive functions of these stromal cells (Calvo et al., 2013). As shown by Ege et al. (2018), targeting actin or Src‐family kinases increases the rate of YAP export in CAFs. Consequently, YAP may become less nuclear and hence less active in the treated CAFs, thereby attenuating the contribution of the CAFs to tumor growth and therapy resistance (Chen and Song, 2019). YAP/TAZ nuclear accumulation may also be suppressed indirectly, by drugs that target signaling pathways responsible for increased nuclear translocation of YAP/TAZ. For example, one may consider in that regard inhibition of the mevalonate or the glucocorticoid receptor pathways (Sorrentino et al., 2014, 2017), for which there are already numerous FDA‐approved drugs. Lastly, as the above studies strengthen the link between the cytoskeleton and YAP/TAZ subcellular localization, existing cytoskeleton‐modulating drugs may be evaluated as YAP/TAZ‐targeted therapies (Piccolo et al., 2013); this is supported by the findings that inhibition of Rho or the actin cytoskeleton attenuates YAP/TAZ nuclear localization and transcriptional activity (Dupont et al., 2011; Ege et al., 2018; Piccolo et al., 2013; Wada et al., 2011).
Hopefully, answers to these questions will shed more light on the involvement of the Hippo pathway in tumorigenesis and open new directions for cancer therapy.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
We thank Yael Aylon for critically reading the manuscript.
References
- Calvo F, Ege N, Grande‐Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G et al (2013) Mechanotransduction and YAP‐dependent matrix remodelling is required for the generation and maintenance of cancer‐associated fibroblasts. Nat Cell Biol 15, 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Loo LS, Chong YF, Huang C and Hong W (2009) TEADs mediate nuclear retention of TAZ to promote oncogenic transformation. J Biol Chem 284, 14347–14358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X and Song E (2019) Turning foes to friends: targeting cancer‐associated fibroblasts. Nat Rev Drug Discovery 18, 99–115. [DOI] [PubMed] [Google Scholar]
- Chen HY, Yu SL, Ho BC, Su KY, Hsu YC, Chang CS, Li YC, Yang SY, Hsu PY, Ho H et al (2015) R331W Missense mutation of oncogene YAP1 is a germline risk allele for lung adenocarcinoma with medical actionability. J Clin Oncol 33, 2303–2310. [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A and Pan D (2007) Elucidation of a universal size‐control mechanism in Drosophila and mammals. Cell 130, 1120–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S et al (2011) Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183. [DOI] [PubMed] [Google Scholar]
- Ege N, Dowbaj AM, Jiang M, Howell M, Hooper S, Foster C, Jenkins RP and Sahai E (2018) Quantitative analysis reveals that actin and Src‐family kinases regulate nuclear YAP1 and its export. Cell Syst 6, 692–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elosegui‐Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz M, Kosmalska AJ, Oria R, Kechagia JZ, Rico‐Lastres P, Le Roux AL et al (2017) Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171, 1397–1410. [DOI] [PubMed] [Google Scholar]
- Gao J, He L, Shi Y, Cai M, Xu H, Jiang J, Zhang L and Wang H (2017) Cell contact and pressure control of YAP localization and clustering revealed by super‐resolution imaging. Nanoscale 9, 16993–17003. [DOI] [PubMed] [Google Scholar]
- Guo L and Teng L (2015) YAP/TAZ for cancer therapy: opportunities and challenges (review). Int J Oncol 46, 1444–1452. [DOI] [PubMed] [Google Scholar]
- Hall A (1998) Rho GTPases and the actin cytoskeleton. Science 279, 509–514. [DOI] [PubMed] [Google Scholar]
- Han SX, Bai E, Jin GH, He CC, Guo XJ, Wang LJ, Li M, Ying X and Zhu Q (2014) Expression and clinical significance of YAP, TAZ, and AREG in hepatocellular carcinoma. J Immunol Res 2014, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey KF, Zhang X and Thomas DM (2013) The Hippo pathway and human cancer. Nat Rev Cancer 13, 246–257. [DOI] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K and Pan D (2005) The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 122, 421–434. [DOI] [PubMed] [Google Scholar]
- Johnson R and Halder G (2014) The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discovery 13, 63–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler M, Speight P, Little D, Di Ciano‐Oliveira C, Szaszi K and Kapus A (2018) Mediated nuclear import and export of TAZ and the underlying molecular requirements. Nat Commun 9, 4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai D, Ho KC, Hao Y and Yang X (2011) Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Can Res 71, 2728–2738. [DOI] [PubMed] [Google Scholar]
- Lau AN, Curtis SJ, Fillmore CM, Rowbotham SP, Mohseni M, Wagner DE, Beede AM, Montoro DT, Sinkevicius KW, Walton ZE et al (2014) Tumor‐propagating cells and Yap/Taz activity contribute to lung tumor progression and metastasis. EMBO J 33, 468–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, Zhao S, Xiong Y and Guan KL (2008) TAZ promotes cell proliferation and epithelial‐mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol 28, 2426–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Adamovich Y, Reuven N and Shaul Y (2007) The Yes‐associated protein 1 stabilizes p73 by preventing Itch‐mediated ubiquitination of p73. Cell Death Differ 14, 743–751. [DOI] [PubMed] [Google Scholar]
- Lin KC, Moroishi T, Meng Z, Jeong HS, Plouffe SW, Sekido Y, Han J, Park HW and Guan KL (2017) Regulation of Hippo pathway transcription factor TEAD by p38 MAPK‐induced cytoplasmic translocation. Nat Cell Biol 19, 996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney JE, Mori M, Szymaniak AD, Varelas X and Cardoso WV (2014) The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev Cell 30, 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning SA, Dent LG, Kondo S, Zhao ZW, Plachta N and Harvey KF (2018) Dynamic fluctuations in subcellular localization of the hippo pathway effector Yorkie in vivo . Curr Biol 28, 1651–1660. [DOI] [PubMed] [Google Scholar]
- Mao B, Hu F, Cheng J, Wang P, Xu M, Yuan F, Meng S, Wang Y, Yuan Z and Bi W (2014) SIRT1 regulates YAP2‐mediated cell proliferation and chemoresistance in hepatocellular carcinoma. Oncogene 33, 1468–1474. [DOI] [PubMed] [Google Scholar]
- Muqbil I, Azmi AS and Mohammad RM (2018) Nuclear export inhibition for pancreatic cancer therapy. Cancers 138, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K, Maehama T, Nishio M, Goto H, Kato W, Omori H, Miyachi Y, Togashi H, Shimono Y and Suzuki A (2016) Targeting the Hippo signalling pathway for cancer treatment. J Biochem 161, 237–244. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Cordenonsi M and Dupont S (2013) Molecular pathways: YAP and TAZ take center stage in organ growth and tumorigenesis. Clin Cancer Res 19, 4925–4930. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Dupont S and Cordenonsi M (2014) The biology of YAP‐TAZ: hippo signaling and beyond. Physiol Rev 94, 1287–1312. [DOI] [PubMed] [Google Scholar]
- Ridley AJ and Hall A (1992) The small GTP‐binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70, 389–399. [DOI] [PubMed] [Google Scholar]
- Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, Manfrin A, Ingallina E, Sommaggio R, Piazza S et al (2014) Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol 16, 357–366. [DOI] [PubMed] [Google Scholar]
- Sorrentino G, Ruggeri N, Zannini A, Ingallina E, Bertolio R, Marotta C, Neri C, Cappuzzello E, Forcato M, Rosato A et al (2017) Glucocorticoid receptor signalling activates YAP in breast cancer. Nat Commun 14073, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strano S, Monti O, Pediconi N, Baccarini A, Fontemaggi G, Lapi E, Mantovani F, Damalas A, Citro G, Sacchi A et al (2005) The transcriptional coactivator Yes‐associated protein drives p73 gene‐target specificity in response to DNA Damage. Mol Cell 18, 447–459. [DOI] [PubMed] [Google Scholar]
- Sun Q, Chen X, Zhou Q, Burstein E, Yang S and Jia D (2016) Inhibiting cancer cell hallmark features through nuclear export inhibition. Signal Transduct Target Ther 16010, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YT, Landesman Y, Acharya C, Calle Y, Zhong MY, Cea M, Tannenbaum D, Cagnetta A, Reagan M, Munshi AA et al (2014) CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia 28, 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K, Itoga K, Okano T, Yonemura S and Sasaki H (2011) Hippo pathway regulation by cell morphology and stress fibers. Development 138, 3907–3914. [DOI] [PubMed] [Google Scholar]
- Wang S, Lu Y, Yin MX, Wang C, Wu W, Li J, Wu W, Ge L, Hu L, Zhao Y et al (2016b) Importin alpha1 mediates Yorkie nuclear import via an N‐terminal Non‐canonical nuclear localization signal. J Biol Chem 291, 7926–7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sinnett‐Smith J, Stevens JV, Young SH and Rozengurt E (2016a) Biphasic regulation of Yes‐associated Protein (YAP) cellular localization, phosphorylation, and activity by G protein‐coupled receptor agonists in intestinal epithelial cells: a novel role for Protein Kinase D (PKD). J Biol Chem 291, 17988–18005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu X, Maglic D, Dill MT, Mojumdar K, Ng PK, Jeong KJ, Tsang YH, Moreno D, Bhavana VH et al (2018) Comprehensive molecular characterization of the hippo signaling pathway in cancer. Cell Rep 25, 1304–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX and Guan KL (2013) The Hippo pathway: regulators and regulations. Genes Dev 27, 355–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanconato F, Battilana G, Cordenonsi M and Piccolo S (2016a) YAP/TAZ as therapeutic targets in cancer. Curr Opin Pharmacol 29, 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanconato F, Cordenonsi M and Piccolo S (2016b) YAP/TAZ at the roots of cancer. Cancer Cell 29, 783–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L et al (2007) Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21, 2747–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y and Yang X (2015) The Hippo pathway in chemotherapeutic drug resistance. Int J Cancer 137, 2767–2773. [DOI] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM et al (2008) TEAD mediates YAP‐dependent gene induction and growth control. Genes Dev 22, 1962–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


