Abstract
This study aimed to evaluate the impact of weather conditions and air pollution on the onset of sudden sensorineural hearing loss (SSNHL). The Korean Health Insurance Review and Assessment Service - National Sample Cohort (HIRA-NSC) from 2002 through 2013 was used. A total of 5,200 participants with SSNHL were matched 1:4 for age, sex, income, region of residence, hypertension, diabetes, and dyslipidemia with 20,800 control participants. Meteorological data included daily mean temperature (°C), daily highest temperature (°C), daily lowest temperature (°C), daily temperature difference (°C), relative humidity (%), ambient atmospheric pressure (hPa), pressure, SO2 (ppm), NO2 (ppm), O3 (ppm), CO (ppm), and PM10 (μg/m3) of a mean of 60 days, 30 days, 14 days, 7 days, and 3 days before SSNHL were analyzed. Hourly measurements were taken from 94 places to assess the temperature, humidity, and atmospheric pressure and from 273 places to determine SO2, NO2, O3, CO, and PM10. Crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) of meteorological data for SSNHL were analyzed using unconditional logistic regression analyses. Subgroup analyses were conducted by age and sex. The mean NO2 and O3 concentrations 14 days before the index date were different in the SSNHL group compared to those in the control group (P < 0.001 for NO2 and P = 0.021 for O3). The adjusted 14-day OR for NO2 (0.1 ppm) exposure was 3.12 in the SSNHL group compared to that in the control group (95% CI = 2.16–4.49, P < 0.001). The increased odds of NO2 exposure for 14 days in the SSNHL group persisted in the age group older than 30 years for both sexes. Other meteorological conditions did not show differences between the SSNHL and control groups. SSNHL was associated with high concentrations of NO2.
Subject terms: Comorbidities, Risk factors
Introduction
Industrialization has contributed to increasing health and economic burdens from air pollution1. Air pollutants, including particulate matter (PM), nitrogen oxide (NO2), and ozone (O3), impact extrapulmonary and pulmonary systems2. Cardiovascular disorders, such as acute myocardial infarction and stroke, are linked to increased levels of air pollutants3–6. Previous studies found that elevated concentrations of PM10 or NO2 were associated with acute myocardial infarction and ischemic stroke3,6. Many cohort studies have demonstrated that the air pollutants SO2, NO2, and PM10 are associated with elevated cardiovascular mortality5. In addition, the air pollutant NO2 and the oxidative potential of PM2.5 contribute to an increased risk of diabetes7. Because air pollutants are exposed as compounds under consistently changing weather conditions, multiple factors need to be considered to investigate their health effects. When exploring the impact of air pollutants on specific diseases, considering conditions such as temperature is crucial because it determines the concentration of air pollutants. For instance, the concentration of O3 peaks when the temperature is highest4. Therefore, this study included constant evaluations of both weather conditions and multiple air pollutant exposures to identify unbiased effects.
Sudden sensorineural hearing loss (SSNHL) is defined as sensorineural hearing loss with sudden onset8. Approximately 35–68% of SSNHL patients had permanent hearing loss in spite of steroid and other treatments9. Approximately 27 per 100,000 persons suffer from SSNHL in the United States each year. In Korea, the incidence of SSNHL was estimated to be approximately 17.76 per 100,000 persons per year10. The cause of SSNHL is elusive and multifactorial. A viral etiology has been suggested with evidence obtained from clinical cases and from temporal bone pathological findings11.
Because viral infection can be influenced by meteorological conditions, a few previous studies proposed an association between SSNHL and meteorological conditions with conflicting results12,13. A retrospective study of hospital patients reported that, of the different meteorological conditions, the onset of SSNHI was associated only with strong wind speeds for 7 days12. Another retrospective study described no significant relationship between the onset of SSNHL and any meteorological conditions, including temperature and atmospheric pressure13. In addition, several recent studies have identified cardiovascular causes of SSNHL14,15. Because cardiovascular diseases are influenced by air pollution, air pollution might have an impact on SSNHL16. Furthermore, a number of recent studies demonstrated an association between hearing loss and air pollutants from cigarette smoking17,18. Current smokers had 1.15 times higher odds of developing hearing loss than nonsmokers (95% confidence intervals [95% CI = 1.09–1.21])18. However, few studies have investigated the impact of air pollution on SSNHL. When the PubMed and EMBASE databases were searched for studies using the keyword phrase ‘(sudden sensorineural hearing loss) AND (pollution)’, no article was retrieved until September 2018.
The present study hypothesized that meteorological conditions (including air pollution) can influence the onset of SSNHL. To confirm this hypothesis, differences in meteorological conditions were analyzed between the SSNHL and the control group.
Results
Age, sex, income level, region of residence, and past medical histories of hypertension, diabetes, and dyslipidemia were precisely matched between the SSNHL and control groups. We described the mean of meteorological and air pollution measurements for 14 days before the index date. Only NO2 and O3 were significantly different (Table 1, P < 0.001 for NO2 and P = 0.021 for O3).
Table 1.
General Characteristics of Participants.
| Characteristics | Total participants | ||
|---|---|---|---|
| Sudden sensory neural hearing loss | Control group | P-value | |
| Age (years old, n, %) | 1.000 | ||
| 5–9 | 27 (0.5) | 108 (0.5) | |
| 10–14 | 65 (1.3) | 230 (1.3) | |
| 15–19 | 138 (2.7) | 552 (2.7) | |
| 20–24 | 149 (2.9) | 596 (2.9) | |
| 25–29 | 254 (4.9) | 1,016 (4.9) | |
| 30–34 | 304 (5.8) | 1,216 (5.8) | |
| 35–39 | 413 (7.9) | 1,652 (7.9) | |
| 40–44 | 480 (9.2) | 1,920 (9.2) | |
| 45–49 | 529 (10.2) | 2,116 (10.2) | |
| 50–54 | 642 (12.3) | 2,568 (12.3) | |
| 55–59 | 599 (11.5) | 2,396 (11.5) | |
| 30–64 | 511 (9.8) | 2,044 (9.8) | |
| 65–69 | 461 (8.9) | 1,844 (8.9) | |
| 70–74 | 342 (6.6) | 1,368 (6.6) | |
| 75–79 | 187 (3.6) | 748 (3.6) | |
| 80–84 | 69 (1.3) | 276 (1.3) | |
| 85+ | 30 (0.6) | 120 (0.6) | |
| Sex (n, %) | 1.000 | ||
| Male | 2,304 (44.3) | 9,216 (44.3) | |
| Female | 2,896 (55.7) | 11,584 (55.7) | |
| Income (n, %) | 1.000 | ||
| 1 (lowest) | 88 (1.7) | 352 (1.7) | |
| 2 | 346 (6.7) | 1,384 (6.7) | |
| 3 | 310 (6.0) | 1,240 (6.0) | |
| 4 | 337 (6.5) | 1,348 (6.5) | |
| 5 | 325 (6.3) | 1,300 (6.3) | |
| 6 | 437 (8.4) | 1,748 (8.4) | |
| 7 | 459 (8.8) | 1,836 (8.8) | |
| 8 | 542 (10.4) | 2,168 (10.4) | |
| 9 | 620 (11.9) | 2,480 (11.9) | |
| 10 | 835 (16.1) | 3,340 (16.1) | |
| 11 (highest) | 901 (17.3) | 3,304 (17.3) | |
| Region of residence (n, %) | 2,430 (46.7) | 9,720 (46.7) | 1.000 |
| Hypertension (n, %) | 1,930 (37.1) | 7,720 (37.1) | 1.000 |
| Diabetes (n, %) | 1,139 (21.9) | 4,556 (21.9) | 1.000 |
| Dyslipidemia (n, %) | 1,636 (31.5) | 6,544 (31.5) | 1.000 |
| Daily mean temperature for 14 days (°C, mean, SD) | 13.0 (9.7) | 13.1 (9.6) | 0.821 |
| Daily highest temperature for 14 days (°C, mean, SD) | 18.2 (9.5) | 18.2 (9.4) | 0.946 |
| Daily lowest temperature for 14 days (°C, mean, SD) | 8.6 (10.1) | 8.7 (10.1) | 0.804 |
| Daily temperature difference for 14 days (°C, mean, SD) | 9.6 (2.3) | 9.6 (2.3) | 0.417 |
| Relative humidity for 14 days (%, mean, SD) | 65.6 (10.6) | 65.8 (10.6) | 0.467 |
| Ambient atmospheric pressure for 14 days (hPa, mean, SD) | 1006.3 (7.5) | 1006.1 (7.6) | 0.078 |
| SO2 for 14 days (ppb, mean, SD) | 5.5 (1.9) | 5.5 (2.0) | 0.851 |
| NO2 for 14 days (ppb, mean, SD) | 24.9 (8.8) | 24.1 (8.6) | <0.001* |
| O3 for 14 days (ppb, mean, SD) | 23.1 (8.7) | 23.4 (8.7) | 0.021* |
| CO for 14 days (ppm, mean, SD) | 0.566 (0.181) | 0.562 (0.186) | 0.148 |
| PM10 for 14 days (μg/m3, mean, SD) | 52.4 (18.1) | 52.1 (18.1) | 0.209 |
SD: standard deviation.
ppb: Parts per billion.
ppm: Part per million ( = 1,000 ppb).
*Chi-square test or independent t-test, significance at P < 0.05.
The adjusted 14-day OR for NO2 (0.1 ppm) exposure for the SSNHL group was 3.12 (95% CI = 2.16–4.49, P < 0.001, Table 2). The daily mean temperature, daily highest temperature, daily lowest temperature, daily temperature difference, relative humidity, ambient atmospheric pressure, SO2, CO, and PM10 did not reach statistical significance (Table 3). We excluded O3 because it was associated with NO2 (Supplemental Table 1).
Table 2.
Adjusted odds ratios (95% confidence intervals) of NO2 for 14 days (0.1 ppm) for sudden sensory neural hearing loss in total and subgroup analyses according to age and sex.
| Participants | N (participants) | Sudden sensory neural hearing loss | |
|---|---|---|---|
| AOR of NO2 | P-value | ||
| Total | 26,000 | 3.12 (2.16–4.49) | <0.001* |
| Age (<30 years old), men | 1,520 | 2.05 (0.45–9.36) | 0.354 |
| Age (<30 years old), women | 1,645 | 0.83 (0.19–3.61) | 0.803 |
| Age (30–59 years old), men | 6,690 | 3.64 (1.76–7.50) | <0.001* |
| Age (30–59 years old), women | 8,145 | 3.96 (2.07–7.56) | <0.001* |
| Age (≥60 years old), men | 3,310 | 4.06 (1.41–11.61) | 0.009* |
| Age (≥60 years old), women | 4,690 | 2.56 (1.08–6.06) | 0.032* |
*Logistic regression model adjusted model for age, sex, income, region of residence, hypertension, diabetes, and dyslipidemia, significance at P < 0.05.
Table 3.
Crude odds ratios (95% confidence intervals) of the meteorological and pollution matter for sudden sensory neural hearing loss.
| Characteristics | Sudden sensory neural hearing loss | |
|---|---|---|
| Crude OR (95% CI) | P-value | |
| Daily mean temperature for 60 days (°C) | 1.00 (1.00–1.00) | 0.793 |
| Daily mean temperature for 30 days (°C) | 1.00 (1.00–1.00) | 0.816 |
| Daily mean temperature for 14 days (°C) | 1.00 (1.00–1.00) | 0.821 |
| Daily mean temperature for 7 days (°C) | 1.00 (1.00–1.00) | 0.748 |
| Daily mean temperature for 3 days (°C) | 1.00 (1.00–1.00) | 0.770 |
| Daily highest temperature for 60 days (°C) | 1.00 (1.00–1.00) | 0.924 |
| Daily highest temperature for 30 days (°C) | 1.00 (1.00–1.00) | 0.964 |
| Daily highest temperature for 14 days (°C) | 1.00 (1.00–1.00) | 0.946 |
| Daily highest temperature for 7 days (°C) | 1.00 (1.00–1.00) | 0.806 |
| Daily highest temperature for 3 days (°C) | 1.00 (1.00–1.00) | 0.800 |
| Daily lowest temperature for 60 days (°C) | 1.00 (1.00–1.00) | 0.760 |
| Daily lowest temperature for 30 days (°C) | 1.00 (1.00–1.00) | 0.771 |
| Daily lowest temperature for 14 days (°C) | 1.00 (1.00–1.00) | 0.804 |
| Daily lowest temperature for 7 days (°C) | 1.00 (1.00–1.00) | 0.765 |
| Daily lowest temperature for 3 days (°C) | 1.00 (1.00–1.00) | 0.788 |
| Daily temperature difference for 60 days (°C) | 1.01 (0.99–1.02) | 0.284 |
| Daily temperature difference for 30 days (°C) | 1.01 (0.99–1.02) | 0.242 |
| Daily temperature difference for 14 days (°C) | 1.01 (0.99–1.02) | 0.417 |
| Daily temperature difference for 7 days (°C) | 1.00 (0.99–1.01) | 0.783 |
| Daily temperature difference for 3 days (°C) | 1.00 (0.99–1.01) | 0.915 |
| Relative humidity for 60 days (%) | 1.00 (1.00–1.00) | 0.436 |
| Relative humidity for 30 days (%) | 1.00 (1.00–1.00) | 0.385 |
| Relative humidity for 14 days (%) | 1.00 (1.00–1.00) | 0.467 |
| Relative humidity for 7 days (%) | 1.00 (1.00–1.00) | 0.885 |
| Relative humidity for 3 days (%) | 1.00 (1.00–1.00) | 0.950 |
| Ambient atmospheric pressure for 60 days (hPa) | 1.00 (1.00–1.00) | 0.067 |
| Ambient atmospheric pressure for 30 days (hPa) | 1.00 (1.00–1.00) | 0.074 |
| Ambient atmospheric pressure for 14 days (hPa) | 1.00 (1.00–1.01) | 0.078 |
| Ambient atmospheric pressure for 7 days (hPa) | 1.00 (1.00–1.01) | 0.079 |
| Ambient atmospheric pressure for 3 days (hPa) | 1.00 (1.00–1.01) | 0.090 |
| SO2 for 60 days (0.1 ppm) | 0.99 (0.10–5.11) | 0.989 |
| SO2 for 30 days (0.1 ppm) | 1.16 (0.24–5.63) | 0.851 |
| SO2 for 14 days (0.1 ppm) | 1.16 (0.25–5.31) | 0.853 |
| SO2 for 7 days (0.1 ppm) | 1.15 (0.27–4.94) | 0.851 |
| SO2 for 3 days (0.1 ppm) | 1.01 (0.27–3.77) | 0.992 |
| NO2 for 60 days (0.1 ppm) | 2.84 (1.96–4.11) | <0.001* |
| NO2 for 30 days (0.1 ppm) | 2.81 (1.97–4.02) | <0.001* |
| NO2 for 14 days (0.1 ppm) | 2.77 (1.96–3.91) | <0.001* |
| NO2 for 7 days (0.1 ppm) | 2.46 (1.77–3.41) | <0.001* |
| NO2 for 3 days (0.1 ppm) | 2.16 (1.61–2.89) | <0.001* |
| O3 for 60 days (0.1 ppm) | 0.63 (0.43–0.93) | 0.020* |
| O3 for 30 days (0.1 ppm) | 0.64 (0.45–0.93) | 0.018* |
| O3 for 14 days (0.1 ppm) | 0.66 (0.47–0.94) | 0.021* |
| O3 for 7 days (0.1 ppm) | 0.70 (0.50–0.98) | 0.037* |
| O3 for 3 days (0.1 ppm) | 0.75 (0.55–1.02) | 0.070 |
| CO for 60 days (1 ppm) | 1.11 (0.93–1.33) | 0.243 |
| CO for 30 days (1 ppm) | 1.11 (0.94–1.32) | 0.226 |
| CO for 14 days (1 ppm) | 1.13 (0.96–1.33) | 0.148 |
| CO for 7 days (1 ppm) | 1.12 (0.96–1.31) | 0.140 |
| CO for 3 days (1 ppm) | 1.13 (0.98–1.29) | 0.096 |
| PM10 for 60 days (10 μg/m3) | 1.02 (0.99–1.04) | 0.164 |
| PM10 for 30 days (10 μg/m3) | 1.00 (1.00–1.00) | 0.162 |
| PM10 for 14 days (10 μg/m3) | 1.00 (1.00–1.00) | 0.209 |
| PM10 for 7 days (10 μg/m3) | 1.00 (1.00–1.00) | 0.291 |
| PM10 for 3 days (10 μg/m3) | 1.00 (1.00–1.00) | 0.332 |
*Logistic regression model, significance at P < 0.05.
We analyzed the odds ratios of meteorological data for sudden sensory neural hearing loss using simple logistic regression analysis. In these results, only NO2 and O3 showed statistical significance (P < 0.05). Therefore, we chose these NO2 and O3 as the independent variables.
In subgroup analyses, NO2 (0.1 ppm) measured over 14 days increased the risk of SSNHL in 30–59-year-old men (AOR = 3.64, 95% CI = 1.76–7.50, P < 0.001) and women (AOR = 3.96, 95% CI = 2.07–7.56, P < 0.001) and in men 60 years or older (AOR = 4.06, 95% CI = 1.41–11.61, P = 0.009) as well as women (AOR = 2.56, 95% CI = 1.08–6.06, P = 0.032) (Table 2). However, these associations did not reach statistical significance among participants younger than 30 years old for both men and women.
Discussion
In the present study, SSNHL patients demonstrated a higher odds of NO2 exposure than the controls (adjusted OR = 3.12, 95% CI = 2.16–4.49, P < 0.001). Other meteorological factors, including temperature, humidity, and atmospheric pressure, as well as air pollutants of SO2, CO, and PM10, did not show a significant difference between the SSNHL and control groups.
Systemic inflammation and oxidative stress induced by NO2 could increase the risk of SSNHL. Inflammation and oxidative stress are also known to be related to SSNHL19. NO2 has been shown to evoke an inflammatory response and to increase susceptibility to infection even in healthy subjects2. The adverse health effects of NO2 were not limited to the duration and amount of exposure, as concluded in a previous review20. A short-term exposure is defined as being exposed to 50 µg NO2/m3 in less than 24 hours, which is associated with an increased rate of hospital admissions and mortality20. In addition, a low concentration below 40 µg NO2/m3 has also been correlated with adverse health outcomes (respiratory diseases, hospital admissions, mortality, and otitis media)20.
NO2 influences intracochlear nitric oxide (NO) concentration, which leads to an alteration in intracochlear neurotransmission and neuromodulation. NO plays a crucial role as a signaling molecule in gap junctions, blood vessels, and the synaptic region of the cochlea21. Thus, elevated NO concentrations can result in hearing impairment21. Similarly, the modulation of the intracochlear NO concentration might influence the risk of SSNHL.
In this study, the cumulative influences of NO2 on SSNHL can be postulated from the lag effects of the 14-day NO2 concentrations. Although the concentration of NO2 at 60, 30, 14, 7, and 3 days before the onset of SSNHL was related to SSNHL, the concentrations of NO2 14 days before the onset of SSNHL were the smallest values based on the Akaike and Baysian information criteria. A previous study reported that the long-term exposure to low-concentration NO2 was related to adverse health outcomes (respiratory diseases, hospital admissions, mortality, and otitis media)20. Moreover, the latency of viral infections could influence the lag effects of NO2 on SSNHL. A population cohort study reported that the lag effects of NO2 were a risk factor for acute upper respiratory infections22. The cumulative 6-day NO2 concentration increased the risk of acute upper respiratory infection (relative risk = 1.25, 95% CI = 1.21–1.29)22. Because viral infection is one of the risk factors for SSNHL23, the lag effects of NO2 on viral infections might affect the lag effects of NO2 on SSNHL observed in this study.
The effect of NO2 on SSNHL was independent of other air pollutants in this study. However, the effects of NO2 on SSNHL could represent the composite effects of air pollutants on SSNHL because NO2 is an indicator of air pollution from traffic in urban areas. Nonetheless, NO2 has been proposed to be an independent contributor to increased cardiovascular and respiratory mortality24,25. A meta-analysis reported that NO2 increased cardiovascular mortality by 1.13-fold (95% CI = 1.09–1.18) and respiratory mortality by 1.20-fold (95% CI = 1.09–1.31), and the results were consistent after considering the effect of PM24. Moreover, another study demonstrated that the effects of NO2 on acute myocardial infarction were higher than the effects of PM10 or O34. However, other air pollutants (e.g., O3 and PM) were not associated with SSNHL in the present study. Although O3 was related to SSNHL, collinearity with NO2 prevented efforts to elucidate the effect of O3 on SSNHL. The health effects of O3 have been controversial in prior studies. A previous study suggested that O3 induced inflammation and increased the risk of lung diseases26. However, O3 also exhibited protective effects against viral infections through virucidal activity27. PM did not show an association with SSNHL in this study. Because the composition of PM can be different depending on the districts, the impact of PM on SSNHL might be mixed and attenuated in this study. A previous study reported that the oxidative potential of PM but not the PM itself was associated with diabetes7. The effects of PM on mortality outcomes (all-cause, cardiovascular, and respiratory causes) were mitigated after considering NO225. The components of PM might have a greater influence on health than the concentration of PM.
The high odds of NO2 exposure in the SSNHL group were consistent in the subgroup analysis based on age and sex. Only in the group of men and women <30 years old was no association found between SSNHL and NO2. This might be due to the relatively small number of SSNHL participants in these young populations. A small sample size or different regional locations of the study groups and possible confounders that were not considered could all explain the different findings in previous studies. In addition, the effects of air pollutants on health problems might be more pronounced in old populations than in young populations. Prior studies have reported a greater influence of NO2 on acute myocardial infarction in old populations4. The reduced metabolism and diminished secretion abilities in older populations might increase their susceptibility to the adverse effects of air pollutants.
The weather conditions of temperature, humidity, and atmospheric pressure were not related to SSNHL in this study. Associations between SSNHL and weather conditions have been controversial. Some previous studies suggested an association between SSNHL and weather conditions12,28. A hospital retrospective study demonstrated that the maximum wind speed was faster within 5 days of onset of SSNHL compared to the days when SSNHL did not occur12. Another study reported that low atmospheric pressure was related to the onset of SSNHL28. However, both studies were conducted with a small number of study participants in one hospital. On the other hand, similar to the present results, there have been a few articles reporting no association between SSNHL and weather conditions13,29. A population cohort study in Taiwan found no evidence of an association between the onset of SSNHL and meteorological conditions of temperature, humidity, and atmospheric pressure29. Although temperature and humidity were related to the incidence of SSNHL before adjusting for seasonality and months, these meteorological conditions were not associated with the incidence of SSNHL after the adjustment29.
This study is the first to assess the association between air pollution and SSNHL. The nationwide, representative cohort population used in this study strengthens the reliability of the present results. In Korea, all the medical records of citizens are legally registered and managed by NHIS. The national health insurance system is operated based on the NHIS data. Thus, no missing participants were anticipated in the NHIS data. NHIS-NSC data were extracted by statisticians, and the representativeness of the data was verified in a previous study30. In addition, the equivalent control group and the adjustment of confounders also increased the reliability of this study. The demographic factors of age, sex, income, and region of residence and the past medical histories of hypertension, diabetes, and dyslipidemia were matched between the SSNHL and control groups. Because this study based on the health claim codes, the unbiased medical accessibility between study and control group was crucial. The medical accessibility was equalized by matching socioeconomic factors of income and region of residence between study and control group in this study. In addition, the medical conditions of hypertension, diabetes, and dyslipidemia were matched between study and control groups to minimize possible confounder effects. The confounding effects of these factors were not sufficiently attenuated with the adjustment in multivariable analysis in our previous study31. This study used the individual data by adjusting these variables, although previous studies that used Poisson analysis did not consider these individual factors. Moreover, to investigate the lag effects and to choose the most suitable models, air pollution concentrations of various durations were analyzed. The meteorological factors were measured hourly, and the daily mean values were analyzed. The accuracy of the meteorological data was guaranteed by the Korean meteorological administration. Lastly, the objective and multiple inclusion criteria for SSNHL were used in this study.
Several limitations should be considered when interpreting the present results. The degree of hearing loss varied among SSNHL participants in this study because of the lack of data regarding the severity of SSNHL in NHIS. In addition, because the diagnosis of SSNHL was based on the ICD-10 codes, it was possible to include cases of acute low frequency hearing loss, which was suggested to have different pathophysiology and prognosis32. Although several confounders were matched and adjusted for, the lifestyle factors of obesity, smoking, and alcohol consumption were not considered in this study. The interaction among complex mixtures of air pollutants could not be excluded, although multiple air pollutants of NO2, SO2, O3, and PM10 were considered in this study. Because PM2.5 has been measured since 2015 in Korea, the present study could not analyze the effect of PM2.5. As in other epidemiologic studies, the potential for misclassification of meteorological exposure is also possible in this study. Because meteorological exposure is estimated by residence rather than by individual patterns of activity and living circumference, the intersubject variability was feasible33. This study could not access information about indoor exposure to air pollutants. For instance, the indoor NO2 exposure from smoking, gas-fired appliances and stoves may influence the present results. Because the meteorological conditions and air pollution differ according to the region, the interpretation of this study might be limited to Korean districts. More studies in other geographical areas need to be conducted to elucidate the specific aspects of each region.
In conclusion, the mean concentration of NO2 before the onset of SSNHL was high in SSNHL patients. Other meteorological conditions and air pollution did not show an association with SSNHL.
Materials and Methods
Participant selection
The Ethics Committee of Hallym University (2017-I102) approved this study. Written informed consent was waived by the Institutional Review Board. All analyses adhered to the guidelines and regulations of the Ethics Committee of Hallym University. The Korean Health Insurance Review and Assessment Service - National Sample Cohort (HIRA-NSC), meteorological, and air pollution data are described in the supplement (Supplemental File 1).
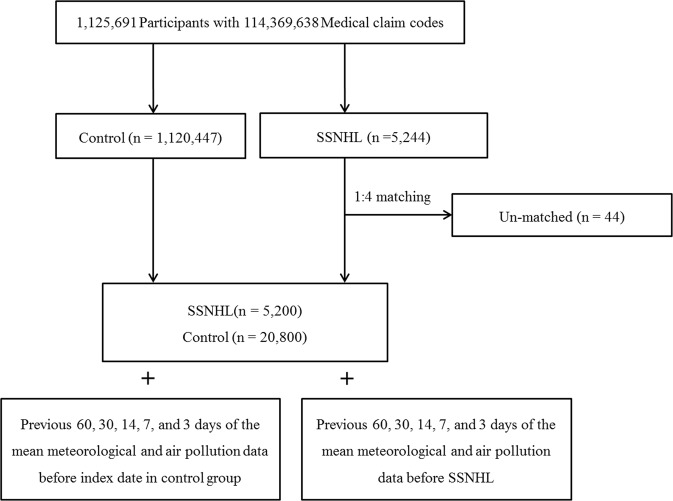
The participants who were diagnosed with SSNHL were selected from 1,125,691 patients with 114,369,638 medical claim codes (n = 5,244). The control group included participants who were never diagnosed with SSNHL from the mother population from 2002 through 2013 (n = 1,120,447). The SSNHL and control groups were matched 1:4 for age, group, sex, income group, region of residence and for past medical histories (hypertension, diabetes, and dyslipidemia). The selection bias was minimized by selecting the control groups using a random number order process. The participants who were deceased before the index date were excluded. The index date was defined as the time when the matched SSNHL participants were included in the study. Forty-four SSNHL participants were excluded because they did not have matched control participants. Conclusively, 5,200 of SSNHL participants were matched 1:4 with 20,800 control participants (Fig. 1).
Figure 1.
A schematic illustration of the participant selection process that was used in the present study. Of a total of 1,125,691 participants, 5,200 SSNHL participants were matched with 20,800 control participants for age, group, sex, income group, region of residence, and past medical histories. Then, SSNHL and control participants were matched with the same meteorological data before the index date.
We analyzed meteorological data over a mean of 60 days, 30 days, 14 days, 7 days, and 3 days before SSNHL (index date). In the matched control group who did not experience SSNHL, we used the same matched date of SSNHL.
Variables
Independent variable
Daily mean temperature (°C), daily highest temperature (°C), daily lowest temperature (°C), daily temperature difference (°C), relative humidity (%), ambient atmospheric pressure (hPa), SO2 (ppm), NO2 (ppm), O3 (ppm), CO (ppm), and PM10 (μg/m3) for 14 days, 10 days, 7 days, 5 days, and 3 days before the index date were defined as the independent variables (Table 3).
Covariate analysis
Age groups were divided into 5-year intervals: 0–4, 5–9, 10–14…, and 85+ years old. A total of 18 age groups were specified. Income groups were classified as 11 classes (class 1 [lowest income]−11 [highest income]). The region of residence was grouped into urban (Seoul, Busan, Daegu, Incheon, Gwangju, Daejeon, and Ulsan) and rural (Gyeonggi, Gangwon, Chungcheongbuk, Chungcheongnam, Jeollabuk, Jeollanam, Gyeongsangbuk, Gyeongsangnam, and Jeju) areas.
The past medical histories were collected using ICD-10 codes. Only the participants who were treated ≥2 times for hypertension (I10 and I15), diabetes (E10-E49), and dyslipidemia (E78) were included to improve the reliability of the diagnoses.
Dependent variable
Sudden sensory neural hearing loss (SSNHL) was selected based on ICD-10 codes (H912). We only included the participants who underwent audiometry testing (claim code: E6931-E6937, F6341-F6348) and who used steroid for treatment.
Statistical analyses
The general characteristics between the SSNHL and control groups were compared using Chi-squared tests. The mean meteorological data from 14 days before the index date were compared using independent t-tests.
To analyze the odds ratio (OR) of meteorological data for SSNHL compared to controls, crude (simple) and adjusted (multiple) logistic regression was used and 95% confidence intervals (CIs) were calculated. The selection of independent variables and the method used to construct the final model are presented in Table 3, Supplemental Tables 1, and 2.
We calculated the single pollutant model for NO2, which was analyzed as the independent variable; age, sex, income, region, hypertension, diabetes, and dyslipidemia were analyzed as covariates; and SSNHL was analyzed as the dependent variable.
For the subgroup analysis, we divided participants by age and sex (young [0–29 years old], middle-aged [30–59 years old], elderly [60+ years old]; men and women). In this analysis, we used a single, combined final model.
Two-tailed analyses were performed, and significance was defined as P values less than 0.05. The SPSS version 22.0 (IBM, Armonk, NY, USA) and SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) were used for the statistical analyses.
Supplementary information
Acknowledgements
This work was supported in part by a research grant (NRF-2018-R1D1A1A02085328 and NRF-2017R1C1B1007696) from the National Research Foundation (NRF) of Korea.
Author Contributions
H.G.C. designed the study, participated in data collection and data interpretation, and revised the manuscript. S.Y.K. and H.G.C. participated in the interpretation of the data and drafted and revised the manuscript. C.M. participated in data analysis, interpretation of data, and revised the manuscript. All authors approved the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-44618-0.
References
- 1.Bai R, Lam JCK, Li VOK. A review on health cost accounting of air pollution in China. Environ Int. 2018;120:279–294. doi: 10.1016/j.envint.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Hesterberg TW, et al. Critical review of the human data on short-term nitrogen dioxide (NO2) exposures: evidence for NO2 no-effect levels. Crit Rev Toxicol. 2009;39:743–781. doi: 10.3109/10408440903294945. [DOI] [PubMed] [Google Scholar]
- 3.Liu H, et al. Air Pollution and Hospitalization for Acute Myocardial Infarction in China. Am J Cardiol. 2017;120:753–758. doi: 10.1016/j.amjcard.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Mustafic H, et al. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA. 2012;307:713–721. doi: 10.1001/jama.2012.126. [DOI] [PubMed] [Google Scholar]
- 5.Dehbi HM, et al. Air pollution and cardiovascular mortality with over 25years follow-up: A combined analysis of two British cohorts. Environ Int. 2017;99:275–281. doi: 10.1016/j.envint.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen ZJ, et al. Stroke and long-term exposure to outdoor air pollution from nitrogen dioxide: a cohort study. Stroke. 2012;43:320–325. doi: 10.1161/STROKEAHA.111.629246. [DOI] [PubMed] [Google Scholar]
- 7.Strak M, et al. Long-term exposure to particulate matter, NO2 and the oxidative potential of particulates and diabetes prevalence in a large national health survey. Environ Int. 2017;108:228–236. doi: 10.1016/j.envint.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Alexander TH, Harris JP. Incidence of sudden sensorineural hearing loss. Otol Neurotol. 2013;34:1586–1589. doi: 10.1097/MAO.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 9.Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss: II. A Meta-analysis. Arch Otolaryngol Head Neck Surg. 2007;133:582–586. doi: 10.1001/archotol.133.6.582. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, et al. A Trend in Sudden Sensorineural Hearing Loss: Data from a Population-Based Study. Audiol Neurootol. 2017;22:311–316. doi: 10.1159/000485313. [DOI] [PubMed] [Google Scholar]
- 11.Linthicum FH, Jr., Doherty J, Berliner KI. Idiopathic sudden sensorineural hearing loss: vascular or viral? Otolaryngol Head Neck Surg. 2013;149:914–917. doi: 10.1177/0194599813506546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seo JH, et al. Meteorological conditions related to the onset of idiopathic sudden sensorineural hearing loss. Yonsei Med J. 2014;55:1678–1682. doi: 10.3349/ymj.2014.55.6.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danielides V, et al. Weather conditions and sudden sensorineural hearing loss. BMC Ear Nose Throat Disord. 2002;2:2. doi: 10.1186/1472-6815-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JY, Hong JY, Kim DK. Association of Sudden Sensorineural Hearing Loss With Risk of Cardiocerebrovascular Disease: A Study Using Data From the Korea National Health Insurance Service. JAMA Otolaryngol Head Neck Surg. 2018;144:129–135. doi: 10.1001/jamaoto.2017.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballesteros F, et al. Is there an overlap between sudden neurosensorial hearing loss and cardiovascular risk factors? Audiol Neurootol. 2009;14:139–145. doi: 10.1159/000171475. [DOI] [PubMed] [Google Scholar]
- 16.Collart P, Dramaix M, Leveque A, Coppieters Y. Short-term effects of air pollution on hospitalization for acute myocardial infarction: age effect on lag pattern. Int J Environ Health Res. 2017;27:68–81. doi: 10.1080/09603123.2016.1268678. [DOI] [PubMed] [Google Scholar]
- 17.Lin YY, et al. Secondhand Smoke is Associated with Hearing Threshold Shifts in Obese Adults. Sci Rep. 2016;6:33071. doi: 10.1038/srep33071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawes P, et al. Cigarette smoking, passive smoking, alcohol consumption, and hearing loss. J Assoc Res Otolaryngol. 2014;15:663–674. doi: 10.1007/s10162-014-0461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masuda M, et al. Correlations of inflammatory biomarkers with the onset and prognosis of idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2012;33:1142–1150. doi: 10.1097/MAO.0b013e3182635417. [DOI] [PubMed] [Google Scholar]
- 20.Latza U, Gerdes S, Baur X. Effects of nitrogen dioxide on human health: systematic review of experimental and epidemiological studies conducted between 2002 and 2006. Int J Hyg Environ Health. 2009;212:271–287. doi: 10.1016/j.ijheh.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Heinrich UR, Helling K. Nitric oxide–a versatile key player in cochlear function and hearing disorders. Nitric Oxide. 2012;27:106–116. doi: 10.1016/j.niox.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Lin YK, et al. Temperature, nitrogen dioxide, circulating respiratory viruses and acute upper respiratory infections among children in Taipei, Taiwan: a population-based study. Environ Res. 2013;120:109–118. doi: 10.1016/j.envres.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia Berrocal JRG, Ramirez-Camacho R, Portero F, Vargas JA. Role of viral and Mycoplasma pneumoniae infection in idiopathic sudden sensorineural hearing loss. Acta Otolaryngol. 2000;120:835–839. doi: 10.1080/000164800750061688. [DOI] [PubMed] [Google Scholar]
- 24.Faustini A, Rapp R, Forastiere F. Nitrogen dioxide and mortality: review and meta-analysis of long-term studies. Eur Respir J. 2014;44:744–753. doi: 10.1183/09031936.00114713. [DOI] [PubMed] [Google Scholar]
- 25.Mills IC, Atkinson RW, Anderson HR, Maynard RL, Strachan DP. Distinguishing the associations between daily mortality and hospital admissions and nitrogen dioxide from those of particulate matter: a systematic review and meta-analysis. BMJ Open. 2016;6:e010751. doi: 10.1136/bmjopen-2015-010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciencewicki Jonathan, Trivedi Shweta, Kleeberger Steven R. Oxidants and the pathogenesis of lung diseases. Journal of Allergy and Clinical Immunology. 2008;122(3):456–468. doi: 10.1016/j.jaci.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciencewicki J, Jaspers I. Air pollution and respiratory viral infection. Inhal Toxicol. 2007;19:1135–1146. doi: 10.1080/08958370701665434. [DOI] [PubMed] [Google Scholar]
- 28.Herbert I, Nolte E, Eichhorn T. [Weather status and incidence of idiopathic facial nerve paralyses, vestibular disorders, Meniere’s attacks and sudden deafness] Laryngol Rhinol Otol. 1987;66:249–250. doi: 10.1055/s-2007-998647. [DOI] [PubMed] [Google Scholar]
- 29.Lin HC, Lee HC, Chao PZ, Wu CS. The effects of weather on the incidence of sudden sensorineural hearing loss: a 5-year population-based study. Audiol Neurootol. 2006;11:165–171. doi: 10.1159/000091268. [DOI] [PubMed] [Google Scholar]
- 30.Lee, J., Lee, J. S., Park, S. H., Shin, S. A. & Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol, 10.1093/ije/dyv319 (2016). [DOI] [PubMed]
- 31.Kim SY, Sim S, Kim HJ, Choi HG. Sudden sensory neural hearing loss is not predictive of myocardial infarction: A longitudinal follow-up study using a national sample cohort. Sci Rep. 2018;8:946. doi: 10.1038/s41598-018-19404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choo OS, et al. Differences in clinical characteristics and prognosis of sudden low- and high-frequency hearing loss. Laryngoscope. 2017;127:1878–1884. doi: 10.1002/lary.26382. [DOI] [PubMed] [Google Scholar]
- 33.Brown KW, Sarnat JA, Suh HH, Coull BA, Koutrakis P. Factors influencing relationships between personal and ambient concentrations of gaseous and particulate pollutants. Sci Total Environ. 2009;407:3754–3765. doi: 10.1016/j.scitotenv.2009.02.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.