Abstract
Midlife obesity is a risk factor for cognitive decline and is associated with the earlier onset of Alzheimer’s disease (AD). Diets high in saturated fat potentiate the onset of obesity, microglial activation, and neuroinflammation. Signaling deficiencies in the hypothalamic peptide orexin and/or orexin fiber loss are linked to neurodegeneration, cognitive impairment, and neuroinflammation. Prior studies show that orexin is neuroprotective, suppresses neuroinflammation, and that treatment with orexin improves cognitive processes in orexin/ataxin-3 (O/A3) mice, a transgenic mouse model of orexin neurodegeneration. Our overall hypothesis is that loss of orexin contributes to high fat diet (HFD)-induced hippocampal neuroinflammation and cognitive decline. To examine this, we tested male O/A3 mice (7–8 mo. of age) in a two-way active avoidance (TWAA) hippocampus-dependent memory task. We tested whether 1) orexin loss impaired cognitive function; 2) HFD worsened cognitive impairment; and 3) HFD increased microglial activation and neuroinflammation. O/A3 mice showed significant impairments in TWAA task learning vs. wild type (WT) mice (increased escapes p<0.05, reduced avoidances p<0.0001). Mice were then placed on HFD (45% total fat, 31.4% saturated fat) or remained on normal chow (NC; 4% total fat and 1% saturated fat), and TWAA was retested at 2 and 4 weeks. Learning impairment was evident at both 2 and 4 weeks in O/A3 mice fed HFD for following diet exposure vs. WT mice on normal chow or HFD (increased escapes, reduced avoidances p<0.05). Additionally, O/A3 mice had increased gene expression of the microglial activation marker Iba-1 (measured via qRT-PCR, p<0.001). Further characterization of the microglial immune response genes in hippocampal tissue revealed a significant increase in CX3 chemokine receptor 1 (CX3CR1), tumor necrosis factor-alpha (TNF-α) and the mitochondria-associated enzyme immune responsive gene-1 (Irg1). Collectively, our results indicate that orexin loss impairs memory, and that HFD accelerates hippocampus-dependent learning deficits and the onset of neuroinflammation.
Keywords: Microglia, Neuroinflammation, Two-Way Active Avoidance Memory Task, Obesity, High Fat Diet
Introduction
Obesity is coupled with peripheral and central chronic low-grade inflammation (Businaro, Ippoliti, Ricci, Canitano, and Fuso, 2012; Uranga, Bruce-Keller, Morrison, Fernandez-Kim, Ebenezer, Zhang, Dasuri, and Keller, 2010). Dietary fats increase the risk of developing obesity and cognitive disorders such as Alzheimer’s disease (AD) (Anstey, Cherbuin, Budge, and Young, 2011; Businaro et al., 2012; Xu, Atti, Gatz, Pedersen, Johansson, and Fratiglioni, 2011). Prolonged overnutrition and excess intake of saturated fatty acids (SFA), specifically palmitic acid (PA) increase neuroinflammation and microglial activation (Cai, 2013; Cai and Liu, 2011; Karmi, Iozzo, Viljanen, Hirvonen, Fielding, Virtanen, Oikonen, Kemppainen, Viljanen, Guiducci, Haaparanta-Solin, Nagren, Solin, and Nuutila, 2010; Thaler, Yi, Schur, Guyenet, Hwang, Dietrich, Zhao, Sarruf, Izgur, Maravilla, Nguyen, Fischer, Matsen, Wisse, Morton, Horvath, Baskin, Tschop, and Schwartz, 2012; Valdearcos, Robblee, Benjamin, Nomura, Xu, and Koliwad, 2014). Neuroinflammation, accompanied with progressive neuronal loss, is known to be heightened in cognitive decline and obesity (Stranahan, Cutler, Button, Telljohann, and Mattson, 2011). Moreover, neuroinflammation and microglial activation represent a common link between age-related neurodegenerative diseases and metabolic related disorders.
Microglia (resident immune cells of the brain) play a dynamic role in responding to changes within the surrounding environment leading to constant transitioning between phenotypes (Perry and Teeling, 2013). Microglia have recently been appreciated to have an important role in cognition (Chung, Welsh, Barres, and Stevens, 2015). In the healthy brain, microglia are constantly surveying the surrounding microenvironment to promote structural formation and elimination of neuronal synapses, a process especially important in the hippocampus (Chung et al., 2015). One mechanism in which this could occur is through orexin signaling. Orexin A and B (OXA; hypocretin 1 and OXB; hypocretin 2 respectively) are ligands for the G-protein coupled receptors, orexin receptor 1 and 2 (OX1R and OX2R respectively). Orexin neurons project throughout the central nervous system (CNS), including the hippocampus, and are responsible for mediating cognitive processes, operant tasks, and fear conditioning (Deadwyler, Porrino, Siegel, and Hampson, 2007; Peyron, Tighe, van den Pol, de Lecea, Heller, Sutcliffe, and Kilduff, 1998; Rolls, Colas, Adamantidis, Carter, Lanre-Amos, Heller, and de Lecea, 2011; Sears, Fink, Wigestrand, Farb, de Lecea, and Ledoux, 2013; Sharf, Sarhan, Brayton, Guarnieri, Taylor, and DiLeone, 2010; Thorpe, Cleary, Levine, and Kotz, 2005). Additionally, orexin signaling is altered in AD patients (Kang, Lim, Bateman, Lee, Smyth, Cirrito, Fujiki, Nishino, and Holtzman, 2009; Roh, Jiang, Finn, Stewart, Mahan, Cirrito, Heda, Snider, Li, Yanagisawa, de Lecea, and Holtzman, 2014). Moreover, we and others have demonstrated that orexin loss impairs memory, supporting that orexin mediates cognitive function (Mavanji, Butterick, Duffy, Nixon, Billington, and Kotz, 2017; Yang, Zou, Xiong, Pascual, Xie, Malik, Xie, Sakurai, and Xie, 2013).
Recent studies highlight novel functions of OXA, including neuroprotection and decreased apoptosis and neuroinflammation via activation of orexin receptors (Butterick, Nixon, Billington, and Kotz, 2012; Duffy, Nixon, and Butterick, 2016; Sokolowska, Urbanska, Bieganska, Wagner, Ciszewski, Namiecinska, and Zawilska, 2014; Sokolowska, Urbanska, Namiecinska, Bieganska, and Zawilska, 2012; Xiong, White, Xu, Yang, Sun, Zou, Pascual, Sakurai, Giffard, and Xie, 2013). Data indicate that orexin-induced neuroprotection could rely upon microglial modulation (Duffy, Yuan, Wisdorf, Billington, Kotz, Nixon, and Butterick, 2015; Xiong et al., 2013). In a cerebral ischemia model, pretreatment with OXA reduces infarct size through a microglial-mediated pathway (Xiong et al., 2013). Additionally, chronic exposure to the potent pro-inflammatory agonist lipopolysaccharide (LPS), reduced orexin signaling indicating orexin has a role in the inflammatory response (Grossberg, Zhu, Leinninger, Levasseur, Braun, Myers, and Marks, 2011). Microglia may also become more sensitive to orexin signaling after activation (Duffy et al., 2015). LPS increases TNF-α (tumor necrosis factor-α) in microglia, but also increases OX1R expression, and OXA treatment prior to LPS exposure reduces TNF-α in microglia (Xiong et al., 2013). We have also demonstrated that PA increases OX1R, and that PA treatment causes microglia to shift toward a pro-inflammatory state (Duffy et al., 2015). However, when microglia are pretreated with OXA prior to PA exposure, the PA-induced pro-inflammatory response is attenuated (Duffy et al., 2015). These data indicate increased sensitivity to OXA may allow orexin-mediated attenuation of the toxic microglial phenotype to a protective anti-inflammatory phenotype.
Our overall hypothesis is that orexin effects on memory depend in part upon immunomodulatory control of microglia. To test this, we used orexin/ataxin-3 (O/A3) mice, a rodent model that gradually loses orexin-producing neurons (~84% by 6 mo age) (Hara, Beuckmann, Nambu, Willie, Chemelli, Sinton, Sugiyama, Yagami, Goto, Yanagisawa, and Sakurai, 2001). The O/A3 mice were maintained on a HFD for 4 weeks. We then sought to determine if orexin loss alters neuroinflammation and cognition.
Methods
Animals
7–8-month-old male wild type (WT) and orexin ataxin-3 (O/A3) mice were obtained from a breeding colony at the Minneapolis VA Health Care System (VAHCS). The colony, maintained by Dr. Catherine Kotz, originated from founder animals obtained from Dr. Masashi Yanagisawa at the University of Texas-Southwestern, bred to wild-type C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME USA). Genotype was confirmed using PCR as described in (Hara et al., 2001). Mice were maintained in a 12:12 hour light/dark cycle in a temperature controlled room(21–22°C), and were group-housed until the beginning of behavioral testing. Normal chow (NC; Teklad 8604 Lab Chow; Harlan, Indianapolis, IN USA) or high fat diet (HFD; Research Diets D12452, 45% fat; New Brunswick, NJ USA) and water were provided ad libitum. Body weights and food intake were measured thrice weekly. Body composition was recorded using an EchoMRI 700 (Echo Medical Systems, Houston, TX USA) before HFD exposure and 14 and 28 days following HFD exposure, as previously described (Nixon, Zhang, Wang, Kuskowski, Novak, Levine, Billington, and Kotz, 2010). Mice were randomly divided into 4 groups and given either HFD or remained on NC (WT+NC, n = 8; WT+HFD, n = 8; O/A3+NC, n = 9; O/A3+HFD n = 11). The experimental protocol was approved by the Institutional Animal Care and Use Committee at the Minneapolis VAHCS.
Two-Way Active Avoidance (TWAA) Task
Mice were placed in the automated two-way shuttle box (size, 45.7 × 20.3 × 30.5 cm; model 75-FSFX-Fusion; AccuScan Instruments, Columbus, OH USA) and allowed to acclimate for 2 min. Mice were able to move freely throughout the shuttle box during acclimation and training trials. Mice were trained on a massed 20-trial shuttle box two-way active avoidance task. The procedures for the conditioned stimulus and unconditioned stimulus were as follows. A tone (5000 Hz; 45 dB) and a pulsatile light (2.5 Hz) were presented as a conditioned stimulus in the compartment with the animal, paired 5 s later with a 0.3 mA scrambled foot shock (unconditioned stimulus) delivered through the floor grid (steel rods 0.5 cm in diameter, spaced 1.5 cm between centers). The “scrambled” nature of the shock prevents the animals from finding a “no-shock” position on the floor. To avoid receiving a foot shock the mouse had 5 s to move to the opposite compartment. If the animal did not move to the other compartment, the unconditioned stimulus was delivered for a maximum of 7 s, and the conditioned stimulus ended with the unconditioned stimulus. While receiving the unconditioned stimulus, if the animal moved to the other compartment, both conditioned and unconditioned stimuli ended immediately. The inter-trial interval was variable with a mean of 40 s. A computer using remote monitoring system software (Fusion software) was used to control experimental protocols and data collection (Datta, Saha, Prutzman, Mullins, and Mavanji, 2005; Mavanji et al., 2017; Noble, Mavanji, Little, Billington, Kotz, and Wang, 2014; Rodriguiz and Wetsel, 2006).
TWAA Data Analysis
To measure performance on the TWAA task, each trial was analyzed for successful shock avoidances and shock latency. Experimental data were analyzed using two-factor (phenotype × diet) ANOVA, followed by Holm-Sidak post-hoc analysis for multiple comparisons. Significance was considered to be achieved where p<0.05. Analyses were performed to determine, a) the differences in acquisition between phenotypes, and b) differences in retention between treatment groups. The improvement of performance between training trials (first session) and test trials (all sessions thereafter) were analyzed for avoidances (crossing after conditioned stimulus, but before shock delivery) and for escape latency (interval between conditioned stimulus and a response). For all experiments, mice underwent 20 trials for training, 24 h later they were retested for TWAA response. Response during the 20 training trials was considered acquisition, while response during the tests 24 h, 14 days, and 28 days later was considered retention. Following 14 d and 28 d of diet exposure mice were retested for TWAA response and body composition. These parameters were also compared between groups during test sessions to see if there was any specific effect of treatment (NC vs. HFD) on memory retention. Statistics were performed using GraphPad Prism 7 (Datta et al., 2005; Noble et al., 2014).
Locomotor activity
Open field chamber tests are commonly used to measure exploration, anxiety, and general locomotor activity. While exploratory activity in a novel environment is expected from rodents, prey animals such as mice can also exhibit anxiety when exposed to an open chamber (Sestakova, Puzserova, Kluknavsky, and Bernatova, 2013). For these tests, mice were placed in a square chamber (27.3 cm × 27.3 cm × 20.3 cm; Med-Associates). The chamber is divided into a grid by infrared beams. An automated system monitors beam breaks to determine exploratory activity. Mice were allowed to explore for 2 h. All tests were conducted 5 h after lights on.
Reverse transcription polymerase chain reaction (qRT-PCR)
Hippocampal tissue was rapidly dissected from WT and O/A3 mice sacrificed during the light phase (5–8 h after lights on), a time period when orexin expression is expected to be low in a nocturnal animal (Martinez, Smale, and Nuñez, 2002). Total RNA was isolated with the aid of TRIzol reagent (Invitrogen). Real-time thermocycling was performed on ABI 7900HT Fast Real Time PCR System (Applied Biosystems) by two-step RT-PCR using SYBR green. Genes analyzed are indicated in table 1. Data analysis was performed using Sequence Detection System software from Applied Biosystems. The experimental Ct (cycle threshold) was calibrated against the endogenous control product GAPDH. Samples were analyzed for relative gene expression by the 2–ΔΔCt method (Pfaffl, 2001).
Table 1.
qPCR Primers (5′→3′)
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| OX1R (NM_198959.2) | CTCATAGCCTTGGTGGGCAA | ACCGACACAGCCTGGAGATA |
| OX2R (NM_198962.3) | CACGGACTATGACGACGAGG | AGAGCCACAACGAACACGAT |
| CX3CR1 (NM_009987) | CCGCCAACTCCATGAACAAC | GGATGAGTCTGACGGCTCTG |
| Iba1 (NM_019467.2) | GGAGATTTCAAAAGCTGATGTGGA | CCTCAGACGCTGGTTGTCTT |
| TNF-α (NM_001278601) | AGGCACTCCCCCAAAAGATG | CCACTTGGTGGTTTGTGAGTG |
| Irg1 (NM_008392.1) | TATGCCAACTACTCCCCCGA | CGGGAAGCTCTTAAAGGCCA |
Results
High fat diet increases obesity in orexin/ataxin-3 mice
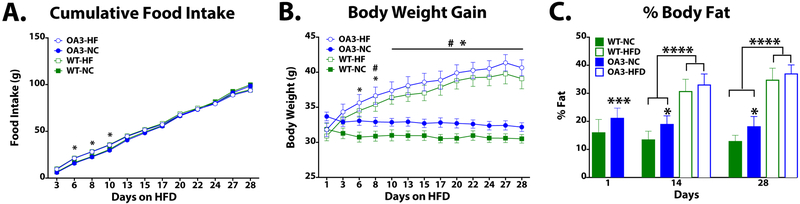
We first sought to determine the effects of HFD on food intake, body weight gain and body composition. WT and O/A3 mice have increased cumulative food intake on days 6–10 (Fig 1A). Body weight gain is significantly increased in O/A3-HFD mice compared to WT-NC beginning at day 6 and continue to be significant through day 28 (p<0.01). WT-HFD mice have significantly increased body weight compared to WT-NC at day 8 through day 28 (p<0.05). Both WT and O/A3 mice have increased body weight compared to O/A3-NC and WT-NC at day 10 through day 28 (Fig 1B). Despite no significant difference in body weight, O/A3-NC mice have increased body fat compared to WT-NC mice on days 1, 14, and 28 (Fig 1C). HFD-fed O/A3 and WT mice have increased body fat compared to NC-fed WT and O/A3 mice on days 14 and 28 (Fig 1C).
Figure 1. High fat diet increases body weight gain and fat mass.
WT and O/A3 mice were given HFD or NC for 28 d. Cumulative food intake was increased after 6, 8, and 10 d HFD exposure regardless of genotype (A, HF-fed mice *p<0.05 vs NC-fed mice). Mice fed HFD had significantly increased body weight gain days 10–28 compared to mice fed NC (B, HF-fed *p<0.001 vs. WT-NC, #p<0.01 vs. O/A3-NC). Chow-fed O/A3 mice have a higher percent body fat than WT mice (OA3-NC *p<0.01, ***p<0.001 vs WT-NC). Following 14 and 28 d on HFD, both WT and O/A3 mice have significantly more body fat than do WT and O/A3 mice maintained on NC (C, HF-fed mice ****p<0.0001 vs. NC-fed mice).
High fat diet reduces locomotor activity but does not impair anxiety like behavior
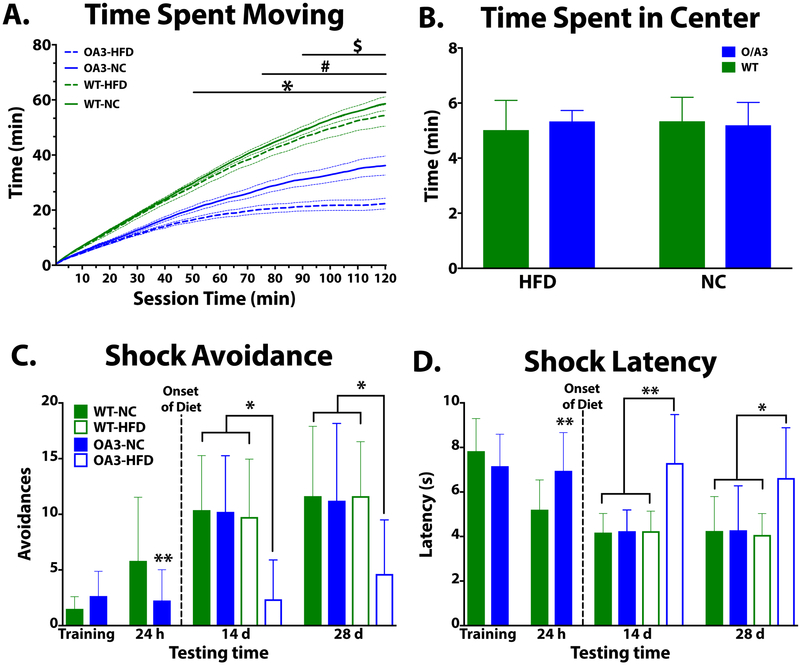
To determine if changes in body fat and weight were due in part to orexin signaling, we analyzed locomotor activity in mice following 4 weeks HFD or NC. We demonstrate that locomotor activity is lower in O/A3-NC and reduced even further in O/A3-HFD mice (Fig 2A; p<0.001). To determine if anxiety-like behavior was altered in response to lack of orexin signaling or HFD, the first 5 min of activity was analyzed. We demonstrate that anxiety-like behavior (open field test) is not impaired in WT or O/A3 mice (Fig 2B).
Figure 2A-B. High fat diet reduces locomotor activity and impairs cognitive function, but does not impair anxiety like behavior, in mice lacking orexin.
In open-field tests (A-B), WT mice move significantly more compared to O/A3 mice fed NC or HFD (A, O/A3-HFD *p<0.05 vs. WT; O/A3-NC #p<0.05 vs. WT; OA3-HFD $p<0.05 vs. OA3-NC). Anxiety-like behavior (B) is not different between strains, as there were no differences in time spent in the chamber center. In Two-Way Active Avoidance task (TWAA, C-D), as indicated by training there is no difference in learning ability between WT and O/A3 mice. However, significant differences in short- and long-term retention were observed. When tested for retention 24 h after training, cognitive impairment in OA/3 is evident via reduced avoidances (C) and increased latency (D, p<0.01 vs. WT-NC). HFD significantly impairs long-term memory in O/A3 mice as indicated by reduced avoidances and increased latency vs. WT mice (C-D; *p<0.05 vs. WT-NC, WT-HFD, O/A3-NC; **p<0.01 vs. WT-NC, WT-HFD, O/A3-NC).
High fat diet impairs cognitive function in mice lacking orexin
Previous studies indicate that orexin is integral in cognitive function (Aitta-Aho, Pappa, Burdakov, and Apergis-Schoute, 2016; Mavanji et al., 2017; Yang et al., 2013). We sought to determine if mice lacking orexin maintained on HFD have impaired cognition. We demonstrated that there was no difference in learning ability between WT and O/A3 mice. When tested 24 h later, cognitive impairment was evident as measured via reduced avoidances and increased latency in O/A3 mice (Fig 2C–D, p<0.01 vs. WT-NC). Mice were then placed on HFD or maintained on NC. Long term memory was tested following 14 and 28 d of HFD exposure. We demonstrate that HFD significantly impairs long term memory in O/A3 mice as indicated by reduced avoidances and increased latency (Fig 2C–D; *p<0.05 vs. WT-NC, WT-HFD, O/A3-NC; **p<0.01 vs. WT-NC, WT-HFD, O/A3-NC).
Inflammatory genes are altered in response to HFD
Our previous data indicate that orexin A attenuates PA-induced microglial inflammation (Duffy et al., 2015). We demonstrate that hippocampal orexin 1 receptor is upregulated in mice fed HFD but orexin 2 receptor is unchanged (Fig. 3A–Fig. 3AB, *p<0.05, **p<0.01 vs. WT-NC). Iba1, a marker of microglial activation, is increased in HFD-fed mice regardless of genotype, and in O/A3-NC mice (Fig 3C, *p<0.05, **p<0.01 vs. WT-NC). Pro-inflammatory marker TNF-α is increased in O/A3 mice (Fig 3D *p<0.05 vs. WT-NC, #p<0.05. ##p<0.01 vs. WT-HFD). CX3CR1 is upregulated following HFD exposure (Fig 3E, *p<0.05, **p<0.01 vs. WT-NC). Irg1 expression is upregulated following HFD exposure (Fig 3F, *p<0.05, **p<0.01 vs. WT-NC).
Figure 3. Inflammatory genes are altered in response to HFD.
HFD but not orexin loss increases orexin 1 receptor (A). Orexin 2 receptor levels are unchanged by diet or genotype (B). HFD and loss of orexin induces microglial activation via increased Iba1 (C). CX3CR1 is upregulated following HFD exposure (D). The pro-inflammatory marker TNF-α is increased in O/A3 mice (E). Irg1 expression is upregulated following HFD exposure (F). In all panels, *p<0.05 vs. WT-NC; **p<0.01 vs WT-NC; #p<0.05 WT-HFD.
Discussion
High fat diets and obesity increase neuroinflammation, neurodegeneration, and cognitive decline (Norden and Godbout, 2013; Stranahan et al., 2011; Uranga et al., 2010; Zhang, Li, Purkayastha, Tang, Zhang, Yin, Li, Liu, and Cai, 2013). While hippocampal neuroinflammation normally increases with age, risk of neuroinflammation and cognitive impairment is exacerbated by chronic consumption of diets high in PA (Norden and Godbout, 2013; Uranga et al., 2010). Orexins are important mediators in cognitive function and metabolic function (Aitta-Aho et al., 2016; Mavanji et al., 2017; Yang et al., 2013). Moreover, pro-inflammatory stimuli have been shown to reduce orexin signaling (Gaykema and Goehler, 2009; Grossberg et al., 2011; Ogawa, Irukayama-Tomobe, Murakoshi, Kiyama, Ishikawa, Hosokawa, Tominaga, Uchida, Kimura, Kanuka, Morita, Hamada, Takahashi, Hayashi, and Yanagisawa, 2016; Xiong et al., 2013). Here, we demonstrate that HFD increases body weight gain and fat mass despite genotype (Fig 1). In mice lacking orexin, increased body weight and fat mass is observed compared to WT mice maintained on NC (Fig 1). The loss of orexin also resulted in reduced locomotor activity and this effect is enhanced with exposure to HFD (Fig 2).
It is well accepted that orexins have a distinct role in the regulation of energy metabolism (Sakurai, 2005), and that loss of orexin signaling contributes to increased obesity in part via reduced energy expenditure and movement (Hara et al., 2001). Orexin has pleiotropic effects in the brain as the neurons project to regions involved in cognition, executive function, and learning and memory (Akbari, Naghdi, and Motamedi, 2007; Nixon and Smale, 2007; Yang et al., 2013). Orexin loss is the causal process in the neurodegenerative disease narcolepsy, and loss of orexin has been implicated in comorbidities such as sleep disturbances or metabolic changes noted in other neurodegenerative diseases such as Parkinson’s, Alzheimer’s, and Huntington’s disease (Fronczek, Overeem, Lee, Hegeman, van Pelt, van Duinen, Lammers, and Swaab, 2007; Fronczek, van Geest, Frolich, Overeem, Roelandse, Lammers, and Swaab, 2012; Petersen, Gil, Maat-Schieman, Bjorkqvist, Tanila, Araujo, Smith, Popovic, Wierup, Norlen, Li, Roos, Sundler, Mulder, and Brundin, 2005; Thannickal, Lai, and Siegel, 2007). While orexin loss has previously been considered largely to be incidental in these latter neurodegenerative diseases, data suggest that orexin loss may actually contribute to disease progression. We and others have demonstrated that OXA exhibits neuroprotective effects (Butterick et al., 2012; Duffy et al., 2016; Duffy et al., 2015; Sokolowska et al., 2014). The loss of orexin could be a mechanism through which the brain becomes more susceptible to neuronal insults and increased degree of neuroinflammation. We demonstrate that mice lacking orexin neurons have increased cognitive deficits, and that exposure to HFD results in impaired long term memory in these mice (Fig 2). We show that both HFD exposure and loss of orexin increase markers of inflammation (Fig 3). Moreover, the upregulation of neuroinflammatory expression indicates that HFD accelerates hippocampal-dependent memory deficits and the onset of neuroinflammation in O/A3 mice. Our results are consistent with prior studies demonstrating upregulation of genes that regulate microglia immune function, including CX3C chemokine receptor 1 (CX3CR1) and the mitochondria-associated enzyme immune responsive gene-1 (Irg1), in response to reduced orexin signaling and pro-inflammatory stimuli (Heydendael, Sengupta, and Bhatnagar, 2012; Lampropoulou, Sergushichev, Bambouskova, Nair, Vincent, Loginicheva, Cervantes-Barragan, Ma, Huang, Griss, Weinheimer, Khader, Randolph, Pearce, Jones, Diwan, Diamond, and Artyomov, 2016). Additionally, the CX3CL1-CX3CR1 system, important in modulating hippocampus-specific memory tasks and synaptic plasticity (Paolicelli, Bisht, and Tremblay, 2014; Sheridan, Wdowicz, Pickering, Watters, Halley, O’Sullivan, Mooney, O’Connell, O’Connor, and Murphy, 2014), is perturbed in HFD exposure, neuroinflammation, aging, and AD (Fuhrmann, Bittner, Jung, Burgold, Page, Mitteregger, Haass, LaFerla, Kretzschmar, and Herms, 2010; Lyons, Lynch, Downer, Hanley, O’Sullivan, Smith, and Lynch, 2009; Morari, Anhe, Nascimento, de Moura, Razolli, Solon, Guadagnini, Souza, Mattos, Tobar, Ramos, Pascoal, Saad, Lopes-Cendes, Moraes, and Velloso, 2014; Paolicelli et al., 2014). The CX3CL1-CX3CR1 system could represent a target for microglial-neuronal cross talk and microglial-mediated inflammation. Moreover, the neuroprotective immunomodulatory effects of orexin occur via CX3CL1-CX3CR1 signaling. Here we show mice exposed to HFD also have increased OX1R expression (Fig 3A). This is in agreement with our previous data demonstrating that OX1R expression is upregulated in microglia in response to PA, indicating that OXA effects primarily occur via OX1R signaling (Duffy et al., 2015; Mavanji et al., 2017). Increased OX1R expression could be a mechanism in which microglia enhance orexin responsivity therefore enhancing the capability to appropriately respond to inflammatory insults.
Future studies will focus on the effects of orexin replacement therapy in O/A3 mice, using exogenous orexin to directly test if orexin improves cognition through a neuronal-glial mechanism. Orexin is necessary for consolidation of hippocampal dependent memory (Yang et al., 2013). Orexin treatment has been shown to improve cognitive performance on a variety of tasks (Aitta-Aho et al., 2016; Deadwyler et al., 2007; Flores, Valls-Comamala, Costa, Saravia, Maldonado, and Berrendero, 2014; Palotai, Telegdy, Ekwerike, and Jaszberenyi, 2014; Sharf et al., 2010; Song, Kim, Kim, Song, and Lee, 2015; Thorpe et al., 2005), potentially via changes in long term potentiation (LTP). Other reports have demonstrated that orexin delivery to the CA1 region of the hippocampus improves cognition via increased LTP (Deadwyler et al., 2007; Yang et al., 2013; Zhao, Zhang, Tang, Ren, Yang, Liu, and Tang, 2014). Microglia have recently been appreciated to have a role in LTP and hippocampal cognition. Additionally, HFDs alter hippocampal LTP and microglial response (Hao, Dey, Yu, and Stranahan, 2016). It is therefore reasonable to predict that orexin effects on cognition depend in part on modulation of microglia. To the best of our knowledge, we are the first to define a pathway linking orexin to phenotypic changes in hippocampal microglia. The data presented here indicate a targetable pathway to reverse diet-induced cognitive decline and treatment for neurodegenerative diseases involving orexin loss.
Supplementary Material
Acknowledgements
This work was funded by the Department of Veterans Affairs BLR&D grants I01 BX001686 and I01 BX004146 (to TAB), Alzheimer’s Disease Association grant AARGD-17–505409 (to TAB), grants from the Center for Veterans Research and Education (to TAB and JPN), and the University of Minnesota MnDRIVE Fellowship in Neuromodulation (to CMD). We thank Dr. Catherine M. Kotz, Ms. Martha Grace, and Dr. Vijayakumar Mavanji (MVAHCS) for generously providing mice used for these studies, guidance on mouse husbandry, and assistance in rodent behavior studies, respectively.
References
- Aitta-Aho T, Pappa E, Burdakov D, & Apergis-Schoute J (2016). Cellular activation of hypothalamic hypocretin/orexin neurons facilitates short-term spatial memory in mice. Neurobiol Learn Mem, 136, 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari E, Naghdi N, & Motamedi F (2007). The selective orexin 1 receptor antagonist SB-334867-A impairs acquisition and consolidation but not retrieval of spatial memory in Morris water maze. Peptides, 28, 650–656. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Cherbuin N, Budge M, & Young J (2011). Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obesity reviews : an official journal of the International Association for the Study of Obesity, 12, e426–437. [DOI] [PubMed] [Google Scholar]
- Businaro R, Ippoliti F, Ricci S, Canitano N, & Fuso A (2012). Alzheimer’s disease promotion by obesity: induced mechanisms-molecular links and perspectives. Curr Gerontol Geriatr Res, 2012, 986823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterick TA, Nixon JP, Billington CJ, & Kotz CM (2012). Orexin A decreases lipid peroxidation and apoptosis in a novel hypothalamic cell model. Neuroscience letters, 524, 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D (2013). Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol Metab, 24, 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, & Liu T (2011). Hypothalamic inflammation: a double-edged sword to nutritional diseases. Ann N Y Acad Sci, 1243, E1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Welsh CA, Barres BA, & Stevens B (2015). Do glia drive synaptic and cognitive impairment in disease? Nat Neurosci, 18, 1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Saha S, Prutzman SL, Mullins OJ, & Mavanji V (2005). Pontine-wave generator activation-dependent memory processing of avoidance learning involves the dorsal hippocampus in the rat. Journal of neuroscience research, 80, 727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler SA, Porrino L, Siegel JM, & Hampson RE (2007). Systemic and nasal delivery of orexin-A (Hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. The Journal of neuroscience : the official journal of the Society for Neuroscience, 27, 14239–14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy CM, Nixon JP, & Butterick TA (2016). Orexin A attenuates palmitic acid-induced hypothalamic cell death. Mol Cell Neurosci, 75, 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy CM, Yuan C, Wisdorf LE, Billington CJ, Kotz CM, Nixon JP, & Butterick TA (2015). Role of orexin A signaling in dietary palmitic acid-activated microglial cells. Neuroscience letters, 606, 140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A, Valls-Comamala V, Costa G, Saravia R, Maldonado R, & Berrendero F (2014). The hypocretin/orexin system mediates the extinction of fear memories. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 39, 2732–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronczek R, Overeem S, Lee SY, Hegeman IM, van Pelt J, van Duinen SG, Lammers GJ, & Swaab DF (2007). Hypocretin (orexin) loss in Parkinson’s disease. Brain, 130, 1577–1585. [DOI] [PubMed] [Google Scholar]
- Fronczek R, van Geest S, Frolich M, Overeem S, Roelandse FW, Lammers GJ, & Swaab DF (2012). Hypocretin (orexin) loss in Alzheimer’s disease. Neurobiol Aging, 33, 1642–1650. [DOI] [PubMed] [Google Scholar]
- Fuhrmann M, Bittner T, Jung CK, Burgold S, Page RM, Mitteregger G, Haass C, LaFerla FM, Kretzschmar H, & Herms J (2010). Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer’s disease. Nat Neurosci, 13, 411–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaykema RP, & Goehler LE (2009). Lipopolysaccharide challenge-induced suppression of Fos in hypothalamic orexin neurons: their potential role in sickness behavior. Brain, behavior, and immunity, 23, 926–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberg AJ, Zhu X, Leinninger GM, Levasseur PR, Braun TP, Myers MG Jr., & Marks DL (2011). Inflammation-induced lethargy is mediated by suppression of orexin neuron activity. The Journal of neuroscience : the official journal of the Society for Neuroscience, 31, 11376–11386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Dey A, Yu X, & Stranahan AM (2016). Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain, behavior, and immunity, 51, 230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, & Sakurai T (2001). Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron, 30, 345–354. [DOI] [PubMed] [Google Scholar]
- Heydendael W, Sengupta A, & Bhatnagar S (2012). Putative genes mediating the effects of orexins in the posterior paraventricular thalamus on neuroendocrine and behavioral adaptations to repeated stress. Brain research bulletin, 89, 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, & Holtzman DM (2009). Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science, 326, 1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmi A, Iozzo P, Viljanen A, Hirvonen J, Fielding BA, Virtanen K, Oikonen V, Kemppainen J, Viljanen T, Guiducci L, Haaparanta-Solin M, Nagren K, Solin O, & Nuutila P (2010). Increased brain fatty acid uptake in metabolic syndrome. Diabetes, 59, 2171–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent EE, Loginicheva E, Cervantes-Barragan L, Ma X, Huang SC, Griss T, Weinheimer CJ, Khader S, Randolph GJ, Pearce EJ, Jones RG, Diwan A, Diamond MS, & Artyomov MN (2016). Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab, 24, 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons A, Lynch AM, Downer EJ, Hanley R, O’Sullivan JB, Smith A, & Lynch MA (2009). Fractalkine-induced activation of the phosphatidylinositol-3 kinase pathway attentuates microglial activation in vivo and in vitro. Journal of neurochemistry, 110, 1547–1556. [DOI] [PubMed] [Google Scholar]
- Martinez GS, Smale L, & Nuñez AA (2002). Diurnal and nocturnal rodents show rhythms in orexinergic neurons. Brain Research, 955, 1–7. [DOI] [PubMed] [Google Scholar]
- Mavanji V, Butterick TA, Duffy CM, Nixon JP, Billington CJ, & Kotz CM (2017). Orexin/hypocretin treatment restores hippocampal-dependent memory in orexin-deficient mice. Neurobiol Learn Mem, 146, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morari J, Anhe GF, Nascimento LF, de Moura RF, Razolli D, Solon C, Guadagnini D, Souza G, Mattos AH, Tobar N, Ramos CD, Pascoal VD, Saad MJ, Lopes-Cendes I, Moraes JC, & Velloso LA (2014). Fractalkine (CX3CL1) is involved in the early activation of hypothalamic inflammation in experimental obesity. Diabetes, 63, 3770–3784. [DOI] [PubMed] [Google Scholar]
- Nixon JP, & Smale L (2007). A comparative analysis of the distribution of immunoreactive orexin A and B in the brains of nocturnal and diurnal rodents. Behav Brain Funct, 3, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon JP, Zhang M, Wang C, Kuskowski MA, Novak CM, Levine JA, Billington CJ, & Kotz CM (2010). Evaluation of a quantitative magnetic resonance imaging system for whole body composition analysis in rodents. Obesity (Silver Spring), 18, 1652–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EE, Mavanji V, Little MR, Billington CJ, Kotz CM, & Wang C (2014). Exercise reduces diet-induced cognitive decline and increases hippocampal brain-derived neurotrophic factor in CA3 neurons. Neurobiol Learn Mem, 114, 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, & Godbout JP (2013). Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol, 39, 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Irukayama-Tomobe Y, Murakoshi N, Kiyama M, Ishikawa Y, Hosokawa N, Tominaga H, Uchida S, Kimura S, Kanuka M, Morita M, Hamada M, Takahashi S, Hayashi Y, & Yanagisawa M (2016). Peripherally administered orexin improves survival of mice with endotoxin shock. Elife, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palotai M, Telegdy G, Ekwerike A, & Jaszberenyi M (2014). The action of orexin B on passive avoidance learning. Involvement of neurotransmitters. Behav Brain Res, 272, 1–7. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bisht K, & Tremblay ME (2014). Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Frontiers in cellular neuroscience, 8, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, & Teeling J (2013). Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin Immunopathol, 35, 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A, Gil J, Maat-Schieman ML, Bjorkqvist M, Tanila H, Araujo IM, Smith R, Popovic N, Wierup N, Norlen P, Li JY, Roos RA, Sundler F, Mulder H, & Brundin P (2005). Orexin loss in Huntington’s disease. Hum Mol Genet, 14, 39–47. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, & Kilduff TS (1998). Neurons containing hypocretin (orexin) project to multiple neuronal systems. The Journal of neuroscience : the official journal of the Society for Neuroscience, 18, 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res, 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguiz RM, & Wetsel WC (2006). Assessments of Cognitive Deficits in Mutant Mice In Levin ED, & Buccafusco JJ (Eds.), Animal Models of Cognitive Impairment. Boca Raton (FL). [PubMed] [Google Scholar]
- Roh JH, Jiang H, Finn MB, Stewart FR, Mahan TE, Cirrito JR, Heda A, Snider BJ, Li M, Yanagisawa M, de Lecea L, & Holtzman DM (2014). Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer’s disease. J Exp Med, 211, 2487–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A, Colas D, Adamantidis A, Carter M, Lanre-Amos T, Heller HC, & de Lecea L (2011). Optogenetic disruption of sleep continuity impairs memory consolidation. Proceedings of the National Academy of Sciences of the United States of America, 108, 13305–13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T (2005). Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep Med Rev, 9, 231–241. [DOI] [PubMed] [Google Scholar]
- Sears RM, Fink AE, Wigestrand MB, Farb CR, de Lecea L, & Ledoux JE (2013). Orexin/hypocretin system modulates amygdala-dependent threat learning through the locus coeruleus. Proceedings of the National Academy of Sciences of the United States of America, 110, 20260–20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestakova N, Puzserova A, Kluknavsky M, & Bernatova I (2013). Determination of motor activity and anxiety-related behaviour in rodents: methodological aspects and role of nitric oxide. Interdiscip Toxicol, 6, 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, Brayton CE, Guarnieri DJ, Taylor JR, & DiLeone RJ (2010). Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement. Biol Psychiatry, 67, 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan GK, Wdowicz A, Pickering M, Watters O, Halley P, O’Sullivan NC, Mooney C, O’Connell DJ, O’Connor JJ, & Murphy KJ (2014). CX3CL1 is up-regulated in the rat hippocampus during memory-associated synaptic plasticity. Frontiers in cellular neuroscience, 8, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowska P, Urbanska A, Bieganska K, Wagner W, Ciszewski W, Namiecinska M, & Zawilska JB (2014). Orexins protect neuronal cell cultures against hypoxic stress: an involvement of Akt signaling. Journal of molecular neuroscience : MN, 52, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowska P, Urbanska A, Namiecinska M, Bieganska K, & Zawilska JB (2012). Orexins promote survival of rat cortical neurons. Neuroscience letters, 506, 303–306. [DOI] [PubMed] [Google Scholar]
- Song J, Kim E, Kim CH, Song HT, & Lee JE (2015). The role of orexin in post-stroke inflammation, cognitive decline, and depression. Molecular brain, 8, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Cutler RG, Button C, Telljohann R, & Mattson MP (2011). Diet-induced elevations in serum cholesterol are associated with alterations in hippocampal lipid metabolism and increased oxidative stress. Journal of neurochemistry, 118, 611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, & Schwartz MW (2012). Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest, 122, 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal TC, Lai YY, & Siegel JM (2007). Hypocretin (orexin) cell loss in Parkinson’s disease. Brain, 130, 1586–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe AJ, Cleary JP, Levine AS, & Kotz CM (2005). Centrally administered orexin A increases motivation for sweet pellets in rats. Psychopharmacology (Berl), 182, 75–83. [DOI] [PubMed] [Google Scholar]
- Uranga RM, Bruce-Keller AJ, Morrison CD, Fernandez-Kim SO, Ebenezer PJ, Zhang L, Dasuri K, & Keller JN (2010). Intersection between metabolic dysfunction, high fat diet consumption, and brain aging. Journal of neurochemistry, 114, 344–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, & Koliwad SK (2014). Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep, 9, 2124–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, White RE, Xu L, Yang L, Sun X, Zou B, Pascual C, Sakurai T, Giffard RG, & Xie XS (2013). Mitigation of murine focal cerebral ischemia by the hypocretin/orexin system is associated with reduced inflammation. Stroke; a journal of cerebral circulation, 44, 764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, & Fratiglioni L (2011). Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology, 76, 1568–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zou B, Xiong X, Pascual C, Xie J, Malik A, Xie J, Sakurai T, & Xie XS (2013). Hypocretin/orexin neurons contribute to hippocampus-dependent social memory and synaptic plasticity in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience, 33, 5275–5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, & Cai D (2013). Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature, 497, 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Zhang R, Tang S, Ren Y, Yang W, Liu X, & Tang J (2014). Orexin-A-induced ERK1/2 activation reverses impaired spatial learning and memory in pentylenetetrazol-kindled rats via OX1R-mediated hippocampal neurogenesis. Peptides, 54, 140–147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.