Abstract
Objectives:
We tested the social action theory hypotheses that (a) psychological stress induced by struggling to control others (agonistic striving) is associated with higher levels of subjective somatic symptoms than stress induced by struggling to control the self (transcendence striving); (b) the association between agonistic striving and symptoms is moderated by the ability to tolerate pain; and (c) associations among agonistic goals, pain tolerance, and subjective symptoms are not explained by personality and affective traits or negative emotional responses to personal stressors.
Methods:
Implicit motives and negative emotional reactivity to recurring personal stressors were assessed by Social Competence Interview in 333 adolescents and adults who participated in longitudinal research on functional abdominal pain at a university medical center. Pain tolerance was assessed by graduated thermal pain protocol; subjective somatic symptoms, and personality/affective traits assessed by questionnaires. The primary outcome measure was the self-reported severity of 35 somatic symptoms often experienced in the absence of diagnosable disease.
Results:
All hypotheses were supported.
Conclusions:
Nonconscious agonistic strivings may increase the perceived frequency and severity of subjective somatic symptoms; this tendency is greatly magnified by difficulty in self-regulating responses to painful stimuli. Implicit agonistic motives and their associations with symptoms are not explained by individual differences in trait neuroticism, anxiety, depression, anger, or low self-esteem or by negative emotional reactivity to a personal stressor. These findings may afford fruitful insights into mechanisms by which stressful social environments undermine health and suggest promising directions for clinical intervention.
Keywords: medically unexplained symptoms, pain regulation, somatization, implicit motives, social action theory
Symptoms that defy adequate medical explanation are common across general/internal medicine and often remain unresolved (Nimnuan, Hotopf, & Wesley, 2001), costing the U.S. health-care system roughly $256 billion in incremental direct costs per year (Barsky, Orav, & Bates, 2005). The unexplained symptoms encountered most often in primary care include pain, fatigue, dizziness, general malaise, and gastrointestinal problems (Brown, 2004); such symptoms often become chronic, causing persistent distress and disability (Smith, Monson, & Ray, 1986). An improved understanding of the factors that contribute to such distress is greatly needed.
Recent theorizing has drawn upon research in cognitive psychology to explain how a person may experience compelling symptoms in the absence of underlying physical pathology. An integrative cognitive approach (e.g., Brown, 2004) focuses on how attention shapes the contents of consciousness to control thought and action. Self-directed somatic-focused attention biases the processing of sensory information by activating memories that shape how a bodily sensation is interpreted (Brown, 2004; Brown et al., 2012). Somatic focus may be augmented by negative emotions (Brown, 2004) and by social-environmental stress (Uchino, Bowen, Carlisle, & Birmingham, 2012); most research has tested the hypothesis that stressful social environments foster somatic distress by inducing negative affect (DeLongis, Folkman, & Lazarus, 1988; Walker, Garber, Smith, Van Slyke, & Claar, 2001). However, the accumulated findings suggest that other mediators are involved. It appears that social milieus extensively influence health via nonaffective pathways, possibly involving “nonconscious” or “implicit” cognitive mechanisms that people are unable or reluctant to report (Lee, Rogge, & Reis, 2010; Uchino et al., 2012; Walker et al., 2001). The challenge is to identify these mediators.
We propose that implicit social-motivational mechanisms contribute to subjective somatic symptoms by heightening symptom focus and biasing sensory processing. We argue that implicit strivings for interpersonal control influence attentional focus and that they may shape, organize, and repeatedly activate the cognitive processing distortions envisaged in the integrative model of unexplained symptoms (Brown, 2004). We suggest specifically that implicit goals induced and sustained by strained social relationships may foster chronic unexplained symptoms by increasing awareness of unpleasant somatic states. Implicit goals may be a means by which unsupportive social environments can influence symptom processing without affective mediation. We now report a study testing the hypothesis that implicit control motives interact with response-regulation capabilities to predict subjective symptom reports and that they can do so independently of negative emotions and affective traits.
A Social Action Theory Perspective
Our proposal derives from the social action theory of chronic psychological stress (Ewart, 2011), which holds that environmentally induced striving for interpersonal control (i.e., “agonistic striving”) fosters recurring social conflict and continuing power struggles that evoke hypervigilant mental states with healthdamaging physiologic and behavioral consequences. Agonistic striving is increased by continued exposure to stressful and unsupportive interpersonal environments in the home, workplace, neighborhood, or school that repeatedly threaten important supportive relationships. We propose that persistent agonistic motives in the form of goals (i.e., mental representations of desired social outcomes) may also contribute to heightened symptom-focused attention and self-reported illness.
Implicit Agonistic Goals
Behavior in recurring stressful situations often is guided by implicit action goals that are not a focus of direct conscious attention (Custers & Aarts, 2010). Implicit goals can be difficult to self-monitor and report because they are nonconscious and may involve intentions that people are unable or unwilling to admit. Whereas explicit self-attributed goals reflect conscious self-representations and beliefs, implicit goals generated automatically in stressful situations are less affected by personal self-schemas and defensive biases (McClelland, 1985). It is important to determine if implicit goals shape stress responses and related health outcomes in ways that explicit self-attributed motives may not.
How can we measure nonconscious goals? A promising approach uses a “situationally grounded” experiential protocol, the Social Competence Interview (SCI; Ewart, Jorgensen, Suchday, Chen, & Matthews, 2002) to assess an individual’s ability to generate goals and action strategies “online” in everyday situations that cause recurring stress. During the first 5 minutes of the 10-minute procedure, the participant is invited to recall and describe a recent stressful situation that exemplifies a recurring personal problem (chronic threat) and to vividly reexperience the thoughts, feelings, sensations, and impulses that the situation evokes. During the second 5 minutes, the interviewer asks the participant to imagine that he or she is a movie director making a film about a person like the participant who experiences a similar problem. The participant is asked to invent a desirable but realistic ending for the film and to create a narrative (story line) that describes how the desired ending might come about. Finally, the participant is asked to consider how the film story and ending might apply to his or her own personal predicament. Implicit motives that foster recurring stress are assessed with behavioral ratings of the imagined film narrative and ending (described below). Nonverbal indices of emotional expressiveness are used to gauge the likelihood that the motive will often induce stress in daily life.
This approach has yielded valuable insights into the nature of chronic stress and its effects on health. SCI narrative assessments in several multiracial samples of urban youth and young adults reveal that people implicitly frame their chronic life dilemmas either as ongoing struggles to get others to change (agonistic striving) or as ongoing struggles to control or change themselves (transcendence striving; Ewart, Elder, Sliwinski, Smyth, & Jorgensen, 2011; Ewart & Jorgensen, 2004). Social action theory proposes that, of the two goal orientations, persistent agonistic striving to influence other people is more likely to foster chronic health-damaging stress because the outcomes of interpersonal strivings are more difficult to predict or control (Ewart, 2011). Research in animals and humans has shown that the magnitude of physiologic responses to stress are modulated by the ability to predict or control threatening events (Bandura, 1997). Struggling to influence or control another person’s behavior involves investing in a goal that the other person ultimately controls. Such struggles easily provoke unwanted reactions (countercontrol) and can generate ongoing power struggles in which the behaviors of each party become increasingly aversive to the other (Ewart, Taylor, Kraemer, & Agras, 1991). Over time, repeated coercive interactions can cause the members of an interacting dyad to become aroused more quickly and to behave more aggressively (Granic & Patterson, 2006). The need to remain perpetually on guard increases the frequency and the strength of stress responses.
Transcendence goals that involve striving to control, change, or improve the self can be highly stressful because one runs the risk of failing to achieve an important personal goal or standard. However, anticipating one’s own behavior is easier than anticipating another’s. In addition, although achievement threats or failures may evoke powerful feelings of anxiety, shame, self-reproach, or despondency, it often is possible to moderate such emotions by altering one’s goals, self-standards, or action strategies. Further, transcendence striving, although distressing, may build personal capabilities and resources that reduce threat exposure (Ewart, 2011).
Research has shown repeatedly that the assessment of implicit goals with the SCI reveals distinctive profiles of stress-inducing motives and related health outcomes that are not evident in people’s explicit self-reports. People are less able or willing to report their agonistic goals than their transcendence goals; implicit agonistic goals assessed with the SCI predict cardiovascular responses in natural settings whereas participants’ explicit self-reported goals assessed with the same goal scale items do not (Ewart, Elder, & Smyth, 2012). In two large community samples, the focus of an implicit threat-related goal and its level of importance (indexed by nonverbal expressiveness indices) combined to generate three distinctive stress syndromes or profiles, each of which characterizes a distinctive cluster containing from 26% to 39% of the participants in each sample (Ewart et al., 2011; Ewart & Jorgensen, 2004). One cluster or “motive profile group”—agonistic striving— consists of individuals who are strongly invested in an ongoing struggle to change or control other people but who show little or no interest in changing or controlling the self. A second motive profile group—transcendence striving—is strongly invested in an ongoing struggle to change or control the self but shows little or no interest in changing or controlling other people. And a third stressed but unexpressive profile group—dissipated striving—shows little or no goal focus and passively wishes that their persistent problem would go away. The agonistic profile is defined by high expressiveness, high “change-others” goal focus, and low “change-self” goal focus; the transcendence striving profile is defined by high expressiveness, low “change-others” goal focus, and high “change-self” goal focus; and the dissipated profile is defined by a lack of goal focus. Ambulatory monitoring of blood pressure levels during normal daily activities indicates that implicit agonistic striving is associated with greater exposure to a threatening interpersonal world (Ewart et al., 2011; Ewart & Jorgensen, 2004). Youths with the agonistic profile exhibit significantly higher levels of ambulatory diastolic pressure during normal daily activities, especially during social interactions (Ewart et al., 2011; Ewart & Jorgensen, 2004).
We now propose that individuals with the agonistic striving profile also may frequently experience distressing somatic symptoms in the absence of identifiable medical pathology. Our hypothesis is based on the consideration that assiduous goal pursuit—agonistic or transcendent—increases self-regulatory demands and thus heightens awareness of conditions or events that could undermine coping by impairing self-regulation. A heightened self-regulatory focus increases somatic attention and influences symptom detection and interpretation by altering higher level cognitive processing of sensory information (cf. Brown, 2004). An alert self-regulatory focus may induce such bias by drawing attention to physical discomforts or symptoms of disability that could weaken social control (Keefe et al., 1997) or that could bolster control through defensive “self-handicapping” (Smith, Snyder, & Perkins, 1983). Implicit strivings may elicit distressing memories that bias sensory processing; this bias often is sustained by negative rumination (Brown, 2004). Although agonistic goals and transcendence goals both may induce alert mental states that bias symptom processing, we predict that agonistic goals will do so to a much greater degree. This prediction is based on the hypothesis that transcendence striving to control the self usually is more feasible than agonistic striving to control others, and agonistic striving more readily generates a continually uncertain and chronically threatening interpersonal world.
Response Modulation
Social action theory proposes that personal response regulation capabilities shape the effect of agonistic motives on stress physiology and behavior. In this view, goals and self-regulatory mechanisms are qualitatively distinct phenomena that combine to influence stress and health. Goals select, organize, and impel behaviors that foster stress exposure. Response regulation activities modulate physiologic and emotional reactions to stressors. Support for this view comes from a study that showed that the association between the agonistic profile and higher blood pressure was significantly greater in agonistic individuals who had greater difficulty regulating anger during an experimental emotion regulation task in the laboratory (Ewart et al., 2011). This interaction effect on blood pressure was replicated when teachers’ ratings of the youths’ self-control skills in the classroom were used to index self-regulatory ability (Ewart et al., 2012). However, group comparisons showed that the self-regulation abilities of the three motive profile groups did not differ. This supports the view that motives and self-regulatory capabilities represent distinctly different mechanisms that interact to shape stress exposure and responding.
These findings suggest the intriguing possibility that the effect of implicit agonistic goals on subjective symptoms might be moderated by self-regulatory resources that enable a person to modulate unpleasant physical sensations. The ability to control somatosensory awareness (Brown et al., 2012) could greatly affect the cognitive processing of aversive stimuli (Brown, 2004). Given that motivation and self-regulation involve different mechanisms, there is no reason to suppose that the three motive profile groups necessarily differ in their ability to regulate somatosensory awareness. However, if persons with the agonistic profile are more often stressed than persons with the other profiles, then it would seem reasonable to expect that an ability to modulate somatosensory awareness would more often be useful to persons with the agonistic profile. Compared with the other motive profile groups, people with the agonistic profile should experience threat-induced symptoms more frequently, and symptom levels in this group should be noticeably higher in individuals who also happen to lack an ability to modulate somatosensory awareness.
The Present Research
The present study extended the social action theory of chronic stress to the domain of subjective somatic symptoms by testing two major hypotheses. The first hypothesis predicted that (a) individuals with the agonistic motive profile would report more somatic symptoms than individuals with the transcendent or dissipated profiles, and (b) the predicted profile difference would be magnified significantly (i.e., moderated) by an individual’s ability tolerate an uncomfortable heat stimulus administered in a graduated pain tolerance test. Support for these predictions would suggest a new social-motivational mechanism by which stressful interpersonal environments may foster subjective somatic illness. The second hypothesis predicted that the expected profile group differences would not be explained by individual differences in trait neuroticism, anxiety, anger, depression, or low self-esteem or by heightened negative emotional reactivity to a personal stressor. Support for this hypothesis would suggest that implicit agonistic goals may represent a cognitive mediating mechanism by which threatening or unsupportive social environments can influence perceived health outcomes independently of negative emotional responses or affective traits (Walker et al., 2001).
The research was conducted with healthy adolescents and adults whose ages ranged from 12 to 31 years. This demographic focus was guided by prior evidence linking implicit agonistic goals to greater stress exposure and hypertension risk in this broad age group and by substantial evidence that stress-related illnesses begin early in life (Ewart, 2004). Although our theoretical model suggests that agonistic striving may bias the processing of a wide array of bodily sensations, the possibility that some participants in the present sample might be vulnerable to developing subjective somatic complaints is suggested by the fact that approximately two thirds had childhood histories of functional abdominal pain (FAP), a prototypic pain condition without significant organic pathology (Walker, Garber, Van Slyke, & Greene, 1995) and the most common recurrent pain complaint in youth (McGrath, 1990).
Method
Participants
The 333 participants had taken part in earlier research on abdominal pain by Dr. Lynn Walker; they later were invited to participate in a new group of studies that included the present research (the other studies addressed different questions and hypotheses). Of the present sample, 215 had a childhood history of FAP; they were recruited from a database of patients who a decade earlier had been evaluated for abdominal pain at a pediatric gastroenterology clinic and enrolled in research at that time (Walker et al., 2001; Walker, Smith, Garber, & Claar, 2005). Eligibility criteria for those studies had included abdominal pain of at least 3 months duration, no chronic illness or disability, and no organic disease diagnosis for abdominal pain from the referring primary care physician. Eligibility criteria for the current study included ≥12 years of age, ≤4 years since initial study enrollment, no evidence of significant organic disease in the initial FAP evaluation, and no significant chronic disease by self-report at follow-up. The remainder of the sample were drawn from a database of schoolchildren who had participated in a “no-FAP” control group when they were between the ages of 8 and 16 years (Walker, Baber, Garber, & Smith, 2008; Walker et al., 2001; Walker et al., 2004)and were followed prospectively using the same procedures as for the FAP group. Current pregnancy and acute minor illness were exclusionary criteria for the present study. For participants <18 years old, a parent provided demographic information including parental occupation and level of education.
Nearly all participants (97%) were Caucasian. Sex and socioeconomic status (SES), assessed based on the adult’s or parent’s occupation and level of education with the Hollingshead Index (1975), did not differ by FAP history (p = .87). Age at follow-up ranged from 12 to 31 years (M = 19.5; SD = 3.4). Participants without a history of FAP were slightly younger (M = 18.1; SD = 2.8) than participants with a history of FAP (M = 20.1, SD = 3.4), t(331) = 5.58, p < 0001.
Procedures
Self-reported symptoms were assessed by telephone or online. The SCI, the thermal pain task, and measures of negative affect were administered in a laboratory at the Vanderbilt University Medical Center. Interviewers were unaware of participants’ FAP history status. All study procedures were approved by the Vanderbilt University Institutional Review Board.
Agonistic Motives
Implicit motives were assessed with the SCI. The SCI’s experiential narrative is described above and in other publications (Ewart et al., 2011, 2002; Ewart, Ditmar, Suchday, & Sonnega, 2007; Ewart et al., 2002; Ewart & Kolodner, 1991). The interview manual is available from the first author. The interviewers—White female graduate students in clinical psychology trained by Dr. Ewart—were unaware ofthe study hypotheses. Implicit goals were assessed from audio recordings by trained coders using a reliable and valid coding system (Ewart & Ditmar, 2006). Examples of agonistic goals include trying to get others to be less demanding/hostile/critical or more friendly/cooperative/sympathetic. Examples of transcendence goals include seeking to overcome a personal defect, improve one’s performance, attain a valued standard, or live up to others’ expectations. The likelihood that a goal will evoke social responses and induce arousal in daily life is indexed by nonverbal indices of forceful, emotionally emphatic expressive speech assessed by an Expressiveness scale; items include “speaks loudly,” “speaks emphatically,” “speaks rapidly,” and “voice easily expresses emotion.” Descriptions of scale content are available in Ewart et al. (2002); the coding manual is available from the first author. Audio recordings of SCI interviews were rated by observers trained by Dr. Ewart; goal items were rated on 5-point scales (1 = not at all to 5 = very much). Internal consistencies of the scales, indicated by Cronbach’s α, all exceeded .78; 3-month temporal stability of SCI goal scales (Pearson r) in an earlier study ranged from r = .40 to r = .79 (Ewart et al., 2002). All interviews were coded by two coders; interrater agreement levels estimated by Pearson coefficients exceeded r = .75. Pairs of coders’ ratings agreed within 1 unit on the 5-point scale on 87% of comparisons.
Thermal Pain Task
A computer-controlled Medoc Thermal NeuroSensory Analyzer (TSA-II, Medoc, Inc., Ramat, Israel) was used to apply heat stimuli to the nondominant ventral forearm using a 30- by 30-mm Peltier thermistor probe as described in Dengler-Crish and colleagues (Dengler-Crish, Bruehl, & Walker, 2011). Heat pain tolerance was determined using an ascending method of limits (Chong & Cros, 2004). A series of four pain tolerance trials was used with the probe applied to a different target site on the arm to avoid local sensitization. Means of the last three trials were computed for data analysis following standard procedures (including elimination ofthe first trial in averaging responses) as described by the protocol’s developers (Fillingim & Edwards, 2005). For each tolerance trial, the probe started at an adaptation temperature of 40°C, with the temperature increasing at a ramp rate of 0.5°C until the participant terminated the trial via mouse click to indicate that the maximum pain tolerance had been reached. The interstimulus interval was 25 seconds. The same procedure was used with adolescents and adults. Participants (and parents) were informed in advance that the maximum temperature that the thermode could reach, 52°C, was approximately the same as the setting that is recommended for hot water heaters in hotels and people’s homes and was safe and could not burn them. The participant could click the mouse to turn off the thermode or could remove the thermode from his or her arm if at any time the discomfort was too great.
Subjective Somatic Symptoms
Subjective symptoms were measured with the Child/Adult Somatization Inventory-35 (C/ASI-35; Walker, Garber, & Green, 1991; Walker & Greene, 1989), which assesses the severity of each of 35 somatic symptoms often experienced in the absence of diagnosable disease (e.g., low energy, nausea, headache, dizziness, fatigue). Symptoms experienced during the past 2 weeks are rated on a 5-point scale (0 = not at all; 4 = a whole lot). Item ratings are summed to yield a total score that can range from 0 to 140, with higher values indicating greater somatic symptom severity. The C/ASI-35 has good internal consistency (α = .86).
Negative Affect
Trait affect.
Trait affect scales assessed traits linked to subjective symptoms in previous research (Campo, 2012): (a) Neuroticism: Neuroticism subscale, NEO Five Factor Inventory (NEO-FFI; Costa & McCrae, 1985); (b) Trait Anxiety: State-Trait Anxiety Inventory, Trait Form (STAI-T; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983); (c) Depression: Center for Epidemiological Studies-Depression Scale (CES-D; Radloff, 1997); (d) Anger: Total Anger Expression scale, State-Trait Anger Expression Inventory (AEI; Spielberger, 1999); and Self-Esteem: Global Competence subscale, Self-Perception Profile for Adults (Messer & Harter, 1986). All of these scales are appropriate for individuals age ≥12 years.
Emotional reactivity.
Assessment of changes in negative emotions evoked by the SCI strengthened the test of our second hypothesis by indexing stress-induced fluctuations in affective states, as distinguished from measuring the prevailing levels of stable affective traits with the questionnaires described above. Immediately before the SCI procedure began, participants rated their negative emotions on 5-point Likert-type scales to indicate the extent to which they felt nervous/worried, scared, annoyed, and upset (0 = not at all; 4 = a whole lot); the mean of the four rating items yielded the prestress Negative Affect score. Immediately after the 10-minute interview, participants completed the same four rating scales a second time to indicate “how you felt during the interview when talking about the problem that causes you stress.” SCI-induced changes in negative affect (emotional reactivity) were computed by subtracting the first Negative Affect score from the second.
Results
The presence of the predicted motive profiles was tested by performing hierarchical and k-means cluster analyses that used participants’ scores on the Expressiveness, Agonistic Goals, and Transcendence Goals scales of the SCI (cf. Ewart et al., 2011). The analysis disclosed that a three-cluster solution yielded the best fit; the cluster (motive group) profiles closely fit the predicted patterns and matched the corresponding motive group profiles that we obtained in two earlier samples (cf. Ewart et al., 2011). The numbers (percent) of study participants in each motive profile group were Agonistic, 76 (23%); Transcendent, 138 (41%); and Dissipated, 119 (36%). The percentages of females in the agonistic and transcendent profile groups (66% and 67%, respectively) did not differ; in the dissipated profile group, the percentage of females (50%) was lower (χ2 = 9.01, p < .01). The groups did not differ with respect to FAP history: Agonistic, 73%; Transcendent, 60%; Dissipated, 65%; χ2 = 4.36, p = .11) nor in the frequency of abdominal pain in the previous 3 months (p = .27).
Analytic Approach
Next, we tested predicted profile group differences in symptom levels with analyses of variance (ANOVAs) that used Type III sums of squares solutions (SAS Institute, Cary, NC). These analyses tested the hypotheses that individuals with the agonistic profile report more symptoms than individuals with the transcendent or dissipated profiles, and that individuals with the agonistic profile who exhibit lower pain tolerance report the most symptoms. We used an analytic approach in which the dependent variable, somatic symptoms, was predicted by age, sex, history of FAP, motive profile group, pain tolerance, and the profile group by pain tolerance interaction. Considering that our sample included males and females, adolescents and adults, and individuals with and without a history of FAP, we conducted initial analyses to determine if these differences might influence the proposed tests of the predicted profile group effects. Social action theory does not hold that implicit agonistic goals will predict subjective symptoms differently (or will interact with pain tolerance differently) in persons of different genders, ages, or FAP histories. Initial analyses of the predicted profile group effects on symptoms that included age, sex, FAP history, and all interactions indicated significant main effects for FAP history and profile group but not for age or sex. On the basis of these empirical findings as well on theory, the statistical analyses were performed using the full study sample. The models testing the predicted profile group differences (Agonistic vs. Transcendent + Dissipated) in symptom reporting, as well as those models testing the profile group by pain tolerance interaction, included FAP history as a covariate. A significant profile group by pain tolerance interaction was evaluated by testing the simple effect of pain tolerance on somatic symptoms within each profile group to see if symptoms were related to pain tolerance within each motive profile and by comparing the simple effects of the three profiles to see if they differed as predicted.
Profile Groups, Pain Tolerance, and Somatic Symptoms
Mean levels of subjective symptoms and maximum pain tolerance for each Profile Group are shown in Table 1. As predicted, symptom reporting differed across the profile groups, as indicated by a significant main effect for Motive Profile, F(2, 330) = 5.71, p = 04. Planned comparisons disclosed that persons with the agonistic profile reported more somatic symptoms than persons with the transcendent profile, t(330) = 2.45, p = .02, or the dissipated profile t(330) = 3.35, p = .001; the latter groups did not differ, t(330) = 1.14, p = .26. However, planned comparisons of the Pain Tolerance means showed that the groups did not differ in their ability to tolerate the heat stimulus, F(2, 330) = 0.29, p = .75. Next, we tested the hypothesis that the agonistic profile and low pain tolerance interact to increase symptoms. A generalized linear model (GLM) analysis revealed significant main effects of Profile Group and Pain Tolerance on symptoms. As predicted, the hypothesized interaction between Profile Group and Pain Tolerance in predicting symptoms was significant, t(347) = 1.76, p = .045. To evaluate this interaction, we tested the simple effects of Pain Tolerance on symptoms separately within each of the three profile groups. These analyses only revealed a significant slope in the Agonistic group, b = −2.61, SE = .91, p < .005; in the Agonistic group, lower levels of Pain Tolerance predicted higher symptom levels. Pain Tolerance was unrelated to symptoms in the Transcendence group, b = .25, SE = .79, p = .75, and the Dissipated group, b = —.18, SE = .81, p = .83.
Table 1.
Maximum Pain Tolerance and Subjective Somatic Symptoms by Motive Profile Group
| Maximum pain tolerance (°C) |
Subjective somatic symptoms (C/ASI) |
|||
|---|---|---|---|---|
| Motive profile | M | SD | M | SD |
| Agonistic (n = 76) | 46.7 | 2.2 | 14.5a | 11.2 |
| Transcendence (n = 138) | 46.8 | 2.0 | 11.3b | 8.7 |
| Dissipated (n = 119) | 46.9 | 2.0 | 10.0b | 7.6 |
Note. Significantly different group means are indicated by different superscripts.
We then compared the significant slope of the Agonistic profile group to the nonsignificant slopes of the other two groups by performing two sets of contrasts. First, two contrasts compared the Agonistic group slope to the slopes of the Transcendence group and the Dissipated group whereas a second contrast compared the Agonistic group slope to the slope that represented the remainder of the sample (i.e., the Transcendence and Dissipated groups combined). This latter contrast maximized statistical power by combining the Transcendence and Dissipated groups’ 257 participants in one comparison.
The first set of contrasts showed that the difference between the slopes of the Agonistic and Dissipated groups was statistically significant, b = 3.44, SE = 1.27, p = .01, and that the difference between the slopes of the Agonistic the Transcendence groups was in the predicted direction but did not achieve significance, b = 1.97, SE = 1.24, p = .11. The contrast involving all study participants (see Figure 1) showed that the Agonistic slope differed as predicted from the combined Transcendence + Dissipated group slope, b = −2.64, SE = 1.07, p = .01.
Figure 1.
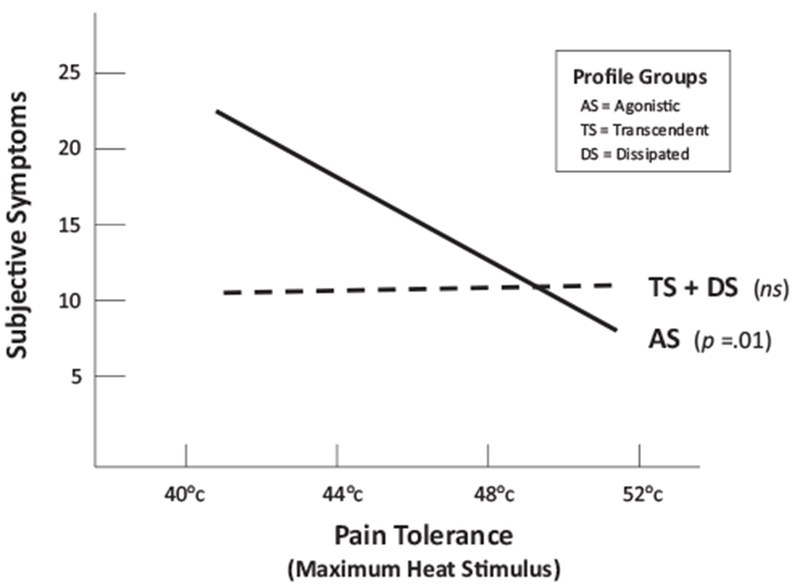
Interaction between Pain Tolerance capability (maximum temperature tolerated on the graduated thermal pain task) and implicit Motive Profile group in predicting Subjective Symptoms experienced during the past 2 weeks (the slope of Agonistic profile group is contrasted with the slope of the Transcendent and Dissipated profile groups combined).
Controlling for Psychological Covariates
Our second hypothesis was that the relationship among agonistic motives, pain tolerance, and subjective symptoms is not explained by affective traits and emotional reactions to personal stress. We tested this hypothesis in two steps. First, we evaluated profile group differences in the mean levels of five symptom-related personality and affective traits as well as SCI indices of negative emotional reactivity to personal stress. Second, we tested the social-motivational model in separate regressions that each included one affect variable as a covariate.
Symptom-related affective traits.
One-way ANOVAs comparing the profile groups on levels of Neuroticism, Anxiety, Depression, Anger Expression, and Self-Esteem yielded nonsignificant results (all values of p > .41), indicating that individuals with the agonistic profile did not differ from individuals with the transcendence or dissipated profiles on these traits. Sample means (SD) for the five traits were Neuroticism, 2.84 (1.30); Anxiety, 36.3 (9.3); Depression, 9.5 (7.5); Anger Expression, 18.6 (14.0); and Self-Esteem, 2.2 (0.57).
Negative emotional reactivity.
One-way ANOVAs comparing the three profile groups on levels of state Negative Affect while waiting to begin the SCI, and change in Negative Affect during the SCI, disclosed significant group differences in levels of Negative Affect experienced while waiting to begin the SCI, F(2, 349) = 3.47, p = .03, as well as in changes in Negative Affect while reexperiencing a personal stressor during the SCI, F(2, 349) = 4.84, p = .01. Profile group comparisons of pre-SCI Negative Affect levels indicated that persons with the Agonistic profile reported significantly higher levels of Negative Affect (M = 0.20, SD = 0.26) while waiting to undergo the SCI than did individuals with the Dissipated profile (M = 0.11, SD = 0.18); t(330) = 2.63, p = .01; but not the Transcendence profile (M = 0.15, SD = 0.21); t(330) = .09. However, the contrast of the Agonistic profile group mean with the combined Transcendence and Dissipated group means was statistically significant; t(330) = 2.41, p = .02. Profile group comparisons of changes in Negative Affect during the SCI indicated that persons with the Agonistic profile reported greater increases in Negative Affect (M = 0.60, SD = 0.66) during the SCI than did individuals with the Transcendence profile (M = 0.41, SD = 0.48) or the Dissipated profile (M = 0.47, SD = 0.56); t(220) = 3.06, p < .001; and t(198) = 2.33, p = .02, respectively.
Controlling for psychological covariates of subjective symptoms.
Finally, we tested the hypothesis that the effects of agonistic striving and pain tolerance on symptoms are not explained by symptom-related affective traits or negative emotional reactivity to personal stress. Separate GLM analyses regressed subjective symptoms (C/ASI scores) on Pain Tolerance, Motive Profile, and the Pain Tolerance by Motive Profile interaction in models that controlled for one of the psychological covariates. Each model also controlled for FAP history. Results of each GLM analysis disclosed that Pain Tolerance, Motive Profile, and the interaction term each independently predicted C/ASI scores after controlling for the covariate. In each model, evaluation of the Pain Tolerance by Motive Profile interaction revealed that, as predicted (a) the negative slope of the Agonistic group was statistically significant but that of the combined Transcendence and Dissipated groups was not, and (b) the slopes differed significantly.
Results of these analyses are summarized in Table 2. Although each covariate predicted subjective somatic symptoms, data in Table 2 show that the agonistic profile and its interaction with pain tolerance consistently accounted for additional variance in symptoms after controlling for each covariate. This pattern of results is consistent with the hypothesis that the effect of agonistic motives and response regulation capabilities on subjective somatic symptoms is not explained by stress-prone affective traits or by negative emotional reactivity to personal stress.
Table 2.
Prediction of Somatic Symptoms by Pain Tolerance in the Agonistic Group and the Combined Transcendence and Dissipated Group, Controlling for Psychological Covariates (Group Differences Are Shown by Group Slopes and Slope Contrasts)
| Covariate effect |
Agonistic group slope |
Dissipated + Transcendent group slope |
Agonistic vs. Transcendent + Dissipated |
|||||
|---|---|---|---|---|---|---|---|---|
| Psychological covariate | b (SE) | t | b (SE) | t | b (SE) | t | b (SE) | t |
| Traits | ||||||||
| Neuroticism | 2.05 (0.35) | 5.80**** | −1.98 (0.85) | −2.31** | 0.03 (0.51) | 0.05 | 2.82(1.07) | 2.63*** |
| Anxiety | 0.38 (0.05) | 7.81**** | −2.20 (0.83) | −2.66*** | −0.05 (0.49) | −0.11 | 3.17 (1.03) | 3.08**** |
| Depression | 0.45 (0.06) | 7.82**** | −2.40 (0.82) | −2.91*** | −0.16(0.49) | −0.32 | 2.93 (1.03) | 2.84*** |
| Anger expression | 0.09 (0.03) | 2.55*** | −2.33 (0.89) | −2.63*** | −0.15 (0.53) | −0.28 | 3.26(1.11) | 2.84*** |
| Self-esteem | −4.33 (0.80) | −5.44**** | −2.61 (0.86) | −3.03*** | −0.47(0.52) | −0.91 | 3.38 (1.08) | 3.14**** |
| Negative emotional reactivity | ||||||||
| Prestress level | 9.31 (2.24) | 4.16**** | −2.20 (0.88) | −2.51*** | −0.08 (0.53) | −0.15 | 2.56(1.10) | 2.32** |
| Stress reactivity | 2.34(0.84) | 2.79*** | −2.40 (0.89) | −2.70*** | −0.15 (0.53) | −0.28 | 2.72(1.12) | 2.43** |
Note. All models control for FAP history and the psychological covariate indicated.
p = .05.
p = .02.
p = .01.
p = .001.
Discussion
This study is the first to link implicit agonistic motives to heightened levels of subjective somatic symptoms and to indicate that this association is magnified in individuals who have difficulty regulating unpleasant bodily sensations. Further, the research supports the hypothesis that agonistic goals and response regulation mechanisms may shape subjective symptoms through processes that are not explained by stress-prone affective traits or by negative emotional reactions to stressful personal experiences. Present findings broaden support for a social action theory of stress-related illness by providing “direct” and “conceptual” replications of prior research in which implicit agonistic goals predicted hypertension risk indexed by higher ambulatory diastolic blood pressure during daily activities. Directly replicated in this study were prior findings that (a) persistent stress fosters agonistic, transcendent, and dissipated striving profiles; (b) these profiles occur across differences of sex, race, and geography; and (c) the agonistic profile is associated with greater emotional reactivity to personal stress. New findings include the discovery that the striving profiles observed in two community samples of adolescents also characterize young adults; the motive profiles obtained here are virtually identical to profiles reported in those studies (cf. Figure 1 in Ewart et al., 2011). Evidence for conceptual replication is seen in the finding that persons with the agonistic profile reported more subjective somatic symptoms than persons with the other striving profiles and the finding that this difference was magnified in persons who had difficulty tolerating an uncomfortable heat stimulus. Indeed, the pattern of profile group slopes depicting this interaction effect on subjective symptoms (see Figure 1) closely matches the pattern of profile group slopes depicting the interaction of agonistic striving with emotion regulation in predicting higher levels of ambulatory blood pressure that Ewart, Elder, and their associates reported previously (cf. Figure 2 in Ewart et al., 2011).
Other new findings include the evidence that the motive profiles are not associated with individual differences in stress-prone traits or in the ability to tolerate heat pain. New also is the related finding that neither stress-prone traits nor emotional reactivity to stressful experiences appear to explain the observed associations among agonistic striving, pain tolerance, and subjective symptoms. The present assessment of trait and state affect allowed us to compare the profile groups with respect to participants’ “usual” trait levels of negative affect and their tendency to experience stress-evoked situational fluctuations in negative emotional states. This analysis showed that, whereas persons with the agonistic profile do not experience more negative affect “on average,” they do react more negatively to personal stressors. Individuals with the agonistic profile reported feeling more (state) negative affect in the laboratory while they waited for the SCI to begin and greater increases in negative affect when reexperiencing a personal stressor during the interview. Thus, implicit agonistic goals are associated with an emotional tendency to react more negatively to personal stressors, but not with a tendency to feel more anxious, angry, or depressed in general. Despite this profile group difference, the agonistic group’s tendency to react more negatively to the SCI stressor did not account for their higher symptom levels.
Indeed, the observed relationships among agonistic striving, negative emotions, and health outcomes represent another conceptual replication. In earlier work testing the social action theory model of hypertension risk, persons with the agonistic striving profile exhibited greater increases in anger and sadness during the SCI as well as more intense anger during a subsequent anger-recall task relative to individuals with the other motive profiles (Ewart et al., 2011). However, regression analyses showed that higher blood pressure was associated with agonistic goals—not stress-induced sadness or anger. The present study yielded similar findings. These results, replicated with different samples and very different health outcomes, support the view that agonistic motives may shape emotions and health outcomes alike.
The absence of profile group differences in heat pain tolerance ability deserves comment. The finding that the agonistic profile group’s performance on the pain tolerance task did not differ from that of the other profile groups may seem to conflict with the finding that the agonistic group reported significantly higher levels of subjective somatic symptoms. However, this pattern of results is entirely consistent with social action theory, which distinguishes between the motivational influences that create threat exposure (e.g., implicit agonistic goals) and the response regulation capabilities (e.g., ability to tolerate physical discomfort) that enable one to ignore unpleasant sensory information. By analogy, a goal that increases exposure to cold viruses (e.g., a goal such as seeking to provide care for young children) can be distinguished from the factors that enable viral resistance (e.g., a healthy immune system). The goals that create virus exposure and the immune regulation factors that enable virus resistance combine to influence illness vulnerability. Thus, just as wanting to care for children does not indicate that one suffers from an immune deficiency, so wanting to influence or control other people (agonistic striving) does not indicate that one is unable to tolerate thermal discomfort.
The present research is limited by its cross-sectional design, which does not establish causation or show the direction of causal influences. Indeed, social action theory suggests that causation likely flows both ways: symptoms often motivate people to try to influence others (Sullivan et al., 2001). Longitudinal and experimental tests of causal mechanisms are needed, including intensive within-person studies investigating the interplay of social and psychological processes using in vivo ecological momentary assessment (EMA). Such research could afford new insights into mechanisms linking social support and health, one of which may involve a perceived ability to influence important others in home and community environments (Ewart, Elder, & Smyth, in press).
Research also is needed to develop clinical applications. The assessment of implicit agonistic strivings with the SCI may disclose sources of subjective symptoms that are not detected by current measures of negative affect, stress, or coping. Agonistic goals may be altered by cognitive-behavioral therapy and social-transactional intervention frameworks that address ongoing everyday interpersonal exchanges in families, work settings, neighborhoods, or schools (Conger & Elder, 1994; Granic & Patterson, 2004). Such intervention may help people identify and alter stress-inducing agonistic struggles.
Acknowledgments
The project described was supported by Award Number R01 HD23264 from the National Institute on Child Health and Development and does not necessarily represent the official views of the National Institute on Child Health and Development or the National Institutes of Health. The project also was supported in part by the Vanderbilt Kennedy Center (P30 HD15052), the Vanderbilt Digestive Disease Research Center (P30 DK058404), and the Vanderbilt CTSA grant (1 UL1 RR024975) from the National Center for Research Resources, National Institutes of Health.
Footnotes
The authors thank Richard Gramzow, who commented on a draft of this article.
Contributor Information
Craig K. Ewart, Department of Psychology, Syracuse University
Gavin J. Elder, Department of Psychology, Syracuse University
Kelsey T. Laird, Department of Psychology and Human Development, Vanderbilt University
Grace D. Shelby, Department of Psychology and Human Development, Vanderbilt University
Lynn S. Walker, Vanderbilt University School of Medicine and the Monroe Carell, Jr. Children’s Hospital at Vanderbilt, Vanderbilt, TN
References
- Bandura A (1997). Self-efficacy: The exercise of control. New York, NY: Freeman. [Google Scholar]
- Barsky AJ, Orav J, & Bates DW (2005). Somatization increases medical utilization and costs independent of psychiatric and medical comorbidity. Archives of General Psychiatry, 62, 903–910. doi: 10.1001/archpsyc.62.8.903 [DOI] [PubMed] [Google Scholar]
- Brown RJ (2004). Psychological mechanisms of medically unexplained symptoms: An integrative conceptual model. Psychological Bulletin, 130, 793–812. doi: 10.1037/0033-2909.130.5.793 [DOI] [PubMed] [Google Scholar]
- Brown RJ, Skehan D, Chapman A, Perry EP, McKenzie KJ, Lloyd DM,. Poliakoff E (2012). Physical symptom reporting is associated with a tendency to experience somatosensory distortion. Psychosomatic Medicine, 74, 648–655. doi: 10.1097/PSY.0b013e3182595358 [DOI] [PubMed] [Google Scholar]
- Campo JV (2012). Annual research review: Functional somatic symp-toms associated with anxiety and depression-developmental psychopa-thology in pediatric practice. Journal of Child Psychology and Psychiatry, 53, 575–592. doi: 10.1111/j.1469-7610.2012.02535.x [DOI] [PubMed] [Google Scholar]
- Chong PS, & Cros DP (2004). Technology literature review; quantitative sensory testing. Muscle-Nerve, 29, 734–747. doi: 10.1002/mus.20053 [DOI] [PubMed] [Google Scholar]
- Conger RD, & Elder GH (1994). Families in troubled times. New York, NY: Aldine de Gruyter. [Google Scholar]
- Costa PT Jr., & McCrae RR (1985). The NEO Personality Inventory Manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Custers R, & Aarts H (2010). The unconscious will: How the pursuit of goals operates outside of conscious awareness. Science, 329, 47–50. doi: 10.1126/science.1188595 [DOI] [PubMed] [Google Scholar]
- DeLongis A, Folkman S, & Lazarus RS (1988). The impact of daily stress on health and mood: Psychological and social resources as mediators. Journal of Personality and Social Psychology, 54, 486–495. doi: 10.1037/0022-3514.54.3.486 [DOI] [PubMed] [Google Scholar]
- Dengler-Crish CM, Bruehl S, & Walker LS (2011). Increased wind-up to heat pain in women with a childhood history of functional abdominal pain. Pain, 152, 802–808. doi: 10.1016/j.pain.2010.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart CK (2004). Social environments, agonistic stress, and elevated blood pressure in urban youth In Portman R, Sorof J, & Ingelfinger J (Eds.), Pediatric hypertension. Totowa, NJ: Humana Press. doi: 10.1007/978-1-59259-797-0_19 [DOI] [Google Scholar]
- Ewart CK (2011). Agonistic striving, emotion regulation, and hypertension risk In Wright RA & Gendolla GHE (Eds.), Motivational perspectives on cardiovascular responses (pp. 267–286). Washington, DC: American Psychological Association. [Google Scholar]
- Ewart CK, & Ditmar MM (2006). Behavioral coding manual for the Social Competence Interview. Syracuse, NY: Syracuse University. [Google Scholar]
- Ewart CK, Ditmar MM, Suchday S, & Sonnega JR (2007). Manual for the Social Competence Interview. Syracuse, NY: Syracuse University. [Google Scholar]
- Ewart CK, Elder GJ, Sliwinski M, Smyth JM, & Jorgensen RS (2011). Do agonistic motives matter more than anger? Three studies of cardiovascular risk in adolescents. Health Psychology, 30, 510–524. doi: 10.1037/a0023127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart CK, Elder GH, & Smyth JM (2012). How implicit motives and everyday self-regulatory abilities shape cardiovascular risk in youth. Annals of Behavioral Medicine, 43, 286–298. doi: 10.1007/s12160-011-9336-3 [DOI] [PubMed] [Google Scholar]
- Ewart CK, Elder GJ, & Smyth JM (in press). How neighborhood stress increases blood pressure in youth: Agonistic striving and subor-dination. Journal of Behavioral Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart CK, & Jorgensen RS (2004). Agonistic interpersonal striving: Social-cognitive mechanism of cardiovascular risk in youth? Health Psychology, 23, 75–85. doi: 10.1037/0278-6133.23.1.75 [DOI] [PubMed] [Google Scholar]
- Ewart CK, Jorgensen RS, Suchday S, Chen E, & Matthews KA (2002). Measuring stress resilience and coping in vulnerable youth: The Social Competence Interview. Psychological Assessment, 14, 339–352. doi: 10.1037/1040-3590.14.3.339 [DOI] [PubMed] [Google Scholar]
- Ewart CK, & Kolodner KB (1991). Social Competence Interview for assessing physiological reactivity in adolescents. Psychosomatic Medicine, 53, 289–304. Retrieved from http://www.psychosomaticmedicine.org/ [DOI] [PubMed] [Google Scholar]
- Ewart CK, Taylor CB, Kraemer CH, & Agras WS (1991). High blood pressure and marital discord: Not being nasty matters more than being nice. Health Psychology, 10, 155–163. doi: 10.1037/0278-6133.10.3.155 [DOI] [PubMed] [Google Scholar]
- Fillingim RB, & Edwards RR (2005). Is self-reported childhood abuse history associated with pain perception among healthy young women and men? Clinical Journal of Pain, 21, 387–397. doi: 10.1097/01.ajp.0000149801.46864.39 [DOI] [PubMed] [Google Scholar]
- Granic I, & Patterson GR (2006). Toward a comprehensive model of anti-social development: A dynamic systems approach. Psychological Review, 113, 101–131. doi: 10.1037/0033-295X.113.1.101 [DOI] [PubMed] [Google Scholar]
- Hollingshead AA (1975). Four-factor index of social status. New Haven, CT: Yale University. [Google Scholar]
- Keefe FJ, Kashikar-Zuck S, Robinson E, Salley A, Beaupre P, Caldwell D,. Haythornthwaite J. (1997). Pain coping strategies that predict patients’ and spouses’ ratings of patients’ self-efficacy. Pain, 73, 191–199. doi: 10.1016/S0304-3959(97)00109-7 [DOI] [PubMed] [Google Scholar]
- Lee S, Rogge RD, & Reis HT (2010). Assessing the seeds of relationship decay: Using implicit evaluations to detect the early stages of disillusionment. Psychological Science, 21, 857–864. doi: 10.1177/0956797610371342 [DOI] [PubMed] [Google Scholar]
- McClelland DC (1985). Human motivation. Glenview, IL: Scott Foresman. [Google Scholar]
- McGrath PA (1990). Pain in children: Nature, assessment, and treatment. New York, NY: Guilford Press. [Google Scholar]
- Messer B, & Harter S (1986). Manual for the Self-Perception Profile. Denver, CO: University of Denver. [Google Scholar]
- Nimnuan C, Hotopf M, & Wesley S (2001). Medically unexplained symptoms: An epidemiological study in seven specialties. Journal of Psychosomatic Research, 51, 361–367. doi: 10.1016/S0022-3999(01)00223-9 [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Smith GR, Monson RA, & Ray DC (1986). Patients with multiple unexplained symptoms: Their characteristics, functional health, and health care utilization. Archives of Internal Medicine, 146, 69–72. doi: 10.1001/archinte.1986.00360130079012 [DOI] [PubMed] [Google Scholar]
- Smith TW, Snyder CR, & Perkins SC (1983). The self-serving function of hypochondriacal complaints: Physical symptoms as selfhandicapping strategies. Journal of Personality and Social Psychology, 44, 787–797. doi: 10.1037/0022-3514.44.4.787 [DOI] [PubMed] [Google Scholar]
- Spielberger CD (1999). State-Trait Anger Expression Inventory-2: Professional manual. Odessa, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, & Jacobs GA (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Sullivan MJL, Thorn B, Haythornthwaite JA, Keefe FJ, Martin M, Bradley LA,. Lefebvre JC (2001). Theoretical perspectives on the relation between catastrophizing and pain. Clinical Journal of Pain, 17, 52–64. doi: 10.1097/00002508-200103000-00008 [DOI] [PubMed] [Google Scholar]
- Uchino BN, Bowen K, Carlisle M, & Birmingham W (2012). Psychological pathways linking social support to health outcomes: A visit with the “ghosts” of research past, present, and future. Social Science & Medicine, 74, 949–957. doi: 10.1016/j.socscimed.2011.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LS, Baber KF, Garber J, & Smith CA (2008). A typology of pain coping strategies in pediatric patients with chronic abdominal pain. Pain, 137, 266–275. doi: 10.1016/j.pain.2007.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LS, Garber J, & Green JW (1991). Somatization symptoms in pediatric abdominal pain patients: Relation to chronicity of abdominal pain and parent somatization. Journal of Abnormal Child Psychology, 19, 379–394. doi: 10.1007/BF00919084 [DOI] [PubMed] [Google Scholar]
- Walker LS, Garber J, Smith CA, Van Slyke DA, & Claar RL (2001). The relation of daily stressors to somatic and emotional symptoms in children with and without recurrent abdominal pain. Journal of Consultingand ClinicalPsychology, 69, 85–91. doi: 10.1037/0022-006X.69.1.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LS, Garber J, Van Slyke DA, & Greene JW (1995). Long-term health outcomes in patients with recurrent abdominal pain. Journal of Pediatric Psychology, 20, 233–245. doi: 10.1093/jpepsy/20.2.233 [DOI] [PubMed] [Google Scholar]
- Walker LS, & Greene JW (1989). Children with recurrent abdominal pain and their parents: More somatic complaints, anxiety, and depression than other patient families? Journal of Pediatric Psychology, 14, 231–243. doi: 10.1093/jpepsy/14.2.231 [DOI] [PubMed] [Google Scholar]
- Walker LS, Smith CA, Garber J, & Claar RL (2005). Testing a model of pain appraisal and coping in children with chronic abdominal pain. Health Psychology, 24, 364–374. doi: 10.1037/0278-6133.24.4.364 [DOI] [PMC free article] [PubMed] [Google Scholar]


