Summary Statement:
In the DRCR.net Protocol I individuals managed with ranibizumab therapy for DME had favorable changes in retinopathy severity at 5-years. Rates of improvement and worsening do not appear altered by a reduction in the number of intravitreous ranibizumab injections for DME during the later years
Purpose:
Explore 5-year changes from baseline in diabetic retinopathy severity among eyes treated with ranibizumab for diabetic macular edema.
Methods:
Diabetic Retinopathy Severity was assessed from study visits and annual fundus photographs among participants in Protocol I (DRCR.net). The proportion of eyes that improved at annual examinations and the cumulative probability of worsening through 5 years were estimated.
Results:
Among 235 participants with non-proliferative diabetic retinopathy (NPDR) at baseline, there were 29%, 28%, and 32% eyes with retinopathy improvement at 1, 3 and 5 years, respectively. Among 111 participants with proliferative diabetic retinopathy (PDR), corresponding improvement percentages were 38%, 35% and 23%. The 5-year cumulative probability of worsening was 18% (95% CI: 14%−25%) among NPDR eyes and 31% (95% CI: 23%−42%) among PDR eyes (P = .01). In years 1, 3, and 5, the mean (SD) number of ranibizumab injections was 8.1 (2.5), 2.2 (2.6), and 1.8 (2.6) for NPDR eyes , and 9.0 (2.8), 2.3 (2.9) and 1.7 (2.6) for PDR eyes. Proportions with improvement or rates of worsening did not change with time.
Conclusion:
Individuals receiving ranibizumab therapy for DME may have favorable changes in DR severity throughout a 5-year period concomitant with sequential reduction in anti-VEGF therapy.
Keywords: Retinopathy, Diabetic Macular Edema, Ranibizumab
INTRODUCTION
Clinical trial results support intravitreous anti-vascular endothelial growth factor (anti-VEGF) agents as first-line therapy to manage vision impairment in eyes with central-involved diabetic macular edema (DME).1–9 Anti-permeability properties of these agents promote anatomic resolution of edema, which favorably affects vision outcomes.10 These agents also retard angiogenesis and may reverse or slow the progression of diabetic retinopathy.11 Pivotal trials evaluating anti-VEGF therapy for DME have reported improvement in diabetic retinopathy severity (DRS) and slower progression of retinopathy through 2 to 3 years among eyes treated with ranibizumab (monthly or using a structured retreatment protocol) or aflibercept (monthly or 5-monthly doses followed by every other month).1,4,12–13
In the first year of anti-VEGF therapy, most eyes with DME experience improved visual acuity (VA) and central retinal thickness. 1,4,12–13 The annual mean number of injections using the DRCR.net re-treatment algorithm is greatest in year 1 and progressively falls through year 5.5 Despite a decline in injection frequency, early beneficial effects on vision and macular thickening are maintained.5 This report evaluates, relative to baseline, the annual cross-sectional proportion of eyes with DRS improvement and the cumulative probability with retinopathy worsening through 5 years of follow up, in the context of sequential reduction in ranibizumab frequency. An exploratory analysis of baseline factors associated with change in DRS also is provided.
METHODS
Study procedures were previously reported.4 The study was conducted between March 2007 and December 2013, and the protocol is available at www.drcr.net (accessed 20 February 2018). The study adhered to the tenets of the Declaration of Helsinki and was approved by multiple institutional review boards. Study participants provided written informed consent. Participants had central-involved DME (central subfield thickness [CST] ≧250 µm on optical coherence tomography [OCT, Stratus; Carl Zeiss Meditec]) and had best-corrected electronic visual acuity (VA) letter scores of 78 through 24 (approximate Snellen equivalent, 20/32 to 20/320). Ranibizumab-assigned eyes received prompt laser at baseline or deferred laser beginning at 24 weeks if DME persisted and was no longer improving.
After completion of the 3-year visit, participants were offered extended follow up through 5 years. Protocol visits occurred every 4 to 16 weeks between the 1-year and 5-year visits, depending on CST and VA response. Assessment of DRS was performed by masked graders (Fundus Photograph Reading Center, Madison, Wisconsin) using standard 7-field color fundus photographs obtained at baseline, 1 year, and annually between years 3 and 5.
Included in this report are 346 of 375 (92%) ranibizumab-assigned eyes. Twenty-nine eyes are excluded from this analysis due to missing (12) or ungradable (3) baseline photographs, or failure to meet Protocol I OCT eligibility criteria (14). Evolution of DR severity was evaluated in two pre-specified subgroups based on DR severity from baseline photographs: non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR). Additional post hoc analyses are reported subdividing the NPDR group into moderate NPDR or better (Early Treatment Diabetic Retinopathy Study [ETDRS] retinopathy levels 10 through 43) versus moderate-severe and severe NPDR (ETDRS levels 47 and 53).
Table 1 provides definitions for improvement, sustained improvement, and worsening in DRS. Events indicating worsening of PDR were collected prospectively and any occurence made an eye ineligible for later improvement. Data from participants who completed a visit but did not have gradable photographs available at the 1-, 3-, 4- or 5-year visit were excluded from the cross-sectional improvement analyses (35, 25, 23, and 19 eyes, respectively), but were included in the analyses for cumulative probability of worsening, as some worsening events were determined based on ophthalmoscopy or treatment for PDR (Table 1).
Table 1.
Definitions for Diabetic Retinopathy Improvement or Worsening During Follow up
| Non-Proliferative Diabetic Retinopathy (NPDR) at baseline | Proliferative Diabetic Retinopathy (PDR) at baseline | |
|---|---|---|
|
Improvement in retinopathy was achieved if both of the following criteria were met during follow up | ||
| Documentation on case report form† | (1) no PRP, vitrectomy, or anti-VEGF injection (to manage PDR or its complications) was performed, and no vitreous hemorrhage, retinal detachment (traction, rhegmatogenous, combined, or unspecified), anterior segment neovascularization, or neovascular glaucoma was identified | |
| Assessment of annual fundus photographs by reading center | (2a) improvement by 2 or more levels on the ETDRS diabetic retinopathy scale vs. baseline | |
| (2b) regression of active PDR (level 61 or higher) to no PDR (level 53 or lower if no prior PRP, or to level 60 if PRP was present at entry) | ||
| Baseline DR severity ineligible for improvement | Microaneurysms only or less (level 10, 12, 14, 15, or 20) |
Inactive PDR (level 60) |
|
Sustained Improvement in retinopathy was achieved if improvement was demonstrated at the 1-year visit and there were gradable photographs at the 3-year visit that confirmed improvement relative to baseline. | ||
|
Worsening of diabetic retinopathy included any of the following events during follow up | ||
| Documentation on case report form† | (1) PRP, vitrectomy, or anti-VEGF injection (to manage PDR or its complications), vitreous hemorrhage, retinal detachment, or neovascularization of the iris or angle or neovascular glaucoma | |
| Assessment of annual fundus photographs by reading center‡ | (2) worsening by 2 or more levels on the ETDRS severity scale vs. baseline |
|
| (3a) worsening from no PDR to PDR (≤ level 53 progressing to level 60 or higher) | (3b) progression from less than high risk PDR to advanced PDR (level 60 to 75 progressing to level 81 or 85) | |
| Baseline DR severity ineligible for worsening | N/A | Advanced PDR (level 81 or 85) |
ETDRS= Early Treatment Diabetic Retinopathy Study; Anti-VEGF=anti-vascular endothelial growth factor.
Eyes that manifested worsening of retinopathy with any PDR related event were considered as non-improvers from that point foward in the improvement analysis.
Cases with missing photograph data or nongradable photographs at a completed follow-up visit were considered as no event for the photograph component in the composite worsening outcome at that annual time point.
Statistical methods
No definitive differences between the deferred and prompt laser treatment groups (P > .05) were identified, therefore this report combines both ranibizumab groups. The cross-sectional results for retinopathy improvement at each annual visit are reported as percentages with exact 95% Clopper-Pearson confidence intervals (CI). Generalized linear regression models were used to test for a difference in percentages improving between the NPDR and PDR subgroups and to test for a trend in the percentage with retinopathy improvement through 5 years.12 Cumulative probabilities of retinopathy worsening throughout 5 years were calculated using the life-table method.13 Proportional hazards regression was used to test for a difference in the rates of worsening between baseline DR subgroups. The Z-test was used to compare hazard rates between time intervals with P-values adjusted using the Hochberg method to account for multiple comparisons.14, 15 Sensitivity analyses for DR improvement and DR worsening were conducted by (1) excluding eyes that underwent cataract surgery during follow up but prior to the visit (improvement analysis) or censoring at the date of surgery (worsening analysis), (2) limiting the definition of improvement or worsening to be based only on photographs (NPDR group only).
Exploratory analyses of 9 variables (Table S1) were performed to identify factors associated with sustained DR improvement through the 3-year visit or of worsening through 5 years. A Poisson regression model with robust variance estimator was used for estimating the relative risk for sustained improvement and excluded all eyes that underwent cataract surgery between baseline and the 3-year visit.16 A Cox proportional hazards model was used for worsening and censored eyes that underwent cataract surgery during follow up at the date of surgery. For both models, initial analyses with including a single factor and baseline DRS level as a covariate were first performed for each factor. Multivariable stepwise selection analysis including factors with P<.10 from the univariable analyses was performed next. Only factors with P-values <.05 were retained in the multivariable model. No adjustments for multiplicity were made. The proportional hazards assumption was verified by testing time-by-baseline characteristic interactions. All P-values are 2-sided. Statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
Among the 346 participants eligible for analysis, NPDR was present in 235 (68%) participants (42% female; average age [SD] 63.9 years [9.2]; 72% white) among whom 89 (38%) had moderate NPDR or better (level 47 or better) and 146 (62%) had moderate-severe to severe NPDR (level 47 or 53). The remaining 111 participants (32%) had PDR at baseline (43% female; average age [SD] 61.0 years [10.2]; 76% white). Seven NPDR (level 20 or less) eyes (3%) and 61 PDR (level 60, PRP without active PDR) eyes (55%) were excluded from the improvement analysis because they could not meet the improvement definition. All eyes in the study were eligible for DR worsening.
Table S2 shows the follow-up status for visits that required fundus photographs. Among participants alive at each time point, completion rates were 96%, 89% and 75% at the 1-, 3- and 5-year visits, respectively. The availability of gradable fundus photographs from patients who remained alive declined from 86% at 1 year to 81% at 3 years and 69% at 5 years. Comparison of participants by 5-year visit completion status showed that non-completers with NPDR were more likely at baseline to have had diabetes longer, use insulin, have a prior cardiovascular event, and be pseudophakic; non-completers with PDR were older, had higher HbA1c, were more likely to have hypertension, prior cataract surgery, and less severe DME (Table S3). The baseline distribution of DRS appeared similar between completers and non-completers within the DR subgroups with one exception: fewer PDR eyes had inactive PDR among the non-completers.
Diabetic Retinopathy Improvement
The proportions of eyes with improvement were similar over time and between the NPDR and PDR subgroups (Figure 1a). At the 5-year visit, 32% (95% CI: 24%- 41%) of the NPDR (N = 137) and 23% (95% CI: 9%- 44%) of the PDR (N = 26) eyes improved. In addition, 15% (95% CI: 7%−28%) of the moderate NPDR or better group (N = 52) improved vs. 42% (95% CI: 32%−54%) of the moderate-severe/severe NPDR eyes (N = 85; P = .002). Similar results were found in sensitivity analyses that excluded eyes with cataract surgery prior to the visit (Figure S1) or limited improvement to a 2-step change on photographs (Figure S2).
Figure 1. Percentage of Ranibizumab-Treated Eyes with Improvement in Diabetic Retinopathy Severity from Baseline.
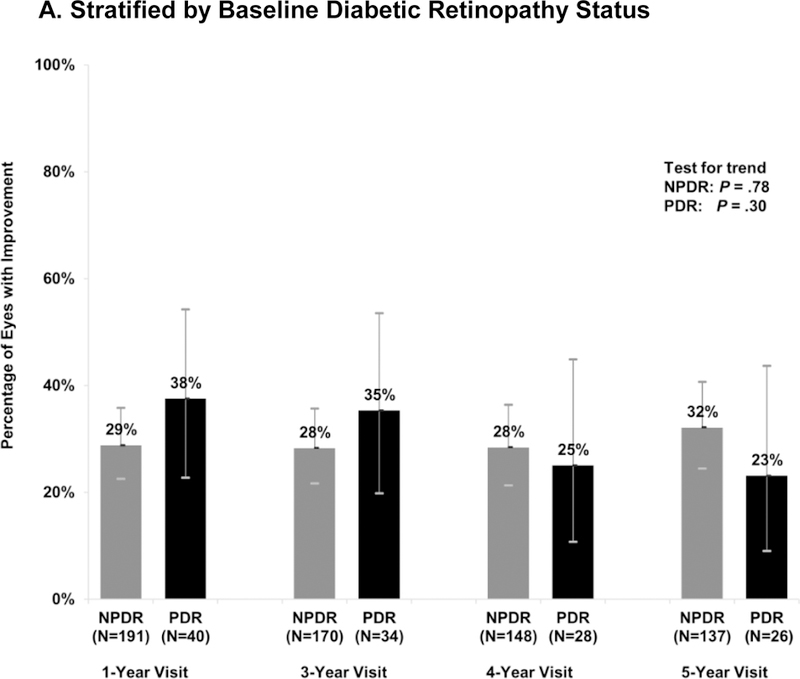
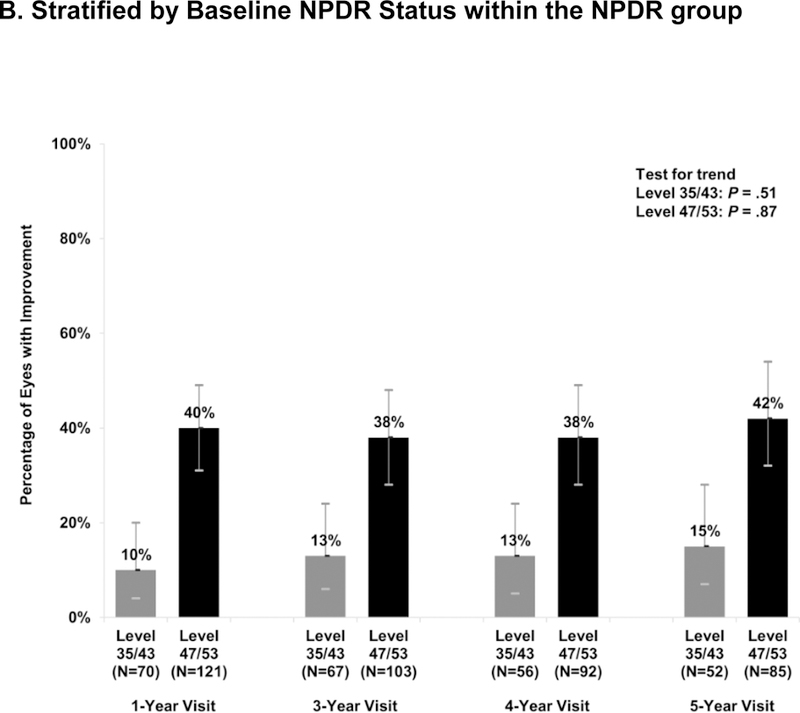
Percentage of eyes demonstrating improvement in diabetic retinopathy at each annual visit at which study photographs were required, by baseline DR subgroup. The number of evaluable study eyes at each visit is listed and error bars represent the Clopper-Pearson exact confidence limits. Percentage of eyes improved were stable over time using the generalized score trend test (test for trend). NPDR = non-proliferative diabetic retinopathy, PDR = proliferative diabetic retinopathy.
Among 70 eyes with improvement at the 1-year visit, 53 (76%) had gradable photographs to assess sustained improvement at the 3-year visit. Improvement was sustained at the 3-year visit in 25 eyes (47%). The proportion with sustained improvement was similar among the baseline NPDR (20 of 43 eyes [47%]) and baseline PDR (5 of 10 eyes [50%])) eyes. Of the 70 eyes improved at 1 year, 11 eyes (16%) had DR worsening relative to their baseline status by the 5th year. Among eyes that did not improve at the 1-year visit, subsequent improvement was seen in 26 of 133 (20%), 22 of 115 (19%) and 26 of 105 (25%) at the 3-, 4-, and 5-year visits. Percentages of eyes improved appeared similar among the DRS subgroups (Table 2).
Table 2.
Late Improvement in Diabetic Retinopathy: Improvement Status at Annual Visits Among Eyes that Did Not Show Improvement in Retinopathy at the 1-Year Visit
| Diabetic Retinopathy Status Between Baseline and Specified Visit | 3-Year Visit [Number Evaluable] |
4-Year Visit [Number Evaluable] |
5-Year Visit [Number Evaluable] |
|---|---|---|---|
|
NPDR at Baseline No Improvement at 1 Year N=136 |
[N=111] |
[N=98] |
[N=89] |
| Late Improvement | 20 (18%) | 18 (18%) | 22 (25%) |
| No Improvement | 91 (82%) | 80 (82%) | 67 (75%) |
|
PDR at Baseline No Improvement at 1 Year N=25 |
[N=22] |
[N=17] |
[N=16] |
| Late Improvement | 6 (27%) | 4 (24%) | 4 (25%) |
| No Improvment | 16 (73%) | 13 (76%) | 12 (75%) |
|
NPDR or PDR at Baseline No Improvement at 1 Year N=161 |
[N=133] |
[N=115] |
[N=105] |
| Late Improvement | 26 (20%) | 22 (19%) | 26 (25%) |
| No Improvment | 107 (80%) | 93 (81%) | 79 (75%) |
Late Improvement: Status of DR severity was improved relative to baseline at the specified annual visit.
No Improvment: Status of DR severity was “not improved” relative to baseline at the specified annual visit.
Note: 2-Year visit is not shown due to limited fundus photographs
Number of Intravitreous Injections and Improvement in Diabetic Retinopathy Severity
Within the improvement cohort, the number of injections administered through each annual visit during the 5-year period was similar between eyes with baseline NPDR (irrespective of severity) and baseline PDR, and also was similar when the comparison was limited to eyes completing the 5-year visit (Table S4). As such, the DRS subgroups were combined in a post hoc analysis exploring relationships between total injection number and improvement or sustained improvement.
With a theoretical maximum number of 63 injections during the 5-year follow up, the cumulative mean (SD) number of injections was 21.2 (13.1) among eyes that had improvement at the 5-year visit vs.16.3 (8.6) among those that had not improved at this visit (P =.005). At each annual visit, eyes that improved received more injections since baseline than eyes that did not improve (P <.05 for each annual visit, Table 3). Overall, the number of annual injections decreased after the 1-year visit.
Table 3.
Cumulative Number of Ranibizumab Injections at Improvement Assessment Visits by Diabetic Retinopathy Improvement Status
| Number of injections between baseline and the specified Visit | * P-value | |||
|---|---|---|---|---|
| **Total no. of injections for each year | Improved | Not Improved | ||
| 1-Year Visit | N=231 | N=70 | N=161 | |
| Mean ± SD | 8.1 ± 2.6 | 9.1 ± 2.6 | 7.6 ± 2.5 | < .001 |
| Median (IQR) | 8 (6, 10) | 10 (7, 11) | 7 (6, 9) | |
| 3-Year Visit | N=204 | N=60 | N=144 | |
| Mean ± SD | 2.2 ± 2.8 | 14.9 ± 7.9 | 12.8 ± 6.3 | .05 |
| Median (IQR) | 1 (0, 4) | 14 (9, 20) | 11 (8, 17) | |
| 4-Year Visit | N=176 | N=49 | N=127 | |
| Mean ± SD | 1.7 ± 2.6 | 17.7 ± 9.5 | 14.4 ± 7.9 | .02 |
| Median (IQR) | 0 (0, 3) | 15 (10, 23) | 13 (8, 19) | |
| 5-Year Visit | N=163 | N=50 | N=113 | |
| Mean ± SD | 1.9 ± 2.7 | 21.2 ± 13.1 | 16.3 ± 8.6 | .005 |
| Median (IQR) | 0 (0, 4) | 17 (11, 30) | 15 (10, 22) | |
SD= standard deviation; IQR= Interquartile range
General linear model used to determine the difference in cumulative number of injections received between eyes with DRS improvement and eyes without DRS improvement
Includes all eyes eligible for improvement at each visit; value represents the number of injections in the 12 month period concluding the day before the specified visit.
The mean (SD) cumulative number of injections through the 3-year visit was 15.7 (7.0) for eyes with improvement at both the 1- and 3-year visits and 12.5 (6.3) injections for eyes that were not improved at both visits (P = .07). There was no association between number of injections and sustained DRS improvement through the 3-year visit (P >.05; Table S5).
Worsening of Diabetic Retinopathy
Eyes with NPDR were less likely to worsen than eyes with PDR. The 5-year cumulative probability of worsening was 18% (95% CI:14%−25%) for the NPDR eyes vs. 31% (95% CI 23%- 42%, P = .01 vs. NPDR) for the PDR eyes (Figure 2a). This was largely driven by the low cumulative probability of worsening among eyes with moderate NPDR or better (11% [95% CI: 5%- 20%], P = .002 vs. PDR) while the rate among the eyes with more severe NPDR (level 47 or 53) approached that of the PDR eyes (24% [95% CI:17%−32%], P = .20 vs. PDR; Figure 2b). There were no detectable differences in the annual rates of worsening between the 1st, 2nd, 3rd, 4th, and 5th years within each DRS group (P > .05). As noted in Table S6, on average, the number of annual injections fell sharply after year 1. The number of ranibizumab injections was not associated with the risk of worsening (Table S7). Results were similar in sensitivity analyses that censored eyes at the date of cataract surgery performed during follow up (Figure S3) or limited worsening to progression documented on photographs (NPDR subgroup only, Figure S4).
Figure 2. Cumulative Probability of Diabetic Retinopathy Worsening Among Eyes Treated with Ranibizumab for Diabetic Macular Edema.
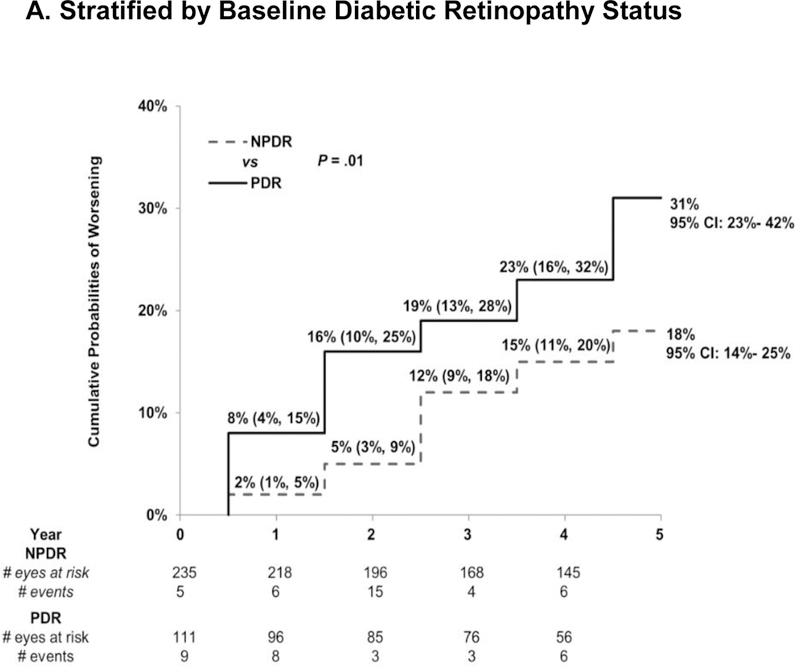
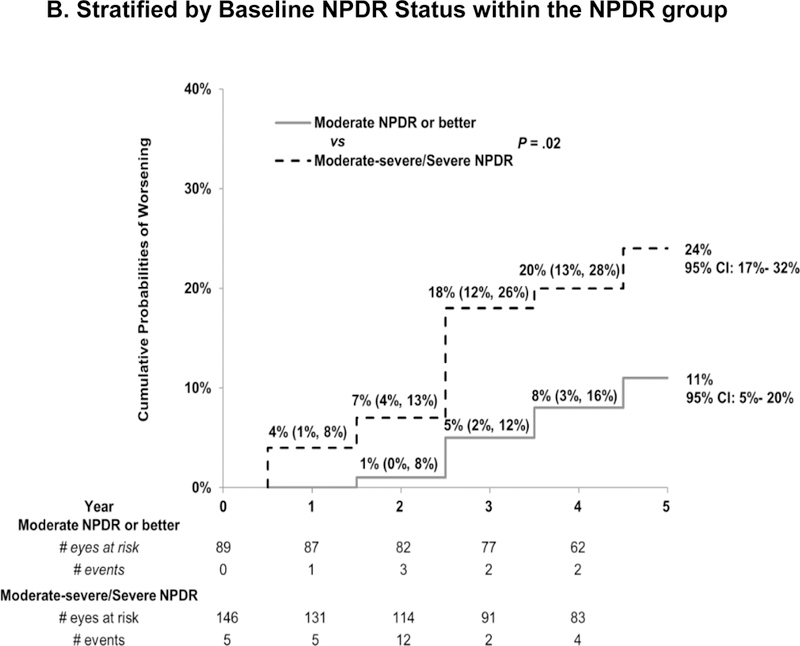
Cumulative probability of worsening of diabetic retinopathy by baseline retinopathy subgroup. There was a difference in the rate of worsening between the NPDR and PDR group [P = .01]. There was no difference in the rate of worsening between the 1st, 2nd, 3rd, 4th, and 5th years within each DR group (P > .05). NPDR=non-proliferative diabetic retinopathy, PDR=proliferative diabetic retinopathy.
Table 4 shows the distribution of the specific event that initially triggered categorization as worsening of retinopathy. Among eyes with baseline NPDR, approximately two-thirds of the events were divided equally between worsening captured on annual photographs (36%) and clinician-reported vitreous hemorrhage (33%). However, 21 of the 36 NPDR eyes that worsened by the 5-year visit eventually met more than one DR worsening criterion. Of note, 16 (44%) of the baseline NPDR eyes that worsened developed neovascularization that was confirmed on photographs. Among eyes with baseline PDR, vitreous hemorrhage accounted for the majority (55%) of the initial worsening events. However, 12 of the 29 PDR eyes that worsened eventually met more than one worsening criterion.
Table 4.
First Event Triggering Worsening by Baseline Diabetic Retinopathy Subgroup among Ranibizumab Assigned Eyes
| First Event Triggering Worsening |
NPDR N=36 |
PDR N=29 |
|---|---|---|
| N (%) | N (%) | |
| Change on Annual Photographs | 13 (36) | 2 (7) |
| Vitreous Hemorrhage | 12 (33) | 16 (55) |
| PRP | 6 (17) | 6 (21) |
| NVI or NVA or NVG | 2 (6) | 0 (0) |
| Retinal Detachment | 0 (0) | 2 (7) |
| Vitrectomy | 0 (0) | 2 (7) |
| PRP and Change on Photographs | 2 (6) | 0 (0) |
| PRP and Vitreous Hemorrhage | 1 (3) | 1 (3) |
| Anti-VEGF to Treat PDR | 0 (0) | 0 (0) |
Anti-VEGF=anti-vascular endothelial growth factor; PDR=proliferative diabetic retinopathy; PRP=panretinal photocoagulation; NPDR= nonproliferative diabetic retinopathy NVI=neovascularization of the iris; NVA=Neovascularization of the angle; NVG=Neovascular glaucoma.
Factors Associated With Sustained Retinopathy Improvement or Worsening
In the multi-variable model, after adjusting for baseline DR level, VA and lens status remained associated with sustained improvement (P < .05, Table S8). For every 5 letters, i.e. 1 line, better baseline VA, the likelihood of sustained improvement decreased by 13% (RR: 0.87; 95% CI: 0.77–0.99; P = .03). There was no difference in sustained improvement between the NPDR and PDR eyes in the multi-variable model (P = .49)
After adjusting only for baseline DR level, cataract surgery prior to entry was associated with lower rates of worsening. In the multivariable model two factors reduced the likelihood of DR worsening: cataract surgery prior to study entry (RR: 0.29; 95% CI: 0.14–0.62; P = .001) and less severe NPDR at entry (RR: 0.16; 95% CI: 0.06–0.47; P < .001) (Table S7).
DISCUSSION
The natural evolution of diabetic retinopathy has been well described. Retinopathy scales based on assessment of disease features present on fundus photographs show an orderly progression of the disease. Moreover, progressive steps on the scale impart escalating risk of advancing to vision threatening retinopathy.17, 18 Management of glycemia, blood pressure, and lipid levels may be instrumental in altering DRS progression rates.19–22 For over 40 years panretinal photocoagulation has been the most effective treatment to stabilize DR until intraocular anti-VEGF agents administered for DME were observed to promote DR regression/improvement and slow progression/worsening. Anti-VEGF agents facilitate reversal of the orderly progression of DR1,2,13
In this exploratory analysis of DRS over a 5-year period in the setting of repeated yet intermittent administration of ranibizumab to manage DME, long-term improvements were seen. Approximately one-quarter of eyes were observed to have less severe DR than at baseline at each annual visit through 5 years, irrespective of baseline DR severity. Although DR improvement was seen in a minority, these observations appear more favorable than the 4-year DR improvement proportions reported in the Wisconsin Epidemiologic Survey.23, 24 In that study, among individuals with diabetes diagnosis before age 30, 7% improved whereas 17% of subjects diagnosed at age 30 or later had improvement at the 4-year exam. 23,24
In the present analysis, the most impressive proportions of improvement were found in the moderate/severe to severe NPDR eyes, a subgroup for whom the natural risk of progression to PDR approaches 50% within 1 year. Importantly, no decline was seen in the proportion of eyes with DRS improvement, across the DR spectrum, as the frequency of anti-VEGF administration to manage DME generally declined over the 5-year period. However, about 50% of individuals with DR improvement at 1 year did not sustain this at 3 years, while others first demonstrated DR improvement only relatively late in their treatment course (e.g., at 4 years). When applying a re-treatment protocol aimed at addressing DME rather than DRS outcomes, DRS improvement at 1 year neither guaranteed that improvement was sustained nor protected from future worsening. Twenty percent with improvement at 1 year eventually worsened relative to their baseline exam. Although there may be a relationship between DRS improvement and larger injection number, the Protocol I study design does not provide a means to identify the optimal number of anti-VEGF injections to achieve DR improvement. As such, diligent monitoring of DRS level is recommended for all eyes, even those for whom DRS regression is noted during the course of anti-VEGF therapy for DME.
Despite receiving anti-VEGF therapy, the cumulative proportion of eyes manifesting DR worsening for the first time increased throughout the 5-year follow up highlighting the need to remain vigilant for disease worsening. No relationship was identified between number of injections or HbA1c, and disease worsening while a relationship between baseline DRS and worsening was confirmed. The cumulative probability of DR worsening was highest among eyes with PDR and lowest for eyes with moderate NPDR or better, as seen in a similar analysis from Protocol T.15. Rates of worsening among eyes in Protocol I did not appear to accelerate in the later years of follow up, in any DRS subgroup, despite a progressive decline in the average annual ranibizumab exposure. Among control eyes in the ETDRS, 54% of NPDR eyes developed PDR and about two-thirds of a combined group consisting of levels 47/53/61 (mild PDR) progressed 2 or more steps at the 5-year visit.18 The 5-year cumulative worsening rates reported herein appear more favorable.
Cataract surgery may confound assessment and evolution of DRS. Surgery may accelerate progression, while a more accurate photographic assessment of DRS level may be facilitated after cataract removal, introducing ascertainment bias.25 Twenty percent of our participants underwent cataract surgery during follow up. Sensitivity analysis censoring eyes at the time of cataract surgery suggested that surgery performed during Protocol I did not substantially affect the proportionof DRS improvement or rates of worsening. Cataract surgery prior to study participation lowered the risk of DR worsening during anti-VEGF therapy. Protocol I excluded those with recent cataract surgery (≤4 months prior to entry). It is possible the window during which cataract surgery may contribute to DR worsening may have expired in these eyes. In addition, the media clarity achieved in pseudophakic eyes may reduce the observed rate of worsening over time by minimizing misclassification of DRS level.
Limitations of this analysis include a decline in participation rates and the percentage of participants with gradable fundus photographs. The reading center assessment of images was an integral component of definitions for improvement and worsening and their absence may have biased our estimates. Rates are reported by DRS subgroup with wide confidence intervals, particularly among the more limited number of eyes with baseline PDR. Additional limitations include the limited subset of participants for whom the issue of sustained improvement could be probed and the differences observed between participants who did not complete the 5-year follow up with those who did.
In summary, individuals managed with ranibizumab therapy for DME simultaneously may have favorable changes in retinopathy severity. Percentage of eyes improved and worsening rates did not appear to be altered by the reduction in ranibizumab administration for DME during the later years of follow up. Confirmation of these longer-term observations of DRS in other datasets is needed.
Supplementary Material
Acknowledgments
Funding/Support: Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, U.S. Department of Health and Human Services EY14231, EY23207, EY18817.
Footnotes
Financial Disclosures: Some authors have financial disclosures to declare, and will submit them with their financial disclosure forms via e-mail when it is requested by the journal.
A complete list of all DRCR.net investigator financial disclosures can be found at www.drcr.net.
Role of the Sponsor: The funding organization (National Institutes of Health) participated in oversight of the conduct of the study and review of the manuscript but not directly in the design or conduct of the study, nor in the collection, management, analysis, or interpretation of the data, or in the preparation of the manuscript.
The Study was completed at 52 sites within the United States a published list of the Diabetic Retinopathy Clinical Research Network investigators and staff participating in this protocol can be found in Ophthalmology 2010;117:1064–1077.e35 with a current list available at www.drcr.net.
REFERENCES
- 1.Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology 2013;120(10):2013–22. [DOI] [PubMed] [Google Scholar]
- 2.Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology 2015;122(10):2044–52. [DOI] [PubMed] [Google Scholar]
- 3.Diabetic Retinopathy Clinical Research N, Elman MJ, Qin H, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology 2012;119(11):2312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010;117(6):1064–77 e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elman MJ, Ayala A, Bressler NM, et al. Intravitreal Ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology 2015;122(2):375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elman MJ, Bressler NM, Qin H, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2011;118(4):609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011;118(4):615–25. [DOI] [PubMed] [Google Scholar]
- 8.Rajendram R, Fraser-Bell S, Kaines A, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol 2012;130(8):972–9. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt-Erfurth U, Lang GE, Holz FG, et al. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology 2014;121(5):1045–53. [DOI] [PubMed] [Google Scholar]
- 10.Chun DW, Heier JS, Topping TM, Duker JS, Bankert JM. A pilot study of multiple intravitreal injections of ranibizumab in patients with center-involving clinically significant diabetic macular edema. Ophthalmology 2006;113(10):1706–12. [DOI] [PubMed] [Google Scholar]
- 11.Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina 2005;25(2):111–8. [DOI] [PubMed] [Google Scholar]
- 12.Piegorsch WW, Bailer AJ. Statistics for environmental biology and toxicology; 1997.
- 13.Armitage P, Berry G, Mathew J. Statistical Methods in Medical Research Vol 4: Blackwell Science; 2002. [Google Scholar]
- 14.Glueck DH. Sample Size Calculations in Clinical Research. 2nd edition by CHOW S-C, SHAO J, and WANG H. Biometrics, 64: 1307–1308. doi: 10.1111/j.1541-0420.2008.01138_10.x; 2008. [DOI] [Google Scholar]
- 15.Hochberg Y A sharper Bonferroni procedure for multiple tests of significance. Biometrika 1988;75(4):800–2. [Google Scholar]
- 16.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 2005;162(3):199–200. [DOI] [PubMed] [Google Scholar]
- 17.The Diabetic Retinopathy Study Research Group. Four risk factors for severe visual loss in diabetic retinopathy. The third report from the Diabetic Retinopathy Study. Arch Ophthalmol 1979;97:654–5. [DOI] [PubMed] [Google Scholar]
- 18.Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 1991;98:823–33. [PubMed] [Google Scholar]
- 19.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317(7160):703–13. [PMC free article] [PubMed] [Google Scholar]
- 20.Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 2007;370(9600):1687–97. [DOI] [PubMed] [Google Scholar]
- 21.Diabetes Control Complications Trial /Epidemiology of Diabetes Interventions Complications Research Group, Lachin JM, White NH, et al. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes 2015;64(2):631–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Group AS, Group AES, Chew EY, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 2010;363(3):233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. IX. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol 1989;107(2):237–43. [DOI] [PubMed] [Google Scholar]
- 24.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. X. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is 30 years or more. Arch Ophthalmol 1989;107(2):244–9. [DOI] [PubMed] [Google Scholar]
- 25.Jaffe GJ, Burton TC, Kuhn E, Prescott A, Hartz A. Progression of nonproliferative diabetic retinopathy and visual outcome after extracapsular cataract extraction and intraocular lens implantation. Am J Ophthalmol 1992;114(4):448–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


