Abstract
Znf703 is an RAR- and Wnt-inducible transcription factor that exhibits a complex expression pattern in the developing embryo: Znf703 mRNA is found in the early circumblastoporal ring, then later throughout the neural plate and its border, and subsequently in the mid/hindbrain and somites. We show that Znf703 has a different and separable function in early mesoderm versus neural crest and placode development. Independent of its early knockdown phenotype on Gdf3 and Wnt8, Znf703 disrupts patterning of distinct neural crest migratory streams normally delineated by Sox10, Twist, and Foxd3 and inhibits otocyst formation and otic expression of Sox10 and Eya1. Furthermore, Znf703 promotes massive overgrowth of SOX2+ cells, disrupting the SoxB1 balance at the neural plate border. Despite prominent expression in other neural plate border-derived cranial and sensory domains, Znf703 is selectively absent from the otocyst, suggesting that Znf703 must be specifically cleared or down-regulated for proper otic development. We show that mutation of the putative Groucho-repression domain does not ameliorate Znf703 effects on mesoderm, neural crest, and placodes. We instead provide evidence that Znf703 requires the Buttonhead domain for transcriptional repression.
Subject terms: Embryology, Xenopus, Pattern formation
Introduction
The neural plate border and non-neural ectoderm comprise a narrow arc of multipotent cells that circumscribe the neural plate1. Although cells of different lineages are comingled in this region early, they eventually segregate into neural, placodal, neural crest, and epidermal fates1–3. Znf703 belongs to a highly conserved family of NET (Noc, Nlz, Elbow, and Tlp-1) zinc finger transcriptional repressors and was previously studied in zebrafish, primarily in the mid/hindbrain4,5. Znf703 was recently shown to be expressed in the neural plate border of Xenopus where it partially overlaps with presumptive neural crest (Snai2)6. We recently identified Znf703 as a target of RARγ7, prompting further investigation into the role of Znf703 in early developmental processes that are sensitive to retinoic acid, such as neural crest and placodal patterning.
Because it is expressed at the neural plate border, Znf703 protein is positioned to interact with a variety of signaling pathways. Znf703 inhibits Wnt and Tgfβ signaling in vitro8,9, and reciprocally, Znf703 is a direct target of Wnt/β-catenin signaling in 293 T human embryonic kidney (HEK) cells, mouse, and Xenopus embryos8,10,11. There is also evidence that Znf703 is a direct target of T (Brachyury) in both mouse and Xenopus12,13. Furthermore, Znf703 is induced by retinoic acid (RA) signaling in early and late zebrafish gastrula embryos14. We recently showed that activation of RARγ1 is required for Znf703 expression at gastrula stage7.
Here we show that Znf703 is strongly modulated by RA throughout development: Znf703 mRNA is vastly expanded dorsally and rostrally by RA, obliterating the mid-hindbrain and neural plate border boundaries. We further show that mis/overexpression of Znf703 blurs the Sox10 expression domain such that normally segregated migratory streams (branchial, hyoid and mandibular) of neural crest are collapsed, aggregated, and devoid of overt patterning. We found that mis/overexpression of Znf703 causes a massive expansion of SOX2+ cells, while inhibiting expression of the placode marker, Eya1 as well as otic Sox10 expression. This results in the shrinkage or disappearance of the hollow ball of ectoderm that delineates an otocyst. Towards Znf703 function, we investigated the highly conserved FKPY domain of Znf703 and found that mutation of FKPY had minimal effect on transcriptional repression and developmental phenotypes compared to wild type, but that the buttonhead domain was indispensable for repression by Znf703. Finally, we show that phenotypes on otic and neural crest development are separable from early loss of mesodermal markers Gdf3 and Wnt8. Hence, despite early phenotypes that could potentially affect neural crest competence, Znf703 is still able to influence neural crest and placode patterning when overexpressed after gastrulation.
Methods
Phylogenetic tree
The Noc-family phylogenetic tree was constructed by aligning sequences with the MAFFT v7.306b (E-INS-i algorithm)15, then creating the tree using default settings, with bootstrap resampling set to 100016. The resultant tree was drawn with FigTree v1.4.2, rooting at the midpoint17.
Embryo microinjection, treatment, and in situ hybridization
All experiments in Xenopus were performed in accordance with the relevant guidelines and regulations, and approved by the Institutional Animal Care and Use Committee of the University of California, Irvine. Xenopus eggs were fertilized in vitro and embryos staged as described18. Embryos were microinjected bilaterally or unilaterally at the two- or four-cell stage with Znf703 (WT, mutant or inducible) mRNA together with 100 pg/embryo β-galactosidase (β-gal) mRNA lineage tracer (LT). Embryos were maintained in 0.1x MBS until appropriate stages. Embryos processed for whole-mount in situ hybridization (WISH) were fixed in MEMFA, stained with magenta-GAL (Biosynth), and then stored in 100% EtOH18. We reverse transcribed (Life Technologies) and sequenced the mRNA we microinjected to verify the identity of each Znf703 mRNA microinjected. For chemical treatments (DEX or TTNPB), embryos were transferred in groups of 25 to 60-mm glass Petri dishes with 10 mL of 0.1X MBS containing chemicals at the following concentrations: 5 μM dexamethasone or corresponding vehicle control (0.05% DMSO); 1 μM TTNPB or corresponding vehicle control (0.1% EtOH).
Whole mount in situ hybridization was performed on microinjected embryos as previously described18. All probes were prepared via PCR amplification of protein coding regions (~500–800 bp) from either cDNA or library clones with a bacteriophage T7 promoter at the 3′ end. Relevant primers are listed in Table S1. Probes were transcribed with MEGAscript® T7 (Life Technologies) in the presence of digoxigenin-11-UTP (Roche). Double WISH was conducted as previously described18. RT-QPCR was conducted as previously described19, and relevant primers are listed in Table S2.
Immunohistochemistry on vibratome sections and whole mounts
Embryos were embedded, sectioned and stained and embedded as described7. Transverse sections were labeled with primary antibody anti-SOX2 E-4 (1:100; Santa Cruz Biotechnology) followed by secondary antibody anti-mouse-647 (1:200; ThermoFisher) and DAPI nuclear stain (1:2000). Whole mount preparations were stained with DAPI, followed by clearing and mounting in Scale A220. Embryos were imaged on the Zeiss LSM880 confocal microscope at 20X magnification as described previously7.
Transient transfection and luciferase assays
pCDG1-Znf703 was constructed by PCR amplification of the protein-coding regions of Xenopus laevis cDNA and cloned into the NcoI-BamHI site of pCDG1. pCDG1-Znf703 mutant constructs were made by two-fragment PCR (primers listed in Table S3) to generate the conservative FKPY→LQAF21, or non-conservative FKPY→AAAA substitutions. pCDG1-Znf703 clones were sequence verified, and linearized with NotI. 5′-capped mRNA was transcribed using T7 mMESSAGE mMACHINE® Kit (Thermo Fisher Scientific). pCMX-Gal-Znf703 constructs were constructed by PCR amplification of pCDG1-Znf703 and cloned into the EcoRI-BamHI site of pCMX-Gal422 (primers listed in Table S4). COS7 cells were transiently transfected with Gal constructs as previously described23. Data are reported as normalized luciferase ± S.E.M. where reduction of luciferase activity compared to Gal4 alone indicates transcriptional repression. Statistical significance was determined using one-way ANOVA and Bonferroni post-hoc test in GraphPad Prism v5.0.
Results
Znf703 is a highly conserved transcriptional repressor that exhibits a complex expression pattern in mesoderm, neural plate, and mid/hindbrain
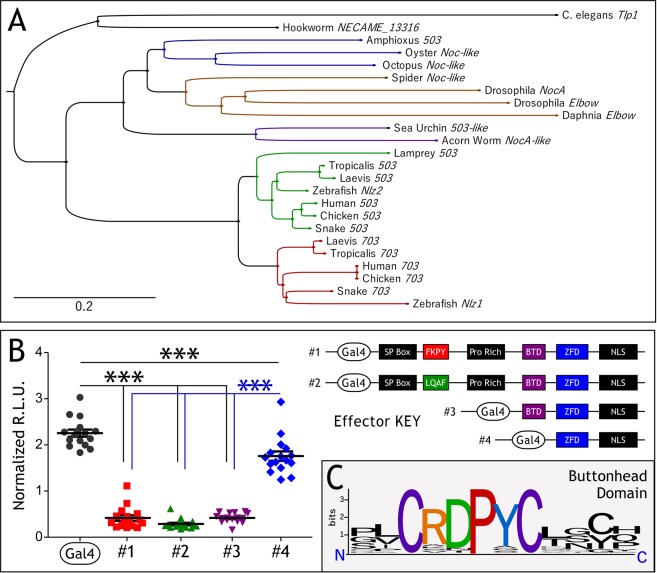
Znf703 and Znf503 are members of the NET (Noc, Nlz, Elbow, and Tlp-1) family of zinc finger transcriptional repressors. We constructed a phylogenic tree of representative members of the NET family and show that orthologs of Znf703 and Znf503 occur in a diverse number of taxonomic groups (Fig. 1A). ZNF703 and ZNF503 proteins have a conserved FKPY motif which was predicted to recruit Groucho based on its similarity to Brinker and Elbow FKPY24,25 and Hucklebein FRPW26. We performed transient transfection assays using four different Gal4 effector constructs, mutating the putative Groucho-interacting domain of Xenopus Znf703 from FKPY to LQAF, or serially deleting the proline rich and buttonhead domains (Fig. 1B). We assayed the ability of these constructs to induce luminescence using a Gal4-luciferase reporter. Reduction of luciferase activity compared to Gal4 alone indicates transcriptional repression by ZNF703. Only deletion past the buttonhead domain relieved repression by ZNF703 (Fig. 1B). The buttonhead domain is highly conserved across all species shown in Fig. 1A, with the two cysteine and proline residues never deviating from the consensus (Fig. 1C). Although neither function nor interacting partners have been characterized for the buttonhead domain, we infer that this domain is likely to be required for transcriptional repression by ZNF703.
Figure 1.
Znf703 is a conserved transcriptional repressor. (A) Phylogenetic tree of Znf703 sequences aligned and constructed with MAFFT (see materials and methods). The scale bar represents the divergence distance of 0.2 amino acid substitutions per site of the Znf703 sequence. The tree segregates organisms appropriately with the exception of Amphioxus. We attempted to add more sequences to improve this unexpected result, but Amphioxus persistently segregated with mollusk sequences. (B) Cos7 cells were transfected with 5:5:1 DNA ratio of reporter (Gal4-Luc): β-gal: effector (Znf703). The y-axes represent relative light units measured by the luminometer normalized to β-gal activity. Basal reporter activity (Gal4 alone) is repressed by Znf703. Mutating the FKPY domain or deletion of the N-terminus up until the Buttonhead (Btd) domain still represses transcriptional activity. Deletion beyond the Btd domain relieves repression. Statistical significance was determined using one-way ANOVA, and Bonferroni post-hoc test in GraphPad Prism v5.0 (***P ≤ 0.001). (C) Conservation of the Btd domain across the animal kingdom, visualized with WebLogo66.
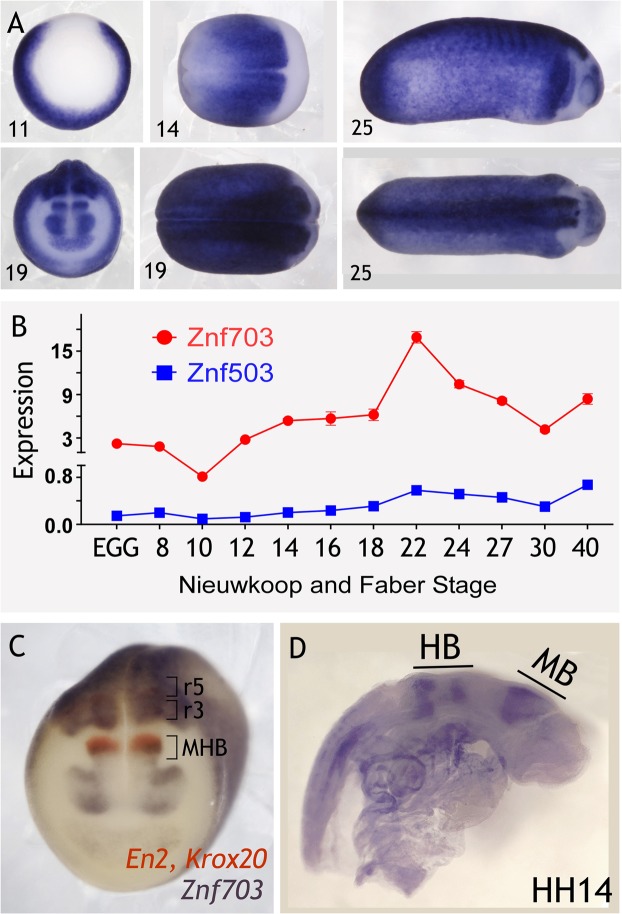
We characterized Xenopus expression of Znf703 (Fig. 2A–C) and Znf503 (Fig. 2B, S1) expression over developmental time. Znf703 and Znf503 are expressed in the circumblastoporal ring of the stage 10 gastrula, but absent from the organizer, reminiscent of Ventx2 or Wnt8 expression domains. At stage 14, Znf703 is expressed broadly in both the neural plate and the border zone, but is absent from the anterior. By stage 19, Znf703 is expressed in the eye/forebrain, absent from r1/r2, then forms a sharp boundary at r3/r4, as shown previously6. We also performed WISH for Znf503 expression, which is weaker and less sharp, but generally shows a similar expression pattern to Znf703, albeit without anterior expression at neurula stage (Fig. S1). Xenbase expression data is not available for X. laevis Znf503, but X. tropicalis shows a 10-to-17-fold magnitude difference in transcript counts between Znf703 and the lower expressed Znf503 at stages 11–12.5. Our QPCR expression analysis in X. laevis verifies that Znf703 is more highly expressed than Znf503 (Fig. 2B).
Figure 2.
Expression of Znf703 and Znf503 across developmental time. (A) Whole mount in situ hybridization of Znf703 expression at Nieuwkoop and Faber developmental stages 11 (vegetal view, dorsal at the top), 14 (dorsal view, anterior on the left), 19 (dorsal and anterior views), and 25 (lateral and dorsal views, anterior on the right). WISH of Znf503 expression can be found in Fig. S1. (B) Double WISH at stage 19 reveals the spatial relationship between Znf703 and midbrain (Engrailed 2) and hindbrain (Krox20) markers. (C) Znf703 expression in a chick embryo at Hamburger Hamilton (HH) Stage 16 (lateral view; anterior on the right). MB = midbrain; HB = hindbrain. (D) QPCR showing Znf703 and Znf503 gene expression averaging two biological replicates over developmental time. Error bars = S.E.M. The y-axis represents 2−ΔCt values (adjusted for primer efficiency), normalized to reference gene, Histone H4.
Double WISH revealed that Znf703 expression overlaps with En2 and Krox2 (Fig. 2C). This mid-hindbrain expression is concordant with zebrafish expression data where Nlz1 is found at both the mid-hindbrain boundary, and the hindbrain, but only as rostral as rhombomere 3, and later expanding to rhombomere 25,14,27. In chick embryos, Znf703 also yields sharp boundaries of expression in the hindbrain at E2.5 (~HH14) (Fig. 2D). The murine ortholog, Zfp703, also shows similar expression to chicken8. These results demonstrate that Znf703 sequence and spatial expression are highly conserved among fish, birds, amphibians, and mammals.
Znf703 is modulated by RA
In Janesick, Tang et al., 2018, we identified Znf703 as a target of RAR signaling by RNA-seq and showed that RARγ1 was required for its expression in the Xenopus gastrula. Treatment at stage 6/7 with 1 µM of the RAR-selective agonist, TTNPB, showed that Znf703 is responsive to modulation of RAR signaling across developmental time (Fig. 3). Znf703 is normally absent from the organizer, but is ectopically expanded in the presence of TTNPB. By stage 16, TTNPB causes Znf703 to be expressed throughout the embryo such that the normal anterior boundary of Znf703 is obliterated–this continues throughout stages 20 and 30. Treatment with the RAR-selective antagonist, AGN193109, elicited only subtle alterations in Znf703 expression (data not shown).
Figure 3.
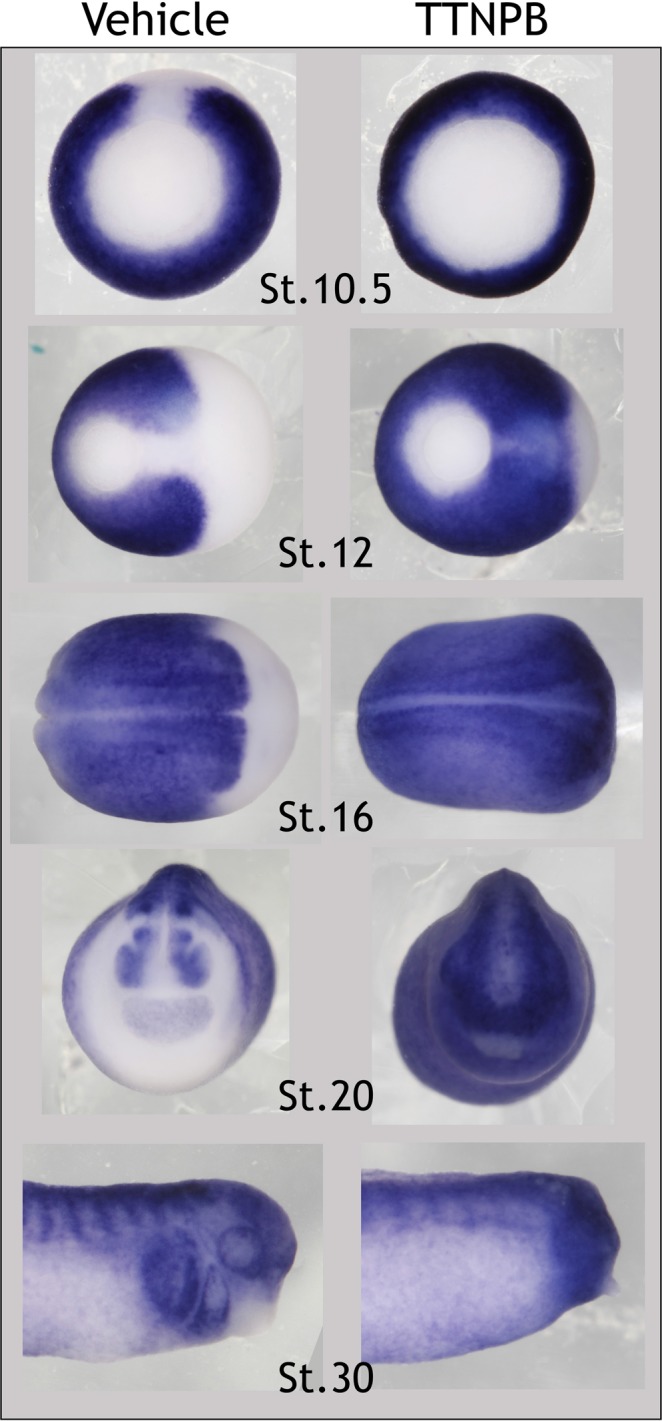
Znf703 expression is modulated by RAR-selective agonist TTNPB. WISH from embryos treated at stage 6/7 with 1 µM TTNPB or control vehicle (0.1% EtOH). TTNPB greatly expands expression of Znf703 into the dorsal and anterior domains, relative to control vehicle. Stage 11 embryos are shown in vegetal view with the dorsal lip at the top. Stage 12 and 16 are shown in dorsal view with anterior to the right. Stage 20 embryos are shown in anterior view. Stage 30 embryos are shown in lateral view with anterior to the right.
Znf703 effect on early mesoderm is separable from its disruption of placode and neural crest patterning
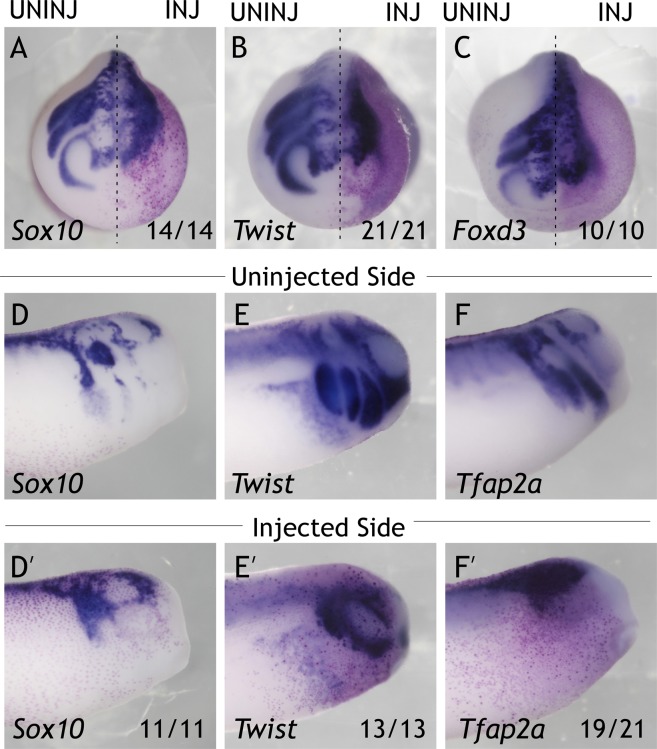
Recent data has shown that modulation of Znf703 activity affects neural crest markers Sox10 and Snai26. When Znf703 is overexpressed, we observed knockdown of early neural crest markers Foxd3 and Tfap2a (Fig. S2). At later stages, Sox10, Twist, and Foxd3 expression domains do not separate into distinct migratory streams, compared to the uninjected side (Fig. 4A–C). Rather, the cells marked by Sox10, Twist, and Foxd3 are aggregated together with no discernible pattern. By tailbud stage, this effect is further manifested in the lack of ventral migration of crest markers into the epibranchial domain (Fig. 4D’-F’). Manipulation of the FKPY (putative Groucho) domain of Znf703 did not affect the overexpression phenotype of Znf703 on the markers we tested (Fig. S3).
Figure 4.
Overexpression of Znf703 mRNA inhibits neural crest migration. Embryos were injected unilaterally at 2- or 4-cell stage with either 0.5 ng Znf703 (A–F’) or control mCherry. (A–C) Znf703 mRNA reduces the lateral and anterior expression of Sox10, Twist, and Foxd3 in stage 19 embryos (shown in anterior view). Injected side is to the right of the dotted line, and is indicated by the magenta β-gal lineage tracer. (D’–F’) Znf703 mRNA inhibits Sox10 patterning, Twist, and Tfap2a in stage 27 embryos (shown in lateral view). (D–F) Uninjected side of the same embryo. Fractions represent the portion of embryos displaying the phenotype.
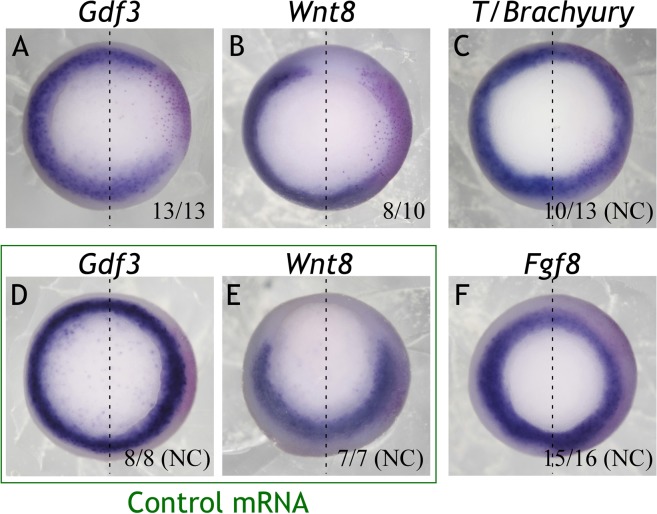
Manipulation of Znf703 yields nearly identical phenotypes in loss-of-function and gain-of-function experiments6. Such outcomes can often be ascribed to the manipulation of a gene that has a different function early versus late in development, and disrupting both functions can confound interpretation of results28. Znf703 is such a gene since it is expressed in early mesoderm (Fig. 2A). Mis/overexpression of Znf703 yields selective loss of mesodermal markers Wnt8 and Gdf3 (Fig. 5A,B) without affecting T/Brachyury or Fgf8 (Fig. 5C,F); control mRNA (mCherry) did not alter Wnt8 or Gdf3 expression (Fig. 5D,E). There is ample evidence that early blastula and gastrula stage events (mesoderm formation, hypoblast signaling, pluripotency retention, etc.) contribute to neural crest and placode development29–33. We hypothesized that early loss of Wnt8 and/or Gdf3 could contribute to the effect of Znf703 on neural crest patterning. To test this hypothesis, we designed dexamethasone (DEX)-inducible34 hGR-Znf703 constructs to separate the effects of Znf703 at gastrula, neurula and tailbud stages (Fig. 6A). A preliminary titration revealed the dose of mRNA (0.2 ng) needed to avoid saturation of the heat-shock protein which tethers hGR-Znf703 outside of the nucleus until DEX binding. This is the dose at which we observed no effect on Sox10 expression in vehicle (DMSO) treated embryos (Fig. S4).
Figure 5.
Overexpression of Znf703 mRNA reduces expression of Gdf3 and Wnt8, but not T or Fgf8. Embryos were injected unilaterally at 2- or 4-cell stage with either 0.5 ng Znf703 or control mCherry mRNA. Injected side is to the right of the dotted line, and is indicated by the magenta ß-gal lineage tracer. (A–C,F) Znf703 mRNA causes loss of Gdf3, knockdown of Wnt8, and weak knockdown or no change of T and Fgf8. Embryos are shown at stage 10.5/11 in vegetal view. (D,E) Control mRNA did not have any effect on Gdf3 or Wnt8. Fractions represent the portion of embryos displaying the phenotype. NC = No change.
Figure 6.
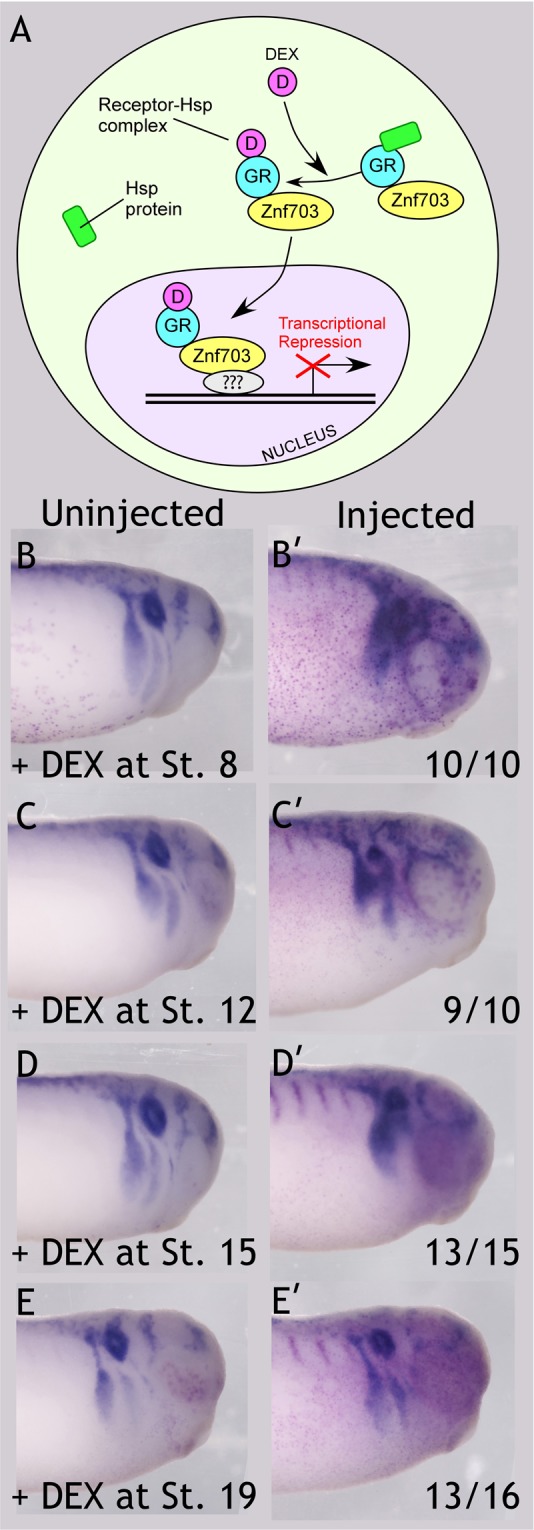
Znf703 impairs Sox10 expression at tailbud stage, when induced after stage 12 up until stage 19. (A) Schematic of ligand-inducible transcriptional repression by hGR-Znf703. In the absence of dexamethasone (DEX), hGR-ZNF703 is tethered in the cytoplasm by HSP90. In the presence of DEX, hGR-ZNF703 is free to enter the nucleus. Question marks indicate that Znf703 is not thought to bind DNA directly. (B–E’) Embryos were injected unilaterally with 0.2 ng hGR-Znf703 mRNA at 2- or 4-cell stage, then treated with 5 µM DEX or 0.05% DMSO vehicle at the stages indicated. Injected side is indicated by the magenta β-gal mRNA lineage tracer. Znf703 blurs the migratory streams of Sox10 expression when Znf703 is induced after gastrulation and prior to stage 19. Little effect on Sox10 is observed when embryos are treated with DEX at stage 19. DMSO treated embryos are pictured in Fig. S4. All embryos are shown in lateral view with anterior on the right, at stage 27. Fractions represent the portion of embryos displaying the phenotype in one time-course experiment from the same clutch of embryos. This experiment was repeated an additional time, and very similar results were obtained.
When Znf703 expression was induced prior to gastrulation (stage 8) we observed loss of Wnt8 and Gdf3 (Fig. S5), confirming that the hGR-Znf703 construct yields the same results as wildtype Znf703. When Znf703 is induced after gastrulation (stage 12 or 15), neural crest gene expression is not noticeably altered at stage 18 (Fig. S6). However, by stage 27, we found that clarity in the segregation of migratory streams is lacking, and the otocyst is compromised (Fig. 6C–D’). This effect on Sox10 was nearly identical to Znf703 mis/overexpression induced before gastrulation (cf Fig. 6B,B’) or non-inducible Znf703 mis/overexpression (Fig. 4D,D’). We observe a slight improvement in the size and posterior-lateral positioning of the otocyst when DEX is provided at stage 15, but epibranchial and lateral line patterning remain perturbed at tailbud stage. From this, we conclude that the early loss of Gdf3 and Wnt8 (Figs 5A,B and S5) by Znf703 mis/overexpression does not significantly impact Sox10 expression later. Hence, Znf703 has a different and separable function in mesoderm versus neural crest development. Finally, treatment with DEX at stage 19 showed minimal effect on Sox10 expression (Fig. 6E,E’).
Znf703 promotes anterior SOX2 expression at the expense of otic development
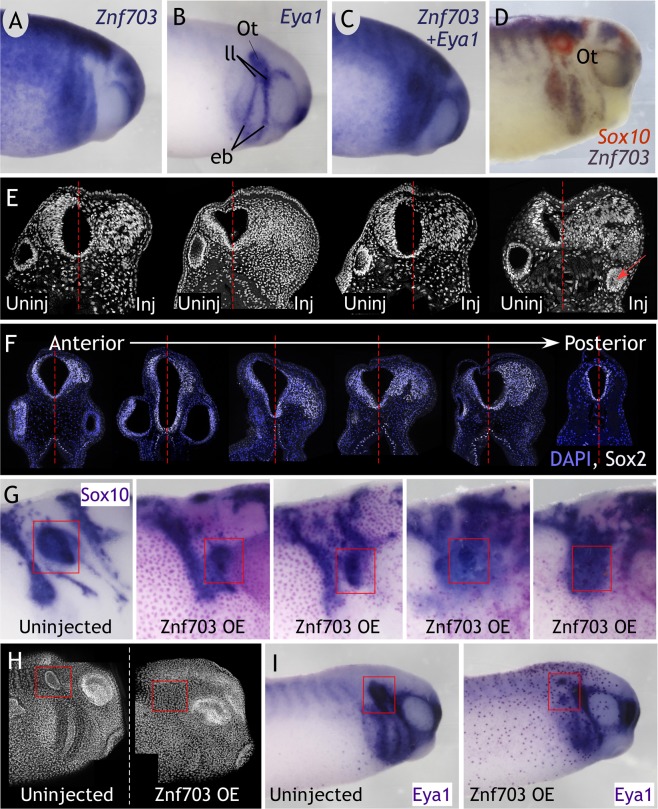
In tailbud embryos, Znf703 is strikingly absent from the otocyst, epibranchial placode, and lateral line, where Eya1 is normally expressed (Fig. 7A–C). Eya1 is a weak probe for double WISH, therefore, we stained with the stronger Sox10 probe along with Znf703 to confirm that Znf703 is completely absent from the otocyst (Fig. 7D). Upon sectioning tailbud-stage embryos microinjected with Znf703 after gastrulation, we consistently observed the absence of an otocyst on the injected side in transverse sections through the head (Fig. 7E,F). Similarly, in laterally-mounted whole embryos, confocal imaging through the surface towards the midline did not reveal an otocyst on the injected side (Fig. 7H), and otic markers Eya1 and Sox10 were significantly reduced (Fig. 7G,I). Concurrently, we observed a massive overgrowth of cells in the anterior neural tube (Fig. 7E), which we determined to be SOX2-positive (Fig. 7F). This proliferative effect is also observed when Znf703 is induced after gastrulation, and is only seen in the anterior domain: the degree of SOX2 expansion diminished in sections taken posterior to the otocyst (Fig. 7F). The fate of these SOX2-expressing cells remains unclear, because Sox2 is widely expressed in development. Perhaps one of the more well-known developmental processes of Sox2 is in the neurogenic lineage, but we found no increase in primary neurons when Znf703 is overexpressed (Fig. S7). Alternatively, increased Sox2 expression could be promoting cell proliferation without driving a specific cell fate.
Figure 7.
Znf703 is normally absent from the otocyst. Znf703 overexpression causes aberrant SOX2 expression and disappearance of otocyst. (A–D) Znf703 is absent from the otocyst as marked by Eya1 and Sox10, and lateral line placode as marked by Eya1, shown in lateral view at stage 27. Ot = otocyst; ll = lateral line; eb = epibranchial placodes. Embryos were injected unilaterally at the 2- or 4-cell stage with 0.5 ng Znf703 mRNA (E,G–I) or 0.2 ng hGR-Znf703 mRNA and treated with DEX at stage 12 (F). (E,F) Maximum intensity projections of Dapi nuclear stain and/or SOX2 from confocal images of transverse sections (expansion of SOX2 observed in 8/8 embryos sectioned). Otocysts were not found on the injected side in 60% of embryos. In the remaining 40% of embryos, the otocyst was reduced in size (red arrow). (G) Sox10 expression marks otocysts that are significantly reduced in size and positioned more rostral/ventral in the head compared to control (Stage 27 embryo in lateral view). (H) Maximum intensity projection of Dapi (false-colored in white) from confocal images through a laterally-mounted stage 27 embryo. (I) Eya1 expression is reduced, particularly in the otic vesicle on the injected side (Stage 27 embryo in lateral view) (15/15 embryos). Red boxes in H and I highlight the otocyst on the uninjected side and its absence on the injected side.
Discussion
Znf703 is an RAR-inducible transcriptional repressor
Znf703 is an intriguing potential effector of cross-talk between RA, Wnt, Noggin, and Nodal signaling. Znf703 has been associated with both Nodal and Noggin6,35 and is a direct target of Wnt pathways8,11. Znf703 is induced by RA in P19 embryonal carcinoma cells and in zebrafish14,36 and was also identified by microarray to be RA-inducible the E8.5 mouse37. The data we have presented further affirms Znf703 as an RA responsive gene in vivo. Whether Znf703 is also a direct target of RAR is an open question. We found no discernable RAR binding site within 20 kilobases upstream or downstream of Znf703, or within the gene itself. Existing ChIP data in mouse in vitro studies do not identify Znf703 as a direct target38–40. This does not prove that the interaction between RAR and Znf703 is indirect, but rather indicates that additional, in vivo, ChIP analysis will be required to conclusively establish whether Znf703 is a direct or indirect target of RA. Furthermore, RAR occupancy on DNA does not necessarily equal transcriptional activation since RARs can act as repressors in the absence of RA41,42.
When Znf703 was originally characterized, it was hypothesized that the FKPY domain was recruiting the transcriptional corepressor Groucho based on its similarity to Brinker and Elbow FKPY24,25 and Hucklebein FRPW26, domains that are required for Groucho interaction. Murine Zfp703 and Zfp503 have been named Zeppo1 and Zeppo29,43, that are undoubtedly referencing the relationship to Groucho and Zeppo Marx. However, deletion analysis in zebrafish showed that there was no effect on the Groucho interaction when the N-terminus and FKPY of Znf703/Zeppo1 were removed5. In addition, the FKPY motif in Brinker did not affect the ability of a stripe2-Brinker transgene to repress dpp25. Similarly, we demonstrated that mutating the FKPY domain did not affect transcriptional repression, nor did it exacerbate or weaken phenotypes associated with wild type Znf703 expression on mesoderm or neural crest markers. Thus, we infer that transcriptional repression by Xenopus Znf703 requires the Buttonhead domain. Future studies will investigate how the Buttonhead domain of Znf703 mediates transcriptional repression during embryonic development and whether a combination of domains is required for maximal repression, in vivo.
Znf703 overexpression hinders neural crest development after gastrulation
Misexpressing or overexpressing a transcriptional repressor from early stages in development can often result in confounding phenotypic effects28. Gain- and loss-of-function analysis of Znf703 both resulted in loss of neural crest genes Snai2 and Sox10 6. We showed that Znf703 inhibits expression of mesodermal markers Gdf3 and Wnt8 at gastrula stage and disrupts neural crest migration and patterning in the neurula and tailbud stage embryo. The dorsolateral marginal zone in Xenopus, where Znf703 is co-expressed with Wnt8 and Gdf3, is thought to be responsible for neural crest induction29–31. Furthermore, if loss of Wnt8 in Znf703-injected embryos is indicative of Wnt levels in the early gastrula, then neural crest competence would be compromised32. Therefore, it is not inconceivable that early deficiency in Wnt8 and Gdf3 expression at gastrula stage would have direct consequences on neural crest later.
To test this possibility, we designed hormone-inducible Znf703 expression constructs. We found that Znf703 is still fully capable of perturbing neural crest and placode patterning at tailbud stage when induced after stage 12, thus demonstrating that Znf703 has a role in early mesoderm development that is separable from its effects on neural crest and placode. A proline- and tyrosine-rich domain in the C-terminus ensures Znf703 and Znf503 nuclear localization4,43,44; however, with only one zinc finger present, it is assumed that these proteins lack the ability to bind DNA directly45. Znf703 and Znf503 are related to the SP family of proteins which can function as both co-activators and co-repressors46. Thus, Znf703 likely behaves as a transcriptional cofactor, potentially with multiple binding partners depending on the cellular context. Based on our results, it is plausible that Znf703 possesses a different interactome in mesoderm versus neural crest, which is an interesting area of future study.
Our hormone-inducible Znf703 experiments clearly resolve early (gastrula) versus late (neurula) events, but we can also propose a role for Znf703 in induction/specification, delamination and migration of the neural crest. When Dex is administered at stage 15, the Znf703 protein is likely to translocate into the nucleus by around stage 18 (two hours later47), when neural crest cells are beginning to segregate and migrate, but induction/specification has already transpired48. At stage 18, we cannot detect any changes in neural crest patterning when Znf703 is induced at stage 12 or stage 15. However, by stage 27, we observed significant loss of defined Sox10 migratory streams. These data are consistent with a model in which Znf703 is primarily affecting delamination/migration, which agrees with its known role in epithelial-mesenchymal transitions (EMTs) and regulation of E-cadherin9,43. Induction of Znf703 beyond stage 19 was no longer detrimental to neural crest patterning. Nevertheless, we did not evaluate bone, cartilage or pigment in tadpoles to completely rule out the role of Znf703 in neural crest differentiation.
Znf703-induced SOX2 expansion at the neural plate border
Our results and those of Hong, 2017 have established that Znf703 is expressed in the neural plate border. The neural plate border is a small region where progenitors of multiple different lineages mingle and signal dynamically over very small distances1. Recently, this was shown at the single cell, molecular level where early neural plate stages show significant overlap of transcription factor expression that later become restricted to separate lineages2. Misexpression of Znf703 expands Sox2 mRNA expression at the open neural plate stage 14/15, and this expansion seemed more pronounced in the anterior6. In agreement with these data, we showed that Znf703 mis/overexpression causes prodigious expansion of the SOX2 protein domain at tailbud stage, observable only in the head.
A preponderance of ectopic SOX2+ expressing cells found in the anterior neural plate of Znf703-overexpressing embryos will undoubtedly upset the balance of intricate signaling and lineage decisions occurring at the neural plate border. Misexpression of Sox2 in quail inhibits Slug expression and neural crest migration49. Sox3 gain-of-function delays neural crest induction, reduces migration, and disrupts branchial cartilage development, while promoting the neural progenitor fate50. The precedent for Sox2/3 overexpression altering the boundary between neural and non-neural ectoderm supports the argument that the SOX2 expansion we observe is causal to the loss of Foxd3 and Tfap2 in the early neurula. Nevertheless, the consequence of copious numbers of SOX2+ cells, induced by Znf703, is not easily interpretable given that the SoxB1 family is notoriously perplexing with respect to its spatial and temporal expression and regulation of proliferation/pluripotency, cell lineage, and differentiation. It is unclear whether extra SOX2+ cells are simply indicative of proliferation, without an associated lineage, or if SOX2 is preferentially driving a specific cell fate, at the expense of another. Inducible Eya1 overexpression phenocopies our results on Sox2, including the failure of the otic vesicle to form Fig. 7 51. These authors concluded that ectopic SoxB1 stabilized a placodal, neurogenic progenitor fate, at the expense of differentiation51. Similarly, we found that Znf703 did not increase N-tubulin expression after gastrulation, therefore, SOX2-expressing cells might commit cells towards a neurogenic lineage, but cannot drive differentiation without additional signals. As a result, the SOX2-expressing cells might later die, since they are unable to differentiate at the correct time and place in the embryo. Alternatively, the ectopic SOX2+ cells contribute to neural crest and epidermal derivatives as found recently in chicken by Roellig and colleagues, 20172. Deciphering the identity of Znf703-induced SOX2+ cells, is an interesting area of future study.
Znf703 and otic development
Our group has a long-standing interest in RA and placode development. We previously found that RA is essential for establishing the posterior-lateral boundary of the preplacodal ectoderm18. Znf703 (orthologous to the invertebrate no ocelli) has been linked to sensorineural development throughout evolution52,53. Nlz morphant zebrafish fail to close the optic fissure in the ventral region of the developing eye54. Znf503 is a negative regulator of Gata355 which is expressed in the preplacodal ectoderm56 and is a critical factor in specifying the prosensory domain of the otocyst57,58. Znf703 is also cistronic to Fgfr1, important for maintaining Sox2+ progenitors in the Organ of Corti59.
In this current study, we noted that Znf703 is almost an inverted image of Eya1, as revealed by double WISH. Transverse sections through Znf703-injected embryos at the r5/r6 level showed otocyst absence or reduction in size, as well as changes in its posterior-lateral position in the head, and subsequent loss of Eya1 and Sox10 otic expression. The implication is that local Znf703 must be specifically cleared or down-regulated in the developing otocyst, otherwise Znf703 would inappropriately repress genes required for ear development. The developing embryo is exquisitely sensitive to RA concentration; therefore, local retinoid levels in the otocyst must be tightly regulated (reviewed in60). Local boundaries of RA during development are often established by opposing Fgf signals61. One possible scenario is that RA induces Znf703 to inhibit Gata3, whose primary target is Fgf1062, thus RA would inhibit FGF signaling via Znf703. A second possibility concerns RA-Wnt interactions. Wnt signaling is required for specifying the otic fate, and when Wnt is inhibited, the placode territory instead becomes epidermal or epibranchial63,64. Others have established Znf703 as a Wnt inhibitor; therefore, Znf703 could regulate the fate decision and/or boundary delineation of the epidermal/epibranchial (low Wnt) versus otic (high Wnt) lineages by modulating Wnt signaling.
Conclusion
Znf703 is an important point of crosstalk among RAR, WNT, and TGFβ (among other) signaling pathways, and exhibits a complex expression pattern early in the circumblastoporal ring and neural plate border, and later in crest, placode, hindbrain, and somites. In this paper, we focused on addressing the role of Znf703 in neural crest and otic development, but Znf703 is likely to affect other lineages at the neural plate border such as anterior placode (e.g., lens) and mesodermal derivatives (somites). Znf703 is poised to interpret local RA levels while, simultaneously, modulating Wnt signaling. Therefore, Znf703 is anticipated to be an important ingredient in many embryonic processes where Wnt-RA crosstalk is required. Znf703 is unlikely to bind DNA directly, and therefore, Znf703 may potentially be promiscuous in its interaction with other transcription factors. Detailed biochemical analysis will be required to understand how Znf703 functions, which co-repressor(s) interacts with Znf703, and which exact domain, or combination of domains are responsible for transcriptional repression. Finally, the translational implications of RA-regulation of Znf703 are intriguing because RA is known to improve outcomes in a limited number of cancers65, but Znf703 acts an oncogene to promote adult luminal B breast cancer in humans35.
Supplementary information
Acknowledgements
This work was supported by grants from the National Science Foundation (IOS-0719576, IOS-1147236) to B.B. We thank Dr. Stefan Heller (Stanford University) and the Stanford Otolaryngology Imaging Core (Lars Becker) for generous use of the vibratome and confocal microscopes which were essential for Figure 7 of this manuscript. A.J. is currently supported by the A.P. Giannini Foundation. We thank Navid Zebarjadi for synthesizing the chicken Znf703 probe.
Author Contributions
A.J. and B.B. conceived the experiments and wrote the manuscript. A.J., B.B. and W.T. executed all the experiments. K.A. aided in cloning the Znf703 constructs and photographing/documenting embryos.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-44722-1.
References
- 1.Groves AK, LaBonne C. Setting appropriate boundaries: fate, patterning and competence at the neural plate border. Dev Biol. 2014;389:2–12. doi: 10.1016/j.ydbio.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roellig, D., Tan-Cabugao, J., Esaian, S. & Bronner, M. E. Dynamic transcriptional signature and cell fate analysis reveals plasticity of individual neural plate border cells. Elife6 (2017). [DOI] [PMC free article] [PubMed]
- 3.Plouhinec JL, et al. A molecular atlas of the developing ectoderm defines neural, neural crest, placode, and nonneural progenitor identity in vertebrates. PLoS Biol. 2017;15:e2004045. doi: 10.1371/journal.pbio.2004045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Runko AP, Sagerstrom CG. Isolation of nlz2 and characterization of essential domains in Nlz family proteins. J Biol Chem. 2004;279:11917–11925. doi: 10.1074/jbc.M310076200. [DOI] [PubMed] [Google Scholar]
- 5.Runko AP, Sagerstrom CG. Nlz belongs to a family of zinc-finger-containing repressors and controls segmental gene expression in the zebrafish hindbrain. Dev Biol. 2003;262:254–267. doi: 10.1016/S0012-1606(03)00388-9. [DOI] [PubMed] [Google Scholar]
- 6.Hong, C. S. & Saint-Jeannet, J. P. Znf703, a novel target of Pax3 and Zic1, regulates hindbrain and neural crest development in Xenopus. Genesis55 (2017). [DOI] [PMC free article] [PubMed]
- 7.Janesick, A., Tang, W., Shioda, T. & Blumberg, B. RARgamma is required for mesodermal gene expression prior to gastrulation in Xenopus. Development145 (2018). [DOI] [PubMed]
- 8.Kumar A, et al. Zfp703 Is a Wnt/beta-Catenin Feedback Suppressor Targeting the beta-Catenin/Tcf1 Complex. Mol Cell Biol. 2016;36:1793–1802. doi: 10.1128/MCB.01010-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slorach EM, Chou J, Werb Z. Zeppo1 is a novel metastasis promoter that represses E-cadherin expression and regulates p120-catenin isoform expression and localization. Genes Dev. 2011;25:471–484. doi: 10.1101/gad.1998111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura, Y., de Paiva Alves, E., Veenstra, G. J. & Hoppler, S. Tissue and stage-specific Wnt target gene expression is controlled subsequent to beta-catenin recruitment to cis-regulatory modules. Development143, 1914–1925 (2016). [DOI] [PMC free article] [PubMed]
- 11.Kjolby, R. A. & Harland, R. M. Genome-wide identification of Wnt/beta-catenin transcriptional targets during Xenopus gastrulation. Dev Biol (2016). [DOI] [PMC free article] [PubMed]
- 12.Lolas M, Valenzuela PD, Tjian R, Liu Z. Charting Brachyury-mediated developmental pathways during early mouse embryogenesis. Proc Natl Acad Sci USA. 2014;111:4478–4483. doi: 10.1073/pnas.1402612111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gentsch GE, et al. In vivo T-box transcription factor profiling reveals joint regulation of embryonic neuromesodermal bipotency. Cell Rep. 2013;4:1185–1196. doi: 10.1016/j.celrep.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andreazzoli M, Broccoli V, Dawid IB. Cloning and expression of noz1, a zebrafish zinc finger gene related to Drosophila nocA. Mech Dev. 2001;104:117–120. doi: 10.1016/S0925-4773(01)00359-8. [DOI] [PubMed] [Google Scholar]
- 15.Katoh K, Kuma K, Toh H, Miyata T. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005;33:511–518. doi: 10.1093/nar/gki198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuraku S, Zmasek CM, Nishimura O, Katoh K. aLeaves facilitates on-demand exploration of metazoan gene family trees on MAFFT sequence alignment server with enhanced interactivity. Nucleic Acids Res. 2013;41:W22–28. doi: 10.1093/nar/gkt389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rambaut, A. FigTree v1.4.2, http://tree.bio.ed.ac.uk/software/figtree (2009).
- 18.Janesick A, Shiotsugu J, Taketani M, Blumberg B. RIPPLY3 is a retinoic acid-inducible repressor required for setting the borders of the pre-placodal ectoderm. Development. 2012;139:1213–1224. doi: 10.1242/dev.071456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janesick A, Tang W, Nguyen TTL, Blumberg B. RARbeta2 is required for vertebrate somitogenesis. Development. 2017;144:1997–2008. doi: 10.1242/dev.144345. [DOI] [PubMed] [Google Scholar]
- 20.Hama H, et al. Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat Neurosci. 2011;14:1481–1488. doi: 10.1038/nn.2928. [DOI] [PubMed] [Google Scholar]
- 21.Bordo D, Argos P. Suggestions for “safe” residue substitutions in site-directed mutagenesis. J Mol Biol. 1991;217:721–729. doi: 10.1016/0022-2836(91)90528-E. [DOI] [PubMed] [Google Scholar]
- 22.Umesono K, Murakami KK, Thompson CC, Evans RM. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991;65:1255–1266. doi: 10.1016/0092-8674(91)90020-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janesick AS, et al. On the Utility of ToxCast and ToxPi as Methods for Identifying New Obesogens. Environ Health Perspect. 2016;124:1214–1226. doi: 10.1289/ehp.1510352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorfman R, Glazer L, Weihe U, Wernet MF, Shilo BZ. Elbow and Noc define a family of zinc finger proteins controlling morphogenesis of specific tracheal branches. Development. 2002;129:3585–3596. doi: 10.1242/dev.129.15.3585. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Levine M, Ashe HL. Brinker is a sequence-specific transcriptional repressor in the Drosophila embryo. Genes Dev. 2001;15:261–266. doi: 10.1101/gad.861201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldstein RE, Jimenez G, Cook O, Gur D, Paroush Z. Huckebein repressor activity in Drosophila terminal patterning is mediated by Groucho. Development. 1999;126:3747–3755. doi: 10.1242/dev.126.17.3747. [DOI] [PubMed] [Google Scholar]
- 27.Sagerstrom CG, Kao BA, Lane ME, Sive H. Isolation and characterization of posteriorly restricted genes in the zebrafish gastrula. Dev Dyn. 2001;220:402–408. doi: 10.1002/dvdy.1119. [DOI] [PubMed] [Google Scholar]
- 28.Janesick A, et al. ERF and ETV3L are retinoic acid-inducible repressors required for primary neurogenesis. Development. 2013;140:3095–3106. doi: 10.1242/dev.093716. [DOI] [PubMed] [Google Scholar]
- 29.Hong CS, Park BY, Saint-Jeannet JP. Fgf8a induces neural crest indirectly through the activation of Wnt8 in the paraxial mesoderm. Development. 2008;135:3903–3910. doi: 10.1242/dev.026229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monsoro-Burq AH, Fletcher RB, Harland RM. Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals. Development. 2003;130:3111–3124. doi: 10.1242/dev.00531. [DOI] [PubMed] [Google Scholar]
- 31.Steventon B, Araya C, Linker C, Kuriyama S, Mayor R. Differential requirements of BMP and Wnt signalling during gastrulation and neurulation define two steps in neural crest induction. Development. 2009;136:771–779. doi: 10.1242/dev.029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buitrago-Delgado E, Nordin K, Rao A, Geary L, LaBonne C. Shared regulatory programs suggest retention of blastula-stage potential in neural crest cells. Science. 2015;348:1332–1335. doi: 10.1126/science.aaa3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trevers KE, et al. Neural induction by the node and placode induction by head mesoderm share an initial state resembling neural plate border and ES cells. Proc Natl Acad Sci USA. 2018;115:355–360. doi: 10.1073/pnas.1719674115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hollenberg SM, Cheng PF, Weintraub H. Use of a conditional MyoD transcription factor in studies of MyoD trans-activation and muscle determination. Proc Natl Acad Sci USA. 1993;90:8028–8032. doi: 10.1073/pnas.90.17.8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holland DG, et al. ZNF703 is a common Luminal B breast cancer oncogene that differentially regulates luminal and basal progenitors in human mammary epithelium. EMBO Mol Med. 2011;3:167–180. doi: 10.1002/emmm.201100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang CW, et al. Identification of a developmentally regulated striatum-enriched zinc-finger gene, Nolz-1, in the mammalian brain. Proc Natl Acad Sci USA. 2004;101:2613–2618. doi: 10.1073/pnas.0308645100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savory JG, Edey C, Hess B, Mears AJ, Lohnes D. Identification of novel retinoic acid target genes. Dev Biol. 2014;395:199–208. doi: 10.1016/j.ydbio.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 38.Mendoza-Parra MA, Walia M, Sankar M, Gronemeyer H. Dissecting the retinoid-induced differentiation of F9 embryonal stem cells by integrative genomics. Mol Syst Biol. 2011;7:538. doi: 10.1038/msb.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moutier E, et al. Retinoic acid receptors recognize the mouse genome through binding elements with diverse spacing and topology. J Biol Chem. 2012;287:26328–26341. doi: 10.1074/jbc.M112.361790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lalevee S, et al. Genome-wide in silico identification of new conserved and functional retinoic acid receptor response elements (direct repeats separated by 5 bp) J Biol Chem. 2011;286:33322–33334. doi: 10.1074/jbc.M111.263681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janesick A, et al. Active repression by RARgamma signaling is required for vertebrate axial elongation. Development. 2014;141:2260–2270. doi: 10.1242/dev.103705. [DOI] [PubMed] [Google Scholar]
- 42.Koide T, Downes M, Chandraratna RA, Blumberg B, Umesono K. Active repression of RAR signaling is required for head formation. Genes Dev. 2001;15:2111–2121. doi: 10.1101/gad.908801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shahi P, et al. The Transcriptional Repressor ZNF503/Zeppo2 Promotes Mammary Epithelial Cell Proliferation and Enhances Cell Invasion. J Biol Chem. 2015;290:3803–3813. doi: 10.1074/jbc.M114.611202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pereira-Castro I, et al. Characterization of human NLZ1/ZNF703 identifies conserved domains essential for proper subcellular localization and transcriptional repression. J Cell Biochem. 2013;114:120–133. doi: 10.1002/jcb.24309. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura M, Choe SK, Runko AP, Gardner PD, Sagerstrom CG. Nlz1/Znf703 acts as a repressor of transcription. BMC Dev Biol. 2008;8:108. doi: 10.1186/1471-213X-8-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura M, Runko AP, Sagerstrom CG. A novel subfamily of zinc finger genes involved in embryonic development. J Cell Biochem. 2004;93:887–895. doi: 10.1002/jcb.20255. [DOI] [PubMed] [Google Scholar]
- 47.Kolm PJ, Sive HL. Efficient hormone-inducible protein function in Xenopus laevis. Dev Biol. 1995;171:267–272. doi: 10.1006/dbio.1995.1279. [DOI] [PubMed] [Google Scholar]
- 48.Theveneau E, Mayor R. Neural crest delamination and migration: from epithelium-to-mesenchyme transition to collective cell migration. Dev Biol. 2012;366:34–54. doi: 10.1016/j.ydbio.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 49.Wakamatsu Y, Endo Y, Osumi N, Weston JA. Multiple roles of Sox2, an HMG-box transcription factor in avian neural crest development. Dev Dyn. 2004;229:74–86. doi: 10.1002/dvdy.10498. [DOI] [PubMed] [Google Scholar]
- 50.Rogers CD, Harafuji N, Archer T, Cunningham DD, Casey ES. Xenopus Sox3 activates sox2 and geminin and indirectly represses Xvent2 expression to induce neural progenitor formation at the expense of non-neural ectodermal derivatives. Mech Dev. 2009;126:42–55. doi: 10.1016/j.mod.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlosser G, et al. Eya1 and Six1 promote neurogenesis in the cranial placodes in a SoxB1-dependent fashion. Dev Biol. 2008;320:199–214. doi: 10.1016/j.ydbio.2008.05.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miwata K, et al. Systematic analysis of embryonic expression profiles of zinc finger genes in Ciona intestinalis. Dev Biol. 2006;292:546–554. doi: 10.1016/j.ydbio.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 53.Cheah PY, et al. The Drosophila l(2)35Ba/nocA gene encodes a putative Zn finger protein involved in the development of the embryonic brain and the adult ocellar structures. Mol Cell Biol. 1994;14:1487–1499. doi: 10.1128/MCB.14.2.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown JD, et al. Expression profiling during ocular development identifies 2 Nlz genes with a critical role in optic fissure closure. Proc Natl Acad Sci USA. 2009;106:1462–1467. doi: 10.1073/pnas.0812017106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shahi P, et al. ZNF503/Zpo2 drives aggressive breast cancer progression by down-regulation of GATA3 expression. Proc Natl Acad Sci USA. 2017;114:3169–3174. doi: 10.1073/pnas.1701690114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao D, et al. Dissecting the differentiation process of the preplacodal ectoderm in zebrafish. Dev Dyn. 2014;243:1338–1351. doi: 10.1002/dvdy.24160. [DOI] [PubMed] [Google Scholar]
- 57.Luo XJ, et al. GATA3 controls the specification of prosensory domain and neuronal survival in the mouse cochlea. Hum Mol Genet. 2013;22:3609–3623. doi: 10.1093/hmg/ddt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duncan JS, Fritzsch B. Continued expression of GATA3 is necessary for cochlear neurosensory development. PLoS One. 2013;8:e62046. doi: 10.1371/journal.pone.0062046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ono K, et al. FGFR1-Frs2/3 signalling maintains sensory progenitors during inner ear hair cell formation. PLoS Genet. 2014;10:e1004118. doi: 10.1371/journal.pgen.1004118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frenz DA, et al. Retinoid signaling in inner ear development: A “Goldilocks” phenomenon. Am J Med Genet A. 2010;152A:2947–2961. doi: 10.1002/ajmg.a.33670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diez del Corral R, et al. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. doi: 10.1016/S0896-6273(03)00565-8. [DOI] [PubMed] [Google Scholar]
- 62.Lillevali K, et al. Gata3 is required for early morphogenesis and Fgf10 expression during otic development. Mech Dev. 2006;123:415–429. doi: 10.1016/j.mod.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 63.Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006;133:865–875. doi: 10.1242/dev.02271. [DOI] [PubMed] [Google Scholar]
- 64.Jansson L, Kim GS, Cheng AG. Making sense of Wnt signaling-linking hair cell regeneration to development. Front Cell Neurosci. 2015;9:66. doi: 10.3389/fncel.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uray IP, Dmitrovsky E, Brown PH. Retinoids and rexinoids in cancer prevention: from laboratory to clinic. Semin Oncol. 2016;43:49–64. doi: 10.1053/j.seminoncol.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.