Abstract
Conditional cell death systems are useful for various aspects of basic science with a wide range of applications, including genetic pest control. We recently demonstrated that expression of the mammalian pro-apoptotic factor, B-cell leukaemia/lymphoma 2-associated X protein (Bax), can induce apoptosis in specific tissues by using tissue specific promoters in silkworm and mosquito. Here, we newly identified a functional promoter in the Asian malaria vector, Anopheles stephensi, which enables gene expression specifically in the testis. We produced a transgenic mosquito line that expresses mouse Bax under the control of this testis-specific promoter. Transgenic mosquito males exhibited aberrant testes without functional sperm and complete sterility, whereas transgenic females maintained normal fecundity. Despite their abnormal testes, the transgenic males maintained normal function of male accessory glands and typical mating behaviour. As a result of mating with these males, females showed refractoriness to further mating. These results suggest that transgenic males induce female sterility via mating. The mosquito is one of the most important disease vectors, and the control of their population benefits global public health. Thus, this Bax-mediated synthetic male-specific sterilization system could be applied to population control of mosquitoes.
Subject terms: Transgenic organisms, Genetic engineering, Apoptosis, Spermatogenesis, Entomology
Introduction
Insect pests are detrimental to health, agricultural production, and economic progress worldwide. Anopheline mosquitoes are competent vectors of malaria, an infectious disease that kills estimated 445,000 people each year worldwide and is an impactful global public health burden1. Mosquito vector control is one of the most effective methods to reduce malaria transmission. However, mosquitoes that are resistant to insecticides have recently been reported and impacted the effectiveness of insecticide sprays and insecticide-treated bed nets2–4. The genetic control of mosquitoes, such as population replacement or suppression with genetically engineered mosquitoes, has the potential to become a novel strategy for vector control. Recently, synthetic gene drive systems designed to spread a desired genetic trait into a population with super-Mendelian inheritance have been developed, and they have the potential to be effective vector control strategies5–8. In the anopheline mosquito, a homing CRISPR/Cas9-based gene drive for population replacement to a malaria resistant phenotype has been studied9. Gene drives also could be used to the method for the population suppression10,11. However, in homing CRISPR/Cas9-based gene-drives, it has been speculated that a non-homologous end joining error in the target allele DNA repair creates a drive-resistant allele and reduces spreading efficiency of the desired phenotype into populations6. Therefore, homing CRISPR/Cas9-based gene drives still need improvement12,13.
Another genetic population control strategy is the release of sterilized mosquito males to reduce the size of field populations, which known as the sterile insect technique (SIT). In classical SIT, male sterilization was attempted by irradiation or chemicals in many mosquito species14. However, irradiation has often caused reduced male mating competitiveness, thereby reducing the effect of SIT. Chemical sterilization has been controversial due to environmental contamination through the predator effect15. The genetic engineered systems for sterilization or carrying a dominant lethal trait in males is a promising alternative to irradiation or chemicals14,16. The gonad is an attractive target tissue to achieve the aim. Therefore, germline-specific promoters have promise for driving the expression of effector genes that cause sterility in germline cells. Previous studies report that the beta2-tubulin gene (b2t) promoter drives testis-specific expression of a transgene in mosquitoes and other dipteran species17–20. The b2t promoter of An. gambiae was also used for inducing dominant lethality on a gene-drive strategy. It is known that a homing endonuclease I-PpoI specifically recognizes and cleaves the ribosomal DNA (rDNA) repeat sequences found solely on the X chromosome in An. gambiae21. In a previous study, I-PpoI was combined with b2t promoter and expressed in the testes. By the expression of I-PpoI in the testes, the shredding of paternal X-chromosome was caused and eggs were predominantly fertilized by Y-bearing sperm, thereby extreme male-biased progeny were produced22. Similarly, it was reported that expression system using Cas9 under the control of b2t promoter and a guide RNA that targeted the X-linked rDNA repeat sequences during spermatogenesis was applicable for induction of X-chromosome shredding and production of the male-biased progeny23. This X-chromosome shredding-based gene-drive system will be effective reducing the population. However, the X-linked rDNA repeat sequence was only conserved among An. gambiae complex species. The effect of X-chromosome shredding using I-PpoI and CRISPR/Cas9 targeting X-linked rDNA would be limited to these species. Therefore, other target genes for X-chromosome shredding found in other mosquito species as well as pest other than mosquitoes are required.
In the present study, we aim to develop a new effector to induce sterility, and applied the mouse B-cell leukaemia/lymphoma 2-associated X protein gene (mBax) as an effector gene inducing sterility of males to testis-specific-expression using the b2t promoter in An. stephensi. Bax is a member of the Bcl-2 family in mammals, and mediates the mitochondrial outer membrane permeabilization, which is an essential event in cell death24–26. In the previous study, we demonstrated that mBax-mediated cell death induction is functional and effective in inhibiting the function of salivary glands in An. stephensi27. Here, we produced transgenic An. stephensi overexpressing mBax under the control of the b2t promoter. The males of the transgenic line had aberrant testes, and sperm were not observed in this tissue. We investigated the fertility of this transgenic mosquito and demonstrated that males are completely sterile. We also demonstrated that these males conferred mating refractoriness on females via mating. Therefore, mBax-mediated cell death during spermatogenesis can completely sterilize An. stephensi males and subsequently sterilize females. The mBax-mediated cell death induction system is functional in several different insect species28,29. Therefore, mBax could be a useful tool for mosquito control as well as overall insect management.
Results
Identification of the beta2-tubulin promoter in An. stephensi
An. stephensi beta2-tubulin gene (Asb2t) was identified in the genomic scaffold sequence of the VectorBase (https://www.vectorbase.org/) by using Protein BLAST. We used the b2t gene of An. gambiae (VectorBase accession number, AGAP008622) and Ae. aegypti (GenBank accession number, DQ833526) as the query sequences. In addition, we used the sequence feature found in the carboxyl terminus of insect testis specific-tubulin to identify b2t30,31. Asb2t (ASTE003208) is located on the genomic scaffold KB664744 and consists of a single exon encoding 446 amino acids (Supplementary Fig. S1A). The amino acid sequence of Asb2t was found to be 97.8% identical to the An. gambiae homologue. In order to confirm the testis specific-expression of Asb2t, we performed RT-PCR analysis in An. stephensi. Expression of Asb2t was confirmed specifically in male pupae and testes of adult males (Supplementary Fig. S1B). The expression was not detected in female pupae and adults. This expression profile is similar to that of other dipteran species, as previously reported18,32.
In order to confirm the region of the Asb2t promoter responsible for inducing testis specific-expression, we cloned a 2,939 bp fragment including the open reading frame of Asb2t, and assembled a piggyBac-based transformation vector containing; a gene cassette the putative 5′ regulatory region of Asb2t (1,253 bp upstream of Asb2t ORF) was placed upstream of a DsRed-monomer gene and an An. gambiae trypsin terminator (Try-term) (Fig. 1a). This transformation vector was injected with piggyBac helper plasmid into An. stephensi embryos, and a transgenic line (B2T-DsRed) was established. In this line, red fluorescence was observed in male pupae and testes of adult males (Fig. 1c–f), whereas no fluorescence was detected in female pupae or adults. Individuals with red fluorescence were observed in the last instar larvae, and then all of them developed to males (Fig. 1b). These results are in agreement with experiments using b2t promoters of An. gambiae and Ae. aegypti17,18. Therefore, the 1,253 bp upstream of the Asb2t ORF functions as the promoter of this testis specific-expression of gene of interest.
Figure 1.
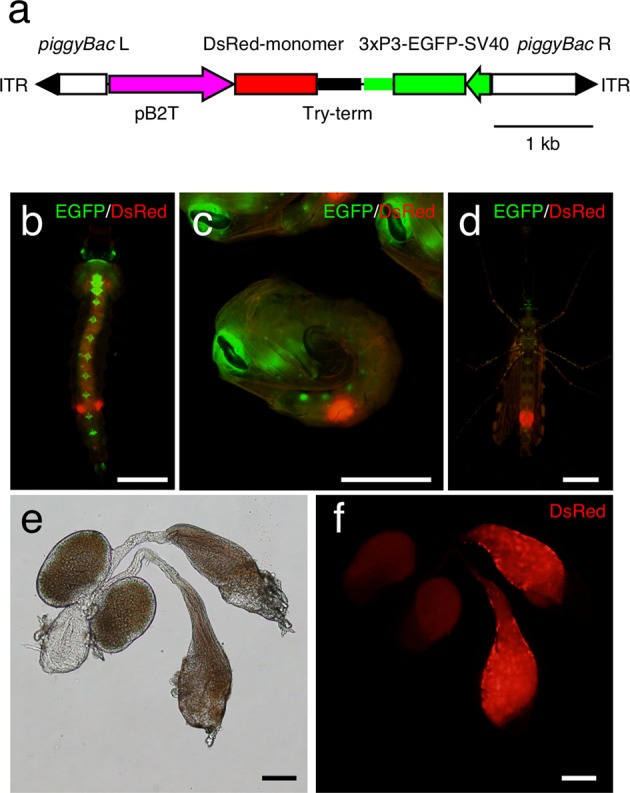
The characterization of B2T-DsRed mosquitoes. (a) Gene construct derived from the piggyBac-based vector, pBac[pB2T-DsRed; 3xP3-EGFP], which contains a piggyBac Left-arm (L) and Right-arm (R) with an inverted terminal repeat (ITR). The DsRed-monomer gene is expressed under the control of An. stephensi beta2-tubulin gene promoter (pB2T) and An. gambiae trypsin terminator (Try-term). The transformation marker, EGFP is expressed under the control of the 3xP3 promoter and SV40 terminator. (b) Last instar larva expressing red fluorescence signals in the abdomen of B2T-DsRed mosquito. These larvae were developed to male pupae. (c,d) Images of pupa and adult B2T-DsRed male mosquitoes, respectively, where the red fluorescence signal was detected only in the sixth abdominal segment. (e,f) Gonad of B2T-DsRed male mosquito. Red fluorescence signal was detected only in the testis. The panels show the merged fluorescence images. Scale bars = 1 mm (b–d), 100 μm (e,f).
Generation of transgenic mosquitoes expressing mBax in the testis
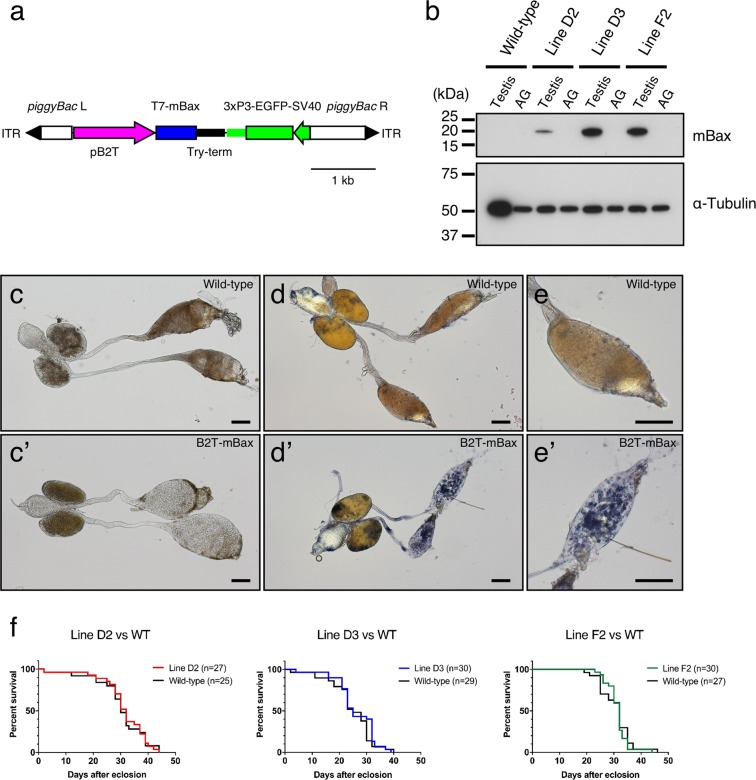
In order to express an mBax gene in the testis of An. stephensi, a piggyBac-based transformation vector containing an mBax gene with T7-tag under the control of the Asb2t promoter and Try-term was generated (Fig. 2a). Three transgenic lines (B2T-mBax, lines D2, D3 and F2) were established (Supplementary Fig. S2). We examined T7-mBax levels in the testes of the B2T-mBax lines using immunoblot analysis with anti-T7 antibody. The T7-mBax protein (Mr = 20 kDa, approximately) was detected in the testes in 12-hour-old males, but not in the accessory glands of each line (Fig. 2b). We then examined the testes in 1-day-old B2T-mBax males. The sperm bundle was not observed in the testes of B2T-mBax mosquitoes, whereas they were observed in wild-type mosquitoes (Fig. 2c and c’). This result suggests that mBax expression causes cell death in spermatocytes, and, as a result, spermatogenesis does not occur normally. In support of this, the testes of B2T-mBax mosquitoes stained with trypan blue, which only stains dead cells (Fig. 2d–e’). This aberrant morphology of the testis was observed in all three transgenic lines, regardless of T7-mBax levels. Together, these data indicate that males of the B2T-mBax mosquito line have no mature sperm and the potential to be sterile.
Figure 2.
The characterization of B2T-mBax mosquitoes. (a) The gene construct derived from the piggyBac-based vector, pBac[pB2T-mBax; 3xP3-EGFP]. The T7-mBax gene is expressed under the control of An. stephensi b2t gene promoter and An. gambiae trypsin terminator. (b) Detection of the T7-mBax protein in the testes of B2T-mBax mosquitoes by immunoblotting with anti-T7 antibodies. An anti-alpha tubulin antibody was used as the loading control. Testes and accessory glands of mosquitoes 12-hour post eclosion were used for analysis. (c) Gonads of 1-day old adult male wild-type mosquitoes and (c’) B2T-mBax mosquitoes. (d) Gonads of 1-day old adult male wild-type mosquitoes and (d’) B2T-mBax mosquitoes stained with trypan blue. (e,e’) The magnified images of the indicated testes stained with trypan blue. Scale bars = 100 μm. (f) Comparison of survival rates between B2T-mBax and wild-type mosquito males. Mosquitoes immediately after eclosion were used in analyses. Transgenic and wild type mosquitoes were taken from a single heterogeneous strain using EGFP selection, respectively. The survival curves of the groups were estimated by Kaplan-Meier methods. No significant differences were observed between B2T-mBax males and wild-type males. (line D2; P = 0.9725, line D3; P = 0.4049, and line F2; P = 0.6079, calculated by the Log-rank test).
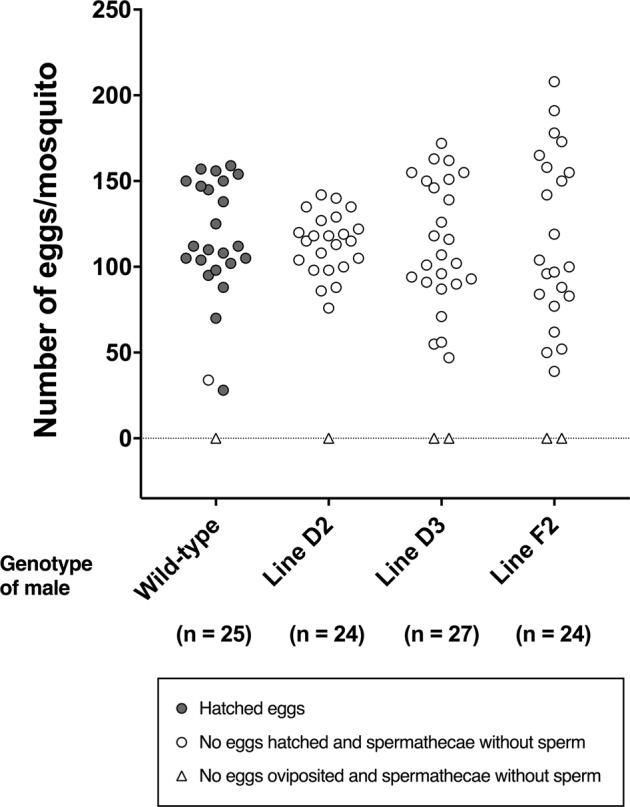
In order to examine the viability of B2T-mBax males, the survival rates of B2T-mBax adult males and wild-type adult males were monitored. No significant differences were observed between B2T-mBax and wild-type mosquitoes (Fig. 2f, Supplementary Fig. S3). These results indicated that the transgene did not affect the viability of males. Next, we examined the fecundity of B2T-mBax females. Female mosquitoes ingest the blood meal to obtain nutrients for egg reproduction. Following ingestion of the blood meal, yolk synthesis begins and egg maturation is completed. Therefore, heterogeneous females of the B2T-mBax lines that mated with wild-type males were allowed to feed on mice. These females oviposited their eggs, which were then hatched (mean of the hatchability; 76 ± 26–92 ± 6%) (Table 1, Supplementary Fig. S4). Furthermore, in hatched larvae from these females, no significant differences were observed between the number of transgenic individuals (EGFP-positive) and that of wild-type individuals (EGFP-negative). These results indicated that B2T-mBax females were able to produce offspring carrying the transgene. Therefore, B2T-mBax mosquito lines can be maintained by mating between heterozygous transgenic females and wild-type males. Three B2T-mBax lines have been stably maintained for more than 20 generations.
Table 1.
Evaluation of female fecundity of B2T-mBax mosquitoes.
| Genotype (Female) | mBax-line D2 | mBax-line D3 | mBax-line F2 |
|---|---|---|---|
| Number of females that examined mating | 30 | 30 | 30 |
| Number of females that examined egg laying | 30 | 29 | 29 |
| Number of females laid eggs (%) | 30 (100) | 27 (93) | 28 (97) |
| Number of females laid eggs that hatched (%) | 30 (100) | 26 (90) | 27 (93) |
| Hatchability*, % | 92 ± 6 | 82 ± 17 | 76 ± 25 |
| Range of hatchability, % | 74–99 | 28–97 | 3–98 |
| Number of eggs per female* | 117.1 ± 28.5 | 119.6 ± 28.8 | 99.3 ± 40.5 |
| Range of number of eggs per female | 45–168 | 54–161 | 7–169 |
| Total number of EGFP positive larvae | 1569 | 1348 | 1046 |
| Total number of EGFP negative larvae | 1674 | 1288 | 1088 |
| Two-tailed paired t-test (EGFP positive larvae vs. EGFP negative larvae) | P = 0.2000 | P = 0.2749 | P = 0.2946 |
Females (n = 10) were crossed to wild-type males (n = 30).
Three independent experiment results were pooled (Total tested females: n = 30).
*The mean ± SD is shown.
Males of the B2T-mBax mosquito line are sterile
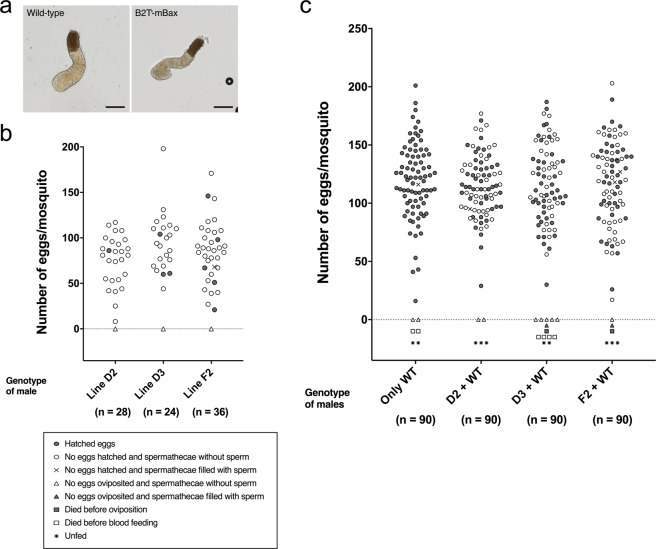
In order to evaluate of sterility of B2T-mBax males, we isolated the wild-type females that were copulated with transgenic line males or wild-type males, allowed them to feed on mouse blood, and then examined oviposition and egg hatching behaviour. The majority of the females that copulated with transgenic or wild type oviposited eggs. However, only eggs laid by females copulated with B2T-mBax males failed to completely hatch, and these females had no sperm in the spermatheca (Fig. 3, Table 2).
Figure 3.
Male sterility of B2T-mBax mosquitoes. Copulated wild-type females with wild type or transgenic males were isolated and collected. Females were allowed to feed on blood within 1–2 day after mating, and then used for the oviposition assay. Each dot corresponds to one female mosquito. Implication of markers is described in the frame.
Table 2.
Evaluation of male sterility of B2T-mBax mosquitoes.
| Genotype (Male) | Wild-type | mBax-line D2 | mBax-line D3 | mBax-line F2 |
|---|---|---|---|---|
| Number of females that examined mating | 25 | 24 | 27 | 24 |
| Number of females that examined egg laying | 25 | 24 | 27 | 24 |
| Number of females laid eggs (%) | 24 (96) | 23 (96) | 25 (93) | 22 (92) |
| Number of females laid eggs that hatched (%) | 23 (92) | 0 (0) | 0 (0) | 0 (0) |
| Hatchability*, % | 91 ± 16 | — | — | — |
| Range of hatchability, % | 20–100 | — | — | — |
| Number of eggs per female* | 114.7 ± 36.2 | 113.5 ± 17.5 | 113.7 ± 36.8 | 116.9 ± 49.6 |
| Range of number of eggs per female | 28–159 | 76–142 | 47–172 | 39–208 |
This table shows the data summarized in the experiment of Fig. 3.
*The mean ± SD is shown.
Moreover, we examined female oviposition and egg hatching behaviour by the mass-mating between thirty wild-type females and thirty transgenic males. Although females placed with B2T-mBax males did not have sperm in the spermatheca, they oviposited eggs, but these eggs did not hatch (Supplementary Fig. S5, Table S1). These results indicate that B2T-mBax line males are completely sterile. On the other hand, eggs laid by females placed with B2T-DsRed line males hatched (Mean of hatchability; 82 ± 23%) (Supplementary Fig. S5, Table S1), and no significant difference was observed in hatchability between females mated with wild-type males and with B2T-DsRed line males. These results suggest that transgene expression in the testis of mosquitoes did not induce the sterility of males, and that the expression of T7-mBax in the testis induces the sterility in males.
Males of the B2T-mBax mosquito line confer mating refractoriness on females
Next, we investigated the response of females copulated with B2T-mBax line males. It is known that mated females are suppressed from further mating by a substance transferred in the mating plug produced by the male accessory glands (MAGs)33. Immediately after mating with B2T-mBax line males, the females retained a mating plug and the form appeared normal (Fig. 4a), suggesting the substance of MAGs functions normally to prevent further mating. In order to evaluate whether females mated with B2T-mBax mosquitoes accepted further mating with other males, we monitored wild-type females that had copulated with B2T-mBax line males and were subsequently placed in a cage containing wild-type mosquito males. After 1 week, these females were allowed to feed blood, and then we investigated oviposition and egg hatching behaviour. A small number of females (4–14%) contained sperm in the spermatheca and their oviposited eggs hatched (Fig. 4b, Table 3). All hatched larvae were EGFP-negative, suggesting larvae were progeny of wild-type males. However, in the majority of females, no sperm was observed in the spermatheca, and, although they oviposited eggs, these eggs did not hatch.
Figure 4.
mBax expression in the testis did not affect the mating behaviour of males. (a) The mating plug dissected from females mated to B2T-mBax and wild type mosquitoes. Scale bars = 100 μm. (b) The mating refractoriness in females by B2T-mBax mosquito males. Copulated wild-type females with B2T-mBax males were isolated and collected. The 8–10 females 2–3 days after mating were pooled, and then placed in cages containing three times wild-type males to the number of females for 1 week. After 1 week, females were allowed to feed blood, and then used for the oviposition assay. Each dot corresponds to one female mosquito. Implication of markers is described in the frame. (c) Analysis of the mating competitiveness of B2T-mBax mosquitoes was performed by crossing wild-type females (n = 30) with wild-type males (n = 15) and B2T-mBax males (n = 15) at the same time for 1 week, and then examining the females using an oviposition assay. In these assays, the wild-type males and transgenic males came from two separately raised populations. Each dot corresponds to one female mosquito. Group of only wild type (Only WT) showed that females were placed with wild-type males (n = 15) for 1 week as the control. Implication of markers is described in the frame. Three pooled independent experiments are shown in each line (total tested females; n = 90).
Table 3.
Evaluation of mating refractoriness in females by B2T-mBax mosquito males.
| Genotype (1st week male) | mBax-line D2 | mBax-line D3 | mBax-line F2 |
|---|---|---|---|
| Number of females that examined mating | 28 | 24 | 36 |
| Number of females that examined egg laying | 28 | 24 | 36 |
| Number of females laid eggs (%) | 27 (96) | 23 (96) | 35 (97) |
| Number of females laid eggs that hatched (%) | 1 (4) | 3 (13) | 5 (14) |
| Hatchability*, % | 86 | 62 ± 40 | 71 ± 21 |
| Range of hatchability, % | — | 16–86 | 41–91 |
| Number of eggs per female* | 75.4 ± 27.6 | 95.4 ± 32.1 | 84.9 ± 32.9 |
| Range of number of eggs per female | 8–117 | 44–198 | 21–171 |
This table shows the data summarized in the experiment of Fig. 4b.
*The mean ± SD is shown.
Moreover, we examined the mating refractoriness in females by mass-mating. A small number of females (1–4%) contained sperm in the spermatheca, and all of their progeny were EGFP-negative (Supplementary Fig. S6, Table S2). After mating to wild-type males, a significant difference in the number of females that oviposited fertile eggs was observed when comparing females from the cage without males and those from the cage containing the transgenic males. These results suggest that female mosquitoes mated with B2T-mBax mosquito males were suppressed in further mating, and that B2T-mBax male mosquitoes retain the ability to suppress the female ability to mating further.
Mating competitiveness of B2T-mBax line mosquito males
Finally, we examined whether B2T-mBax mosquito males were capable of competition with wild-type males for mating. Virgin wild-type females were placed in a cage containing an equal number of transgenic and wild type males, which came from separately raised populations. After one week, we investigated the oviposition behaviour of the females and their egg hatching behaviour. About half of the females (47–52%) oviposited fertile eggs (Fig. 4c, Table 4), and these females contained the sperm in spermatheca. All hatched larvae were EGFP-negative, suggesting the larvae were progeny of wild-type males. Significant differences were observed between the number of females that oviposited fertile eggs in the cage containing only wild-type males and the cage containing the transgenic and wild type males. Similar results were also found by using transgenic and wild type males raised in the same population until pupation (Supplementary Fig. S7, Table S3). These results suggest that B2T-mBax males have the ability to compete with wild-type males for mating under these experimental conditions.
Table 4.
Evaluation of mating competitiveness of B2T-mBax mosquito males.
| Genotype (Male) | Wild-type only | mBax-line D2 | mBax-line D3 | mBax-line F2 |
|---|---|---|---|---|
| Number of females that examined mating | 90 | 90 | 90 | 90 |
| Number of females that examined egg laying | 86 | 87 | 83 | 86 |
| Number of females laid eggs (%) | 84 (98) | 85 (98) | 77 (93) | 84 (98) |
| Number of females laid eggs that hatched (%) | 83 (97) | 45** (52) | 43** (52) | 40** (47) |
| Hatchability*, % | 83 ± 20 | 84 ± 22 | 85 ± 16 | 85 ± 19 |
| Range of hatchability, % | 6–99 | 1–100 | 28–99 | 4–100 |
| Number of eggs per female* | 119.8 ± 32.7 | 115.0 ± 26.0 | 116.3 ± 33.2 | 116.7 ± 36.1 |
| Range of number of eggs per female | 16–201 | 29–177 | 30–187 | 17–203 |
This table shows the data summarized in the experiment of Fig. 4c.
*The mean ± SD is shown.
**A significant difference was observed between the number of females that oviposited fertile eggs in the cage containing only wild-type males and in the cage containing the transgenic and wild type males (P < 0.0001, calculated by Fisher’s exact test).
Discussion
Depletion of the germ cells in male anopheline mosquitoes is reasonable approach to control the population of these disease-spreading insects. In line with this aspect, we assumed that Bax would be a suitable effector gene. Bax is one of the Bcl-2 family proteins and known to function as pro-apoptotic factor. The Bax-mediated mechanism of action has recently been clarified. In mammals, Bax and Bak (Bcl-2 homologues antagonistic killer) form oligomers resulting in pores on the mitochondrial outer membrane, a process known to be involved in apoptosis and the potentiation of interferon response through mtDNA efflux25,26,34. Insects have homologues of mammalian Bax; however, it is unclear whether they have same physiological function. Previous study and our recent works found that mouse Bax acts as a pro-apoptotic factor in insects, and that expression of mBax can induce ablation of cells in specific tissues in fly, silkworm, and mosquito27–29. In the present study, we newly identified a testis-specific promoter in An. stephensi, and established lines expressing mBax under the control of this promoter. These transgenic lines, carrying the mBax construct, specifically expressed the gene in the testes. Males of these lines contain aberrant testes without normal sperm. We found that females copulated with B2T-mBax males oviposited eggs, but all of the eggs did not hatch, suggesting that these eggs were not fertilized. Therefore, B2T-mBax line males are completely sterile. In anopheline mosquitoes, oviposition behaviour of females is stimulated by male accessory gland substances transferred during mating33. Normal morphology of the male accessory glands in B2T-mBax mosquitoes was observed using microscopic analysis. Our results indicated that the males of B2T-mBax lines retain the ability to stimulate oviposition behaviour in the accessory glands. The substances of the male accessory glands also stimulated the refractoriness of copulated females from further mating33. We demonstrated that males of B2T-mBax mosquitoes inhibited the further mating in females. Furthermore, mating plugs similar to those found in females mated with wild-type males were observed in females mated with B2T-mBax males. Therefore, our results indicated that males of B2T-mBax mosquitoes retain normal function of accessory glands. The expression of mBax in testes is effective for the sterilization of males and the induction of female sterility for long-term via the mating refractoriness observed in An. stephensi. For genetic control of Anopheles mosquitoes resulting in population suppression, it is imperative that sterile males induce refractoriness to further mating for the lifespan of the female35. Another crucial criterion to ensuring the success of genetically induced population suppression is that sterile males must be competitive with wild males for mating14,16. In this study, B2T-mBax males appeared to have competitiveness with wild-type males in mating. Since mosquitoes were forced to mate in small cages in this study, further analysis in mass-mating in large spaces would be required for defining the competitiveness of transgenic line males to mate with females in the wild36,37.
A previous study reported that spermless males were generated by RNAi-mediated knockdown of a gene involving germ cell differentiation, zero population growth (zpg), in An. gambiae38. The zpg-silenced males were sterile, and females copulated by these males exhibited refractoriness to further mating. The zpg-silenced females were also sterile38,39, and therefore, a male-specific gene knockout system would be required for development of SIT that targets zpg for the induction of sterility. On the other hand, in the present study, our testis-specific mBax expression system induces male-specific sterility in An. stephensi. The females of transgenic lines expressing mBax driven by the b2t promoter showed fecundity; therefore, these transgenic lines could be maintained by mating between transgenic females and wild-type males. Further studies are required to evaluate whether the expression of mBax affects female fecundity and viability by analyzing the competitiveness of transgenic and wild type females in near-natural environments.
Findings from our previous studies lead us to take note that the Bax gene is a remarkable effector for investigation of the functions of particular tissues27,28. Here, the great advantage of this genetic system was shown by the induction of complete sterility even in heterozygotes. For the application of our Bax-mediated cell death system, strict regulation of Bax expression is absolutely required because this gene has high pro-apoptotic activity. In this regard, we demonstrated the male-specific sterilization of An. stephensi by combination of mBax and a newly testis-specific tubulin promoter. We accomplished this without affecting basic biological processes of females, and it was suggested that this cell death system is applicable for effective mosquito control. The use of a dominant sterility gene, such as mBax, dramatically limits the massive production of males for SIT because the lines can only be maintained through heterozygous females. In terms of both the necessity and direction of the practical development of sterile male strains, it would be expected that a convenient sorting system for males expressing mBax, such as genetic sexing be developed40–43. The tetracycline-mediated expression system (tet-off) is preferable for the generation of homozygous individuals44. Furthermore, gene regulatory systems, such as site-specific gene integration and gene-drive, are required for further application and practical usage of Bax. Comparative analysis of mBax-expressing males with RIDL and I-PpoI sterile males modelling different scenarios would also be required.
In conclusion, mBax is an available effector gene for genetic sterility without affecting other biological processes. Cell death by mBax was inducible in D. melanogaster as well as An. stephensi29. Since the b2t promoter is effective in many dipteran species, this sterilization system is a useful tool for the control of many mosquito species and other dipteran insect pests17–20. Efficacy of an mBax-mediated cell death system has been also demonstrated in a silkworm, which is beyond the insect order28. This technology promises to provide a versatile tool in the management of beneficial insects as well as in the control of disease vectors and crop pests.
Methods
Ethics statement
All mouse procedures were approved by the Institutional Animal Experiment Committee of Jichi Medical University (Number: 16255 and 17142) and in accordance with the Institutional Regulation for Animal Experiments and Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology.
Animals
The An. stephensi mosquito SDA500 strain was maintained at 26 °C, 60–80% relative humidity, and under conditions of 13-h light/11-h dark at Jichi Medical University. Larvae were fed with fish food, Hikari (Kyorin, Himeji, Japan). Adults were fed on filter paper soaked with a 5% fructose (Nacalai Tesque, Kyoto, Japan). Female BALB/c mice were obtained from Japan SLC (Hamamatsu, Shizuoka, Japan). Mosquito tissues were observed using an inverted microscope, IX73 or SZX7 stereoscopic microscope equipped with a DP73 digital camera (Olympus, Tokyo, Japan).
Plasmid construction
The An. stephensi beta2-tubulin gene (Asb2t) was amplified by PCR from An. stephensi genomic DNA using the primers AsB2TPL: ACTGCCGCAGCAGACGGACACATGCCA and AsB2T3’genomeR: GTACACACTCTCTACAGAGCCGGAGCCTTG. The resulting fragments were cloned into a pGEM-T-Easy vector (Promega, Madison, WI, USA) to generate an AsB2T-full plasmid, which was then sequenced.
The DsRed-monomer gene was amplified by PCR from the pDsRed-monomer-C1 plasmid (Takara Bio USA, Mountain View, CA, USA) using the primers DsRedmonoL-NcoI: CAACCATGGACAACACCGAGGACGTCA and DsRedmonoR-SalI: GCGTCGACCTGGGAGCCGGAGTGGC. The resulting DsRed-monomer containing DNA fragment was digested with NcoI and SalI, and then cloned into the NcoI/SalI site of pBiEx-3 vector (Merck KGaA, Darmstadt, Germany) to generate pBiEx-DsRedmono. Fragments of the DsRed-monomer with an S-Tag and His-Tag were amplified by PCR from pBiEx-DsRedmono using the primers DsRedmonoL-NcoI and pBiExR-NdeI: GGGCATATGTTAGTGATGGTGATGGTGATG and then digested with NcoI and NdeI. Fragments of the Asb2t promoter (pB2T) were amplified by PCR from the AsB2T-full plasmid using the primers AsB2TPL-EcoRI: TTGAATTCACTGCCGCAGCAGACGGA and AsB2TPR-NcoI: GGGCCATGGTTTGCAAACTGCAAACAG and then digested with EcoRI and NcoI. PCR reactions were performed using KOD plus neo polymerase (Toyobo, Tokyo, Japan). These fragments were cloned into the EcoRI/NdeI site of pSL-tryter27 to generate pSL-pAsB2T-DsRedmono. The pB2T-DsRed-monomer fragment was excised from pSL-pAsB2T-DsRedmono by digestion with AscI and FseI, and was then cloned into the AscI/FseI site of pBac[3xP3-EGFPaf]45 to generate the pBac[pB2T-DsRed; 3xP3-EGFP] transformation vector.
The mBax gene with a T7-tag was amplified by PCR from a pEF-BOS-T7 vector46 using the primers pB2T-mBax-startL: TGCAGTTTGCAAACCATGGCCAGCATGACTGGTGG and TrypolyA-mBax-stopR: GCCGAGATCGCATGCTCAGCCCATCTTCTTCCAGA, and the resulting gene fragment was cloned between pB2T and Try-term of pBac[pB2T-DsRed; 3xP3-EGFP] using the GENEART seamless cloning and assembly kit (Thermo Fisher Scientific, Waltham, MA, USA) to generate the pBac[pB2T-mBax; 3xP3-EGFP] transformation vector. Procedures for the microinjection of vectors into embryos, screening of transgenic individuals, and generation of homozygous lines have been described previously47. We used homozygous transgenic B2T-DsRed mosquitoes in all experiments.
Maintenance of transgenic lines
To maintain B2T-mBax lines, 100–150 heterozygous transgenic females were mated with 100–150 wild-type males in small cages in every generation. After blood meals, wild-type females were allowed to lay eggs in a plastic cup arranged with conically folded filter paper and filled with water at the bottom. Transgenic females expressing EGFP were screened at the pupal stage under a stereoscopic microscope, and then used for mass-mating to obtain the next generation.
Southern blotting
The isolation of genomic DNA from An. stephensi and subsequent Southern blot analysis were performed as described previously48. Briefly, genomic DNA was digested with MspI, separated on a 0.8% agarose gel, and then transferred to a Hybond-N+ membrane (GE Healthcare UK Ltd., Buckinghamshire, UK). Probe labelling and signals detection were performed using AlkPhos Direct Labelling Reagents and CDP-Star Detection Reagent (GE Healthcare UK Ltd.) according to the supplier’s protocol.
SDS-PAGE and immunoblotting
A rabbit anti-T7 tag monoclonal antibody (T7 tag, D9E1X XP rabbit mAb, #13246, Cell Signaling, MA, USA) and rabbit anti-alpha-tubulin monoclonal antibody (11H10 mAb, #2125, Cell Signaling) were used as primary antibodies for immunoblotting. Groups of 10 pairs of testes or accessory glands were homogenized by a plastic homogenizer in 100 µl of sample buffer (Nacalai) containing 5% 2-mercaptoethanol and were then boiled at 95 °C for 3 min. Ten microliters of each sample (equivalent to 1 pair of testes or accessory glands) was separated on a 4–12% NuPAGE gel (Thermo Fisher Scientific) and then transferred to an Immobilon-P Membrane (Merck). Membranes were blocked with T-TBS (20 mM Tris-HCl, 137 mM NaCl, pH 7.4, 0.05% Tween-20) containing 5% skimmed milk (Megmilk Snow Brand, Tokyo, Japan). Membranes were incubated with primary antibodies. The polypeptides recognized by primary antibodies were detected with either horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (#7074, Cell Signaling). The detection of HRP-labelled antibodies was performed by exposing membranes on Hyperfilm-ECL using ECL Prime Western Blotting Detection Reagents (all GE Healthcare UK Ltd.) according to the supplier’s protocol.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using TRIzol and purified using the PureLink RNA mini kit (all Life Technologies). Five hundred nanograms of total RNA was reverse-transcribed from each sample using the High-Capacity cDNA Reverse Transcription kit (Life Technologies). cDNA was used for the PCR amplification of the An. stephensi b2t and An. stephensi ribosomal protein S7 (rpS7) genes using Taq DNA polymerase (New England Biolabs). The primers AsB2T3′L: 5′-ACAGGTGAATAAACGATGGCCAGCATGACTGGTGG-3′ and AsB2T3′R: 5′-GCCGAGATCGCATGCTCAGCCCATCTTCTTCCAGA-3′ were used to amplify b2t. The primers AnsS7L: 5′-GGCGATCATCATCTACGTGC-3′ and AnsS7R: 5′-CGGTCTCTTCTGCTTGTTGG-3′ were used to amplify rpS7.
Isolation of copulated females
One wild-type virgin female 4–7 days after eclosion was entered into a cage containing over 100 wild type or transgenic males 2–7 days post eclosion and after 1–3 hours in the dark. The mating females were observed, and then isolated by an aspirator. Females mating over 20 s were used in subsequent assays. Females 1–2 days after mating were allowed to feed on the mice and were then used for the oviposition assay. The mating plug was observed with under the inverted IX 73 (Olympus) microscope and dissected within 1 hour after mating.
In evaluation of refractoriness to further mating by B2T-mBax line males, 8–10 females were pooled 1–2 days after mating with B2T-mBax males. Then, the females were placed into a cage containing three times the number of wild-type males to females. After 1 week, females were allowed to feed on mice and then used for the oviposition assay.
Mass mating
Thirty virgin wild-type females 1–2 days after eclosion and thirty virgin males 1–2 days after eclosion from each line were placed in cages (size; 15 cm × 26 cm × 26 cm) and mated for 1 week. After 1 week, females were allowed to feed on mice and then used for the oviposition assay. Male mosquitoes were removed before blood-feedings.
In evaluation of mating refractoriness, wild-type females mated with B2T-mBax males for 1 week, as described above. After 1 week, the males were replaced with thirty virgin wild-type males and then remated for 1 week.
To evaluate the mating competitiveness of B2T-mBax mosquitoes, wild-type females were placed in cages containing fifteen wild-type males and fifteen transgenic males for 1 week, as described above.
To determine fecundity of B2T-mBax mosquito females, ten virgin females and thirty virgin males were placed in cages for 1 week, as described above.
Three independent experiments were performed and pooled in each assay.
Oviposition assay
Females were individually placed into single plastic cups arranged with a conical folded filter paper and filled with ~30 ml water at the bottom 4–7 days after blood feeding. After 2 days, the spermatheca of females was dissected, and then the presence of sperm was checked. The eggs and hatched larvae were counted. The unhatched eggs were defined as sterile eggs 4 days post laying. In calculation of hatchability, only females that oviposited hatched eggs and more than one larva were used.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 7 software (GraphPad software, Inc., La Jolla, CA, USA). Comparison of egg hatchability between mating with wild type and transgenic males was performed using a Mann-Whitney U test. The number of EGFP positive- and negative-larvae from B2T-mBax females were compared with a two-tailed paired t-test. The number of females that oviposited fertile eggs were compared with a Fisher’s exact test. Adult survival curves were generated by Kaplan-Meier methods and compared by the Log-rank test.
Accession numbers for genes
The following genes were analysed in this study: mouse Bax (mBax, GenBank NM_007527.3), An. stephensi ribosomal protein S7 (rpS7, GenBank AF539918.2).
Supplementary information
Acknowledgements
We thank C. Seki (Jichi Medical University) for the excellent assistance with counting eggs and larvae. We thank K. Watano (Jichi Medical University) for rearing the mosquitoes and mice. This study was funded by grants from JSPS KAKENHI Grant Number 16K18824 to DSY and JSPS KAKENHI Grant Number 17K08161 to MS.
Author Contributions
D.S.Y. and M.S. conceived and designed the study. D.S.Y. and M.S. performed the experiments and analysed the data. K.K. contributed to the plasmid construction. K.K., H.S., H.M. and H.K. contributed to data analysis and discussion. D.S.Y. and M.S. wrote the manuscript. K.K., H.S., H.M. and H.K. contributed to the revised manuscript. D.S.Y. supervised the study.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Daisuke S. Yamamoto and Megumi Sumitani contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-44480-0.
References
- 1.WHO. World malaria report, 2017. (WHO, 2017).
- 2.Churcher, T. S., Lissenden, N., Griffin, J. T., Worrall, E. & Ranson, H. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. Elife5, e16090, 10.7554/eLife.16090 (2016). [DOI] [PMC free article] [PubMed]
- 3.Gatton ML, et al. The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution. 2013;67:1218–1230. doi: 10.1111/evo.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hemingway J, et al. Averting a malaria disaster: will insecticide resistance derail malaria control? Lancet. 2016;387:1785–1788. doi: 10.1016/S0140-6736(15)00417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Champer J, Buchman A, Akbari OS. Cheating evolution: engineering gene drives to manipulate the fate of wild populations. Nat Rev Genet. 2016;17:146–159. doi: 10.1038/nrg.2015.34. [DOI] [PubMed] [Google Scholar]
- 6.Esvelt KM, Smidler AL, Catteruccia F, Church GM. Concerning RNA-guided gene drives for the alteration of wild populations. Elife. 2014;3:e03401. doi: 10.7554/eLife.03401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gantz VM, Bier E. The mutagenic chain reaction: a method for converting heterozygous to homozygous mutations. Science. 2015;348:442–444. doi: 10.1126/science.aaa5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchman A, Marshall JM, Ostrovski D, Yang T, Akbari OS. Synthetically engineered Medea gene drive system in the worldwide crop pest Drosophila suzukii. Proc Natl Acad Sci USA. 2018;115:4725–4730. doi: 10.1073/pnas.1713139115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gantz VM, et al. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc Natl Acad Sci USA. 2015;112:E6736–6743. doi: 10.1073/pnas.1521077112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammond A, et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat Biotechnol. 2016;34:78–83. doi: 10.1038/nbt.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.KaramiNejadRanjbar M, et al. Consequences of resistance evolution in a Cas9-based sex conversion-suppression gene drive for insect pest management. Proc Natl Acad Sci USA. 2018;115:6189–6194. doi: 10.1073/pnas.1713825115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Champer J, et al. Reducing resistance allele formation in CRISPR gene drive. Proc Natl Acad Sci USA. 2018;115:5522–55275. doi: 10.1073/pnas.1720354115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noble C, Olejarz J, Esvelt KM, Church GM, Nowak MA. Evolutionary dynamics of CRISPR gene drives. Sci Adv. 2017;3:e1601964. doi: 10.1126/sciadv.1601964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benedict MQ, Robinson AS. The first releases of transgenic mosquitoes: an argument for the sterile insect technique. Trends Parasitol. 2003;19:349–355. doi: 10.1016/S1471-4922(03)00144-2. [DOI] [PubMed] [Google Scholar]
- 15.Scott, M. J. & Benedict, M. Q. In Genetic Control of Malaria and Dengue (ed. Adelman, Z. N.) 31–54 (Academic Press, 2016).
- 16.Alphey L, et al. Sterile-insect methods for control of mosquito-borne diseases: an analysis. Vector Borne Zoonotic Dis. 2010;10:295–311. doi: 10.1089/vbz.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catteruccia F, Benton JP, Crisanti A. An Anopheles transgenic sexing strain for vector control. Nat Biotechnol. 2005;23:1414–1417. doi: 10.1038/nbt1152. [DOI] [PubMed] [Google Scholar]
- 18.Smith RC, Walter MF, Hice RH, O’Brochta DA, Atkinson PW. Testis-specific expression of the beta2 tubulin promoter of Aedes aegypti and its application as a genetic sex-separation marker. Insect Mol Biol. 2007;16:61–71. doi: 10.1111/j.1365-2583.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 19.Zimowska GJ, Nirmala X, Handler AM. The beta2-tubulin gene from three tephritid fruit fly species and use of its promoter for sperm marking. Insect Biochem Mol Biol. 2009;39:508–515. doi: 10.1016/j.ibmb.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Scolari F, et al. Fluorescent sperm marking to improve the fight against the pest insect Ceratitis capitata (Wiedemann; Diptera: Tephritidae) N Biotechnol. 2008;25:76–84. doi: 10.1016/j.nbt.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Windbichler N, Papathanos PA, Crisanti A. Targeting the X chromosome during spermatogenesis induces Y chromosome transmission ratio distortion and early dominant embryo lethality in Anopheles gambiae. PLoS Genet. 2008;4:e1000291. doi: 10.1371/journal.pgen.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galizi R, et al. A synthetic sex ratio distortion system for the control of the human malaria mosquito. Nat Commun. 2014;5:3977. doi: 10.1038/ncomms4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galizi R, et al. A CRISPR-Cas9 sex-ratio distortion system for genetic control. Sci Rep. 2016;6:31139. doi: 10.1038/srep31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 25.Große L, et al. Bax assembles into large ring-like structures remodeling the mitochondrial outer membrane in apoptosis. The EMBO journal. 2016;35:402–413. doi: 10.15252/embj.201592789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salvador-Gallego R, et al. Bax assembly into rings and arcs in apoptotic mitochondria is linked to membrane pores. The EMBO journal. 2016;35:389–401. doi: 10.15252/embj.201593384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto DS, Sumitani M, Kasashima K, Sezutsu H, Matsuoka H. Inhibition of malaria infection in transgenic anopheline mosquitoes lacking salivary gland cells. PLoS Pathog. 2016;12:e1005872. doi: 10.1371/journal.ppat.1005872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sumitani M, et al. Establishment of a specific cell death induction system in Bombyx mori by a transgene with the conserved apoptotic regulator, mouse Bcl-2-associated X protein (mouse Bax) Insect Mol Biol. 2015;24:671–680. doi: 10.1111/imb.12192. [DOI] [PubMed] [Google Scholar]
- 29.Gaumer S, Guenal I, Brun S, Theodore L, Mignotte B. Bcl-2 and Bax mammalian regulators of apoptosis are functional in Drosophila. Cell death and differentiation. 2000;7:804–814. doi: 10.1038/sj.cdd.4400714. [DOI] [PubMed] [Google Scholar]
- 30.Kawasaki H, Sugaya K, Quan GX, Nohata J, Mita K. Analysis of alpha- and beta-tubulin genes of Bombyx mori using an EST database. Insect Biochem Mol Biol. 2003;33:131–137. doi: 10.1016/S0965-1748(02)00184-4. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen MG, Gadagkar SR, Gutzwiller L. Tubulin evolution in insects: gene duplication and subfunctionalization provide specialized isoforms in a functionally constrained gene family. BMC Evol Biol. 2010;10:113. doi: 10.1186/1471-2148-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kemphues KJ, Raff EC, Raff RA, Kaufman TC. Mutation in a testis-specific beta-tubulin in Drosophila: analysis of its effects on meiosis and map location of the gene. Cell. 1980;21:445–451. doi: 10.1016/0092-8674(80)90481-X. [DOI] [PubMed] [Google Scholar]
- 33.Klowden MJ. Sexual receptivity in Anopheles gambiae mosquitoes: absence of control by male accessory gland substances. J Insect Physiol. 2001;47:661–666. doi: 10.1016/S0022-1910(00)00127-X. [DOI] [PubMed] [Google Scholar]
- 34.McArthur Kate, Whitehead Lachlan W., Heddleston John M., Li Lucy, Padman Benjamin S., Oorschot Viola, Geoghegan Niall D., Chappaz Stephane, Davidson Sophia, San Chin Hui, Lane Rachael M., Dramicanin Marija, Saunders Tahnee L., Sugiana Canny, Lessene Romina, Osellame Laura D., Chew Teng-Leong, Dewson Grant, Lazarou Michael, Ramm Georg, Lessene Guillaume, Ryan Michael T., Rogers Kelly L., van Delft Mark F., Kile Benjamin T. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science. 2018;359(6378):eaao6047. doi: 10.1126/science.aao6047. [DOI] [PubMed] [Google Scholar]
- 35.Gabrieli, P., Marois, E. & Catteruccia, F. In Transgenic insects: techniques and applications (ed. Benedict, M. Q.) 188–207 (CABI, 2014).
- 36.Facchinelli L, et al. Stimulating Anopheles gambiae swarms in the laboratory: application for behavioural and fitness studies. Malar J. 2015;14:271. doi: 10.1186/s12936-015-0792-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Achinko D, et al. Swarming and mating activity of Anopheles gambiae mosquitoes in semi-field enclosures. Med Vet Entomol. 2016;30:14–20. doi: 10.1111/mve.12143. [DOI] [PubMed] [Google Scholar]
- 38.Thailayil J, Magnusson K, Godfray HC, Crisanti A, Catteruccia F. Spermless males elicit large-scale female responses to mating in the malaria mosquito Anopheles gambiae. Proc Natl Acad Sci USA. 2011;108:13677–13681. doi: 10.1073/pnas.1104738108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magnusson K, et al. Transcription regulation of sex-biased genes during ontogeny in the malaria vector Anopheles gambiae. PLoS One. 2011;6:e21572. doi: 10.1371/journal.pone.0021572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Concha C, et al. A transgenic male-only strain of the New World screwworm for an improved control program using the sterile insect technique. BMC Biol. 2016;14:72. doi: 10.1186/s12915-016-0296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamada H, et al. Genetic sex separation of the malaria vector, Anopheles arabiensis, by exposing eggs to dieldrin. Malar J. 2012;11:208. doi: 10.1186/1475-2875-11-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu G, et al. Female-specific insect lethality engineered using alternative splicing. Nat Biotechnol. 2007;25:353–357. doi: 10.1038/nbt1283. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Zhongjie, Niu Baolong, Ji Dongfeng, Li Muwang, Li Kai, James Anthony A., Tan Anjiang, Huang Yongping. Silkworm genetic sexing through W chromosome-linked, targeted gene integration. Proceedings of the National Academy of Sciences. 2018;115(35):8752–8756. doi: 10.1073/pnas.1810945115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lycett GJ, Kafatos FC, Loukeris TG. Conditional expression in the malaria mosquito Anopheles stephensi with Tet-On and Tet-Off systems. Genetics. 2004;167:1781–1790. doi: 10.1534/genetics.104.028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horn C, Wimmer EA. A versatile vector set for animal transgenesis. Dev Genes Evol. 2000;210:630–637. doi: 10.1007/s004270000110. [DOI] [PubMed] [Google Scholar]
- 46.Kasashima K, Terashima K, Yamamoto K, Sakashita E, Sakamoto H. Cytoplasmic localization is required for the mammalian ELAV-like protein HuD to induce neuronal differentiation. Genes Cells. 1999;4:667–683. doi: 10.1046/j.1365-2443.1999.00292.x. [DOI] [PubMed] [Google Scholar]
- 47.Catteruccia F, et al. Stable germline transformation of the malaria mosquito Anopheles stephensi. Nature. 2000;405:959–962. doi: 10.1038/35016096. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook, J. & Russell, D. W. Molecular cloning: a laboratory manual. 3rd edn, (Cold Spring Harbor Laboratory Press, 2001).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.