Fritz and Lenardo discuss the basic science and clinical discoveries of immune checkpoint blockade, which boosts antitumor immunity and increases survival of patients with cancer.
Abstract
Since the early 20th century, immunologists have investigated mechanisms that protect vertebrates from damaging immune responses against self-antigens by mature lymphocytes, i.e., peripheral tolerance. These mechanisms have been increasingly delineated at the molecular level, ultimately culminating in new therapeutics that have revolutionized clinical oncology. Here, we describe basic science and clinical discoveries that converge mainly on two molecules, CTLA-4 and PD-1, that were recognized with the 2018 Nobel Prize in Physiology or Medicine awarded to James Allison and Tasuku Honjo. We discuss their investigations and those of many others in the field that contravene tolerance through checkpoint inhibition to boost immune killing of malignant cells. We also discuss the mechanisms underlying each therapy, the efficacy achieved, and the complications of therapy. Finally, we hint at research questions for the future that could widen the success of cancer immunotherapy.
Introduction
T lymphocytes as cytotoxic agents against cancer cells
One of the infrequently heralded breakthroughs of the 1960s was the observation that cells of the adaptive immune system could be divided into two broad functional classes: B and T lymphocytes or, simply, B and T cells (Miller, 1961; Cooper et al., 1966). We will focus on T cells, which are important for immunotherapy because they secrete cytokines and generate cytotoxic reactions against other cells that are infected with viruses or are cancerous (Miller and Mitchell, 1967; Masopust et al., 2007). The body has a large repertoire of T cells, each with a unique TCR that recognizes antigen as short peptides bound to MHC proteins on the surface of APCs. These antigen/MHC complexes, especially when unique to tumor cells, are the key signal for T cells to attack. By either enhancing the initial recognition and immune response to cancer antigens or thwarting peripheral tolerance “checkpoints,” or both, cancer immunotherapies generate and sustain tumoricidal immunity.
Peripheral tolerance of T cells
Tolerance is the ability of T cells to generally ignore antigens endogenous or harmless to the host and mount strong reactions only to foreign and pathogenic antigens. Failure of tolerance can cause a range of autoimmune diseases and great human suffering, although most people go through life without much obvious or permanent damage from T cell immunity. Mechanisms have evolved in T cells to ensure specific and controlled responses that involve tolerance (limited responsiveness) to self. One mechanism to prevent autoimmune responses is to eliminate autoreactive T cells during development, i.e., central tolerance. Other mechanisms restrain, neutralize, or eliminate mature T cells in the periphery when they engage antigens, i.e., peripheral tolerance (Miller and Morahan, 1992; Lenardo et al., 1999). To begin, MHC-presented peptides will generally activate naive T cells only if “costimulatory” signals are received through CD28 or allied molecules. The ligands for CD28, B7-1 (CD80), and B7-2 (CD86) are restricted to specific “professional” APCs and are induced by pathogen-specific signals operating through TLRs and other sensors for molecules from dangerous microbes. Hence, the incoming signal is evaluated for a likely correspondence to pathogens, and a go/no-go decision is made. This is a true “checkpoint” for T cell reactivity. In fact, strong stimulation through the TCR without costimulation paralyzes T cells in a nonresponsive state called “anergy.” Anergy may contribute to peripheral tolerance to antigens seen again and again, a key feature of “self” antigens.
To promote a therapeutic anticancer response, CD8+ T cells that are strongly activated by tumor antigens must be unrestrained by negative regulators. A fundamental problem in biological systems is that a priori information is often lacking about how much stimulus will be encountered in order to gauge an appropriately measured reaction. Given that the immune system is confronted daily with rapidly growing microorganisms, it is a constant challenge to ensure an effective pathogen response while limiting overkill that damages host tissues. Evolution has countered with cybernetic or feedback control systems in which the initial stimulus triggers negative regulators that dampen the response (Lenardo et al., 1999). As described below, these negative regulators are proportionately engaged by the strength of stimulation, and have been called “checkpoints” since they detect, resist, and reverse overactivation. By creating negative feedback, immune checkpoints vouchsafe more uniform and controlled immune reactions to prevent collateral damage.
Immune checkpoint therapy
Cytotoxic T lymphocyte–associated protein 4 (CTLA-4) biology
CTLA-4 is a member of the CD28 family of receptors that is induced on the cell surface on conventional T cells by antigen activation and constitutively expressed on regulatory T (T reg) cells, a specialized subset of CD4+ T cells that can arrest T cell responses (Sansom, 2000). It negatively regulates costimulatory signaling and powerfully enforces peripheral tolerance. CD28 and CTLA-4 compete for binding to B7-1 and B7-2 on APCs, including B lymphocytes, dendritic cells, and other immune cells. As the cousin of CD28, which provides the critical cosignal required for TCR-mediated proliferation, survival, and cytokine production, CTLA-4 has evolved to counterbalance these costimulatory signals since it can bind B7-1/B7-2 more tightly, but delivers negative rather than costimulatory signals to the T cell (Fig. 1; Walker and Sansom, 2011). CTLA-4 is part of a built-in tolerance algorithm involving its induction with a delay, but in proportion to T cell activation. This allows it to suppress apparently spontaneous T cell reactions against self-antigens and diminish the intensity of responses to foreign antigens. Genetic CTLA-4 deficiency in mice and humans wreaks immune havoc with severe lymphoproliferation and clonal senescence, lymphocytic infiltration into the lungs, brain, and gastrointestinal tract, autoimmunity, hypogammaglobulinemia, respiratory tract infections, and enteropathy. Loss of costimulation control is the culprit, since immunopathology can be circumvented in CTLA-4–deficient mice by either blocking B7 ligand interactions with a soluble CTLA-4–immunoglobulin fusion protein or by crossing CTLA-4–deficient mice to B7-deficient mice (Tivol et al., 1997; Mandelbrot et al., 1999). Not surprisingly, therapy with anti–CTLA-4 causes colitis as a major side effect (Tivol et al., 1995; Waterhouse et al., 1995; Kuehn et al., 2014; Schubert et al., 2014). By contrast, clinical improvement has been observed when abatacept, a Food and Drug Administration (FDA)–approved CTLA-4–immunoglobulin fusion protein drug, is administered to humans suffering from CTLA-4–haploinsufficiency with autoimmunity and infiltration disease, a genetic deficiency of CTLA-4 (Lee et al., 2016). The effectiveness of soluble CTLA-4 is because it competes for CD28 ligands on the surface of APCs. These soluble forms, like CTLA-4 itself, associate with B7-1 and B7-2 with higher affinity and avidity than CD28 (van der Merwe et al., 1997; Collins et al., 2002). Imaging analyses pinpoint CTLA-4 accumulation in the immunological synapse, where it interferes with CD28 recruitment and downstream signaling (Yokosuka et al., 2010). Other studies show CTLA-4 consorting with the TCR in lipid rafts to form lattice-like structures that inhibit CD28 signaling (Darlington et al., 2002).
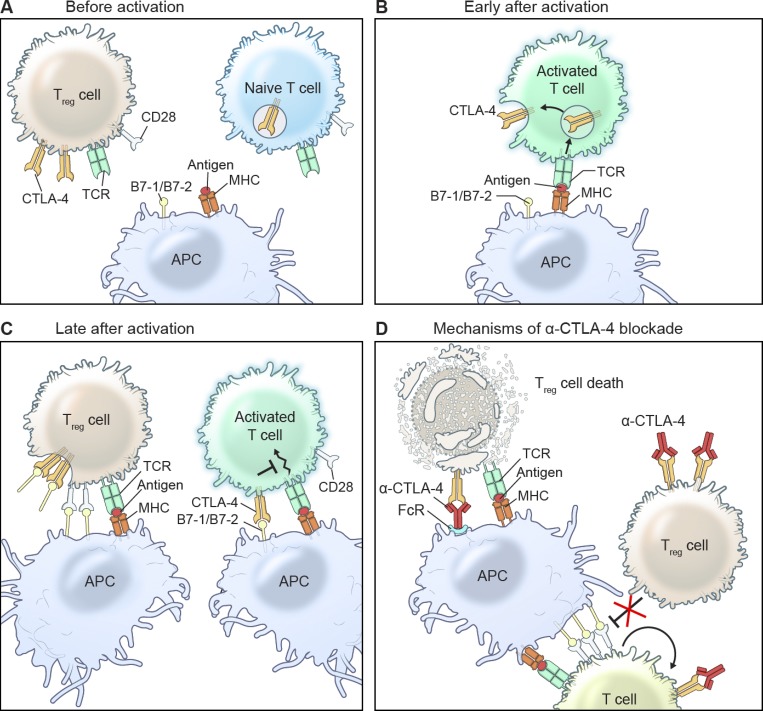
Figure 1.
Mechanisms of CTLA-4 function and inhibition. (A–C) CTLA-4 is up-regulated on activated T cells and inhibits further T cell activation by competing with CD28 for B7 ligation. T reg cells, which constitutively express CTLA-4, trans-endocytose B7 ligands on APCs to prevent CD28 costimulation. (D) Blocking CTLA-4 results in enhanced CD28-B7 ligation, which unleashes T cell expansion and effector function. By inhibiting CTLA-4 on T reg cells, B7 trans-endocytosis is suppressed, resulting in increased APC potency. CTLA-4 blockade is also thought to cause T reg cell depletion through ADCC by cells expressing Fcγ receptors.
In addition to encumbering CD28 signaling, CTLA-4 engagement induces negative signals (Krummel and Allison, 1996; Walunas et al., 1996). The cytoplasmic domain of CTLA-4 attracts several members of the PP2A family of serine/threonine phosphatases that disengage downstream TCR signaling (Lee et al., 1998; Chuang et al., 2000). CTLA-4 can also run interference by interacting directly with the TCR and prevent its tyrosine phosphorylation following stimulation (Lee et al., 1998). Finally, CTLA-4 may strike at the heart of the proliferation machinery by decreasing ERK activity and increasing JNK in T cells (Calvo et al., 1997; Schneider et al., 2002). Hence, CTLA-4 counteracts several internal signaling nodes to impede activation and proliferation of T cells.
Several studies suggest that additional functions of CTLA-4 may be T cell–extrinsic (Walker and Sansom, 2011). Hints initially came from the surprising observation that RAG2-deficient mice transplanted with a mixture of normal and CTLA-4–deficient bone marrow do not develop lymphoproliferative disease (Bachmann et al., 1999). Something from the normal marrow apparently suppressed the unrestrained immune reactions typically caused by CTLA-4–deficient bone marrow. Later studies showed that T reg cells suppress dendritic cell function by decreasing B7-1 and B7-2 expression (Misra et al., 2004; Oderup et al., 2006; Wing et al., 2008; Qureshi et al., 2011). This led to the fascinating discovery that CTLA-4 could pluck B7-1 and B7-2 from the surface of APCs and send them to lysosomes within the T reg cells, a process called trans-endocytosis (Qureshi et al., 2011; Kong et al., 2014). This leaves the APCs without costimulatory ability.
CTLA-4 deficiency in mice and humans debilitates the tolerance mechanisms described above, thereby fooling T cells into activation and migration into organs as though they were chasing an infection (Wing et al., 2008). All of the cell-intrinsic and -extrinsic tolerance mechanisms required CTLA-4 to evolve a multifaceted toolbox to restrain T cells following antigen engagement. However, the fact that CTLA-4 operates at the cell surface suggested a simple strategy for boosting T cell immunity by using a CTLA-4 inhibitory antibody. This inspired a new mode of cancer treatment that could work in the absence of any knowledge of tumor antigens (Leach et al., 1996).
Development of CTLA-4 blockade for cancer therapy
Allison and colleagues first demonstrated that administering CTLA-4 blocking antibodies in mice prevented tumor establishment and induced the rejection of preestablished tumors. They also demonstrated immunological memory; mice continued to be able to reject tumor cells if they had once rejected the tumor with anti–CTLA-4 therapy. Moreover, rejection depended on immunogenicity of the tumor (Leach et al., 1996; Kwon et al., 1997). The preclinical success in experimental animals led to the development and testing of fully humanized monoclonal antibodies against CTLA-4 that prevent its interaction with B7 ligands (tremelimumab and ipilimumab) in humans with advanced cancer (Hoos et al., 2010). Early clinical trials of CTLA-4 blockade showed disease regression in patients with various tumor types (Hodi et al., 2003; Phan et al., 2003; Ribas et al., 2005; Eroglu et al., 2015). These studies also showed that there was a price to be paid for breaching tolerance, e.g., severe autoimmune reactions that led to early termination of certain studies (Phan et al., 2003). A large phase III trial demonstrated that ipilimumab increased the median overall survival of metastatic melanoma patients to 10.0 mo compared with 6.4 mo in patients treated with the peptide vaccine gp100 (Hodi et al., 2010). Notably, later follow-up studies revealed that ∼20% of patients treated with ipilimumab survived for ≥3 yr (Schadendorf et al., 2015). Ipilimumab also compared favorably to standard chemotherapy (Robert et al., 2011). The FDA approved ipilimumab for the treatment of metastatic melanoma in 2011. Given the success of ipilimumab, it is puzzling and frustrating that tremelimumab has not had the same efficacy. In a phase III trial, tremelimumab was no better in advanced melanoma patients than standard chemotherapy (Ribas et al., 2013). Clinical trials testing tremelimumab in other cancer types (mesothelioma and non–small cell lung cancer) were also unsuccessful. The discrepancy between ipilimumab and tremelimumab may be due to mechanistic or structural differences in the antibody molecules (Furness et al., 2014; He et al., 2017). To date, tremelimumab monotherapy has not obtained FDA approval, although combinatorial therapies with tremelimumab are being evaluated. Overall, inhibiting CTLA-4 with ipilimumab has caused a paradigm shift in cancer therapy by successfully mobilizing the immune response against tumors. As an orthogonal approach, checkpoint immunotherapy has been effective in tumors like melanoma that responded poorly to conventional chemotherapy. Although this form of immunotherapy has been a groundbreaking achievement, it is not a panacea. Not all patients or cancer types respond, underscoring the need for a greater basic understanding of antitumor immunity.
Mechanisms of CTLA-4 blockade in tumor regression
The simplest explanation behind anti–CTLA-4 immunotherapy is that T cells exist that recognize tumor antigens, including neoantigens resulting from somatic gene mutations in the tumor cell genomes. Possibly because these antigens are too close to self-antigens or they are presented by poorly costimulatory tumor cells, or other reasons, cytotoxic T cells need a kick-start from CTLA-4 blockade. There are many variations of this theme, but the key idea is that taking the brakes off T cell activation causes greater tumoricidal action. Ipilimumab has been shown to mask the epitope that binds to B7 ligands, suggesting that CTLA-4 blockade inhibits the crucial interaction that normally suppresses T cell responses (Ramagopal et al., 2017). Preventing CTLA-4–B7 interactions not only fortifies effector T cells but may also modulate the suppressive function and/or presence of T reg cells (Fig. 1).
One pertinent question is how this relates to the classical idea of tumor immunosurveillance. In other words, is the eradication of malignant cells a primary capability that the immune system has evolved to do, or are we artificially borrowing the cytolytic power that protects against infections? One observation to consider is that neither primary nor acquired immunodeficiency leads to an outbreak of solid tumors. For example, the AIDS epidemic, which has affected millions of people worldwide, is not associated with an excess of common epithelial cancers (Grulich et al., 2007). In fact, malignancies that usually arise can be attributed to oncogenic viruses that are uncontrolled. On the other hand, breaching peripheral T cell tolerance by anti–CTLA-4 therapy causes a whole range of intense immune reactions that injure host and cancer cells alike. Nonetheless, a firm answer to this question will require additional molecular investigation of immune responses during checkpoint therapy.
Enhanced T effector function
CTLA-4 blockade unleashes potent antitumor T cell responses, but the specificity and phenotype of these T cells under different treatment settings are not fully understood. Recent studies have indicated that immunotherapy can bolster specific T cell responses to neoantigens, peptides unique to the cancer genome arising from spontaneous or carcinogen-derived mutations in DNA (van Rooij et al., 2013). McGranahan et al. (2016) demonstrated that melanoma and non–small cell lung cancer patients with the highest burden of clonal neoantigens had the most durable responses to checkpoint blockade. Other studies have also reported a correlation between mutational load and response to immunotherapy (Snyder et al., 2014; Rizvi et al., 2015; Van Allen et al., 2015). The implication of these studies, albeit indirect, is that a higher burden of mutations translates into more tumor-specific neoantigens, which can then be targeted by T cells. Obviously, there are a lot of links in this hypothetical mechanistic chain. Neoantigens require greater investigation through genomic and biochemical approaches. Personalized vaccines against neoantigens are becoming a viable therapeutic option and were the subject of recent review (Sahin and Türeci, 2018). This strategy could be conjoined with checkpoint inhibition to further boost antitumor immune responses and improve patient outcome.
The phenotypic profile of T cells that infiltrate tumors during checkpoint blockade has been partly characterized using mass spectrometry–driven methods and shows differential expression of markers involved in activation, development, and exhaustion (Fehlings et al., 2017; Wei et al., 2017). For example, neoantigen-specific CD8+ T cells in tumor-bearing mice that received either anti–CTLA-4 or anti–programmed cell death protein-1 (PD-1); PD-1 therapy had reduced expression of markers associated with T cell dysfunction (PD-1 and glucocorticoid-induced TNFR-related protein) and increased markers of T cell activation and development (stem cell antigen-1; Fehlings et al., 2017). Tumor immune infiltrates in human melanoma contain CD8+ T cells with an exhausted phenotype expressing PD-1 and T cell immunoglobulin mucin 3 (TIM3), and, curiously, a subset of effector CD4+ T cells that express PD-1 and inducible T cell costimulator (ICOS), markers that typically define T follicular helper cells (Wei et al., 2017). Also, the phenotypic profile of intratumoral T cells in human melanoma patients differed depending on the type of checkpoint blockade. Both anti–CTLA-4 and anti–PD-1 therapy induced the expansion of CD8+ T cells expressing PD-1 and TIM3, while anti–CTLA-4 therapy caused a selective increase in CD4+, ICOS+ effectors. These T helper cell 1–like CD4+ cells have been reported in multiple tumor types following ipilimumab treatment (Liakou et al., 2008; Chen et al., 2009; Carthon et al., 2010; Chaput et al., 2017), and may enhance CD8+ T cell cytotoxicity and memory development. Thus, CTLA-4 blockade increases effector capabilities, particularly cytotoxicity, of antigen-specific CD8+ T cells, and alters T cell differentiation.
Reduced frequency and function of T reg cells
Blockade of CTLA-4 on T reg cells may also give free rein to antitumor T cell responses by reducing both T reg cell frequency and suppressive function (Peggs et al., 2009). Studies in mice and humans suggest that anti–CTLA-4 therapy depletes T reg cells at the tumor site, although the precise mechanisms are unclear (Liakou et al., 2008; Selby et al., 2013; Simpson et al., 2013; Tarhini et al., 2014; Romano et al., 2015). By contrast, T reg cells in secondary lymphoid tissues are increased (Maker et al., 2005; Quezada et al., 2006; O’Mahony et al., 2007; Kavanagh et al., 2008). These differences could be explained by higher expression of CTLA-4 by intratumoral T reg cells or increased effector cells in the tumor that mediate T reg cell death, but we don’t yet have a compelling explanation for this paradox. T reg cell depletion and antitumor activity are dependent on Fcγ receptors and not C3 expression (Selby et al., 2013; Simpson et al., 2013), suggesting antibody-dependent cell-mediated cytotoxicity (ADCC) is involved rather than complement-mediated lysis. IgG1, the isotype of ipilimumab, has high affinity for FcγRIIIa (CD16a), the Fc receptor mediating ADCC (Bruhns et al., 2009). Interestingly, cancer patients with a high-affinity polymorphism in this receptor had greater responsiveness to ipilimumab therapy (Arce Vargas et al., 2018). This lends more weight to the idea that ipilimumab works partly by ADCC. Next-generation ipilimumab antibodies that have increased ADCC activity are currently being tested in preclinical models (Korman, A.J., J. Engelhardt, J. Loffredo, J. Valle, R. Akter, R. Vuyyuru, N. Bezman, P. So, R. Graziano, K. Tipton, et al. 2017. AACR Annual Meeting. Abstr. SY09-01).
T reg cells constitutively express high surface CTLA-4, making them potent antagonists of T cell activation (Read et al., 2000; Wing et al., 2008; Qureshi et al., 2011; Hou et al., 2015). Thus, inhibiting CTLA-4 on T reg cells wipes out one of the strongest tolerance enforcers. This is dramatically illustrated in mice engineered to have CTLA-4–deficient T reg cells. Depleting CTLA-4 on that compartment alone causes intense lymphoproliferation and fatal autoimmunity, while heightening antitumor immunity (Wing et al., 2008). Anti–CTLA-4 antibody treatment in mice also neutralizes T reg cells (Read et al., 2000; Takahashi et al., 2000). T reg cells are elevated in many human cancers, and this loosely correlates with a worse prognosis (Facciabene et al., 2012). Suppression of T reg cells has been attempted with both anti-CD25, to starve them of their principal growth factor IL-2, or anti–CTLA-4 (Facciabene et al., 2012). Maker et al. (2005) reported that CD25+ T reg cells isolated from patients treated with anti–CTLA-4 antibody retained suppressive function in vitro; thus, if T reg cells survive treatment, at least some of their functions remain intact.
PD-1/PD-L1 biology
PD-1 was first discovered by Honjo and colleagues as a member of the immunoglobulin gene superfamily thought to be involved in programmed cell death. It is a transmembrane protein expressed by select thymocyte subsets and T and B lymphocytes, especially after antigen receptor stimulation (Okazaki et al., 2002). However, detailed studies showed that its biological function was to enfeeble T cell function not by death but by old age. When engaged, it mediates a complex epigenetic and transcriptional transformation in T cells, creating a new hyporesponsive phenotype called “exhaustion” that resembles senescence (Ishida et al., 1992; Agata et al., 1996; Okazaki and Honjo, 2007; Pauken and Wherry, 2015). PD-1 is triggered by the B7 homologues, PD-L1 (B7-H1) and PD-L2 (B7-H2), that constitutively reside on nonlymphoid tissues and can be up-regulated in immune cells by proinflammatory cytokines such as IFN-γ and TNF (Fig. 2; Dong et al., 1999; Freeman et al., 2000; Latchman et al., 2001; Tseng et al., 2001). The critical role for PD-1 in peripheral tolerance was shown in knockout mice that developed lupus-like arthritis and glomerulonephritis (C57BL/6) or autoantibody-mediated dilated cardiomyopathy and thrombosis (BALB/c) depending on the strain (Nishimura et al., 1999, 2001). Tolerance induction by PD-1 is a lymphocyte-intrinsic effect involving phosphorylation of the immunoreceptor tyrosine-based switch motif in its cytoplasmic tail and subsequent recruitment of Src homology 2 (SH2) domain–containing tyrosine phosphatase 2 (SHP-2) in T and B cells (Okazaki et al., 2001; Sheppard et al., 2004). However, in contrast to PD-1–deficient mice (Juneja et al., 2017), T cells deficient in SHP-2 did not have enhanced antitumor immunity, suggesting other pathways contributed to PD-1 suppression (Rota et al., 2018). Indeed, PD-1 can inhibit phosphorylation of TCR/B cell receptor proximal (CD3ζ, Zap70, Igβ, and Syk) and distal (PKCθ, PLCγ, and ERK1/2) signaling molecules, which prevents calcium mobilization and reprograms T cell metabolism from glycolysis toward fatty acid β-oxidation (Okazaki et al., 2001; Zaugg et al., 2011; van der Windt et al., 2012). Importantly, PD-L1 and PD-L2 are also expressed on myeloid cells present in the tumor microenvironment, and can be up-regulated on malignant cells themselves (Kuang et al., 2009; Zhang et al., 2009). This type of immunoediting sets up a protective barrier to antitumor immunity by exhausting PD-1 antitumor T cells that approach (Wherry and Kurachi, 2015). Thus, breaking this mechanism of tumor escape was proposed as a promising strategy for cancer immunotherapy, and paved the way for further examination of inhibiting PD-1/PD-L1 to unleash antitumor immunity (Iwai et al., 2002).
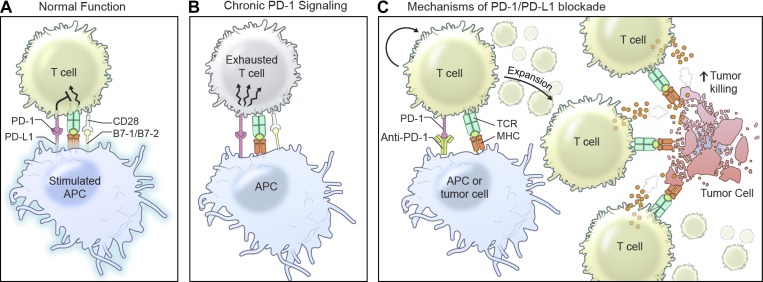
Figure 2.
Mechanisms of PD-1 function and inhibition. (A and B) PD-1 is expressed by activated T cells and binds to PD-L1 and PD-L2 on APCs or other nonimmune cells to inhibit T cell signaling through a poorly defined mechanism. Chronic T cell signaling results in exhaustion and an inability to properly function. (C) PD-1/PD-L1 inhibition unleashes the expansion of CD4+ and CD8+ T cell populations, resulting in increased memory formation and cytotoxic T cell responses.
Development of PD-1/PD-L1 blockade for cancer therapy
The discovery that PD-1 ligation induces T cell exhaustion meant that inhibiting its engagement might improve immunity against infections or cancer. Preclinical models demonstrated that PD-L1 blockade increased the duration of dendritic cell–T cell interactions and promoted T cell activation in vivo (Fife et al., 2009). Moreover, anti–PD-L1 treatment in tumor-burdened mice resulted in enhanced tumor-specific T cell responses and inhibition of tumor growth (Dong et al., 2002; Iwai et al., 2002, 2005). The effectiveness of PD-1/PD-L1 inhibition in tumor regression encouraged clinical tests in humans with advanced cancer. The fully human PD-1 (nivolumab) and PD-L1 (MDX-1105 and atezolizumab) monoclonal blocking antibodies showed exciting regression in patients with diverse incurable cancers, including melanoma, non–small cell lung cancer, and renal cell carcinoma (Brahmer et al., 2012; Topalian et al., 2012; Powles et al., 2014). In multiple clinical trials, anti–PD-1 therapy resulted in an overall response rate of 30–40% in patients with otherwise fatal melanoma (Hamid et al., 2013; Robert et al., 2015a; Weber et al., 2015). One especially hopeful finding was a 26% response rate in melanoma patients whose disease had progressed on ipilimumab (Robert et al., 2014). The FDA approved anti–PD-1 antibodies, nivolumab and pembrolizumab, for melanoma in 2014, and since then, for other malignancies. Atezolizumab became the first PD-L1 inhibitor to receive FDA approval for the treatment of urothelial cancer in 2016 despite an overall response rate of only 15%, which nonetheless exceeded the historical control rate of 10% (Rosenberg et al., 2016). Other PD-L1 inhibitors (avelumab and durvalumab) have also been approved, and treatments with these inhibitors have expanded to include non–small cell lung cancer and Merkel cell carcinoma of the skin.
Mechanisms of PD-1/PD-L1 blockade in tumor regression
PD-1 engagement on activated T cells puts the brakes on by causing cell cycle arrest, suppression of T cell migration, and reduced cytolytic mediators (Patsoukis et al., 2015). This has likely evolved to tune down the inflammatory and tissue-destructive capacity of T cells if they remain activated. In the face of an ever-growing tumor, however, PD-1 would blunt the desired antitumor response. Evidence suggests that inhibiting the PD-1/PD-L1 signaling pathway reinvigorates tumor-specific T cells at the tumor site (Fig. 2). For instance, metastatic melanoma patients responding to anti–PD-1 therapy (pembrolizumab) show exuberant proliferation of intratumoral CD8+ T cells correlating with a decrease in tumor size (Tumeh et al., 2014). Moreover, CD8+ T cells (CXCR5+PD-1+ICOS+) expand in response to PD-1 blockade, which correlates with responsiveness to anti–PD-1 therapy (Quigley et al., 2007; Kim et al., 2010; Im et al., 2016). Up-regulated PD-L1 in tumor cell lines following IFN-γ treatment is associated with increased apoptosis of tumor-specific T cells, resulting in greater tumorigenesis in vivo (Dong et al., 2002). Thus, PD-1/PD-L1 inhibition not only promotes expansion, migration, and cytolytic activity of tumor-specific T cells but also may prevent their demise. Not surprisingly, boosting T cell responses with anti-PD-1/PD-L1 therapy was most effective when tumors expressed high levels of PD-L1 (Herbst et al., 2014). Also, PD-L1 expression by infiltrating immune cells (dendritic cells, macrophages, and T cells) was particularly important for responsiveness to anti–PD-L1 therapy in multiple cancer types (Herbst et al., 2014).
Although it is chiefly CD8+ T cells that recover function and proliferate after anti–PD-1/PD-L1 therapy, CD4+ T cells may also be rescued. The classic conception holds that CD4+ T cells are required to produce stimulatory cytokines for primary CD8+ T cell responses and/or CD8+ memory formation (Williams and Bevan, 2007). Activated CD4+ T cells also secrete IFN-γ and chemokines that enhance vascular permeability and migration of cytotoxic T cells and antibodies into peripheral tissues (Nakanishi et al., 2009; Iijima and Iwasaki, 2016). Blocking PD-1 has a variety of CD4+ T cell effects. Takeuchi et al. (2018) identified three specialized phenotypes of CD4+ memory T cells that expand in melanoma patients responding to PD-1 blockade. Additionally, CD4+CD57+ T cells, a senescent subset with limited proliferative capacity but high IFN-γ production, are decreased in biopsies from patients responding to anti–PD-1 therapy, but increased in nonresponders (Ribas et al., 2016). PD-L1 blockade during chronic viral infections causes a surfeit of PD-1+ T reg cells and reduces CD8+ T cell responsiveness. To counteract this, depletion of CD4+ T cells or T reg cells in combination with PD-L1 blockade revives exhausted CD8+ T cells and reduces viral load (Penaloza-MacMaster et al., 2014). Collectively, CD4+ T cell help during PD-1 blockade may improve efficacy, but more studies are needed to define the impact of CD4+ T cells in tumor regression in the context of anti–PD-1/PD-L1 therapy.
Adverse events from immune checkpoint blockade
The cost of breaking tolerance is that checkpoint blockade treatment is accompanied by immune-related adverse events in ≤80% of patients (Postow et al., 2018). Globally tampering with immune homeostasis causes autoimmune and inflammatory side effects involving the skin (pruritus and cutaneous rash), gastrointestinal tract (colitis and diarrhea), endocrine glands (hypophysitis and thyroid dysfunction) and liver (autoimmune hepatitis), although any organ system can be affected. The toxicity of anti–CTLA-4 therapy mirrors that of patients with loss-of-function mutations in CTLA-4 or the lipopolysaccharide-responsive and beige-like anchor protein, a critical regulator of CTLA-4 cell surface expression. These include autoantibody-mediated cytopenias, lymphocytic infiltration, and organ-specific autoimmunity (Kuehn et al., 2014; Lo et al., 2015). Effective management of these systemic disorders involves continuous monitoring and multidisciplinary collaboration to promptly diagnose and treat complications.
The adverse events resulting from CTLA-4 blockade differ from PD-1/PD-L1 inhibition in frequency, distribution, and toxicity because of the basic idea that CTLA-4 and PD-1 maintain tolerance in different ways. Anti–CTLA-4 therapy more commonly causes high-grade toxicity, especially severe and life-threatening colitis, whereas PD-1 inhibition has more tolerable side effects such as pneumonitis and hypothyroidism (Hodi et al., 2010; Robert et al., 2015b). Not surprisingly, patients who received ipilimumab and nivolumab combination therapy experienced a greater frequency (55%) of adverse events compared with either nivolumab (16.3%) or ipilimumab (27.3%) alone, although dual therapy was more successful therapeutically (see text box; Larkin et al., 2015). Severe, life-threatening reactions necessitate discontinuing therapy. Patients experiencing milder autoimmune reactions to immunotherapy often receive glucocorticoids to mitigate their symptoms. However, glucocorticoid treatment can be ineffective and has its own side effects, so other interventions have been deployed. Infliximab, an anti–TNF-α antibody commonly used for inflammatory bowel disease, was recently reported to completely resolve nivolumab-induced colitis (Yanai et al., 2017). An IL-17 inhibitor, already used for rheumatologic disease, has also been considered (Harbour et al., 2015); however, it is possible that IL-17 blockade could shackle antitumor efficacy (Esfahani and Miller, 2017). Notably, a retrospective study found that cancer outcomes were unchanged by immunosuppressive therapies necessitated by adverse events, which supports an optimistic view that effective immunotherapy need not be sacrificed by controlling side effects (Horvat et al., 2015).
Combinatorial therapies
Combining different treatment modalities designed to attack different vulnerabilities of the cancer cell and achieve synergism, i.e., combinatorial therapies, is a powerful concept in cancer treatment. Although checkpoint blockade revolutionized cancer therapy, not all patients respond or undergo complete remission, leaving room for new ideas. Combinatorial cancer therapy got a second wind from checkpoint inhibitors because they could be conjoined with conventional treatments as well as each other, both at the outset of newly diagnosed disease and when conventional modalities fail. This has improved response rates and long-term survival of cancer patients in several different trials. So augmenting T cell responsiveness during early activation as well as preventing a nonresponsive cell fate may synergize. Advanced melanoma patients treated with both ipilimumab and nivolumab responded at a rate of 61% compared with 11% in patients treated only with ipilimumab. Astoundingly, patients receiving combination therapy had a 22% complete response rate compared with no response in monotherapy patients (Postow et al., 2015). Even more amazing is that combinations outperformed monotherapy even in patients with PD-L1–negative tumors, with an added 18 mo of survival (Larkin et al., 2015). The remarkable increase in efficacy using ipilimumab plus nivolumab led to FDA approval in 2015 to treat melanoma and, more recently, for renal cell carcinoma. These combinatorial successes confirm that CTLA-4 and PD-1 blockade circumvent peripheral tolerance through distinct molecular pathways, allowing synergy.
Other types of cancer therapies, notably radiation, may also help to improve clinical responses when combined with checkpoint blockade. Radiation damages tumor cells and generates neoantigens that are taken up by APCs to activate T cells in the lymph node (Ngwa et al., 2018). Although this results in an increased diversity of the intratumoral TCR repertoire, radiotherapy may also impede antitumor immunity through PD-L1 up-regulation on tumor cells. Thus, radiation in combination with anti–PD-L1 antibody may further boost antitumor immunity, and preclinical trials have shown increased efficacy with this treatment strategy (Dovedi et al., 2014; Twyman-Saint Victor et al., 2015). Immune checkpoint blockade has also been shown to enhance the abscopal effect of radiation in patients with metastatic cancer (Postow et al., 2012; Golden et al., 2013; Ngwa et al., 2018). The success of different combination therapies reveals the complexity of antitumor immunity as well as the immunosuppressive nature of the tumor microenvironment.
Conclusions
Over the past decade, immunotherapy has emerged as a new pillar of cancer treatment and provides renewed hope for reducing morbidity and mortality of this complex disease. Although the concept of using the immune system to fight cancer was theorized by Paul Ehrlich over a century ago (Strebhardt and Ullrich, 2008), a deep understanding of the mechanisms underlying antitumor immunity, particularly the role of T cells in immunity, was needed to achieve clinical success (Miller and Mitchell, 1967). The discovery that CTLA-4 negatively regulates T cell signaling (Walunas et al., 1996), as well as the observation that inhibiting CTLA-4 results in rejection of preestablished tumors in mice (Leach et al., 1996), were the underpinnings of an immunotherapeutic strategy that was the first to extend long-term survival of patients with advanced melanoma. Inhibition of PD-1, another negative regulator of T cells, proved to be an even more effective therapeutic than anti–CTLA-4. However, not all cancer types can be vanquished yet with checkpoint blockade or other types of cancer immunotherapy. Thus, while basic science research has changed the face of clinical oncology, efforts must continue to uncover molecular pathways that drive immune function and homeostasis. Although the baton of immunotherapy has been passed into the clinic, the history of this field has taught us that strong basic science efforts in the laboratory today are crucial to produce innovations at the bedside tomorrow.
Acknowledgments
We thank Ryan Kissinger for the illustrations. We thank and apologize to all researchers in this field who may have authored related studies that were not cited due to space limitations. We thank the Henry Kunkel Society for the honor of writing this review.
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. J.M. Fritz was supported by the Postdoctoral Research Associate Training Program of the National Institute of General Medical Sciences.
The authors declare no competing financial interests.
Author contributions: J.M. Fritz and M.J. Lenardo designed, wrote, and edited the manuscript. All authors read and approved the manuscript prior to submission.
References
- Agata Y., Kawasaki A., Nishimura H., Ishida Y., Tsubata T., Yagita H., and Honjo T.. 1996. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 8:765–772. 10.1093/intimm/8.5.765 [DOI] [PubMed] [Google Scholar]
- Arce Vargas F., Furness A.J.S., Litchfield K., Joshi K., Rosenthal R., Ghorani E., Solomon I., Lesko M.H., Ruef N., Roddie C., et al. TRACERx Melanoma. TRACERx Renal. TRACERx Lung consortia . 2018. Fc Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies. Cancer Cell. 33:649–663.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M.F., Köhler G., Ecabert B., Mak T.W., and Kopf M.. 1999. Cutting edge: lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J. Immunol. 163:1128–1131. [PubMed] [Google Scholar]
- Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.J., Topalian S.L., Hwu P., Drake C.G., Camacho L.H., Kauh J., Odunsi K., et al. 2012. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366:2455–2465. 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhns P., Iannascoli B., England P., Mancardi D.A., Fernandez N., Jorieux S., and Daëron M.. 2009. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 113:3716–3725. 10.1182/blood-2008-09-179754 [DOI] [PubMed] [Google Scholar]
- Calvo C.R., Amsen D., and Kruisbeek A.M.. 1997. Cytotoxic T lymphocyte antigen 4 (CTLA-4) interferes with extracellular signal-regulated kinase (ERK) and Jun NH2-terminal kinase (JNK) activation, but does not affect phosphorylation of T cell receptor zeta and ZAP70. J. Exp. Med. 186:1645–1653. 10.1084/jem.186.10.1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthon B.C., Wolchok J.D., Yuan J., Kamat A., Ng Tang D.S., Sun J., Ku G., Troncoso P., Logothetis C.J., Allison J.P., and Sharma P.. 2010. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin. Cancer Res. 16:2861–2871. 10.1158/1078-0432.CCR-10-0569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput N., Lepage P., Coutzac C., Soularue E., Le Roux K., Monot C., Boselli L., Routier E., Cassard L., Collins M., et al. 2017. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 28:1368–1379. 10.1093/annonc/mdx108 [DOI] [PubMed] [Google Scholar]
- Chen H., Liakou C.I., Kamat A., Pettaway C., Ward J.F., Tang D.N., Sun J., Jungbluth A.A., Troncoso P., Logothetis C., and Sharma P.. 2009. Anti-CTLA-4 therapy results in higher CD4+ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proc. Natl. Acad. Sci. USA. 106:2729–2734. 10.1073/pnas.0813175106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang E., Fisher T.S., Morgan R.W., Robbins M.D., Duerr J.M., Vander Heiden M.G., Gardner J.P., Hambor J.E., Neveu M.J., and Thompson C.B.. 2000. The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity. 13:313–322. 10.1016/S1074-7613(00)00031-5 [DOI] [PubMed] [Google Scholar]
- Collins A.V., Brodie D.W., Gilbert R.J., Iaboni A., Manso-Sancho R., Walse B., Stuart D.I., van der Merwe P.A., and Davis S.J.. 2002. The interaction properties of costimulatory molecules revisited. Immunity. 17:201–210. 10.1016/S1074-7613(02)00362-X [DOI] [PubMed] [Google Scholar]
- Cooper M.D., Raymond D.A., Peterson R.D., South M.A., and Good R.A.. 1966. The functions of the thymus system and the bursa system in the chicken. J. Exp. Med. 123:75–102. 10.1084/jem.123.1.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington P.J., Baroja M.L., Chau T.A., Siu E., Ling V., Carreno B.M., and Madrenas J.. 2002. Surface cytotoxic T lymphocyte-associated antigen 4 partitions within lipid rafts and relocates to the immunological synapse under conditions of inhibition of T cell activation. J. Exp. Med. 195:1337–1347. 10.1084/jem.20011868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Zhu G., Tamada K., and Chen L.. 1999. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 5:1365–1369. 10.1038/70932 [DOI] [PubMed] [Google Scholar]
- Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B., Roche P.C., Lu J., Zhu G., Tamada K., et al. 2002. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8:793–800. 10.1038/nm730 [DOI] [PubMed] [Google Scholar]
- Dovedi S.J., Adlard A.L., Lipowska-Bhalla G., McKenna C., Jones S., Cheadle E.J., Stratford I.J., Poon E., Morrow M., Stewart R., et al. 2014. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 74:5458–5468. 10.1158/0008-5472.CAN-14-1258 [DOI] [PubMed] [Google Scholar]
- Eroglu Z., Kim D.W., Wang X., Camacho L.H., Chmielowski B., Seja E., Villanueva A., Ruchalski K., Glaspy J.A., Kim K.B., et al. 2015. Long term survival with cytotoxic T lymphocyte-associated antigen 4 blockade using tremelimumab. Eur. J. Cancer. 51:2689–2697. 10.1016/j.ejca.2015.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani K., and Miller W.H. Jr. 2017. Reversal of Autoimmune Toxicity and Loss of Tumor Response by Interleukin-17 Blockade. N. Engl. J. Med. 376:1989–1991. 10.1056/NEJMc1703047 [DOI] [PubMed] [Google Scholar]
- Facciabene A., Motz G.T., and Coukos G.. 2012. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 72:2162–2171. 10.1158/0008-5472.CAN-11-3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlings M., Simoni Y., Penny H.L., Becht E., Loh C.Y., Gubin M.M., Ward J.P., Wong S.C., Schreiber R.D., and Newell E.W.. 2017. Checkpoint blockade immunotherapy reshapes the high-dimensional phenotypic heterogeneity of murine intratumoural neoantigen-specific CD8+ T cells. Nat. Commun. 8:562 10.1038/s41467-017-00627-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife B.T., Pauken K.E., Eagar T.N., Obu T., Wu J., Tang Q., Azuma M., Krummel M.F., and Bluestone J.A.. 2009. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat. Immunol. 10:1185–1192. 10.1038/ni.1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G.J., Long A.J., Iwai Y., Bourque K., Chernova T., Nishimura H., Fitz L.J., Malenkovich N., Okazaki T., Byrne M.C., et al. 2000. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192:1027–1034. 10.1084/jem.192.7.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness A.J., Vargas F.A., Peggs K.S., and Quezada S.A.. 2014. Impact of tumour microenvironment and Fc receptors on the activity of immunomodulatory antibodies. Trends Immunol. 35:290–298. 10.1016/j.it.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Golden E.B., Demaria S., Schiff P.B., Chachoua A., and Formenti S.C.. 2013. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol. Res. 1:365–372. 10.1158/2326-6066.CIR-13-0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grulich A.E., van Leeuwen M.T., Falster M.O., and Vajdic C.M.. 2007. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 370:59–67. 10.1016/S0140-6736(07)61050-2 [DOI] [PubMed] [Google Scholar]
- Hamid O., Robert C., Daud A., Hodi F.S., Hwu W.J., Kefford R., Wolchok J.D., Hersey P., Joseph R.W., Weber J.S., et al. 2013. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 369:134–144. 10.1056/NEJMoa1305133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour S.N., Maynard C.L., Zindl C.L., Schoeb T.R., and Weaver C.T.. 2015. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc. Natl. Acad. Sci. USA. 112:7061–7066. 10.1073/pnas.1415675112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., Chai Y., Qi J., Zhang C.W.H., Tong Z., Shi Y., Yan J., Tan S., and Gao G.F.. 2017. Remarkably similar CTLA-4 binding properties of therapeutic ipilimumab and tremelimumab antibodies. Oncotarget. 8:67129–67139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst R.S., Soria J.C., Kowanetz M., Fine G.D., Hamid O., Gordon M.S., Sosman J.A., McDermott D.F., Powderly J.D., Gettinger S.N., et al. 2014. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 515:563–567. 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi F.S., Mihm M.C., Soiffer R.J., Haluska F.G., Butler M., Seiden M.V., Davis T., Henry-Spires R., MacRae S., Willman A., et al. 2003. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc. Natl. Acad. Sci. USA. 100:4712–4717. 10.1073/pnas.0830997100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi F.S., O’Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C., et al. 2010. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363:711–723. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoos A., Ibrahim R., Korman A., Abdallah K., Berman D., Shahabi V., Chin K., Canetta R., and Humphrey R.. 2010. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin. Oncol. 37:533–546. 10.1053/j.seminoncol.2010.09.015 [DOI] [PubMed] [Google Scholar]
- Horvat T.Z., Adel N.G., Dang T.O., Momtaz P., Postow M.A., Callahan M.K., Carvajal R.D., Dickson M.A., D’Angelo S.P., Woo K.M., et al. 2015. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J. Clin. Oncol. 33:3193–3198. 10.1200/JCO.2015.60.8448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T.Z., Qureshi O.S., Wang C.J., Baker J., Young S.P., Walker L.S., and Sansom D.M.. 2015. A transendocytosis model of CTLA-4 function predicts its suppressive behavior on regulatory T cells. J. Immunol. 194:2148–2159. 10.4049/jimmunol.1401876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima N., and Iwasaki A.. 2016. Access of protective antiviral antibody to neuronal tissues requires CD4 T-cell help. Nature. 533:552–556. 10.1038/nature17979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im S.J., Hashimoto M., Gerner M.Y., Lee J., Kissick H.T., Burger M.C., Shan Q., Hale J.S., Lee J., Nasti T.H., et al. 2016. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 537:417–421. 10.1038/nature19330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y., Agata Y., Shibahara K., and Honjo T.. 1992. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895. 10.1002/j.1460-2075.1992.tb05481.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai Y., Ishida M., Tanaka Y., Okazaki T., Honjo T., and Minato N.. 2002. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA. 99:12293–12297. 10.1073/pnas.192461099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai Y., Terawaki S., and Honjo T.. 2005. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int. Immunol. 17:133–144. 10.1093/intimm/dxh194 [DOI] [PubMed] [Google Scholar]
- Juneja V.R., McGuire K.A., Manguso R.T., LaFleur M.W., Collins N., Haining W.N., Freeman G.J., and Sharpe A.H.. 2017. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J. Exp. Med. 214:895–904. 10.1084/jem.20160801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh B., O’Brien S., Lee D., Hou Y., Weinberg V., Rini B., Allison J.P., Small E.J., and Fong L.. 2008. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 112:1175–1183. 10.1182/blood-2007-11-125435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.J., Verbinnen B., Tang X., Lu L., and Cantor H.. 2010. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature. 467:328–332. 10.1038/nature09370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong K.F., Fu G., Zhang Y., Yokosuka T., Casas J., Canonigo-Balancio A.J., Becart S., Kim G., Yates J.R. III, Kronenberg M., et al. 2014. Protein kinase C-η controls CTLA-4-mediated regulatory T cell function. Nat. Immunol. 15:465–472. 10.1038/ni.2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel M.F., and Allison J.P.. 1996. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J. Exp. Med. 183:2533–2540. 10.1084/jem.183.6.2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang D.M., Zhao Q., Peng C., Xu J., Zhang J.P., Wu C., and Zheng L.. 2009. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J. Exp. Med. 206:1327–1337. 10.1084/jem.20082173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn H.S., Ouyang W., Lo B., Deenick E.K., Niemela J.E., Avery D.T., Schickel J.N., Tran D.Q., Stoddard J., Zhang Y., et al. 2014. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 345:1623–1627. 10.1126/science.1255904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon E.D., Hurwitz A.A., Foster B.A., Madias C., Feldhaus A.L., Greenberg N.M., Burg M.B., and Allison J.P.. 1997. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc. Natl. Acad. Sci. USA. 94:8099–8103. 10.1073/pnas.94.15.8099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.J., Cowey C.L., Lao C.D., Schadendorf D., Dummer R., Smylie M., Rutkowski P., et al. 2015. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 373:23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchman Y., Wood C.R., Chernova T., Chaudhary D., Borde M., Chernova I., Iwai Y., Long A.J., Brown J.A., Nunes R., et al. 2001. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2:261–268. 10.1038/85330 [DOI] [PubMed] [Google Scholar]
- Leach D.R., Krummel M.F., and Allison J.P.. 1996. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 271:1734–1736. 10.1126/science.271.5256.1734 [DOI] [PubMed] [Google Scholar]
- Lee K.M., Chuang E., Griffin M., Khattri R., Hong D.K., Zhang W., Straus D., Samelson L.E., Thompson C.B., and Bluestone J.A.. 1998. Molecular basis of T cell inactivation by CTLA-4. Science. 282:2263–2266. 10.1126/science.282.5397.2263 [DOI] [PubMed] [Google Scholar]
- Lee S., Moon J.S., Lee C.R., Kim H.E., Baek S.M., Hwang S., Kang G.H., Seo J.K., Shin C.H., Kang H.J., et al. 2016. Abatacept alleviates severe autoimmune symptoms in a patient carrying a de novo variant in CTLA-4. J. Allergy Clin. Immunol. 137:327–330. 10.1016/j.jaci.2015.08.036 [DOI] [PubMed] [Google Scholar]
- Lenardo M., Chan K.M., Hornung F., McFarland H., Siegel R., Wang J., and Zheng L.. 1999. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol. 17:221–253. 10.1146/annurev.immunol.17.1.221 [DOI] [PubMed] [Google Scholar]
- Liakou C.I., Kamat A., Tang D.N., Chen H., Sun J., Troncoso P., Logothetis C., and Sharma P.. 2008. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc. Natl. Acad. Sci. USA. 105:14987–14992. 10.1073/pnas.0806075105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo B., Zhang K., Lu W., Zheng L., Zhang Q., Kanellopoulou C., Zhang Y., Liu Z., Fritz J.M., Marsh R., et al. 2015. AUTOIMMUNE DISEASE. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science. 349:436–440. 10.1126/science.aaa1663 [DOI] [PubMed] [Google Scholar]
- Maker A.V., Attia P., and Rosenberg S.A.. 2005. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J. Immunol. 175:7746–7754. 10.4049/jimmunol.175.11.7746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelbrot D.A., McAdam A.J., and Sharpe A.H.. 1999. B7-1 or B7-2 is required to produce the lymphoproliferative phenotype in mice lacking cytotoxic T lymphocyte-associated antigen 4 (CTLA-4). J. Exp. Med. 189:435–440. 10.1084/jem.189.2.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D., Vezys V., Wherry E.J., and Ahmed R.. 2007. A brief history of CD8 T cells. Eur. J. Immunol. 37(Suppl 1):S103–S110. 10.1002/eji.200737584 [DOI] [PubMed] [Google Scholar]
- McGranahan N., Furness A.J., Rosenthal R., Ramskov S., Lyngaa R., Saini S.K., Jamal-Hanjani M., Wilson G.A., Birkbak N.J., Hiley C.T., et al. 2016. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 351:1463–1469. 10.1126/science.aaf1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.F. 1961. Immunological function of the thymus. Lancet. 2:748–749. 10.1016/S0140-6736(61)90693-6 [DOI] [PubMed] [Google Scholar]
- Miller J.F., and Mitchell G.F.. 1967. The thymus and the precursors of antigen reactive cells. Nature. 216:659–663. 10.1038/216659a0 [DOI] [PubMed] [Google Scholar]
- Miller J.F., and Morahan G.. 1992. Peripheral T cell tolerance. Annu. Rev. Immunol. 10:51–69. 10.1146/annurev.iy.10.040192.000411 [DOI] [PubMed] [Google Scholar]
- Misra N., Bayry J., Lacroix-Desmazes S., Kazatchkine M.D., and Kaveri S.V.. 2004. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J. Immunol. 172:4676–4680. 10.4049/jimmunol.172.8.4676 [DOI] [PubMed] [Google Scholar]
- Nakanishi Y., Lu B., Gerard C., and Iwasaki A.. 2009. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 462:510–513. 10.1038/nature08511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngwa W., Irabor O.C., Schoenfeld J.D., Hesser J., Demaria S., and Formenti S.C.. 2018. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer. 18:313–322. 10.1038/nrc.2018.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H., Nose M., Hiai H., Minato N., and Honjo T.. 1999. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 11:141–151. 10.1016/S1074-7613(00)80089-8 [DOI] [PubMed] [Google Scholar]
- Nishimura H., Okazaki T., Tanaka Y., Nakatani K., Hara M., Matsumori A., Sasayama S., Mizoguchi A., Hiai H., Minato N., and Honjo T.. 2001. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 291:319–322. 10.1126/science.291.5502.319 [DOI] [PubMed] [Google Scholar]
- O’Mahony D., Morris J.C., Quinn C., Gao W., Wilson W.H., Gause B., Pittaluga S., Neelapu S., Brown M., Fleisher T.A., et al. 2007. A pilot study of CTLA-4 blockade after cancer vaccine failure in patients with advanced malignancy. Clin. Cancer Res. 13:958–964. 10.1158/1078-0432.CCR-06-1974 [DOI] [PubMed] [Google Scholar]
- Oderup C., Cederbom L., Makowska A., Cilio C.M., and Ivars F.. 2006. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology. 118:240–249. 10.1111/j.1365-2567.2006.02362.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T., and Honjo T.. 2007. PD-1 and PD-1 ligands: from discovery to clinical application. Int. Immunol. 19:813–824. 10.1093/intimm/dxm057 [DOI] [PubMed] [Google Scholar]
- Okazaki T., Maeda A., Nishimura H., Kurosaki T., and Honjo T.. 2001. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc. Natl. Acad. Sci. USA. 98:13866–13871. 10.1073/pnas.231486598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T., Iwai Y., and Honjo T.. 2002. New regulatory co-receptors: inducible co-stimulator and PD-1. Curr. Opin. Immunol. 14:779–782. 10.1016/S0952-7915(02)00398-9 [DOI] [PubMed] [Google Scholar]
- Patsoukis N., Bardhan K., Chatterjee P., Sari D., Liu B., Bell L.N., Karoly E.D., Freeman G.J., Petkova V., Seth P., et al. 2015. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun. 6:6692 10.1038/ncomms7692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken K.E., and Wherry E.J.. 2015. SnapShot: T Cell Exhaustion. Cell. 163:1038–1038.e1. [DOI] [PubMed] [Google Scholar]
- Peggs K.S., Quezada S.A., Chambers C.A., Korman A.J., and Allison J.P.. 2009. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 206:1717–1725. 10.1084/jem.20082492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penaloza-MacMaster P., Kamphorst A.O., Wieland A., Araki K., Iyer S.S., West E.E., O’Mara L., Yang S., Konieczny B.T., Sharpe A.H., et al. 2014. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J. Exp. Med. 211:1905–1918. 10.1084/jem.20132577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan G.Q., Yang J.C., Sherry R.M., Hwu P., Topalian S.L., Schwartzentruber D.J., Restifo N.P., Haworth L.R., Seipp C.A., Freezer L.J., et al. 2003. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl. Acad. Sci. USA. 100:8372–8377. 10.1073/pnas.1533209100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow M.A., Callahan M.K., Barker C.A., Yamada Y., Yuan J., Kitano S., Mu Z., Rasalan T., Adamow M., Ritter E., et al. 2012. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 366:925–931. 10.1056/NEJMoa1112824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow M.A., Chesney J., Pavlick A.C., Robert C., Grossmann K., McDermott D., Linette G.P., Meyer N., Giguere J.K., Agarwala S.S., et al. 2015. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 372:2006–2017. 10.1056/NEJMoa1414428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow M.A., Sidlow R., and Hellmann M.D.. 2018. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 378:158–168. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- Powles T., Eder J.P., Fine G.D., Braiteh F.S., Loriot Y., Cruz C., Bellmunt J., Burris H.A., Petrylak D.P., Teng S.L., et al. 2014. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 515:558–562. 10.1038/nature13904 [DOI] [PubMed] [Google Scholar]
- Quezada S.A., Peggs K.S., Curran M.A., and Allison J.P.. 2006. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J. Clin. Invest. 116:1935–1945. 10.1172/JCI27745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley M.F., Gonzalez V.D., Granath A., Andersson J., and Sandberg J.K.. 2007. CXCR5+ CCR7- CD8 T cells are early effector memory cells that infiltrate tonsil B cell follicles. Eur. J. Immunol. 37:3352–3362. 10.1002/eji.200636746 [DOI] [PubMed] [Google Scholar]
- Qureshi O.S., Zheng Y., Nakamura K., Attridge K., Manzotti C., Schmidt E.M., Baker J., Jeffery L.E., Kaur S., Briggs Z., et al. 2011. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 332:600–603. 10.1126/science.1202947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramagopal U.A., Liu W., Garrett-Thomson S.C., Bonanno J.B., Yan Q., Srinivasan M., Wong S.C., Bell A., Mankikar S., Rangan V.S., et al. 2017. Structural basis for cancer immunotherapy by the first-in-class checkpoint inhibitor ipilimumab. Proc. Natl. Acad. Sci. USA. 114:E4223–E4232. 10.1073/pnas.1617941114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S., Malmström V., and Powrie F.. 2000. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295–302. 10.1084/jem.192.2.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A., Camacho L.H., Lopez-Berestein G., Pavlov D., Bulanhagui C.A., Millham R., Comin-Anduix B., Reuben J.M., Seja E., Parker C.A., et al. 2005. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J. Clin. Oncol. 23:8968–8977. 10.1200/JCO.2005.01.109 [DOI] [PubMed] [Google Scholar]
- Ribas A., Kefford R., Marshall M.A., Punt C.J., Haanen J.B., Marmol M., Garbe C., Gogas H., Schachter J., Linette G., et al. 2013. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J. Clin. Oncol. 31:616–622. 10.1200/JCO.2012.44.6112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A., Shin D.S., Zaretsky J., Frederiksen J., Cornish A., Avramis E., Seja E., Kivork C., Siebert J., Kaplan-Lefko P., et al. 2016. PD-1 Blockade Expands Intratumoral Memory T Cells. Cancer Immunol. Res. 4:194–203. 10.1158/2326-6066.CIR-15-0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi N.A., Hellmann M.D., Snyder A., Kvistborg P., Makarov V., Havel J.J., Lee W., Yuan J., Wong P., Ho T.S., et al. 2015. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 348:124–128. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C., Thomas L., Bondarenko I., O’Day S., Weber J., Garbe C., Lebbe C., Baurain J.F., Testori A., Grob J.J., et al. 2011. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364:2517–2526. 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- Robert C., Ribas A., Wolchok J.D., Hodi F.S., Hamid O., Kefford R., Weber J.S., Joshua A.M., Hwu W.J., Gangadhar T.C., et al. 2014. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 384:1109–1117. 10.1016/S0140-6736(14)60958-2 [DOI] [PubMed] [Google Scholar]
- Robert C., Long G.V., Brady B., Dutriaux C., Maio M., Mortier L., Hassel J.C., Rutkowski P., McNeil C., Kalinka-Warzocha E., et al. 2015a Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372:320–330. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- Robert C., Schachter J., Long G.V., Arance A., Grob J.J., Mortier L., Daud A., Carlino M.S., McNeil C., Lotem M., et al. KEYNOTE-006 investigators . 2015b Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 372:2521–2532. 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- Romano E., Kusio-Kobialka M., Foukas P.G., Baumgaertner P., Meyer C., Ballabeni P., Michielin O., Weide B., Romero P., and Speiser D.E.. 2015. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc. Natl. Acad. Sci. USA. 112:6140–6145. 10.1073/pnas.1417320112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg J.E., Hoffman-Censits J., Powles T., van der Heijden M.S., Balar A.V., Necchi A., Dawson N., O’Donnell P.H., Balmanoukian A., Loriot Y., et al. 2016. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 387:1909–1920. 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota G., Niogret C., Dang A.T., Barros C.R., Fonta N.P., Alfei F., Morgado L., Zehn D., Birchmeier W., Vivier E., and Guarda G.. 2018. Shp-2 Is Dispensable for Establishing T Cell Exhaustion and for PD-1 Signaling In Vivo. Cell Reports. 23:39–49. 10.1016/j.celrep.2018.03.026 [DOI] [PubMed] [Google Scholar]
- Sahin U., and Türeci Ö.. 2018. Personalized vaccines for cancer immunotherapy. Science. 359:1355–1360. 10.1126/science.aar7112 [DOI] [PubMed] [Google Scholar]
- Sansom D.M. 2000. CD28, CTLA-4 and their ligands: who does what and to whom? Immunology. 101:169–177. 10.1046/j.1365-2567.2000.00121.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadendorf D., Hodi F.S., Robert C., Weber J.S., Margolin K., Hamid O., Patt D., Chen T.T., Berman D.M., and Wolchok J.D.. 2015. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J. Clin. Oncol. 33:1889–1894. 10.1200/JCO.2014.56.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Mandelbrot D.A., Greenwald R.J., Ng F., Lechler R., Sharpe A.H., and Rudd C.E.. 2002. Cutting edge: CTLA-4 (CD152) differentially regulates mitogen-activated protein kinases (extracellular signal-regulated kinase and c-Jun N-terminal kinase) in CD4+ T cells from receptor/ligand-deficient mice. J. Immunol. 169:3475–3479. 10.4049/jimmunol.169.7.3475 [DOI] [PubMed] [Google Scholar]
- Schubert D., Bode C., Kenefeck R., Hou T.Z., Wing J.B., Kennedy A., Bulashevska A., Petersen B.S., Schäffer A.A., Grüning B.A., et al. 2014. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat. Med. 20:1410–1416. 10.1038/nm.3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby M.J., Engelhardt J.J., Quigley M., Henning K.A., Chen T., Srinivasan M., and Korman A.J.. 2013. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol. Res. 1:32–42. 10.1158/2326-6066.CIR-13-0013 [DOI] [PubMed] [Google Scholar]
- Sheppard K.A., Fitz L.J., Lee J.M., Benander C., George J.A., Wooters J., Qiu Y., Jussif J.M., Carter L.L., Wood C.R., and Chaudhary D.. 2004. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 574:37–41. 10.1016/j.febslet.2004.07.083 [DOI] [PubMed] [Google Scholar]
- Simpson T.R., Li F., Montalvo-Ortiz W., Sepulveda M.A., Bergerhoff K., Arce F., Roddie C., Henry J.Y., Yagita H., Wolchok J.D., et al. 2013. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med. 210:1695–1710. 10.1084/jem.20130579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A., Makarov V., Merghoub T., Yuan J., Zaretsky J.M., Desrichard A., Walsh L.A., Postow M.A., Wong P., Ho T.S., et al. 2014. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371:2189–2199. 10.1056/NEJMoa1406498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebhardt K., and Ullrich A.. 2008. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat. Rev. Cancer. 8:473–480. 10.1038/nrc2394 [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tagami T., Yamazaki S., Uede T., Shimizu J., Sakaguchi N., Mak T.W., and Sakaguchi S.. 2000. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 192:303–310. 10.1084/jem.192.2.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Tanemura A., Tada Y., Katayama I., Kumanogoh A., and Nishikawa H.. 2018. Clinical response to PD-1 blockade correlates with a sub-fraction of peripheral central memory CD4+ T cells in patients with malignant melanoma. Int. Immunol. 30:13–22. 10.1093/intimm/dxx073 [DOI] [PubMed] [Google Scholar]
- Tarhini A.A., Edington H., Butterfield L.H., Lin Y., Shuai Y., Tawbi H., Sander C., Yin Y., Holtzman M., Johnson J., et al. 2014. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One. 9:e87705 10.1371/journal.pone.0087705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tivol E.A., Borriello F., Schweitzer A.N., Lynch W.P., Bluestone J.A., and Sharpe A.H.. 1995. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 3:541–547. 10.1016/1074-7613(95)90125-6 [DOI] [PubMed] [Google Scholar]
- Tivol E.A., Boyd S.D., McKeon S., Borriello F., Nickerson P., Strom T.B., and Sharpe A.H.. 1997. CTLA4Ig prevents lymphoproliferation and fatal multiorgan tissue destruction in CTLA-4-deficient mice. J. Immunol. 158:5091–5094. [PubMed] [Google Scholar]
- Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Carvajal R.D., Sosman J.A., Atkins M.B., et al. 2012. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366:2443–2454. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng S.Y., Otsuji M., Gorski K., Huang X., Slansky J.E., Pai S.I., Shalabi A., Shin T., Pardoll D.M., and Tsuchiya H.. 2001. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp. Med. 193:839–846. 10.1084/jem.193.7.839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumeh P.C., Harview C.L., Yearley J.H., Shintaku I.P., Taylor E.J., Robert L., Chmielowski B., Spasic M., Henry G., Ciobanu V., et al. 2014. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 515:568–571. 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman-Saint Victor C., Rech A.J., Maity A., Rengan R., Pauken K.E., Stelekati E., Benci J.L., Xu B., Dada H., Odorizzi P.M., et al. 2015. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 520:373–377. 10.1038/nature14292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Allen E.M., Miao D., Schilling B., Shukla S.A., Blank C., Zimmer L., Sucker A., Hillen U., Foppen M.H.G., Goldinger S.M., et al. 2015. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 350:207–211. 10.1126/science.aad0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe P.A., Bodian D.L., Daenke S., Linsley P., and Davis S.J.. 1997. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J. Exp. Med. 185:393–403. 10.1084/jem.185.3.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt G.J., Everts B., Chang C.H., Curtis J.D., Freitas T.C., Amiel E., Pearce E.J., and Pearce E.L.. 2012. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 36:68–78. 10.1016/j.immuni.2011.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij N., van Buuren M.M., Philips D., Velds A., Toebes M., Heemskerk B., van Dijk L.J., Behjati S., Hilkmann H., El Atmioui D., et al. 2013. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J. Clin. Oncol. 31:e439–e442. 10.1200/JCO.2012.47.7521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L.S., and Sansom D.M.. 2011. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol. 11:852–863. 10.1038/nri3108 [DOI] [PubMed] [Google Scholar]
- Walunas T.L., Bakker C.Y., and Bluestone J.A.. 1996. CTLA-4 ligation blocks CD28-dependent T cell activation [published erratum appears in J Exp Med. 1996;184:301]. J. Exp. Med. 183:2541–2550. 10.1084/jem.183.6.2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse P., Penninger J.M., Timms E., Wakeham A., Shahinian A., Lee K.P., Thompson C.B., Griesser H., and Mak T.W.. 1995. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 270:985–988. 10.1126/science.270.5238.985 [DOI] [PubMed] [Google Scholar]
- Weber J.S., D’Angelo S.P., Minor D., Hodi F.S., Gutzmer R., Neyns B., Hoeller C., Khushalani N.I., Miller W.H. Jr., Lao C.D., et al. 2015. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 16:375–384. 10.1016/S1470-2045(15)70076-8 [DOI] [PubMed] [Google Scholar]
- Wei S.C., Levine J.H., Cogdill A.P., Zhao Y., Anang N.A.S., Andrews M.C., Sharma P., Wang J., Wargo J.A., Pe’er D., and Allison J.P.. 2017. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell. 170:1120–1133.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry E.J., and Kurachi M.. 2015. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15:486–499. 10.1038/nri3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.A., and Bevan M.J.. 2007. Effector and memory CTL differentiation. Annu. Rev. Immunol. 25:171–192. 10.1146/annurev.immunol.25.022106.141548 [DOI] [PubMed] [Google Scholar]
- Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T., Miyara M., Fehervari Z., Nomura T., and Sakaguchi S.. 2008. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 322:271–275. 10.1126/science.1160062 [DOI] [PubMed] [Google Scholar]
- Yanai S., Nakamura S., and Matsumoto T.. 2017. Nivolumab-Induced Colitis Treated by Infliximab. Clin. Gastroenterol. Hepatol. 15:e80–e81. 10.1016/j.cgh.2016.09.017 [DOI] [PubMed] [Google Scholar]
- Yokosuka T., Kobayashi W., Takamatsu M., Sakata-Sogawa K., Zeng H., Hashimoto-Tane A., Yagita H., Tokunaga M., and Saito T.. 2010. Spatiotemporal basis of CTLA-4 costimulatory molecule-mediated negative regulation of T cell activation. Immunity. 33:326–339. 10.1016/j.immuni.2010.09.006 [DOI] [PubMed] [Google Scholar]
- Zaugg K., Yao Y., Reilly P.T., Kannan K., Kiarash R., Mason J., Huang P., Sawyer S.K., Fuerth B., Faubert B., et al. 2011. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev. 25:1041–1051. 10.1101/gad.1987211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Gajewski T.F., and Kline J.. 2009. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 114:1545–1552. 10.1182/blood-2009-03-206672 [DOI] [PMC free article] [PubMed] [Google Scholar]