Abstract
Systemic autoimmune diseases are characterized by specific targeting of a limited group of ubiquitously expressed autoantigens by the immune system. This chapter examines the mechanisms underlying their selection as immune targets. Initiation of autoimmune responses likely reflects the presentation of antigens with distinct structure not previously encountered by the immune system, in a pro-immune context (injury, malignancy or infection). Causes of modified structure include somatic mutation, and post-translational modifications (including citrullination and proteolysis). Many autoantigens are components of multimolecular complexes, and some of the additional components may provide adjuvant activity. Propagation of autoimmune responses appears to reflect an active interaction between the immune response and the target tissues, in a mutually reinforcing cycle in which immune effector pathways generate additional autoantigen, which feed further immune response. We propose that this resonance may be a critical principle underlying disease propagation, with specific autoantigens functioning as the hubs around which amplification occurs.
Keywords: Immunodominance, Autoantibodies, modified structure
I. Introduction: a conceptual framework
The induction of an immune response against self antigens, which damages self tissues, is a central feature of autoimmune diseases. Over the past 50 years, there has been significant progress in defining the molecules which are targeted in autoimmune diseases, largely using high titer autoantibodies as probes (1). The resulting catalogue of autoantigens, together with careful clinical descriptions of the distinct disease features associated with such responses, has provided a critical framework to begin to understand the mechanisms responsible for antigen selection in autoimmune diseases (2).
It is noteworthy that the antigenic targets of the highly driven immune responses in autoimmune diseases are very limited and quite specific. Indeed, out of tens of thousands of possible molecular targets, it is estimated that less than 300 molecules are associated with self-sustaining and precise target tissue damage. This specificity, while not absolute, has nevertheless allowed autoantibody responses to become clinically useful markers and/or predictors of phenotype. The mechanisms underlying this striking specificity remain unclear for many of the autoantigens, but there is accumulating data which suggests that properties of the antigens themselves (such as structure, adjuvant properties and multimeric nature) play central roles in antigen selection. This chapter focuses exclusively on autoantigens in human autoimmune diseases, with particular emphasis on various autoimmune rheumatic syndromes. Although these human processes are extremely complex across multiple dimensions, the ability of patients and investigators to capture and express the nuance of disease phenotype and course, coupled to the ability of the immune system to capture and remember discrete molecular events provides clarifying insights into the biology underlying autoimmune responses and their resulting pathologies.
This chapter takes an “autoantigen-centric” look at human autoimmune diseases, coupling specificity and kinetics of the immune response and disease phenotype (including unusual confluence of clinical events), to studies of antigen structure and expression in different tissues targeted in various human autoimmune diseases. The complexity of this biology, and the available data to date, strongly suggest that a single feature is unlikely to unify all autoantigens. Rather, available data indicates that several subgroups of similar molecules exist. Analysis of these subgroups highlights that shared properties within members of the categories may guide their selection as autoantigens.
II. Autoantigens in different types of autoimmune disease
Autoimmune diseases have traditionally been classified by whether they affect a specific tissue (e.g. beta cells of the pancreatic islets in insulin dependent diabetes mellitus, or receptors at the neuromuscular junction in myasthenia gravis), or affect multiple tissues (e.g. muscle, skin, and lung in dermatomyositis/synthetase syndromes; or skin, joint, kidney, bone marrow elements and nervous system in systemic lupus erythematosus). Interestingly, autoantigens targeted in the tissue-specific autoimmune processes are often tissue-restricted (e.g. islet cell autoantigens in insulin-dependent diabetes mellitus (3), or components of the acetylcholine receptor in myasthenia gravis (4), while the autoantigens defined in the systemic processes are generally antigens which are ubiquitously expressed in multiple cell types and tissues (5).
II.1. Kinetics of development of specific autoantibodies: Changes in antigens targeted at different disease stages
Over the past 15 years, there have been significant advances in understanding the kinetics of onset of the systemic autoimmune diseases. Previously, the assumption was that since autoantibodies are present at disease diagnosis, they arise close in time to this event. Although there were early indications that autoantibodies might precede full-blown clinical disease, it was not until the landmark study of Harley and colleagues that conclusions could be based on relevant data. Using the United States Department of Defense (DOD) serum repository (which collects and stores serum from active military recruits unrelated to diagnosis or disease), these investigators demonstrated that patients with SLE frequently have autoantibodies present for long periods before the first disease symptom or manifestation (6). Interestingly, the antibodies found in patients who were evolving to an SLE diagnosis could be divided into 2 broad groups: (i) antibodies present for several years before the first symptom (e.g. antinuclear antibodies (ANA), anti-phospholipid antibodies (APL), as well as anti-Ro antibodies) and (ii) antibodies that were present at low frequency prior to symptom development, but become much more frequent around the time of diagnosis (e.g. Anti-Sm, anti-RNP and to a lesser extent anti-DNA). The presence of an immune response recognizing autoantigens prior to development of symptoms has also been observed for other tissue-specific and systemic autoimmune diseases (e.g. type I diabetes (7, 8), RA (9, 10, 11). Although there was evidence in these other conditions that the number of autoantigens and epitopes within targeted antigens increased towards the appearance of disease (12), no clear specificity change that was suggested in the SLE studies was appreciated.
These important observations suggest that an immune response against autoantigens precedes the development of self-sustaining tissue damage, and that a quantitative and/or qualitative change in the autoimmune response coincides (or culminates) with the onset of clinical symptoms. The two distinct kinetic phases in the development of autoimmune diseases – reporting on events at initiation and propagation - might occur along a continuum, or could be discrete events. It has been proposed that the established second phase (propagation) might represent a feedforward loop, in which enhanced autoantigen expression in the target tissue, or immune effector pathway-induced antigen generation play important roles (13, 2). Interestingly, the antibodies appearing in SLE in this propagation phase (6) include molecules which also have the capacity to ligate Toll-like receptors (TLRs; see section III.5).
III. Shared characteristics of autoantigens targeted in the systemic autoimmune diseases
In spite of the extraordinary diversity of autoantigens (they are found in any subcellular location, and include numerous different molecular species), the cataloguing of large numbers of autoantigens across the systemic autoimmune diseases has elucidated several important shared characteristics. These have provided critical insights into the mechanisms underlying their selection as targets of the autoimmune response, and are also the basis of the considerable diagnostic power of autoantibodies.
III.1. Autoantigens in the systemic autoimmune diseases are frequently ubiquitously expressed molecules that function in conserved pathways
A prominent feature of many autoantigens targeted in the systemic autoimmune diseases (a representative group is listed in Table 1) is their lack of restricted expression. Indeed, the vast majority are expressed in many different cell types and tissues. (It should be noted that this is not a universal feature, and there are well-described examples of a minority of autoantigens which are expressed in a more restricted fashion - e.g. neutrophil antigens which are targeted in SLE and vasculitis). There is also diversity in the type of molecule targeted - the range includes DNA-protein complexes (14-16), RNA-protein complexes (17-20), phospholipid protein complexes (21), simple protein antigens (22) and carbohydrate antigens (23). Additionally, targeted molecules are found in a variety of subcellular locations, including nuclear, cytoplasmic, or associated with various organelles or cell membranes (reviewed in (24)).
TABLE 1:
Partial listing of autoantigens targeted with phenotypic implications in the autoimmune rheumatic diseases
| Disease | Autoantigen | Phenotypic features |
|---|---|---|
| Autoimmune myopathies | Aminoacyl tRNA synthetases | “Anti-synthetase syndrome” – myopathy, ILD, nonerosive arthritis, fever, and mechanic’s hands |
| Mi-2 (CHD4) | Dermatomyositis-specific; associated with more severe skin rash, better response to steroid therapy | |
| Signal recognition particle (SRP) | Associated with immune-mediated necrotizing myopathy, rapidly progressive disease course, and severe muscle weakness | |
| Transcriptional intermediary factor 1-gamma (TIF1-γ; TRIM 33) | Less likely to have systemic features; associated with more extensive skin involvement and cancer | |
| Nuclear matric protein-2 (NXP2; MORC3) | Associated with cancer and calcinosis | |
| 3-hydroxy-3-methylgultaryl-coA reductase (HMGCR) | Associated with immune-mediated necrotizing myopathy | |
| Melanoma-associated differentiation gene-5 (MDA5) | Dermatomyositis with mild/absent muscle disease; high frequency of interstitial lung disease; cutaneous ulcers and palmar papules | |
| Small ubiquitin-like modifier activating enzymes SAE-1 and SAE-2 | Found in dermatomyositis patients- often with skin manifestations before muscle involvement, and frequently with dysphagia | |
| Scleroderma | Topoisomerase-1 | Associated with diffuse cutaneous scleroderma, pulmonary fibrosis |
| Centromere proteins A, B & C (“CENPs”) | Associated with limited cutaneous scleroderma and the CREST syndrome (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly and telangiectasia) | |
| Fibrillarin; Component of the small nucleolar U3 (snoRNP) complex | Associated with an increased risk of pulmonary arterial hypertension | |
| Nucleophosmin (NPM; B23) | Associated with pulmonary arterial hypertension | |
| RNA polymerases I, II & III, multiple components | Diffuse cutaneous involvement. For POLR3, higher risk of renal crisis and cancer | |
| PMSCL; RNA exosome complex consisting of 10 proteins, the most commonly targeted are EXOSC9 & EXOSC10 | Overlap syndrome (scleroderma/polymyositis) | |
| Ku 70/80 components of DNA-dependent protein kinase | Associated with muscle & joint involvement in scleroderma patients | |
| Sjogren’s syndrome | Ro52 (TRIM 21) | Extensive lymphocytic salivary gland infiltration & exocrine gland hypofunction. Also targeted in SLE, myositis and scleroderma |
| Ro60 | Extensive lymphocytic salivary gland infiltration & exocrine gland hypofunction. Also in SLE | |
| La (SS-B) | Extensive lymphocytic salivary gland infiltration & exocrine gland hypo-function. Also in SLE | |
| Gamma interferon-inducible protein-16 (IFI16) | Associates with more severe disease in Sjogren’s. Also targeted in scleroderma & SLE | |
| Vasculitis | Proteinase-3 (PR3) | Targeted in small-vessel necrotizing vasculitis (eg Granulomatosis with polyangiitis (GPA)) |
| Myeloperoxidase (MPO) | Targeted in small vessel necrotizing vasculitis with renal focus (e.g. microscopic polyangiitis (MPA)) | |
| RA | ACPA (anti-citrullinated protein antibodies) | Recognize multiple citrullinated autoantigens; High specificity for RA |
| Peptidyl arginine-deiminase-4 (PAD4) | Associated with anti-CCP antibodies and more severe disease | |
| SLE | ds DNA | Diagnostic of SLE, associated with lupus nephritis |
| Components of the Sm splicing ribonucleoprotein (subunits A-G, most commonly targeted are B, B’ & D) | Specific for SLE | |
| U1-RNP | Targeted in SLE; also in myositis and scleroderma | |
| Ribosomal protein P | CNS lupus, including psychosis, depression and neuropathy | |
| Cardiolipin, Anionic phospholipid/protein complexes | Associated with anti-phospholipid syndrome (thrombosis, pregnancy loss, thrombocytopenia) | |
| N-methyl-D-aspartate (NMDA) receptor | Bind DNA and subunits of NMDA receptor, associated with cognitive abnormalities in SLE |
The targeting frequency of different autoantigens falls into 2 broad groups: (i) antigens that are frequent targets of the immune response in a specific patient population (i.e. targeted in >10% of patients with a particular phenotype), and (ii) antigens that are infrequent targets of the immune response in a specific phenotype. Examples of frequently targeted autoantigens include the citrullinated antigens in Rheumatoid Arthritis (70–90% (25)), nucleosomes in patients with SLE (30–50% (26, 27)), Ro52/Ro60 targeted in Sjogren’s syndrome (50–70% (28, 29)), proteinase-3 in 70–90% of patients with Granulomatosis with polyangiitis (formerly Wegener’s granulomatosis) (30, 31), and topoisomerase-1 in 25–50% of patients with diffuse scleroderma (32). The high frequency of these immune responses in the outbred human population with autoimmune diseases is particularly noteworthy, since even in inbred mouse strains susceptible to spontaneous development of SLE, only 30–50% of genetically identical animals generate particular autoantibodies, likely reflecting important stochastic factors influencing whether specific molecules become targeted in autoimmune responses (33). Immune responses to the infrequently targeted autoantigens might similarly have very high specificity for phenotype, but in these instances, the antigens are targeted in rare phenotypes, or in very infrequent subgroups of larger phenotypes (e.g. MDA5 is targeted in a rare but clinically specific mucocutaneous syndrome associated with severe interstitial lung disease (34, 35), or alanyl-tRNA synthetase, which is a rare but highly specific target in ~3% of patients with the synthetase syndrome (36).
These features of autoantigens suggest that a limited group of possible molecules (often ubiquitously expressed) are selected as autoantigens, and that the targeted molecules share structural/biochemical features (e.g. binding of nucleic acid, specific post-translational modifications). The distinct form of the autoantigens that are selected may reflect something about the physiological state that initiates the autoimmune response. Furthermore, the striking association of immune responses against specific autoantigens with clinical phenotype suggests that target tissue antigen and immune-mediated tissue dysfunction are related.
In this regard, it is important to point out that the initial methods used to define autoantigens in systemic autoimmune diseases may have shaped what was found. Thus, for tissue-specific processes such as IDDM and thyroid disease, damage was focused on specific tissues, and screening for tissue-specific antigens was pursued. In contrast, for systemic autoimmune processes where the tissue targets were less obvious and more challenging to obtain for screening, transformed cell lines (usually epithelial) were used to define autoantigens recognized by patient sera. It is possible that the prominent autoantigens defined in systemic autoimmune diseases to date therefore reflect those molecules whose expression overlaps with transformed cells, and that there are tissue-specific and cell state-specific autoantigens that have not yet been defined. This will be an important priority for future studies, as the additional molecules yet-to-be defined might have significant diagnostic and mechanistic implications.
III.2. There is a striking association of immune responses to some autoantigens with specific clinical phenotypes.
Perhaps the most striking feature is that immune responses to specific autoantigens are associated with distinct clinical phenotypes affecting specific tissues (see Table 1), in spite of the fact that the autoantigens themselves are not expressed in a tissue-specific manner. For example, high titer autoantibody responses to nucleosomes or Sm ribonucleoproteins are associated with SLE (37); autoantibodies to topoisomerase-1 are associated with the diffuse form of scleroderma (and particularly with interstitial lung disease) (38); and autoantibodies against the aminoacyl-tRNA synthetases are associated with myositis, interstitial lung disease, and an unusual skin rash affecting the palmar creases of the hands (39). For autoantigens where immune response is associated with distinct clinical phenotypic features, detecting the autoantibodies has become useful for diagnosis and prediction.
The fact that autoantigens which function in centrally important cellular pathways active in many cell types, are targeted in distinct phenotypes is unexpected. If tissue-specific phenotypes result from immune effector pathways targeting these specific molecules, the finding suggests that antigen expression/availability/functional impact in different sites is distinct. The mechanisms underlying this specificity remain obscure, but their definition constitutes one of the most important challenges in the autoimmune rheumatic diseases. Several broad areas may be relevant, including those related to the microenvironment-specific expression and/or structure of the antigen (e.g. enhanced antigen expression or modification in tissues under specific circumstances), and particular sensitivity of some cells/tissues to specific effector pathways and mechanisms (e.g distinct patterns of MHC expression, sensitivity or resistance to key immune effector pathways, including autoantibodies). Interestingly, there are genetic diseases where abnormalities in a component of multimolecular machines which function in shared pathways that have important functions in all tissues (e.g. ribosomopathies), can have strikingly tissue-specific phenotypes (40).
It is important to note that there is an additional group of autoantigens targeted by the immune response where there is no association with a specific phenotype. This group may represent a distinct type of interaction between the immune response and target tissue – that is, one where the immune response recognizes that specific antigen but does not participate actively in generating tissue damage/dysfunction.
III.3. Many of the frequently targeted molecules are components of multimolecular complexes.
Nucleic acids (either DNA or RNA) are frequent components of autoantigen complexes. In many cases, multiple different components of these multimolecular complexes are independently recognized by autoantibodies (41). This feature is prominent across many of the rheumatic disease phenotypes. In SLE patients, various nucleoprotein complexes including those catalyzing mRNA splicing are prominent targets of the immune response. In this example, patients independently target multiple components of splicing snRNP particles, including U1-A/C proteins, U1–70k, and multiple Sm proteins (including B/B’, D1 and D3). There are many additional examples in lupus and other autoimmune rheumatic diseases, including histones in the nucleosome in SLE; components of DNA repair pathways in autoimmune myopathies and SLE (42-44); the signal recognition particle (SRP) (45), aminoacyl-tRNA synthetases (46), and components of the nucleosome remodeling and deacetylation (NuRD) complex in autoimmune myopathies (16); components of the PM-SCl exosome complex in scleroderma/polymyositis (47), and multiple components of RNA polymerase-1, −2 and −3 complexes (48-50) and the centromere in scleroderma (51). Interestingly, the nucleic acid component of the complex can also be independently recognized by the autoimmune response (52).
It is noteworthy that so many autoantigens function within multimolecular machines that mediate and/or regulate pathways involved in DNA replication, DNA repair, RNA splicing and processing, gene expression and protein translation. This has suggested that the distinct physiological or pathological states in which these processes are particularly active may be an important focus of the autoimmune response. The targeting of multiple components of multimolecular complexes is of particular relevance in light of the known ability of the immune response to spread from the first epitope targeted to additional epitopes within the same molecule (intramolecular spreading), or even to additional molecules within a complex (intermolecular spreading). This immunological design framework likely represents an adaptation that allows the immune system to maintain control of infectious and malignant challenges, which are constantly pressured to evolve away from the initial immune response (53).
In this context, the recent finding (see section IV.2 below) that the immune response in a subset of scleroderma patients with antibodies to the large subunit of RNA polymerase-3 may be initiated by a somatic mutation in that antigen in the patient’s cancer is of interest (54). RNA polymerase-positive scleroderma patients (including patients with mutations detectable in their cancer) frequently target multiple components of the RNA polymerase-3 complex, suggesting that an immune response initiated against one component of the complex spreads to others in the same complex. This in turn may provide some protection against the cancer losing the epitope driving disease, possibly through chromosomal loss. Such spreading to multiple components of complexes that play central roles in cellular division, survival and homeostasis might therefore have important biological benefits if the immune response against self antigens is being initiated by cancer.
It remains unclear in the human rheumatic diseases whether there is a uniform evolution of the immune response to multicomponent complexes in autoimmune diseases (i.e. always beginning with the same one component, and subsequently involving additional components), or whether initiation can begin with any component, and then evolve to engage the entire complex. It is also unclear whether the same components are always targeted in all patients, and whether the fine specificity of such immune responses are associated with distinct phenotypic features. These questions would be best addressed in disease cohorts (e.g. military cohorts using banked samples) studied during disease evolution.
III.4. Autoantigens are enriched in subcellular structures, including those induced during various physiologic processes (e.g cell death, NETosis)
The observation that multiple components of multimolecular machines are targeted by the immune system in systemic rheumatic diseases does not give insights into the circumstances under which these groups of antigens become visible to the immune response. Instead, for many of these intracellular autoantigens, various processes of cell damage and death are relevant in this regard. An interesting feature of the systemic disease autoantigens is their clustering and enrichment in specific subcellular domains during apoptosis (55). For example, the splicing ribonucleoproteins are clustered in coarse nuclear speckles in cells, but become aggregated and enriched in apoptotic bodies at the surface of cells during programed cell death. Although there has been an expectation that the antigens in systemic autoimmune diseases would be enriched in the specific target tissue associated with that immune response, the details of the immune effector pathways which cause tissue damage vary strikingly between different diseases, and perhaps even within the same disease between different target tissues.
When immune complex deposition plays an important role in pathology (rather than other effector pathways, including cytotoxic lymphocyte killing or cytokine effects), the specificity of injury may be more strongly influenced by the sites of deposition of immune complexes, which may not reflect locally generated antigen. For example, recent data in SLE has suggested that several well-defined lupus autoantigens may originate in inflammatory cells rather than cells resident in the skin (56). These include mature or immature myeloid cells (57). Interestingly, while mature neutrophils express several neutrophil-specific autoantigens (e.g. LL37, elastase, MPO), as well as several ubiquitously-expressed antigens (e.g. histones, nucleosomes, Ro), these cells are not a prominent source of other frequently targeted lupus autoantigens, including splicing ribonucleoproteins and PARP. It is likely that the immature, low-density granulocytes that are typical of SLE express the full spectrum of lupus autoantigens (58).
The finding that many autoantigens exist in macromolecular clusters, which undergo higher order organization during various processes (including cell death) is of great interest. Several of the clustered autoantigens are also structurally modified during these physiological processes (reviewed below), raising the possibility that circumstances exist in vivo in which the group of molecules prominently targeted by the immune system in autoimmunity become clustered, concentrated and structurally modified. While there is not yet direct data which addresses whether the generation of these antigen clusters can induce autoimmunity, the similarities to the types of repetitive, pathogen-derived antigens which the immune system evolved to respond to, is striking. A key determinant of the immune outcome of these pathogen-derived antigens is the context within which the molecule is seen, particularly whether it is directly or indirectly associated with the capacity to ligate pathogen-associated molecular pattern (PAMP)-receptors. Significant data has accrued suggesting that this capacity may also be critical for these autoantigen-containing forms.
III.5. Many frequently targeted autoantigens are able to activate innate and adaptive immune systems simultaneously
One of the features of the frequently targeted antigens across the spectrum of the autoimmune rheumatic diseases is the striking enrichment of nucleic acid binding. For example, targeted autoantigens include the protein components of chromatin, splicing ribonucleoproteins, aminoacyl tRNA synthetases, the exosome, numerous components of diverse DNA repair pathways, the ribosome, nucleolar autoantigens, topoisomerases, components of DNA and RNA polymerases, as well as components of nucleosome remodeling and deacetylation machinery. Significant recent data has demonstrated that the property of being able to bind nucleic acid plays important roles in selecting autoantigens, at least for several RNA- and DNA-containing autoantigens targeted in SLE.
In initial studies by Marshak-Rothstein, Shlomchik and colleagues, B cell signaling was strikingly augmented by autoantigens which could co-ligate endosomal TLRs and the B cell antigen receptor (59, 60). Subsequent studies demonstrated that the ability of autoantigens to bind to TLR 7 or TLR 9 plays important roles in determining the target of the immune response in autoimmune processes. Thus TLR-9 deficiency prevents the development of anti-DNA antibodies in a lupus mouse model (61, 62). Unexpectedly, SLE clinical disease is augmented in these animals, suggesting that TLR-9 ligation might have additional regulatory roles (61). TLR 7 deficiency both prevents the generation of an antibody response against splicing ribonucleoproteins, and also markedly decreases the severity of lupus. Interestingly, the effects of TLR-9 deficiency on disease severity appear to be B cell intrinsic, and TLR-9 deficient B cells form antibody-secreting cells more readily than their normal counterparts (63).
The capacity to ligate and activate endosomal TLRs is therefore an essential determinant of the immunogenicity of DNA- and RNA-containing lupus autoantigens. This property is likely broadly applicable to additional nucleic acid containing autoantigens in other systemic rheumatic autoimmune diseases. In addition to the TLRs as sensors of nucleic acid, there are multiple cytosolic nucleic acid sensors (e.g. MDA5, STING) which may also be relevant to sensing nucleic acid-containing autoantigens (64-66).
Since it is likely that TLRs evolved to focus on pathogen-derived nucleic acids, much of the structural specificity of the TLRs is directed at forms of nucleic acid more likely to be found in bacteria and viruses. Understanding whether specific forms of nucleic acid in human cells are relevant to disease propagation in autoimmune diseases remains an important priority. It is of interest that nucleic acids in various forms are also found in extracellular settings; this likely represents both physiological and pathological release of nucleic acids from cells. In most circumstances, these intracellular components released from host cells do not initiate a significant immune response to autoantigens, suggesting that there are additional features of the nucleic acid autoantigens (such as modification of structure, or efficiency of clearance and/or degradation) which may influence their ability to initiate an immune response. Some of these additional features may reflect distinct elements of genetic susceptibility (e.g. rapid, non-inflammatory clearance of apoptotic cells enhanced by IgM (67) and C1q (68, 69), rapid DNA degradation by DNAase (70), or 8-hydroxyguanosine modification of DNA preventing degradation by TREX (66), but there are certainly additional pathways that may increase immunogenicity of self. Defining the circumstances in which the autoantigen forms most likely to ligate TLRs and cytoplasmic nucleic acid sensors are generated and preserved to subsequently engage these sensors and activate type I interferon synthesis, remain a high priority. Certainly, such pathways may be of significance for prevention and therapy.
III.6. Autoantigen-virus complexes
Autoantigens may be also found in complexes with viral components during viral infection, creating opportunities for intermolecular spread to the self-components. This is particularly prominent for viral nucleic acids, which can bind directly to several autoantigens. For example, the autoantigen La binds to Rubella virus RNA (71) and to Japanese encephalitis virus RNA (72), hnRNP-K binds to EBNA2 (73), calreticulin binds to rubella and denque virus dsRNA (74, 75), and most recently, it has been demonstrated that DNA from human DNA viruses (including herpes simplex virus (HSV) and cytomegalovirus (CMV) is bound by IFI-16 (76, 77), a prominent autoantigen in Sjögren’s syndrome and SLE (78). There is also evidence that Herpesvirus episomal dsDNA genomes bind to and activate IFI16, leading to activation of IFNβ and the inflammasome (79-81). Ebstein Barr virus (EBV) is a member of the Herpesvirus family, which has been associated with childhood SLE and proposed as an SLE initiator.
There are also examples of viral proteins binding to autoantigens. For example, p53 has been described as an autoantigen in several autoimmune diseases, including lupus and scleroderma (82-84). p53 is a target of many viral proteins, including SV40 large T antigen (85), and HPV16/18 E6 proteins (86). In a mouse study by Reeves and colleagues, complexes of SV40 large T antigen and murine p53 induced an immune response to p53, which could subsequently be boosted with murine p53 alone (87). Although these do not provide mechanistic proof of a causal pathway to targeting specific autoantigens in the setting of viral infections, they do highlight a plausible pathway whereby complexes of viral components and autoantigens formed during infection might initiate immune responses against the self antigen. These immune responses could subsequently be driven even in the absence of the viral initiator.
This segregation of possible initiating events from the events which drive subsequent tissue damage complicates the study of spontaneous autoimmunity in humans. Defining the specific targets of the earliest autoimmune response, prior to spreading to additional self components, remain challenges that likely will provide important insights.
III.7. Autoantibody responses frequently recognize native or specific post-translationally modified autoantigen forms.
As noted above, many autoantigens were initially described using immunoprecipitation-based assays using radiolabeled extracts of transformed cultured cells. Extensive data has since demonstrated that many autoantigens are recognized predominantly/exclusively in their native forms, which are assumed to be biologically relevant. Consistent with this, where enzymatically active molecules are targeted, autoantibodies frequently recognize the catalytically active of the antigen, and can inhibit their function (88). The preference for native antigens is also evident in the preferential recognition of some autoantigens in assay formats that use the native protein form (e.g. immunoprecipitation and ELISA). For example, only ~10–15% of human dermatomyositis sera positive for Mi-2 or MDA5 autoantibodies by immunoprecipitation are also positive by immunoblotting; for anti-IFI16 antibodies in Sjogren’s and SLE patient sera, this is only ~5–10%. Additionally, multiple autoantigens are only recognized by the immune system in post-translationally modified forms. Relevant modifications include phosphorylated (89, 90, 49), acetylated (91, 92), iso-aspartylated (93), citrullinated (94, 95) and carbamylated (96) autoantigens.
There are several important consequences to the fact that conformationally distinct and post-translationally modified forms of autoantigens are frequently targeted by the immune response. Firstly, for autoantigen identification, assays that use a relevant form of the antigen in a native conformation have been the most successful. Antigen detection formats that utilize non-native or small peptides may miss a significant proportion of autoantigens. Secondly, it strongly implies that only specific forms of the autoantigens are driving the ongoing immune response, suggesting again that specific physiological and pathological states are providing the autoantigen most relevant to pathogenesis. Post-translational modification of autoantigens may play a role in initiating and driving autoimmunity by generating conformational changes which may alter autoantigen processing (see below). The mechanisms leading to targeting of the native forms of the antigen are less clear; these are discussed below.
IV. Modification of autoantigen structure: Role in disease initiation and propagation
IV.1. Effects of altered autoantigen structure on antigen processing and presentation to T cells
Much of the discussion above highlights several important observations about autoantigens gleaned from extensive studies using autoantibodies as probes. Firstly, autoantigens are limited in number, and share important features such as being components of multimolecular complexes and subcellular structures. Secondly, despite the apparently ubiquitous expression of the targeted molecules, there is an association of immune responses against specific antigens (or groups of antigens) with distinct clinical phenotypes. Thirdly, autoantibodies recognize modified antigens in some patients. Since the immune responses against these antigens are T cell-dependent and appear to be driven by the self-antigens themselves, it is essential to consider these features of autoantigens in the context of CD4 T cell immune responses, and as possible participants in the amplifying loops that sustain autoimmune diseases over long periods of time.
A very effective intellectual framework within which to view the role of antigen structure in the initiation of autoimmunity derives from the extensive, elegant work of Eli Sercarz and colleagues (97). They observed that during the natural processing of whole protein antigens, only a few of the potential antigenic determinants that could be generated by proteolysis and binding to MHC class II were actually selected for presentation as peptides within the MHC groove. The determinants which are processed and efficiently presented within the groove of MHC class II were termed ‘dominant’, whereas those that are not presented (the majority of determinants), and were termed ‘cryptic’. For self antigens, only dominant epitopes are presented during development of T cell tolerance in the thymus; the CD4 T cell repertoire that recognizes the cryptic self is therefore preserved (97).
Numerous mechanisms account for these distinct outcomes, including intrinsic peptide affinities for MHC class II, protein folding, the formation of protein complexes with other molecules, and effects of post-translational modifications (98). Since the output of processing and presentation of a specific antigen in a given individual is unchanged in most contexts, T cells recognizing the cryptic self seldom encounter their specific self-antigens. However, processes or circumstances that modify generation of dominant and cryptic epitopes may allow a determinant to emerge to activate CD4 T cells which has not been previously tolerized. For example, modification of early proteolytic events in the antigen processing pathway may destroy a dominant epitope, and/or allow emergence of a cryptic epitope not previously presented effectively, and therefore not tolerized. Additionally, modification or mutation of specific amino acids might create new dominant epitopes. Under such circumstances, an effective T cell response against the self antigen can be initiated. This T cell immune response can diversify, often through antibody/B cell-directed processing, to focus on the wild type unmodified antigen, which might drive subsequent, amplifying the responses to self antigen (99).
There are several excellent examples of this phenomenon. (i) Mamula and colleagues demonstrated cyanogen bromide mediated cleavage of cytochrome C can liberate cryptic CD4 T cell determinants in mouse cytochrome c, and initiate a T cell response against the native antigen (100). Interestingly, when mouse and human cytochromes c are co-injected into mouse, there is an early T cell response against human cytochrome c, which drives a cross-reactive B cell response that recognizes both the human and mouse antigens (99). Autoreactive B cells, likely through capture and altered processing of the mouse cytochrome c, initiate a subsequent CD4 T cell response to the mouse antigen (101), which no longer requires the foreign antigen. (ii) Watts and colleagues demonstrated a central role for early proteolytic cleavage mediated by asparagine –endopeptidase (AEP) in directing subsequent proteolytic processing by cathepsins. Inhibition of AEP or augmentation of AEP can modify the peptides loaded into MHC class II, and create the opportunity to activate self reactive CD4 positive T cells (102). (iii) Similarly, binding of HIV gp120 to CD4 can modify the processing of CD4 and generate autoimmunity against epitopes in CD4 not previously tolerized (103). (iv) Even subtle changes in antigen chirality (e.g. replacement of Asp with iso-Asp) can result in activation of T cell responses to the modified molecule, which can then spread to native molecule, and drive autoimmune responses (104), (105).
There is growing evidence that modified autoantigen structure plays a role in initiating the specific immune responses observed in the human rheumatic diseases. Although direct data capturing autoantigen structure at the very beginning of disease remains limited and is difficult to obtain, there is significant indirect data supporting this construct.
IV.2. Somatically mutated autoantigens in cancer
Human diseases can provide critical clues to their origins, particularly where unusual events occur simultaneously and unexpectedly. Coupled with the striking specificity of the immune response observed in different rheumatic phenotypes, and the powerful memory of the immune response, these unique events may allow much of the multidimensional complexity of the autoimmune rheumatic diseases to be neutralized. In this regard, the co-occurrence of cancer and rheumatic disease, and its association with novel immune responses have provided important opportunities to probe the origins of specific disease subgroups.
Extensive clinical descriptions of associations between autoimmune rheumatic diseases and cancers have been made over the past 50 years. These associations are interesting both in their magnitude and kinetics, and initial anecdotal reports were subsequently confirmed by multiple population-based studies. In dermatomyositis (DM), approximately 15–20% of patients have cancer-associated DM (defined as having cancer and DM appearing within 3 years of each other). Although the incidence of cancer is lower in patients with polymyositis (PM), multiple studies have demonstrated that this association is robust. While cancers are observed in other rheumatic phenotypes, they are less frequent. For example, in ANCA-associated vasculitis, renal cell cancers have been observed in ~5% of incident cases. In scleroderma, cancer occurs at some time in the disease course in ~10% of cases, but a smaller proportion of scleroderma patients (<5%) have clustering of cancer and scleroderma diagnosis within 3 years of each of each other. The kinetics of cancer onset in the autoimmune rheumatic diseases is particularly interesting. For example, in some antibody subgroups in DM and scleroderma, a clear clustering of cancers and autoimmune phenotype is evident. Appearance of cancer is maximal in the period −2 years to +2 years from diagnosis of the autoimmune disease, but continues until year +5, although at decreased frequency (106, 107).
Although the association between cancer and autoimmune rheumatic phenotype was initially described as an association with phenotype rather than specific immune response, increasing data suggests that particular immune responses in myositis and scleroderma are more likely to be associated with cancer, particularly with a short cancer-autoimmunity interval. Early observations in DM suggested that patients in whom DM was associated with cancer did not have the classically defined myositis autoantibodies. For example antibodies against Mi-2 and histidyl tRNA synthetase are found infrequently in the cancer-associated group. Significant advances have recently been made in defining autoantibodies enriched in patients with cancer-associated DM. For example, autoantibodies recognizing a protein doublet of 145kDa and 155 kDa were demonstrated to be enriched in cancer-associated DM. These autoantigens have been demonstrated to be TIF-1γ and TIF-1α (108). Recently, NXP2 autoantibodies were also shown to be found at higher frequencies in patients with cancer-associated DM. Together, 83% of patients with cancer-associated DM have autoantibodies recognizing either NXP2 or TIF-1γ (109).
Recent studies suggest that similar findings are also present in other autoimmune rheumatic diseases. For example, there is a serological subgroup of scleroderma patients in whom scleroderma and cancer occur in close proximity. Thus when patients with antibodies to the large subunit of RNA polymerase-3 (RPC1) also have a cancer diagnosed, the median interval between cancer and scleroderma is −1.2 years. This is in contrast to scleroderma patients with cancer in the setting of other autoantibodies, where the median interval between cancer and scleroderma is ~ +12 years. Interestingly, when the distribution of RPC-1 in the tumors of scleroderma patients was examined, the pattern observed in patients with RPC-1 autoantibodies was distinct, with striking nucleolar staining of RPC-1 being noted in the majority of these cancers, but not in cancers from scleroderma patients with other autoantibodies, including centromere and topoisomerase-1 (110).
This striking association of short interval between scleroderma and cancer with a particular autoantibody specificity suggested that something about the antigen in that patient’s cancer might play a role in initiating this specific immune response. A series of experiments was pursued to address this (54). Patients with scleroderma and cancer in whom the autoantibody specificities were known and the cancers available for study were selected. POLR3A (the gene that encodes RPC-1) was sequenced, and the striking, finding was that three of eight patients with RPC −1 autoantibodies had evidence of somatic missense mutations in POLR3A, compared to 0 of eight patients with other antibody specificities. Notably, one of these somatic mutations was subclonal, that is, was present in only a subset of the neoplastic cells.
This raised the possibility that cells containing these mutations may have been selected against during tumor cell growth, perhaps even disappearing as a result of an immune response. Since the most common way to lose a mutant allele in human cancers is a gross chromosomal event that results in loss of the gene and surrounding chromosomal region (loss of heterozygosity, LOH), the investigators searched for LOH of the POLR3A gene. This was detected in five of eight patients with antibodies to RPC-1, but was not found in patients with other autoantibody specificities (54). Together with the observation that POLR3A is mutated very infrequently in the cancers sequenced to date (<0.7% in the COSMIC database), these data strongly suggested that mutation might generate novel forms of antigens not previously tolerized.
These investigators therefore went on to directly address whether T cell and B cell immune responses against the wild type and mutant forms of RPC-1 could be defined. In the 3 patients with identified mutations, autoantibodies recognized wild type and mutated RPC-1 similarly. In two of the three patients, somatic mutations generated peptides predicted to fit with high affinity into one of that patient’s MHC class II alleles. In both patients, CD4 T-cell responses recognizing the mutated antigen were demonstrated. The frequency of antibody specific T cells varied between 1:600 and 1:5000. In one patient, the T cell response also recognized the wild type peptide. In this case, the T cells responding to the wild type and mutant antigens were clearly distinct, as next generation sequencing of T cell receptors stimulated by the different peptides showed that these TCRs were non-overlapping. In the third patient, mutant peptide did not fit into any of the patient’s MHC class II alleles with high affinity, and no T-cell immune response to either the wild type or the mutant peptide was observed. Interestingly, in this case, the mutation appeared to create a cathepsin cleavage site, which might influence processing of the antigen and presentation of another peptide of RPC-1 (54).
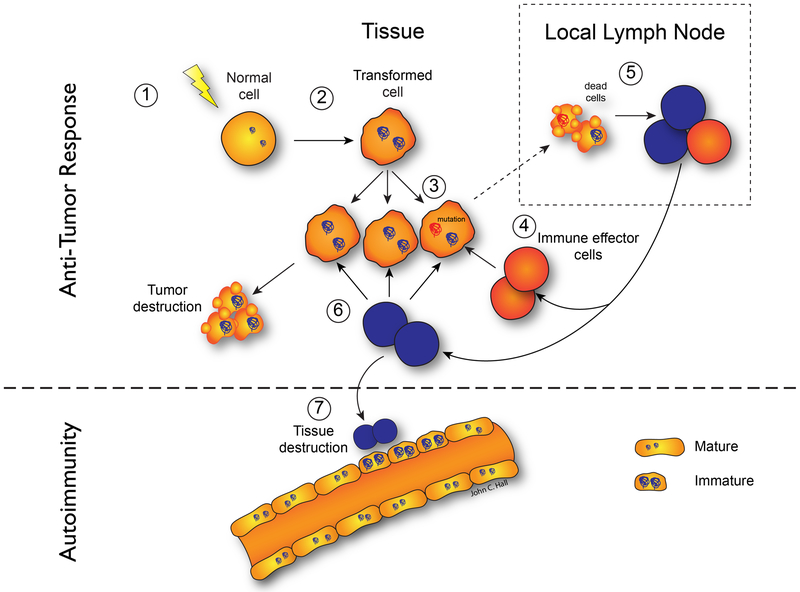
Together, these findings are consistent with many previous studies demonstrating that while small differences in antigens can initiate an antigen-specific CD4 T cell immune response, the antibody response fails to discriminate the minor structural changes that initiated that CD4 T cell response. These studies suggest that many cases of cancer-associated autoimmunity may reflect an immune response initiated against mutated autoantigens present in the patient’s cancer. It is noteworthy that this immune response spreads to the wild type autoantigen, likely driving the autoimmune phenotype (54). A model for this is presented in Figure 1. It will be important to define whether similar principles underlie the other examples of cancer occurring close to the onset of an autoimmune process, including DM, Granulomatosis with polyangiitis (previously Wegener’s granulomatosis), and lymphoma in SLE.
Figure 1. Model for cancer-induced autoimmunity.
Transformation of normal cells (1) may result in gene expression patterns that resemble immature cells involved in tissue healing (2). Occasionally, autoantigens become mutated (3); these are not driver mutations, and not all cancer cells have them. The first immune response is directed against the mutated form of the antigen (4), and may spread to the wild-type version (5). Immune effector cells directed against the mutant (depicted in red) delete exclusively cancer cells containing the mutation (6). Immune effector cells directed against the wild type (depicted in blue) delete cancer cells without the mutation and also cross-react with the patient’s own tissues (particularly immature cells expressing high levels of antigen, found in damaged/repairing tissue) (7). Once autoimmunity has been initiated, the disease is self-propagating. Immature cells (expressing high antigen levels) that repair the immune-mediated injury can themselves become the targets of the immune response, sustaining an ongoing cycle of damage/repair that provides the antigen source that fuels the autoimmune response. Reproduced with permission from (138).
While somatic mutation is a hallmark of cancer, somatic mutations clearly accrue in cells as a function of age, number of stem cell divisions, and cumulative exposure to mutagens. Recent studies have demonstrated that immune responses which emerge after checkpoint inhibitor therapy in cancer are largely directed against the mutanome in that patient (111). Since somatic mutation of autoantigens might occur in the absence of malignant change, it is possible that non-malignant processes may also generate the somatic mutanome that serves as an initiating stimulus for autoimmunity (112, 113). Since the immune response initiated by a somatic mutation can spread to the wild type version of the antigen (54), self-sustaining autoimmunity could be driven even if the mutation is not expressed.
The observation that patients with an immune response to RPC-1 exhibit significant LOH at the POLR3A locus strongly suggests the existence of immune editing, with the anti-RPC1 immune response exerting significant negative pressure against the cancer, and shaping loss of heterozygosity. This may also provide insights into the group of scleroderma patients with antibodies against RPC-1, in whom cancer does not emerge (~75–80% of RPC-1 positive patients). It is possible that the anti-cancer immune response in these patients, which begins with the mutated antigen and spreads to include the wild type antigen, effectively eliminates the cancer, or maintains it in equilibrium (114).
It is important to note that the mechanisms whereby the immune response initiated by the cancer cross-react with soft tissue remain unclear. As noted below, a key to understanding this latter phenomenon will be the definition of autoantigen expression in relevant target tissues.
IV.3. Modification of autoimmune rheumatic disease autoantigen structure by various post-translational processes, including citrullination and limited proteolysis
IV.3.1. Preferential binding of modified antigens targeted in rheumatoid arthritis to RA-associated HLA-DRB1 alleles.
Significant evidence for a role for post-translational modification of autoantigen structure is emerging from recent studies in RA (115), where there is an association between particular HLA-DRB1 alleles and RA that to the shared epitope hypothesis almost 30 years ago (116). A large haplotype association study has attributed most of the DR-associated risk to positions 11, 13, 71, and 74 of the HLA-DRβ1 polypeptide chain encoded by SE alleles (117), strongly suggesting that the shared epitope permits binding and presentation of autoantigenic peptides. It is therefore of great interest that, in addition to an association with RA, these shared-epitope alleles are also strikingly associated with the generation of autoantibodies which recognize epitopes containing deiminated arginine (citrulline) (118). These autoantibodies have a high specificity for RA (25).
In recent studies from the group at Monash, it was demonstrated using several peptides derived from well-defined citrullinated RA autoantigenic epitopes (including three from vimentin and one from aggrecan) that citrulline and not arginine was accommodated within the electropositive P4 pocket of the shared epitope MHC class II allele, HLA-DRB1*04:01. Interestingly, the electronegative P4 pocket of the RA-resistant HLA-DRB1*04:02 allele interacted with either arginine or citrulline-containing epitopes (115). Thus the P4 pocket of HLA-DRB1*04:01 is highly suited to preferentially accommodate citrulline over the corresponding arginine. Citrullinated peptides can be generated in abundance during specific inflammatory effector pathways, especially membranolysis mediated by cytotoxic lymphocyte granules (perforin) or the complement pathway (membrane attack complex) (119). It is therefore possible that citrullination modifies autoantigens, providing a novel set of peptides in the context of HLA-DR shared epitope molecules not previously tolerized, thus inducing the critical specificities that drive RA. There is also evidence that other post-translational modifications of specific autoantigens, including phosphorylation, acetylation, and ubiquitination, may be recognized specifically by autoantibodies, suggesting that the citrullination paradigm might be more broadly applied. For the more common post-translational modifications, which occur during multiple physiological and pathological perturbations, making mechanistic inferences will not be possible studying the human disease alone.
IV.3.2. Autoantigens targeted in systemic autoimmune diseases are frequently modified by limited proteolysis catalyzed by caspases and granzymes.
The studies of Watts and colleagues reviewed above demonstrate that early proteolytic cleavage mediated by proteases (in that case, by AEP) has important consequences in terms of directing subsequent proteolytic processing by cathepsins, and modifying the peptides loaded into MHC class II. This is particularly interesting in light of accumulating evidence that the majority of autoantigens targeted in systemic autoimmune diseases, which are diverse in terms of structure, function, or distribution, are frequently susceptible to cleavage by granzyme B (GrB) (120). The latter is a highly specific, fastidious protease which is abundant in cytotoxic lymphocyte granules, and has a major function in immune effector pathways. Indeed, there is strong evidence implicating the cytotoxic lymphocyte granule pathway in the pathogenesis of systemic autoimmune diseases, with activated cytotoxic lymphocytes present in target tissues and effector function positively correlated with disease activity in several diseases (e.g. myositis, SLE, RA) (121).
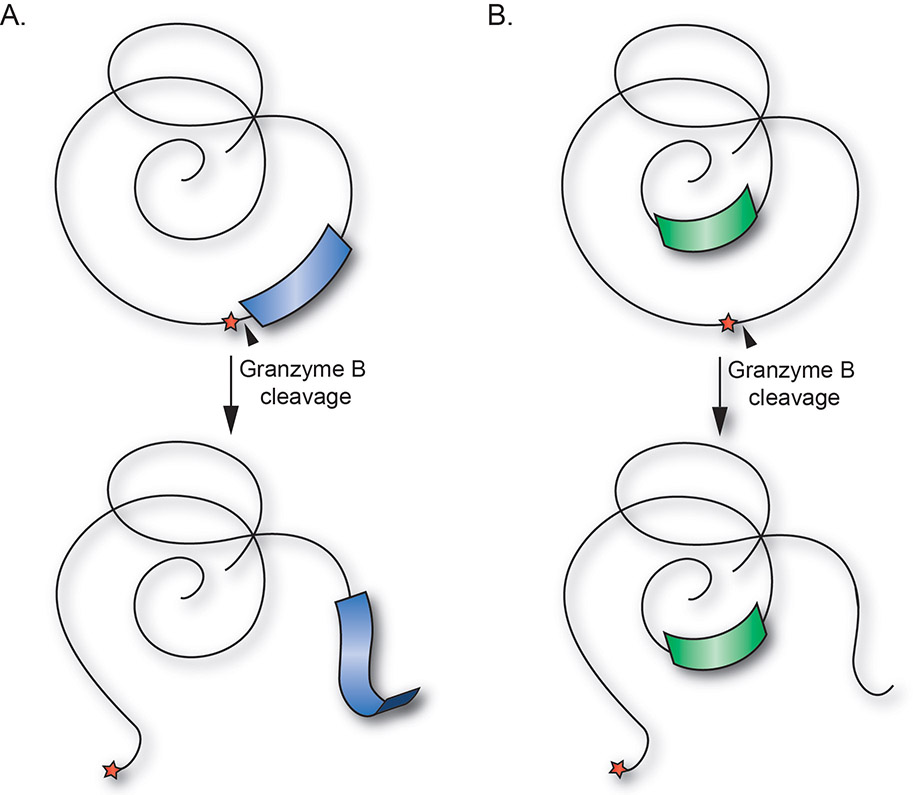
Interestingly, analysis of available data shows that GrB cleavage sites are often situated in unstructured regions of antigens, adjacent to structured domains. Immune epitopes are also frequently located in close proximity to GrB cleavage sites, suggesting that cleavage by GrB may modulate presentation of epitopes from adjacent structured regions. It has been proposed that GrB-mediated cleavage of autoantigens uncovers novel pathways of antigen presentation, allowing the emergence and presentation of previously cryptic epitopes (121), as is outlined in Figure 2.
Figure 2. Cleavage of autoantigens at GrB cleavage sites located in unstructured loops may liberate cryptic epitopes derived from structural elements.
(A) Proteolysis by GrB (black triangle) occurring in unstructured loops or linker regions of autoantigens (red star), may enhance presentation of cryptic epitopes derived from adjacent structural elements (blue). (B) GrB cleavage may also induce structural changes leading to increased presentation of cryptic epitopes derived from previously hidden regions of autoantigens (green). Reproduced with permission from (121).
Since GrB is an important component of a major cytotoxic effector pathway, it is possible that activated CD8 T cell and NK-mediated cytotoxicity pathways continue generating the forms of autoantigens that feed the CD4 T cell immune response raised against cleavable autoantigens. Data showing that cleavage by GrB can affect the epitopes subsequently presented compared to the whole antigen is not yet available. The observation that human and mouse GrB have distinct cleavage site specificities underscores the need to address the question in humans (122). These studies will be challenging, and will require a GrB-cleavable human autoantigen, which is frequently targeted in a phenotypically distinct autoimmune disease, with an immune response known to be restricted by specific MHC class II alleles. Establishing a mechanistic function for granzymes in the self-amplifying pathway of autoimmunity could have important therapeutic implications (see below).
V. Augmented expression of autoantigens in response to various immune effector pathways and tissue repair creates specificity and chronicity through immune:target resonance.
One of the central enigmas in understanding the specific targeting of ubiquitously expressed antigens in systemic autoimmune processes is the striking association of those immune responses with phenotype. There is an increasing appreciation that the immune response and target tissue are not independent entities in this regard, but rather represent interacting and mutually reinforcing forces, resonating around target tissue-specific and stimulus-specific autoantigen expression. We term these interactions immune:target “resonance”.
In physics, resonance is a phenomenon that occurs when a given system is driven by another vibrating system or external force to oscillate with greater amplitude at a specific preferential frequency. At resonant frequencies, small periodic driving forces have the ability to produce large amplitude oscillations. Applying these concepts to autoimmunity, several broad areas appear to be relevant, related to either the antigen arm or the immune effector arm. Antigen-related considerations would include the microenvironment-specific expression of high levels of an antigen, or of structurally modified antigens. On the immune side, possibilities would include particular sensitivity of some cells/tissues to specific immune effector pathways and mechanisms. For example, these could be distinct patterns of MHC expression, as well as sensitivity or resistance to key immune effector pathways, including autoantibodies.
Since the chronic, self-sustaining, immune-mediated injury likely resonates around specific autoantigen “hubs”, a key to understanding these mutually reinforcing interactions will be defining autoantigen expression in the relevant target tissue in humans in vivo, and defining the pathways responsible for any enhanced expression. Although limited information is currently available, several examples are presented.
V.1. Enhanced autoantigen expression
V.1.1 Autoimmune myositis autoantigens are expressed at low levels in normal resting muscle, but are expressed at high levels in myositis muscle, where their levels are enhanced particularly in regenerating cells (123). Levels of these autoantigens are also very low in many other unperturbed tissues (e.g. normal lung, normal breast), while cancers derived from these tissues frequently express high levels of these molecules. Interestingly, while normal, mature muscle cells express very low levels of MHC class I (making them poor targets for CD8 cytotoxic T cells), regenerating and perturbed muscle cells express high levels of MHC class I (124). These elements create the components needed to establish resonance, which could occur as follows: (i) An immune response is directed against autoantigens expressed in regenerating muscle cells (this immune response is potentially cancer-induced, possibly initiated by mutated autoantigen in a cancer – see section IV); (ii) the immune response causes immune-mediated muscle damage. In turn, this induces muscle regeneration, MHC class I expression, and consequently more damage. Thus additional autoantigen is generated that feeds/sustains/propagates the autoimmune response. Defining the in vivo cell types and differentiation states which express the autoantigens targeted in other systemic autoimmune diseases remains a high priority.
V.1.2 DM muscle and skin are highly enriched in type I and type II interferon producing cells. Especially noteworthy in muscle cells is the strikingly increased expression of several interferon induced autoantigens including TRIM21 (induced by both type I and II IFN), as well as MDA5 and IFIT3 (predominantly type I IFN-induced (125)). The induction of autoantigens by immune effector pathways active in the target tissue creates opportunities for resonance.
V.1.3 In a recently described autoimmune necrotizing myopathy syndrome induced by exposure to statins, the autoantigen targeted was discovered to be the enzyme responsible for cholesterol biosynthesis (hydroxymethylglutaryl-coA reductase, HMGCR), which is strongly induced by exposure to statins (126). In this syndrome, there is a marked enrichment of HLA DRB1*11:01in both white and African American patients (OR 10.4 and 26 respectively compared to non-disease, race-matched controls) (127). HMGCR expression is increased in regenerating muscle cells, even in the absence of stain exposure. The authors propose that stain exposure induces robust expression of HMGCR. In patients who have HLA DRB1*11:01, this initiates an immune response against HMGCR, which, in the setting of statin therapy, initially causes muscle damage and healing. Since regenerating cells express the antigen even when statins are removed, this may continue to drive the process, until immunosuppression decreases ongoing muscle damage. Defining the essential elements of the immune effector pathways that generate this unusual necrotizing pathology in the absence of vigorous inflammatory infiltrates remains a high priority.
V.1.4 Although the targeting of citrullinated autoantigens in RA is a prominent and specific feature of this disease, the source of citrullinated autoantigens, and the pathways responsible for their generation, remain unclear. Several studies have demonstrated that members of the peptidyl arginine-deiminase (PAD) family expressed at high levels in monocytes and neutrophils (PAD-2 and PAD-4) are present in RA synovial fluid, where citrullinated proteins are also enriched (128). Citrullination in RA joints is unusual both in magnitude and pattern. The levels of citrullination in synovial fluid cells from some RA patients is high when compared to neutrophils stimulated ex vivo with a variety of stimuli known to activate PADs. In terms of pattern, citrullination is not focused on just a few prominent citrullinated substrates, but rather affects proteins across the entire range of molecular weights, a pattern that has been termed hypercitrullination (119).
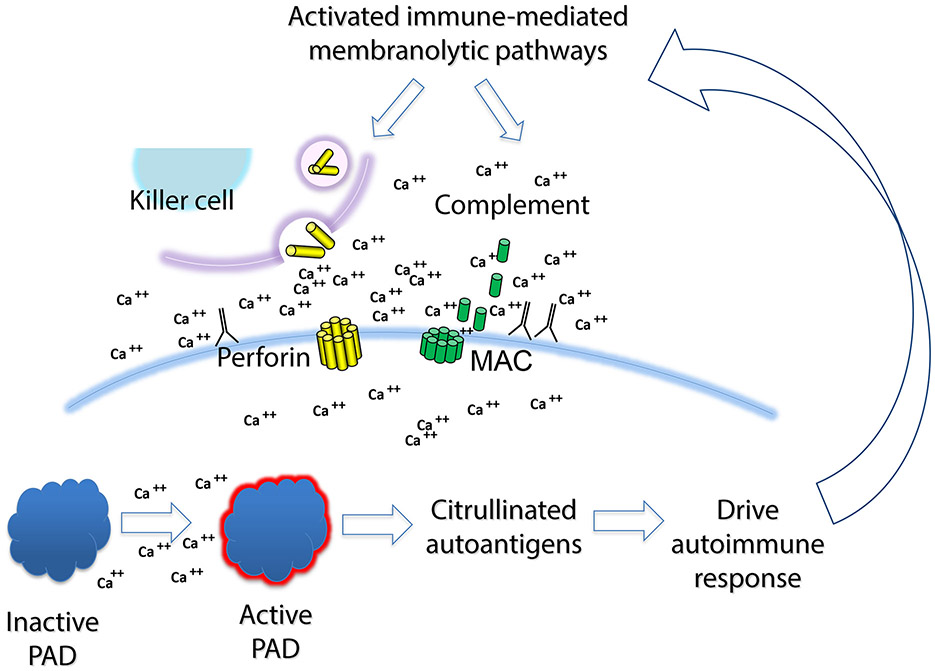
The only stimuli to date that have been defined to induce cellular hypercitrullination in human neutrophils engage immune-mediated membranolytic pathways, and include perforin and the membrane attack complex of complement (see Figure 3). Interestingly, there is evidence for activity of both of these pathways in active RA. Indeed, cytotoxic lymphocytes expressing GrB are one of the best predictors of erosive disease in RA (129). Intriguingly, the evidence points yet again to resonance between the immune effector and autoantigen expression pathways, with mutual reinforcement. Thus, immune-mediated membranolytic effector pathways appear to play a role in inducing antigen expression in cells in the RA joint, which drive additional immune response.
Figure 3: Immune-mediated membranolytic pathways generate citrullinated autoantigens, which further drive the immune response and subsequent antigen generation in RA.
Activated cytotoxic lymphocytes (NK cells and CTL) express perforin and granzymes, and induce membranolysis of cells within the synovium (including synoviocytes and infiltrating myelomonocytic cells). Activation of the classical or alternate pathway of complement can induce the formation of the membrane attack complex (MAC), and complement-mediated membranolysis. In both cases, membranolysis induces calcium flux, with a spike in cytoplasmic calcium concentrations towards the millimolar range. This activates PAD4 enzymatic function, and generation of citrullinated autoantigens, which after uptake and processing by antigen-presenting cells, can further feed the afferent arm of the immune response. It is proposed that the effector arm of this response induces more membranolysis, further feeding the cycle.
V.2. Augmented generation of autoantigens through antibody effects
Another recent example of immune system-enhanced generation of autoantigen is the identification of antibodies which activate the function of the PADs. In addition to targeting citrullinated autoantigens, ~30–40% of patients with RA also generate autoantibodies that recognize the PAD4 enzyme itself (130-131). Interestingly, autoantibodies against PAD4 are associated with the most erosive form of RA, which appears less responsive to therapy with anti-TNF agents (130). The PAD enzymes have a striking requirement for calcium ions, with optimal enzyme activity occurring at the unphysiologic concentration of >5 mM (132). The reasons underlying this high calcium requirement remain unclear. PAD4 autoantibodies from the most erosive subgroup of RA patients have a striking ability to augment the activity of the PAD4 enzyme several orders of magnitude by decreasing calcium requirements into the physiologic range. It has been proposed that when cells in the RA synovium release PAD4 (possibly in the setting of induced cytotoxicity) in the presence of these activating autoantibodies, there is an associated increase in the generation of citrullinated autoantigens, which drive the immune response, and additional immune effector function (133).
It is likely that similar “resonant” connections between immune effector pathways, autoantigen generation, and continued activation of immunity against self antigens exist for many other autoimmune rheumatic diseases. A key to defining these connections will be the study of phenotype-specific autoantigens in normal and the perturbed target tissue from patients.
V3. Tissue damage and dysfunction initiated by transient access of autoantibodies to hidden autoantigens.
While many of the autoantigens targeted in systemic autoimmune diseases are intracellular, the recent observations of Diamond and colleagues raise an important additional possibility for focusing specific immune-mediated injury on specific cells and tissues. These investigators observed that some anti-DNA antibodies crossreact with the N-methyl-D-aspartic acid (NMDA) receptor, specifically the NR2A and NR2B subunits (134). This receptor is expressed in neurons throughout the brain, but is at highest density within cells of the hippocampus, amygdala, and hypothalamus. The neurons in the CNS are normally protected from brain-reactive Abs by the blood-brain barrier (BBB); however, a breach in the barrier’s integrity exposes neurons to potentially pathogenic antibodies (135). Furthermore, additional neuronal dysfunction and damage can induce further disturbance of the BBB, further allowing immune access, and augmenting the injury through inducing neuronal cell death (136). Diamond and colleagues propose that in lupus patients, autoantibodies lack access to these receptors at baseline. In settings of stress (where there are transient changes in permeability of the BBB), antibodies gain access to cells expressing the relevant receptors, and induce transient dysfunction of irreversible neural cell death (137). The fact that episodes of CNS dysfunction in SLE may become repeated after the patient first experiences them suggests that the threshold for subsequent driving of injury is decreased, potentially due to changes on the immune (e.g. changed specificity to include additional neuron-specific targets) or target tissue side.
VI. Model
The striking association of specific immune responses with distinct clinical phenotypes in the autoimmune rheumatic diseases has provided important tools for diagnosis and prediction in these diseases. Understanding the mechanisms underlying the selection of this limited group of autoantigens will provide important insights into the initiation and propagation of autoimmunity.
Initiation of autoimmune responses likely reflects the presentation of antigens with distinct structure not previously encountered by the immune system, in a pro-immune context (injury, malignancy or infection). Causes of modified structure include somatic mutation, and various post-translational modifications (including citrullination and proteolysis). Many autoantigens are components of multimolecular complexes, and some of the additional components may provide adjuvant activity. The immune response also frequently spreads to these additional components.
There is also growing evidence that the target tissues in autoimmune rheumatic diseases are not just passive bystanders being damaged by an active immune response. Rather, they may play more active roles in a mutually reinforcing series of events in which immune effector pathways generate additional autoantigen, which feed further immune response. This type of resonance may be a critical principle underlying disease propagation and amplification, with specific autoantigens functioning as the hubs around which amplification occurs. The power of phenotype-specific autoantigens to elucidate disease mechanisms in the autoimmune rheumatic disease is enormous; studies in the target tissue in human will be critical to unleashing this potential.
Acknowledgements:
Drs Rosen and Casciola-Rosen are supported by NIH grant DE-12354. The authors and their research are supported by the Jerome L. Greene Foundation, the Scleroderma Research Foundation, and the Donald B. and Dorothy L. Stabler Foundation.
LITERATURE CITED
- 1.Tan EM. 1997. Autoantibodies and autoimmunity: A three-decade perspective. A tribute to henry G. kunkel. Ann. N. Y. Acad. Sci. 815 : 1–14 [DOI] [PubMed] [Google Scholar]
- 2.Suber TL, Casciola-Rosen L, Rosen A. 2008. Mechanisms of disease: Autoantigens as clues to the pathogenesis of myositis. Nat. Clin. Pract. Rheumatol. 4 : 201–9 [DOI] [PubMed] [Google Scholar]
- 3.Srikanta S, Eisenbarth GS. 1986. Islet cell antigens. initial studies of their biology and function. Mol. Biol. Med. 3 : 113–27 [PubMed] [Google Scholar]
- 4.Satyamurti S, Drachman DB, Slone F. 1975. Blockade of acetylcholine receptors: A model of myasthenia gravis. Science. 187 : 955–7 [DOI] [PubMed] [Google Scholar]
- 5.Tan EM. 1989. Antinuclear antibodies: Diagnostic markers for autoimmune diseases and probes for cell biology. Adv. Immunol. 44 : 93–151 [DOI] [PubMed] [Google Scholar]
- 6.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, et al. 2003. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 349 : 1526–33 [DOI] [PubMed] [Google Scholar]
- 7.Gorsuch AN, Spencer KM, Lister J, McNally JM, Dean BM, et al. 1981. Evidence for a long prediabetic period in type I (insulin-dependent) diabetes mellitus. Lancet. 2 : 1363–5 [DOI] [PubMed] [Google Scholar]
- 8.Bruining GJ, Molenaar JL, Grobbee DE, Hofman A, Scheffer GJ, et al. 1989. Ten-year follow-up study of islet-cell antibodies and childhood diabetes mellitus. Lancet. 1 : 1100–3 [DOI] [PubMed] [Google Scholar]
- 9.Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, et al. 2004. Specific autoantibodies precede the symptoms of rheumatoid arthritis: A study of serial measurements in blood donors. Arthritis Rheum. 50 : 380–6 [DOI] [PubMed] [Google Scholar]
- 10.Kokkonen H, Mullazehi M, Berglin E, Hallmans G, Wadell G, et al. 2011. Antibodies of IgG, IgA and IgM isotypes against cyclic citrullinated peptide precede the development of rheumatoid arthritis. Arthritis Res. Ther. 13 : R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gan RW, Trouw LA, Shi J, Toes RE, Huizinga TW, et al. 2015. Anti-carbamylated protein antibodies are present prior to rheumatoid arthritis and are associated with its future diagnosis. J. Rheumatol. 42 : 572–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, et al. 2012. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 7 : e35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen A, Casciola-Rosen L. 2009. Autoantigens in systemic autoimmunity: Critical partner in pathogenesis. J. Intern. Med. 265 : 625–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stollar BD. 1967. Studies on nucleoprotein determinants for systemic lupus erythematosus serum. J. Immunol. 99 : 959–65 [PubMed] [Google Scholar]
- 15.Mohan C, Adams S, Stanik V, Datta SK. 1993. Nucleosome: A major immunogen for pathogenic autoantibody-inducing T cells of lupus. J. Exp. Med. 177 : 1367–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Targoff IN, Reichlin M. 1985. The association between mi-2 antibodies and dermatomyositis. Arthritis Rheum. 28 : 796–803 [DOI] [PubMed] [Google Scholar]
- 17.Reichlin M, Mattioli M. 1972. Correlation of a precipitin reaction to an RNAprotein antigen and a low prevalence of nephritis in patients with systemic lupus erythematosus. N. Engl. J. Med. 286 : 908–11 [DOI] [PubMed] [Google Scholar]
- 18.Schur PH, Monroe M. 1969. Antibodies to ribonucleic acid in systemic lupus erythematosus. Proc. Natl. Acad. Sci. U. S. A. 63 : 1108–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardin JA, Craft JE. 1987. Patterns of autoimmunity to nucleoproteins in patients with systemic lupus erythematosus. Rheum. Dis. Clin. North Am. 13 : 37–46 [PubMed] [Google Scholar]
- 20.Bernstein RM, Morgan SH, Chapman J, Bunn CC, Mathews MB, et al. 1984. Anti-jo-1 antibody: A marker for myositis with interstitial lung disease. Br. Med. J. (Clin. Res. Ed). 289 : 151–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris EN, Gharavi AE, Hughes GR. 1985. Anti-phospholipid antibodies. Clin. Rheum. Dis. 11 : 591–609 [PubMed] [Google Scholar]
- 22.Gupta SK, Niles JL, McCluskey RT, Arnaout MA. 1990. Identity of wegener’s autoantigen (p29) with proteinase 3 and myeloblastin. Blood. 76 : 2162. [PubMed] [Google Scholar]
- 23.Grader-Beck T, Boin F, von Gunten S, Smith D, Rosen A, Bochner BS. 2011. Antibodies recognising sulfated carbohydrates are prevalent in systemic sclerosis and associated with pulmonary vascular disease. Ann. Rheum. Dis. 70 : 2218–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan EM. 1991. Autoantibodies in pathology and cell biology. Cell. 67 : 841–2 [DOI] [PubMed] [Google Scholar]
- 25.van Venrooij WJ, van Beers JJ, Pruijn GJ. 2011. Anti-CCP antibodies: The past, the present and the future. Nat. Rev. Rheumatol. 7 : 391–8 [DOI] [PubMed] [Google Scholar]
- 26.Koffler D, Carr R, Agnello V, Thoburn R, Kunkel HG. 1971. Antibodies to polynucleotides in human sera: Antigenic specificity and relation to disease. J. Exp. Med. 134 : 294–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes GR. 1975. Frequency of anti-DNA antibodies in SLE. RA and other diseases. experience wiht the ammonium sulphate precipitation technique. Scand. J. Rheumatol. Suppl. 11 : 42–51 [DOI] [PubMed] [Google Scholar]
- 28.Malladi AS, Sack KE, Shiboski SC, Shiboski CH, Baer AN, et al. 2012. Primary sjogren’s syndrome as a systemic disease: A study of participants enrolled in an international sjogren’s syndrome registry. Arthritis Care. Res. (Hoboken). 64 : 911–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elkon KB, Gharavi AE, Hughes GR, Moutsoupoulos HM. 1984. Autoantibodies in the sicca syndrome (primary sjogren’s syndrome). Ann. Rheum. Dis. 43 : 243–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kallenberg CG, Mulder AH, Tervaert JW. 1992. Antineutrophil cytoplasmic antibodies: A still-growing class of autoantibodies in inflammatory disorders. Am. J. Med. 93 : 675–82 [DOI] [PubMed] [Google Scholar]
- 31.Jennette JC, Falk RJ. 1990. Antineutrophil cytoplasmic autoantibodies and associated diseases: A review. Am. J. Kidney Dis. 15 : 517–29 [DOI] [PubMed] [Google Scholar]
- 32.Steen VD, Powell DL, Medsger TA Jr. 1988. Clinical correlations and prognosis based on serum autoantibodies in patients with systemic sclerosis. Arthritis Rheum. 31 : 196–203 [DOI] [PubMed] [Google Scholar]
- 33.Eisenberg RA, Craven SY, Warren RW, Cohen PL. 1987. Stochastic control of anti-sm autoantibodies in MRL/mp-lpr/lpr mice. J. Clin. Invest. 80 : 691–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato S, Hoshino K, Satoh T, Fujita T, Kawakami Y, et al. 2009. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: Association with rapidly progressive interstitial lung disease. Arthritis Rheum. 60 : 2193–200 [DOI] [PubMed] [Google Scholar]
- 35.Fiorentino D, Chung L, Zwerner J, Rosen A, Casciola-Rosen L. 2011. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): A retrospective study. J. Am. Acad. Dermatol. 65 : 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Targoff IN, Arnett FC. 1990. Clinical manifestations in patients with antibody to PL-12 antigen (alanyl-tRNA synthetase). Am. J. Med. 88 : 241–51 [DOI] [PubMed] [Google Scholar]
- 37.Harley JB, Gaither KK. 1988. Autoantibodies. Rheum. Dis. Clin. North Am. 14 : 43–56 [PubMed] [Google Scholar]
- 38.Greidinger EL, Flaherty KT, White B, Rosen A, Wigley FM, Wise RA. 1998. African-american race and antibodies to topoisomerase I are associated with increased severity of scleroderma lung disease. Chest. 114 : 801–7 [DOI] [PubMed] [Google Scholar]
- 39.Targoff IN. 2006. Myositis specific autoantibodies. Curr. Rheumatol. Rep. 8 : 196–203 [DOI] [PubMed] [Google Scholar]
- 40.Armistead J, Triggs-Raine B. 2014. Diverse diseases from a ubiquitous process: The ribosomopathy paradox. FEBS Lett. 588 : 1491–500 [DOI] [PubMed] [Google Scholar]
- 41.White PJ, Gardner WD, Hoch SO. 1981. Identification of the immunogenically active components of the sm and RNP antigens. Proc. Natl. Acad. Sci. U. S. A. 78 : 626–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller S, Briand JP, Barakat S, Lagueux J, Poirier GG, et al. 1994. Autoantibodies reacting with poly(ADP-ribose) and with a zinc-finger functional domain of poly(ADP-ribose) polymerase involved in the recognition of damaged DNA. Clin. Immunol. Immunopathol. 73 : 187–96 [DOI] [PubMed] [Google Scholar]
- 43.Mimori T, Akizuki M, Yamagata H, Inada S, Yoshida S, Homma M. 1981. Characterization of a high molecular weight acidic nuclear protein recognized by autoantibodies in sera from patients with polymyositis-scleroderma overlap. J. Clin. Invest. 68 : 611–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casciola-Rosen LA, Pluta AF, Plotz PH, Cox AE, Morris S, et al. 2001. The DNA mismatch repair enzyme PMS1 is a myositis-specific autoantigen. Arthritis Rheum. 44 : 389–96 [DOI] [PubMed] [Google Scholar]
- 45.Reeves WH, Nigam SK, Blobel G. 1986. Human autoantibodies reactive with the signal-recognition particle. Proc. Natl. Acad. Sci. U. S. A. 83 : 9507–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathews MB, Bernstein RM. 1983. Myositis autoantibody inhibits histidyl-tRNA synthetase: A model for autoimmunity. Nature. 304 : 177–9 [DOI] [PubMed] [Google Scholar]
- 47.Bluthner M, Bautz FA. 1992. Cloning and characterization of the cDNA coding for a polymyositis-scleroderma overlap syndrome-related nucleolar 100-kD protein. J. Exp. Med. 176 : 973–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reimer G, Rose KM, Scheer U, Tan EM. 1987. Autoantibody to RNA polymerase I in scleroderma sera. J. Clin. Invest. 79 : 65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Satoh M, Ajmani AK, Ogasawara T, Langdon JJ, Hirakata M, et al. 1994. Autoantibodies to RNA polymerase II are common in systemic lupus erythematosus and overlap syndrome. specific recognition of the phosphorylated (IIO) form by a subset of human sera. J. Clin. Invest. 94 : 1981–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuwana M, Kaburaki J, Mimori T, Tojo T, Homma M. 1993. Autoantibody reactive with three classes of RNA polymerases in sera from patients with systemic sclerosis. J. Clin. Invest. 91 : 1399–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Earnshaw W, Bordwell B, Marino C, Rothfield N. 1986. Three human chromosomal autoantigens are recognized by sera from patients with anti-centromere antibodies. J. Clin. Invest. 77 : 426–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deutscher SL, Keene JD. 1988. A sequence-specific conformational epitope on U1 RNA is recognized by a unique autoantibody. Proc. Natl. Acad. Sci. U. S. A. 85 : 3299–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burke KP, Cox AL. 2010. Hepatitis C virus evasion of adaptive immune responses: A model for viral persistence. Immunol. Res. 47 : 216–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joseph CG, Darrah E, Shah AA, Skora AD, Casciola-Rosen LA, et al. 2014. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science. 343 : 152–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Casciola-Rosen LA, Anhalt G, Rosen A. 1994. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J. Exp. Med. 179 : 1317–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, et al. 2011. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3 : 73ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaplan MJ. 2011. Neutrophils in the pathogenesis and manifestations of SLE. Nat. Rev. Rheumatol. 7 : 691–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, et al. 2003. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197 : 711–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. 2002. Chromatin-IgG complexes activate B cells by dual engagement of IgM and toll-like receptors. Nature. 416 : 603–7 [DOI] [PubMed] [Google Scholar]
- 60.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, et al. 2005. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/toll-like receptor 7 engagement. J. Exp. Med. 202 : 1171–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. 2006. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 25 : 417–28 [DOI] [PubMed] [Google Scholar]
- 62.Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. 2006. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J. Exp. Med. 203 : 553–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nundel K, Green NM, Shaffer AL, Moody KL, Busto P, et al. 2015. Cell-intrinsic expression of TLR9 in autoreactive B cells constrains BCR/TLR7-dependent responses. J. Immunol. 194 : 2504–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCartney SA, Vermi W, Lonardi S, Rossini C, Otero K, et al. 2011. RNA sensor-induced type I IFN prevents diabetes caused by a beta cell-tropic virus in mice. J. Clin. Invest. 121 : 1497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Funabiki M, Kato H, Miyachi Y, Toki H, Motegi H, et al. 2014. Autoimmune disorders associated with gain of function of the intracellular sensor MDA5. Immunity. 40 : 199–212 [DOI] [PubMed] [Google Scholar]
- 66.Gehrke N, Mertens C, Zillinger T, Wenzel J, Bald T, et al. 2013. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity. 39 : 482–95 [DOI] [PubMed] [Google Scholar]
- 67.Kim SJ, Gershov D, Ma X, Brot N, Elkon KB. 2002. I-PLA(2) activation during apoptosis promotes the exposure of membrane lysophosphatidylcholine leading to binding by natural immunoglobulin M antibodies and complement activation. J. Exp. Med. 196 : 655–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Korb LC, Ahearn JM. 1997. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: Complement deficiency and systemic lupus erythematosus revisited. J. Immunol. 158 : 4525–8 [PubMed] [Google Scholar]
- 69.Botto M, Dell’Agnola C, Bygrave AE, Thompson EM, Cook HT, et al. 1998. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 19 : 56–9 [DOI] [PubMed] [Google Scholar]
- 70.Pawaria S, Moody K, Busto P, Nundel K, Choi CH, et al. 2015. Cutting edge: DNase II deficiency prevents activation of autoreactive B cells by double-stranded DNA endogenous ligands. J. Immunol. 194 : 1403–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pogue GP, Hofmann J, Duncan R, Best JM, Etherington J, et al. 1996. Autoantigens interact with cis-acting elements of rubella virus RNA. J. Virol. 70 : 6269–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vashist S, Bhullar D, Vrati S. 2011. La protein can simultaneously bind to both 3’- and 5’-noncoding regions of japanese encephalitis virus genome. DNA Cell Biol. 30 : 339–46 [DOI] [PubMed] [Google Scholar]
- 73.Gross H, Hennard C, Masouris I, Cassel C, Barth S, et al. 2012. Binding of the heterogeneous ribonucleoprotein K (hnRNP K) to the epstein-barr virus nuclear antigen 2 (EBNA2) enhances viral LMP2A expression. PLoS One. 7 : e42106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakhasi HL, Ramanujam M, Atreya CD, Hobman TC, Lee N, et al. 2001. Rubella virus glycoprotein interaction with the endoplasmic reticulum calreticulin and calnexin. Arch. Virol. 146 : 1–14 [DOI] [PubMed] [Google Scholar]
- 75.Khadka S, Vangeloff AD, Zhang C, Siddavatam P, Heaton NS, et al. 2011. A physical interaction network of dengue virus and human proteins. Mol. Cell. Proteomics. 10 : M111.012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Content J, De Wit L, Poupart P, Opdenakker G, Van Damme J, Billiau A. 1985. Induction of a 26-kDa-protein mRNA in human cells treated with an interleukin-1-related, leukocyte-derived factor. Eur. J. Biochem. 152 : 253–7 [DOI] [PubMed] [Google Scholar]
- 77.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, et al. 2010. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 11 : 997–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baer AN, Petri M, Sohn J, Rosen A, Casciola-Rosen L. 2015. Antibodies to interferon-inducible protein-16 in primary sjogren’s syndrome are associated with markers of more severe disease. Arthritis Care. Res. (Hoboken). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ansari MA, Singh VV, Dutta S, Veettil MV, Dutta D, et al. 2013. Constitutive interferon-inducible protein 16-inflammasome activation during epstein-barr virus latency I, II, and III in B and epithelial cells. J. Virol. 87 : 8606–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dutta D, Dutta S, Veettil MV, Roy A, Ansari MA, et al. 2015. BRCA1 regulates IFI16 mediated nuclear innate sensing of herpes viral DNA and subsequent induction of the innate inflammasome and interferon-beta responses. PLoS Pathog. 11 : e1005030. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Ansari MA, Dutta S, Veettil MV, Dutta D, Iqbal J, et al. 2015. Herpesvirus genome recognition induced acetylation of nuclear IFI16 is essential for its cytoplasmic translocation, inflammasome and IFN-beta responses. PLoS Pathog. 11 : e1005019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hara T, Ogawa F, Muroi E, Komura K, Takenaka M, et al. 2008. Anti-p53 autoantibody in systemic sclerosis: Association with limited cutaneous systemic sclerosis. J. Rheumatol. 35 : 451–7 [PubMed] [Google Scholar]
- 83.Mimura Y, Yazawa N, Tada Y, Ihn H, Tamaki K. 2007. Anti-p53 antibodies in patients with systemic sclerosis. Int. J. Dermatol. 46 : 549–50 [DOI] [PubMed] [Google Scholar]
- 84.Herkel J, Mimran A, Erez N, Kam N, Lohse AW, et al. 2001. Autoimmunity to the p53 protein is a feature of systemic lupus erythematosus (SLE) related to anti-DNA antibodies. J. Autoimmun. 17 : 63–9 [DOI] [PubMed] [Google Scholar]
- 85.Tan TH, Wallis J, Levine AJ. 1986. Identification of the p53 protein domain involved in formation of the simian virus 40 large T-antigen-p53 protein complex. J. Virol. 59 : 574–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Werness BA, Levine AJ, Howley PM. 1990. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 248 : 76–9 [DOI] [PubMed] [Google Scholar]
- 87.Dong X, Hamilton KJ, Satoh M, Wang J, Reeves WH. 1994. Initiation of autoimmunity to the p53 tumor suppressor protein by complexes of p53 and SV40 large T antigen. J. Exp. Med. 179 : 1243–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang DC, Dang CV, Arnett FC. 1984. Rat liver histidyl-tRNA synthetase. purification and inhibition by the myositis-specific anti-jo-1 autoantibody. Biochem. Biophys. Res. Commun. 120 : 15–21 [DOI] [PubMed] [Google Scholar]
- 89.Neugebauer KM, Merrill JT, Wener MH, Lahita RG, Roth MB. 2000. SR proteins are autoantigens in patients with systemic lupus erythematosus. importance of phosphoepitopes. Arthritis Rheum. 43 : 1768–78 [DOI] [PubMed] [Google Scholar]
- 90.Kamachi M, Le TM, Kim SJ, Geiger ME, Anderson P, Utz PJ. 2002. Human autoimmune sera as molecular probes for the identification of an autoantigen kinase signaling pathway. J. Exp. Med. 196 : 1213–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu CL, Tangsombatvisit S, Rosenberg JM, Mandelbaum G, Gillespie EC, et al. 2012. Specific post-translational histone modifications of neutrophil extracellular traps as immunogens and potential targets of lupus autoantibodies. Arthritis Res. Ther. 14 : R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pieterse E, Hofstra J, Berden J, Herrmann M, Dieker J, van der Vlag J. 2015. Acetylated histones contribute to the immunostimulatory potential of neutrophil extracellular traps in systemic lupus erythematosus. Clin. Exp. Immunol. 179 : 68–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doyle HA, Aswad DW, Mamula MJ. 2013. Autoimmunity to isomerized histone H2B in systemic lupus erythematosus. Autoimmunity. 46 : 6–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, et al. 2000. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 43 : 155–63 [DOI] [PubMed] [Google Scholar]
- 95.Nogueira L, Sebbag M, Vincent C, Arnaud M, Fournie B, et al. 2001. Performance of two ELISAs for antifilaggrin autoantibodies, using either affinity purified or deiminated recombinant human filaggrin, in the diagnosis of rheumatoid arthritis. Ann. Rheum. Dis. 60 : 882–7 [PMC free article] [PubMed] [Google Scholar]
- 96.Shi J, van de Stadt LA, Levarht EW, Huizinga TW, Hamann D, et al. 2014. Anti-carbamylated protein (anti-CarP) antibodies precede the onset of rheumatoid arthritis. Ann. Rheum. Dis. 73 : 780–3 [DOI] [PubMed] [Google Scholar]
- 97.Sercarz EE, Lehmann PV, Ametani A, Benichou G, Miller A, Moudgil K. 1993. Dominance and crypticity of T cell antigenic determinants. Annu. Rev. Immunol. 11 : 729–66 [DOI] [PubMed] [Google Scholar]
- 98.Lanzavecchia A 1995. How can cryptic epitopes trigger autoimmunity? J. Exp. Med. 181 : 1945–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mamula MJ, Lin RH, Janeway CA Jr, Hardin JA 1992. Breaking T cell tolerance with foreign and self co-immunogens. A study of autoimmune B and T cell epitopes of cytochrome c. J. Immunol. 149 : 789–95 [PubMed] [Google Scholar]
- 100.Mamula MJ. 1993. The inability to process a self-peptide allows autoreactive T cells to escape tolerance. J. Exp. Med. 177 : 567–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lin RH, Mamula MJ, Hardin JA, Janeway CA Jr. 1991. Induction of autoreactive B cells allows priming of autoreactive T cells. J. Exp. Med. 173 : 1433–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Manoury B, Mazzeo D, Fugger L, Viner N, Ponsford M, et al. 2002. Destructive processing by asparagine endopeptidase limits presentation of a dominant T cell epitope in MBP. Nat. Immunol. 3 : 169–74 [DOI] [PubMed] [Google Scholar]
- 103.Salemi S, Caporossi AP, Boffa L, Longobardi MG, Barnaba V. 1995. HIVgp120 activates autoreactive CD4-specific T cell responses by unveiling of hidden CD4 peptides during processing. J. Exp. Med. 181 : 2253–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Doyle HA, Yang ML, Raycroft MT, Gee RJ, Mamula MJ. 2014. Autoantigens: Novel forms and presentation to the immune system. Autoimmunity. 47 : 220–33 [DOI] [PubMed] [Google Scholar]
- 105.Mamula MJ, Gee RJ, Elliott JI, Sette A, Southwood S, et al. 1999. Isoaspartyl post-translational modification triggers autoimmune responses to self-proteins. J. Biol. Chem. 274 : 22321–7 [DOI] [PubMed] [Google Scholar]
- 106.Hill CL, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, et al. 2001. Frequency of specific cancer types in dermatomyositis and polymyositis: A population-based study. Lancet. 357 : 96–100 [DOI] [PubMed] [Google Scholar]
- 107.Buchbinder R, Forbes A, Hall S, Dennett X, Giles G. 2001. Incidence of malignant disease in biopsy-proven inflammatory myopathy. A population-based cohort study. Ann. Intern. Med. 134 : 1087–95 [DOI] [PubMed] [Google Scholar]
- 108.Fujimoto M, Hamaguchi Y, Kaji K, Matsushita T, Ichimura Y, et al. 2012. Myositis-specific anti-155/140 autoantibodies target transcription intermediary factor 1 family proteins. Arthritis Rheum. 64 : 513–22 [DOI] [PubMed] [Google Scholar]
- 109.Fiorentino DF, Chung LS, Christopher-Stine L, Zaba L, Li S, et al. 2013. Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1gamma. Arthritis Rheum. 65 : 2954–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shah AA, Rosen A, Hummers L, Wigley F, Casciola-Rosen L. 2010. Close temporal relationship between onset of cancer and scleroderma in patients with RNA polymerase I/III antibodies. Arthritis Rheum. 62 : 2787–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kreiter S, Vormehr M, van de Roemer N, Diken M, Lower M, et al. 2015. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 520 : 692–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shen W, Clemente MJ, Hosono N, Yoshida K, Przychodzen B, et al. 2014. Deep sequencing reveals stepwise mutation acquisition in paroxysmal nocturnal hemoglobinuria. J. Clin. Invest. 124 : 4529–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Poduri A, Evrony GD, Cai X, Walsh CA. 2013. Somatic mutation, genomic variation, and neurological disease. Science. 341 : 1237758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schreiber RD, Old LJ, Smyth MJ. 2011. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science. 331 : 1565–70 [DOI] [PubMed] [Google Scholar]
- 115.Scally SW, Petersen J, Law SC, Dudek NL, Nel HJ, et al. 2013. A molecular basis for the association of the HLA-DRB1 locus, citrullination, and rheumatoid arthritis. J. Exp. Med. 210 : 2569–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gregersen PK, Silver J, Winchester RJ. 1987. The shared epitope hypothesis. an approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 30 : 1205–13 [DOI] [PubMed] [Google Scholar]
- 117.Raychaudhuri S, Sandor C, Stahl EA, Freudenberg J, Lee HS, et al. 2012. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat. Genet. 44 : 291–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. 1998. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J. Clin. Invest. 101 : 273–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Romero V, Fert-Bober J, Nigrovic PA, Darrah E, Haque UJ, et al. 2013. Immune-mediated pore-forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis. Sci. Transl. Med 5 : 209ra150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Casciola-Rosen L, Andrade F, Ulanet D, Wong WB, Rosen A. 1999. Cleavage by granzyme B is strongly predictive of autoantigen status: Implications for initiation of autoimmunity. J. Exp. Med. 190 : 815–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Darrah E, Rosen A. 2010. Granzyme B cleavage of autoantigens in autoimmunity. Cell Death Differ. 17 : 624–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Casciola-Rosen L, Garcia-Calvo M, Bull HG, Becker JW, Hines T, et al. 2007. Mouse and human granzyme B have distinct tetrapeptide specificities and abilities to recruit the bid pathway. J. Biol. Chem. 282 : 4545–52 [DOI] [PubMed] [Google Scholar]
- 123.Casciola-Rosen L, Nagaraju K, Plotz P, Wang K, Levine S, et al. 2005. Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy. J. Exp. Med. 201 : 591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Englund P, Nennesmo I, Klareskog L, Lundberg IE. 2002. Interleukin-1alpha expression in capillaries and major histocompatibility complex class I expression in type II muscle fibers from polymyositis and dermatomyositis patients: Important pathogenic features independent of inflammatory cell clusters in muscle tissue. Arthritis Rheum. 46 : 1044–55 [DOI] [PubMed] [Google Scholar]
- 125.Hall JC, Casciola-Rosen L, Berger AE, Kapsogeorgou EK, Cheadle C, et al. 2012. Precise probes of type II interferon activity define the origin of interferon signatures in target tissues in rheumatic diseases. Proc. Natl. Acad. Sci. U. S. A. 109 : 17609–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mammen AL, Chung T, Christopher-Stine L, Rosen P, Rosen A, et al. 2011. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 63 : 713–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mammen AL, Gaudet D, Brisson D, Christopher-Stine L, Lloyd TE, et al. 2012. Increased frequency of DRB1*11:01 in anti-hydroxymethylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Care. Res. (Hoboken). 64 : 1233–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Foulquier C, Sebbag M, Clavel C, Chapuy-Regaud S, Al Badine R, et al. 2007. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum. 56 : 3541–53 [DOI] [PubMed] [Google Scholar]
- 129.Smeets TJ, Dolhain RJ, Breedveld FC, Tak PP 1998. Analysis of the cellular infiltrates and expression of cytokines in synovial tissue from patients with rheumatoid arthritis and reactive arthritis. J. Pathol. 186 : 75–81 [DOI] [PubMed] [Google Scholar]
- 130.Halvorsen EH, Haavardsholm EA, Pollmann S, Boonen A, van der Heijde D, et al. 2009. Serum IgG antibodies to peptidylarginine deiminase 4 predict radiographic progression in patients with rheumatoid arthritis treated with tumour necrosis factor-alpha blocking agents. Ann. Rheum. Dis. 68 : 249–52 [DOI] [PubMed] [Google Scholar]
- 131.Harris ML, Darrah E, Lam GK, Bartlett SJ, Giles JT, et al. 2008. Association of autoimmunity to peptidyl arginine deiminase type 4 with genotype and disease severity in rheumatoid arthritis. Arthritis Rheum. 58 : 1958–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Arita K, Hashimoto H, Shimizu T, Nakashima K, Yamada M, Sato M. 2004. Structural basis for ca(2+)-induced activation of human PAD4. Nat. Struct. Mol. Biol. 11 : 777–83 [DOI] [PubMed] [Google Scholar]
- 133.Darrah E, Giles JT, Ols ML, Bull HG, Andrade F, Rosen A. 2013. Erosive rheumatoid arthritis is associated with antibodies that activate PAD4 by increasing calcium sensitivity. Sci. Transl. Med 5 : 186ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. 2001. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat. Med. 7 : 1189–93 [DOI] [PubMed] [Google Scholar]
- 135.Huerta PT, Kowal C, DeGiorgio LA, Volpe BT, Diamond B. 2006. Immunity and behavior: Antibodies alter emotion. Proc. Natl. Acad. Sci. U. S. A. 103 : 678–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Faust TW, Chang EH, Kowal C, Berlin R, Gazaryan IG, et al. 2010. Neurotoxic lupus autoantibodies alter brain function through two distinct mechanisms. Proc. Natl. Acad. Sci. U. S. A. 107 : 18569–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kowal C, Degiorgio LA, Lee JY, Edgar MA, Huerta PT, et al. 2006. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc. Natl. Acad. Sci. U. S. A. 103 : 19854–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shah AA, Casciola-Rosen L, Rosen A. 2015. Review: Cancer-induced autoimmunity in the rheumatic diseases. Arthritis Rheumatol. 67 : 317–26 [DOI] [PMC free article] [PubMed] [Google Scholar]