This review provides a concise overview of pro- and anti-inflammatory approaches in current tumor therapy.
Abstract
A link between chronic inflammation and development of tumors is well established. Moreover, it has become evident that tumorigenesis is not a cell autonomous disease, and an inflammatory microenvironment is a prerequisite of basically all tumors, including those that emerge in the absence of overt inflammation. This knowledge has led to the development of anti-inflammatory concepts to treat and prevent cancer. In contrast, immunotherapies, in particular checkpoint inhibitors, representing the most significant progress in the therapy of several malignancies depend on the presence of a pro-inflammatory “hot” environment. Here, we discuss pro- and anti-inflammatory concepts for the treatment of cancer.
Introduction
The clinical connection of inflammation and cancer reaches back to the late 19th century, when Rudolf Virchow postulated sites of chronic inflammation as origin of neoplastic malignancies after he had noticed the presence of leukocyte infiltrates in cancerous tissues (Balkwill and Mantovani, 2001). Nearly at the same time, the German physician Wilhelm Busch employed an inflammatory immune response as a treatment for cancer, partially curing a patient suffering from soft-tissue sarcoma of the neck with an erysipelas infection. He was followed by the American bone surgeon William Coley, who used a mixture of heat-killed bacteria, later called “Coley’s toxins,” to successfully treat sarcomas (Coley, 1893), making him the father of immunotherapy. These historic examples depict vividly what we know today: while inflammation can promote carcinogenesis, it may as well be used for tumor therapy. Initially, the underlying mechanisms were completely unknown, and the original forms of pro-inflammatory therapy bore severe side effects. During the following century, radiation therapy and chemotherapy emerged, and because cancer was increasingly considered a cell-intrinsic genetic disease, new treatment modalities focused on killing tumor cells directly, while “inflammatory” therapies were neglected (Fig. 1; Faguet, 2015). This view has changed again over the last two decades. It became clear that cancer resembles complex organs, consisting of tumor cells and host-derived stroma, which is composed of resident as well as recruited cells (Hanahan, 2014; Weinberg, 2014). Thus, it has become unequivocally evident that tumor development depends on the intricate reciprocal interplay of mutagenized tumor cells with their local and distant microenvironment (Balkwill and Mantovani, 2012; Quail and Joyce, 2013).
Figure 1.
Time course from first documented cancer cases to modern therapy. Ab, antibody; ABL, Abelson murine leukemia viral oncogene homologue 1; AML, acute myeloid leukemia; CAR, chimeric antigen receptor; CML, chronic myeloid leukemia; CTCL, cutaneous T cell lymphoma; RA, rheumatoid arthritis; T-VEC, talimogene laherparepvec; VEGF, vascular endothelial growth factor.
Chronic inflammation shapes the tumor microenvironment, affecting cell plasticity through epithelial–mesenchymal transition, dedifferentiation, polarization of immune cells, ROS, cytokines, epigenetic mechanisms, miRNAs, and complex regulatory cascades in tumor and stromal cells (Varga and Greten, 2017). Curiously, not all inflammatory diseases or persistent infections are correlated to increased cancer risk, and although allergic diseases also embody a state of constant or recurring inflammation, this type of inflammation may be even inversely correlated with cancer progression (Turner et al., 2006; Kozłowska et al., 2016). Thus, an important open question remains why certain organs with ongoing inflammation, such as rheumatoid arthritis or myocarditis, are not susceptible to tumor induction. The formation of inflammation-induced reactive oxygen or nitrogen species, produced by activated myeloid cells, that can directly mediate DNA damage and chromosomal instability in neighboring cells (Canli et al., 2017) cannot account for this phenomenon, considering that this would occur in all types of organs. Interestingly, organs with high tumor incidence in the context of chronic inflammation are those that usually interact closely with microbial products or directly with microbiota, pointing to the role of the microenvironment, potentially carcinogenic microbe-derived metabolites, or host immune responses in cancer initiation.
In addition to cytotoxic therapies that induce a pro-inflammatory response (Grivennikov et al., 2010), surgery can act in an immunomodulating way, contributing to the outgrowth of metastases even when surgery is performed years after removal of a primary tumor. Here, the concept of premetastatic niches and circulating tumor cells (CTCs) is considered to play an important role, and dormant CTCs seem to be essential for the formation of metastases upon surgery (Murthy et al., 1989; Demicheli et al., 2008; Tohme et al., 2017; Castaño et al., 2018). One reason for this is the loss of tumor-derived angiogenesis inhibitors after removal of the primary tumor; others may comprise shedding of mediators that promote wound healing and neoangiogenesis to promote the outgrowth of formerly dormant CTC or micrometastases (Hofer et al., 1998; Demicheli et al., 2008). Through the surgery itself, inflammatory cells and cytokines are released into the blood, helping to create premetastatic niches, where CTCs can settle and prosper (Lim et al., 2013; Peinado et al., 2017). These findings already give some insight into the complex nature of inflammatory processes connected to tumor development, progression, and classical treatment.
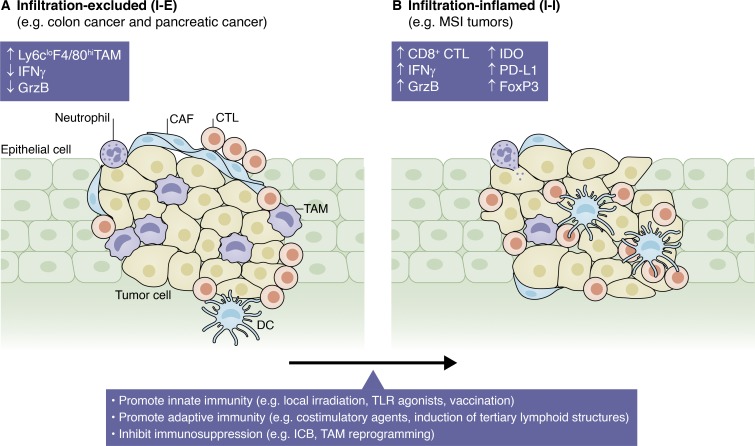
Although the importance of the tumor microenvironment for tumor progression is undisputed, most current cytotoxic treatments or recently developed small-molecule inhibitors target specific signaling pathways within tumor cells. Undoubtedly, several of these promising new compounds have proven extremely successful (McCubrey et al., 2012; Hengel et al., 2017; Whittaker et al., 2017). However, many patients that initially benefit from these very effective compounds rapidly develop therapy resistance, leading to even more aggressive tumors. The clinical approval of antiangiogenic tumor therapy nearly 15 yr ago marked a breakthrough paradigm change as the first clinically effective antistroma therapy (Ferrara et al., 2004). However, the efficacy of antiangiogenic therapies continues to be limited, and the mechanism of action (vascular regression vs. vascular normalization) is poorly understood (Klement et al., 2000; Jain, 2001). Today, a variety of molecular pathways and stroma cells are targeted ranging from epigenetic factors, hypoxia, neoangiogenesis, and cytokines over tumor and tumor-associated cells to the microbiota of the gut. Although certain anti-inflammatory therapies show very promising results in various malignancies, the real breakthrough of the last years was the development and clinical approval of antibody-based immunotherapies targeting CTLA-4 or PD-1/PD-L1. While immune checkpoint blockade (ICB) is clinically very effective, leading to durable treatment responses in a few tumor entities, the majority of tumor patients do not respond for a wide range of potential reasons (Chen and Mellman, 2017). So-called infiltrated–inflamed tumors are considered “hot” tumors that contain a high number of infiltrating cytotoxic lymphocytes expressing PD-1 and that usually respond well to ICB. In contrast, infiltration-excluded tumors are characterized by accumulation of CTLs along the border of the tumor mass and a lack of CTL infiltration into the tumor core. These tumors are generally considered “cold” tumors with poor sensitivity to ICB (Fig. 2; Gajewski, 2015). Several promising strategies have been suggested to change a cold into a hot tumor, which can render these tumors sensitive to ICB (Table 1), yet there is certainly an unmet need to unravel further mechanisms that would allow such conversion. Moreover, it remains one of the biggest challenges to identify biomarkers that will allow assessment of individual patients’ ICB sensitivity or that will help to decide about the most promising combinatorial approach for an individual patient.
Figure 2.
Tumor immune statuses. Infiltration-excluded tumors (left) accumulate cytotoxic T lymphocytes (CTLs) around their margins, where they interact with Ly6cloF4/80hi TAMs or are impeded by cancer-associated fibroblasts (CAFs). Infiltration-inflamed tumors (right) show activated PD1+ CTLs in their core that express IFNγ and granzyme B (GrzB). They may form tertiary lymphoid structures and are generally associated with good prognosis and response to ICB. Therefore, therapies aim to promote this state. DC, dendritic cell; IDO, indoleamine (2,3)-dioxygenase; MSI, microsatellite instability.
Table 1. Mechanisms to improve anti-tumor response and immune infiltration with selected examples.
| Promote innate immunity | ||
|---|---|---|
| Local inflammation | Local irradiation | Liu et al., 2018 |
| Oncolytic viruses | Raja et al., 2018 | |
| Macrophage/DC activation | CD40 | Vonderheide, 2018 |
| TLR or RLR agonists | Li et al., 2017 | |
| Pro-inflammatory cytokines | IFNα | Medrano et al., 2017 |
| Promote phagocytosis | CD47/SIRPα | Liu et al., 2017 |
| Vaccination | DC vaccination | Palucka and Banchereau, 2013 |
| Peptide vaccination | Kumai et al., 2017 | |
| Tumor cell vaccination | Srivatsan et al., 2014 | |
| Promote adaptive immunity | ||
| Induction of tertiary lymphoid structures | LIGHT | Johansson-Percival et al., 2017 |
| Costimulatory agents | CD40, CD137, GITR, ICOS, OX40 | Dempke et al., 2017 |
| T cell activation | IL-2 | Jiang et al., 2016 |
| Pegylated IL-10 | Naing et al., 2018 | |
| STING | Rivera Vargas et al., 2017 | |
| Inhibition of immunosuppression | ||
| ICB | CTLA-4, PD-1/PD-L1 | Seidel et al., 2018 |
| IDO, KIR, LAG-3, Tim-3, VISTA | Dempke et al., 2017 | |
| TAM reprogramming | BTK | Gunderson et al., 2016 |
| Class IIa HDAC | Guerriero et al., 2017 | |
| CD40 | Majety et al., 2018 | |
| Blocking immunosuppressive cytokines | TGFβ | Haque and Morris, 2017 |
DC, dendritic cell; ICOS, inducible costimulator; IDO, indoleamine (2,3)-dioxygenase; KIR, killer cell immunoglobulin-like receptor; RLR, RIG-I-like receptor.
Inhibiting inflammation
Because tumors and their constant smoldering inflammation resemble and are promoted by chronic inflammatory diseases, it seems logical to employ anti-inflammatory drugs. Aspirin is one of the oldest anti-inflammatory drugs. Its anticoagulant properties were noted in the 1950s, and its metastasis-reducing capacity was tested in animal models in the 1970s, when its suppressive effect on prostaglandin production had been discovered (Henschke et al., 1977). More evidence of aspirin or other nonsteroidal anti-inflammatory drugs, targeting cyclooxygenases, came from the observation that taking these drugs as pain killers for cancer-elicited pain or therapy side effects had an overall survival benefit. Today, several clinical trials are still ongoing, showing modest results in different kinds of cancer, such as breast, prostate, and especially colon, although the positive effect on cardiovascular-caused death, which could influence these results, should be kept in mind (Jacobs et al., 2014; Chen and Holmes, 2017; Frouws et al., 2017).
Neutralizing pro-inflammatory cytokines or blocking their receptors represents a more direct targeted approach. In 1993, the first IL-1 receptor antagonist was approved for the treatment of rheumatoid arthritis (anakinra from Amgen). Since then, its application has been extended to a variety of other diseases. Today, several mediators blocking or neutralizing the IL-1 pathway (e.g., antibodies, soluble receptors, and small-molecule inhibitors) are in use or being tested for cancer treatment (Dinarello et al., 2012; Molgora et al., 2018). In a recently completed trial, the IL-1β blocking antibody canakinumab was shown to ameliorate inflammation in patients suffering from atherosclerosis (Canakinumab Anti-inflammatory Thrombosis Outcomes Study; CANTOS). Interestingly, a secondary analysis of the obtained data including a 5-yr follow-up revealed that canakinumab-receiving patients showed a significant dose–dependent reduction in lung cancer incidence and mortality compared with placebo-treated patients (Ridker et al., 2017). Similar positive results of blocking inflammatory IL-1 pathways were observed in different murine models of breast cancer in both primary tumors and metastases formation (Guo et al., 2016; Dagenais et al., 2017). In contrast, a recent preclinical study could demonstrate an IL-1β–dependent suppression of metastasis-initiating cancer cells, which was lost upon its neutralization. This was underscored by database analysis that showed a beneficial effect of high levels of IL-1β on overall survival of breast cancer patients with lymph node metastases (Castaño et al., 2018). Admittedly, the murine studies differed in model and cells used and the suppressive effect was restricted to early stages of metastases outgrowth. Although most of the available data support the concept to block IL-1, there is no doubt that further research is needed, as well as for other pro-inflammatory cytokines that are targeted in anticancer therapy, including IL-6 (Kitamura et al., 2017), IL-23, TNFα, and CC-chemokine ligand 2 (CCL2), to dampen inflammation and/or leukocyte recruitment (Todoric et al., 2016).
Interestingly, although IL-17 is known to be one of the most potent inflammatory cytokines and the culprit in many autoimmune diseases, patients suffering from different kind of cancers showed prolonged survival when they expressed high levels of IL-17 (Qian et al., 2017). While IL-17 was originally considered to promote neovascularization and tumor cell proliferation, its anti-tumorigenic function, achieved by, e.g., activation of tumoricidal T cells, natural killer cells, or neutrophils and upholding barrier integrity, is undisputable (Kryczek et al., 2009; Wang et al., 2014; Fabre et al., 2016). Since more and more is known about the molecular mechanisms linking inflammation and tumor progression, the respective intracellular signaling cascades constitute a novel target for therapeutic intervention. This is practiced indirectly by cytokine blocking antibodies mentioned above, many of which impinge on protumorigenic STAT3 or NF-κB signaling. Several direct STAT3 inhibitors are currently tested in clinical trials (Johnson et al., 2018). Unfortunately, various side effects, including development of neutrophilia and elevated IL-1β serum levels (Greten et al., 2007; Mankan et al., 2011), led to the discontinuation of IKKβ inhibitor development by many pharmaceutical companies.
Inducing inflammation and modulating immune cell activation
Less than 25% of all patients respond to immune-oncology compounds. Exhaustion of T cells, PD-1/PD-L1 gene amplification, MHC-I/II mutations, β-catenin overexpression, and other reasons have been described to be responsible for such resistance (Dempke et al., 2017). One concept to improve response to ICB has simply been the combination of anti–CTLA-4 plus anti–PD-1/PD-L1. This prolongs survival of metastatic melanoma patients and led to Food and Drug Administration approval of nivolumab (anti–PD-1) plus ipilimumab (anti–CTLA-4) in 2015 (Fig. 1). Yet, while single administration of anti–CTLA-4 induces PD-L1 expression on tumor cells (Hu-Lieskovan and Ribas, 2017), the advantage of additional CTLA-4 blockade is often only moderate, accompanied by more severe cytotoxic side effects compared with PD-1 blockade alone.
One of the greatest obstacles in immune therapy is the immune-deserted or cold state of certain tumors that lack an appropriate anti-tumor immune response (Chen and Mellman, 2017). To increase T cell infiltration into tumors, various combination therapies now aim to induce a pro-inflammatory response that would overcome T cell exclusion, turning tumors into hot tumors (Fig. 2). One of these approaches comprises the likewise relatively novel oncolytic viruses. Talimogene laherparepvec is the first approved oncolytic virus for use in melanoma. Its lytic traits, together with the expression of GM-CSF, lead to increased tumor cell lysis and release of danger signals and antigens, promoting T cell responses, which showed beneficial effects in combination with anti–CTLA-4 or anti–PD-1 therapy (Zamarin et al., 2014; Ribas et al., 2017; Chesney et al., 2018). Further attempts to increase T cell activity include irradiation, GM-CSF–expressing tumor vaccine GVAX, or cytostatics as combinatorial therapies (Robert et al., 2011; van den Eertwegh et al., 2012; Le et al., 2013; Rech et al., 2018). Not only does irradiation induce a local inflammatory milieu with increased IFNγ production, cell death, antigen release, and broadened T cell receptor repertoire, its combination with PD-1 blockade can exert an abscopal effect, with tumor regression in non-irradiated secondary tumors (Liu et al., 2018). Furthermore, irradiation-induced DNA damage activates pattern recognition receptors like the cytosolic DNA sensor cyclic GMP-AMP synthase, which further activates STING (stimulator of interferon genes) and leads to a type I IFN response, responsible for anti-tumor immunity (Deng et al., 2014). Interestingly, cyclic GMP-AMP synthase/STING seems to be essential for anti–PD-L1 and anti–CTLA-4 to act when combined with irradiation in murine models of melanoma (Harding et al., 2017; Wang et al., 2017). Another promising approach comprises direct interference with cytokines. TGFβ promotes T cell exclusion and is correlated with poor prognosis (Calon et al., 2012, 2015; Tauriello et al., 2018). Blocking TGFβ along with PD-1 provided excellent results in preclinical models of colorectal and mammary carcinoma (Mariathasan et al., 2018; Tauriello et al., 2018). While the anti-inflammatory cytokine IL-10 is one of the main mediators secreted by regulatory T cells to inhibit tumor-specific immune responses, the contradicting observation that block or deficiency of IL-10 increases tumor growth led to the development of PEGylated IL-10 (Pegilodecakin; Oft, 2014; Naing et al., 2016). Interestingly, while dampening pro-inflammatory macrophages and Th17 cells, a simultaneous induction of anti-tumorigenic CD8 T cell responses and CD8-derived IFNγ was observed following administration of Pegilodecakin in mice. More importantly, Pegilodecakin promoted expansion of underrepresented T cell clones as well as LAG-3+ PD-1+ CD8+ T cells, which are further induced by anti–PD-1 in various solid tumors (Naing et al., 2018). Intratumoral applications of TNFα “TNFerade” seemed not to be as effective as initially hoped, and clinical trials have been stopped despite first promising results (Kali, 2015). To date, IL-2 and IFNα are the only cytokines approved for use in cancer. They aim for increased T cell proliferation and MHC-I/HLA expression by tumor cells (Lee and Margolin, 2011).
Several other compounds that trigger innate and adaptive immune responses have now found their way into the clinics and are being tested in combination with ICB. Apart from Toll- and RIG-like receptor or STING activation (Li et al., 2017) current strategies employ inhibitory compounds targeting V-domain immunoglobulin suppressor of T cell activation (VISTA), TIM-3, LAG-3, indoleamine (2,3)-dioxygenase, or killer cell immunoglobulin-like receptor as well as costimulatory antibodies including CD40, OX40, inducible costimulator, CD137, or glucocorticoid-induced TNFR family-related gene (GITR). Initial concerns regarding the potential development of cytokine-release syndromes, autoimmune reactions, and hyperimmune stimulation have not been confirmed in early phase I/II clinical trials so far (Dempke et al., 2017). Moreover, cyclin-dependent kinase (CDK) inhibitors targeting CDK4 and CDK6 (Goel et al., 2017; Deng et al., 2018), histone deacetylase (HDAC), or DNA methyltransferase inhibitors (Fraga et al., 2005; Goel and Boland, 2012; Lee and Huang, 2013) showed promising results while maintaining tolerable side effects. Several recent excellent reviews have summarized these results in greater detail (Hu-Lieskovan and Ribas, 2017; Patel and Minn, 2018).
PD-1–PD-L1 therapies may also function through a direct effect on macrophages, since PD-1 expression can be observed on tumor-associated macrophages (TAMs), where its engagement dampens tumor cell phagocytosis and acts in an immunosuppressive manner on CD8 T cells (Gordon et al., 2017; Wang et al., 2018). Interestingly, blocking either PD-1 or PD-L1 may even have distinct effects on macrophage activation that could be targeted synergistically (Hartley et al., 2018).
TAMs with an M2-like phenotype comprise one cell type that is associated with poor prognosis in many solid cancers (Shabo et al., 2008; Kurahara et al., 2011). Their recruitment can be inadvertently induced by affected tissue following chemotherapy (DeNardo et al., 2011). Inhibitors targeting colony-stimulating factor-1 receptor (CSF-1R), a critical macrophage survival factor, were shown to have positive outcomes in xenograft models of glioblastoma, by reprogramming the M2-like phenotype (Pyonteck et al., 2013). CSF-1R–targeting antibodies showed clinical response in diffuse-type giant cell tumor of human patients (Ries et al., 2014). Alternative targets that are involved in polarization of TAMs comprise Bruton tyrosine kinase (BTK) and PI3Kγ. In a preclinical model of pancreatic ductal adenocarcinoma, the BTK inhibitor ibrutinib reprogrammed macrophages toward a M1 phenotype and thereby stimulated CD8+ T cells in a macrophage-dependent manner (Gunderson et al., 2016). Inhibition of PI3Kγ in macrophages prolongs NF-κB activation and inhibits C/EBPβ to induce an immunostimulatory program that enhances cytotoxic CD8+ T cell recruitment and activation, thus improving anti-tumor function (Kaneda et al., 2016). A recent report demonstrated reprogramming of TAMs and recruitment of non-TAM macrophages by class IIa HDAC inhibition, resulting in cytotoxic T cell response and regression of breast tumors and metastases (Guerriero et al., 2017). Although the inhibitor was acting specifically on myeloid cells, the exact mechanism was not yet clear. TAM reprogramming into an immune-stimulating phenotype can also be achieved by agonistic CD40 therapy, which enhances macrophage activation and activity (Beatty et al., 2011; Vonderheide, 2018) and which showed promising results in combination with the aforementioned CSF-1R blockade (Hoves et al., 2018; Perry et al., 2018), checkpoint inhibitors, and chemo- or radiotherapy (Byrne and Vonderheide, 2016; Bajor et al., 2018; Rech et al., 2018). Another approach to enhance macrophage function consists of blocking antiphagocytic signals from tumor cells. CD47 depicts the most advanced target in this category. Blocking the interaction with its ligand SIRPα leads to increased phagocytosis and tumor regression in several models (Chao et al., 2010; Goto et al., 2014; Gholamin et al., 2017; Liu et al., 2017; Métayer et al., 2017). Currently, different strategies aiming at TAM recruitment, function, or activation are tested in clinical trials (Ruffell and Coussens, 2015; Cannarile et al., 2017). Among these are mediators, blocking the CCL2/CCR2 axis, that prevent chemoattraction of monocytes/macrophages and their subsequent effects including metastasis (Lim et al., 2016). An interesting discovery was that the cytostatic trabectedin exerts some of its function by selective depletion of monocytes and macrophages apart from its direct effect on tumor cells (Germano et al., 2013). Of note, however, was the observation that termination of anti-CCL2 treatment caused a dramatic increase of metastases and death in syngeneic breast cancer models (Bonapace et al., 2014). Furthermore, macrophages exert critical Fc-mediated effector functions like antibody-dependent cellular cytotoxicity of, for example, anti-CTLA-4–targeted T reg cells (Simpson et al., 2013), which should be considered in the context of a clinical use of monocyte/macrophage blocking agents.
While the concept to employ bacteria for cancer therapy was initiated by Wilhelm Busch and William Coley, nowadays the microbiome, and particularly the intestinal microbiome, has to be considered for tumor therapy due to its important role in host physiology and metabolism. Either through changes in the microbial composition (dysbiosis) or through a barrier defect, the microbiome has a significant impact on the development of the immune system (Chung et al., 2012) as well as carcinogenesis (Quante et al., 2013). Importantly, however, the microbiome can also directly affect the efficacy of various cancer therapies (Zitvogel et al., 2018), including administration of platinum-based agents (Iida et al., 2013), alkylating agents such as cyclophosphamide (Viaud et al., 2013), innate immunity modulators such as TLR9 stimulating CpG-DNA (Iida et al., 2013), or immune checkpoint inhibitors (Sivan et al., 2015; Vétizou et al., 2015; Gopalakrishnan et al., 2018). Furthermore, intratumor Gammaproteobacteria can metabolize the chemotherapeutic gemcitabine to an inactive form, dampening its efficacy (Geller et al., 2017). So far, mechanistic studies clearly defining the exact mechanisms for the beneficial or detrimental effects of microbes in cancer immunotherapy are mostly missing, and conclusions rather depend on correlative results showing effects, or the lack thereof, on antibiotic treatments or gnotobiotic experiments. Furthermore, it remains to be proven that these studies, which have been mostly performed in mice using syngeneic cancer cell lines that had previously been immune edited, can be recapitulated in (humanized) models of spontaneous tumorigenesis. Although there is clear evidence that the microbiome has an immunostimulatory function, it also remains to be defined which and if individual bacteria, or rather bacterial communities, are responsible for this and whether direct stimulation of T cell receptors, engagement of pattern recognition receptors, or system metabolic effects are the key drivers (Zitvogel et al., 2018). Nevertheless, these recent studies underscore the potentially adverse effects of antibiotic use in patients during anticancer therapy, and they further suggest that, for example, pre-existing diet-dependent changes in the intestinal microbiome are not only important for the development of tumors (Arkan, 2017), but may also affect the responsiveness to chemo- or immunotherapy to a much greater extent than previously anticipated. However, a better understanding of the microbiome-induced effects on the host during cancer therapy as well as the identification of a possibly beneficial microbiome (Tanoue et al., 2019) may lead to concepts aiming at altering the microbiome by either fecal transplantation, supplementation of distinct bacterial strains, or targeted antibiotic therapy.
Conclusion
Over the last years, the field of cancer-related inflammation has tremendously expanded, and a multitude of different cellular and molecular mechanisms have been discovered, so far nicely illustrating the intricate interaction of immune cells, vascular cells, stromal cells, and tumor cells and the influence of various external factors. Essentially all immune cells have been shown to be involved in the different stages of tumorigenesis, and this has unraveled various exciting new strategies for tumor therapy, some of which we have highlighted above. Individualized multimodal combinatorial approaches targeting both tumor cell intrinsic and extrinsic pathways will most likely represent the future of modern cancer therapy. The biggest challenge will be the timely identification of the most efficient combination for each individual cancer patient and the identification of biomarkers that will allow prediction of immunotherapeutic response. Better characterization of individual immune and stromal cells in the tumor microenvironment using single-cell analysis that will particularly help to address the plasticity of these cells will be important. Development of technological platforms that allow interactions of T cells with matched tumor organoids in a personalized manner to assess killing efficiency (Dijkstra et al., 2018) will prove extremely helpful in this regard. Nevertheless, novel, original approaches that will help overcome T cell exclusion from tumors remain an unmet need. In this context, CRISPR-mediated genetic in vivo screens represent a promising approach, as they have recently unraveled pathways that may lead to innovative concepts that could render unresponsive cold tumors sensitive to ICB (Manguso et al., 2017; Ishizuka et al., 2019). Another important task and the basis for proper preclinical validation of novel immunotherapeutic concepts is the development of improved in vivo tumor models and the use of humanized mice that enable an adequate recapitulation of tumor evolution and that sufficiently take into consideration other external factors such as age, diet, and the microbiome. However, also recently developed microfluidic human organs-on-chips that can be used to model cancer cell behavior within human-relevant tissue and organ microenvironments in vitro (Sontheimer-Phelps et al., 2019) represent a promising alternative to evaluate personalized therapy responses.
Acknowledgments
Work in the laboratory of F.R. Greten is supported by institutional funds from the Georg-Speyer-Haus, by the LOEWE Center Frankfurt Cancer Institute funded by the Hessen State Ministry for Higher Education, Research and the Arts (III L 5 - 519/03/03.001 - (0015)), as well as grants from the Deutsche Forschungsgemeinschaft (FOR2438: Gr1916/11-1; SFB 815, 1177, and 1292). The Institute for Tumor Biology and Experimental Therapy, Georg-Speyer-Haus, is funded jointly by the German Federal Ministry of Health and the Ministry of Higher Education, Research and the Arts of the State of Hessen.
The authors declare no competing financial interests.
Author contributions: The manuscript was written and edited by B. Ritter and F.R. Greten.
References
- Arkan M.C. 2017. The intricate connection between diet, microbiota, and cancer: A jigsaw puzzle. Semin. Immunol. 32:35–42. 10.1016/j.smim.2017.08.009 [DOI] [PubMed] [Google Scholar]
- Bajor D.L., Mick R., Riese M.J., Huang A.C., Sullivan B., Richman L.P., Torigian D.A., George S.M., Stelekati E., Chen F., et al. . 2018. Long-term outcomes of a phase I study of agonist CD40 antibody and CTLA-4 blockade in patients with metastatic melanoma. OncoImmunology. 7:e1468956 10.1080/2162402X.2018.1468956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F., and Mantovani A.. 2001. Inflammation and cancer: back to Virchow? Lancet. 357:539–545. 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- Balkwill F.R., and Mantovani A.. 2012. Cancer-related inflammation: common themes and therapeutic opportunities. Semin. Cancer Biol. 22:33–40. 10.1016/j.semcancer.2011.12.005 [DOI] [PubMed] [Google Scholar]
- Beatty G.L., Chiorean E.G., Fishman M.P., Saboury B., Teitelbaum U.R., Sun W., Huhn R.D., Song W., Li D., Sharp L.L., et al. . 2011. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 331:1612–1616. 10.1126/science.1198443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonapace L., Coissieux M.M., Wyckoff J., Mertz K.D., Varga Z., Junt T., and Bentires-Alj M.. 2014. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 515:130–133. 10.1038/nature13862 [DOI] [PubMed] [Google Scholar]
- Byrne K.T., and Vonderheide R.H.. 2016. CD40 Stimulation Obviates Innate Sensors and Drives T Cell Immunity in Cancer. Cell Reports. 15:2719–2732. 10.1016/j.celrep.2016.05.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calon A., Espinet E., Palomo-Ponce S., Tauriello D.V., Iglesias M., Céspedes M.V., Sevillano M., Nadal C., Jung P., Zhang X.H., et al. . 2012. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell. 22:571–584. 10.1016/j.ccr.2012.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calon A., Lonardo E., Berenguer-Llergo A., Espinet E., Hernando-Momblona X., Iglesias M., Sevillano M., Palomo-Ponce S., Tauriello D.V., Byrom D., et al. . 2015. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat. Genet. 47:320–329. 10.1038/ng.3225 [DOI] [PubMed] [Google Scholar]
- Canli Ö., Nicolas A.M., Gupta J., Finkelmeier F., Goncharova O., Pesic M., Neumann T., Horst D., Löwer M., Sahin U., and Greten F.R.. 2017. Myeloid Cell-Derived Reactive Oxygen Species Induce Epithelial Mutagenesis. Cancer Cell. 32:869–883.e5. 10.1016/j.ccell.2017.11.004 [DOI] [PubMed] [Google Scholar]
- Cannarile M.A., Weisser M., Jacob W., Jegg A.M., Ries C.H., and Rüttinger D.. 2017. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J. Immunother. Cancer. 5:53 10.1186/s40425-017-0257-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaño Z., San Juan B.P., Spiegel A., Pant A., DeCristo M.J., Laszewski T., Ubellacker J.M., Janssen S.R., Dongre A., Reinhardt F., et al. . 2018. IL-1β inflammatory response driven by primary breast cancer prevents metastasis-initiating cell colonization. Nat. Cell Biol. 20:1084–1097. 10.1038/s41556-018-0173-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao M.P., Alizadeh A.A., Tang C., Myklebust J.H., Varghese B., Gill S., Jan M., Cha A.C., Chan C.K., Tan B.T., et al. . 2010. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 142:699–713. 10.1016/j.cell.2010.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D.S., and Mellman I.. 2017. Elements of cancer immunity and the cancer-immune set point. Nature. 541:321–330. 10.1038/nature21349 [DOI] [PubMed] [Google Scholar]
- Chen W.Y., and Holmes M.D.. 2017. Role of Aspirin in Breast Cancer Survival. Curr. Oncol. Rep. 19:48 10.1007/s11912-017-0605-6 [DOI] [PubMed] [Google Scholar]
- Chesney J., Puzanov I., Collichio F., Singh P., Milhem M.M., Glaspy J., Hamid O., Ross M., Friedlander P., Garbe C., et al. . 2018. Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination With Ipilimumab Versus Ipilimumab Alone in Patients With Advanced, Unresectable Melanoma. J. Clin. Oncol. 36:1658–1667. 10.1200/JCO.2017.73.7379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H., Pamp S.J., Hill J.A., Surana N.K., Edelman S.M., Troy E.B., Reading N.C., Villablanca E.J., Wang S., Mora J.R., et al. . 2012. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 149:1578–1593. 10.1016/j.cell.2012.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley W.B. 1893. The Treatment of Malignant Tumors by Repeated Innoculations of Erysipelas: With a Report of Ten Original Cases. Am. J. Med. Sci. 10:487–511. 10.1097/00000441-189305000-00001 [DOI] [PubMed] [Google Scholar]
- Dagenais M., Dupaul-Chicoine J., Douglas T., Champagne C., Morizot A., and Saleh M.. 2017. The Interleukin (IL)-1R1 pathway is a critical negative regulator of PyMT-mediated mammary tumorigenesis and pulmonary metastasis. OncoImmunology. 6:e1287247 10.1080/2162402X.2017.1287247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demicheli R., Retsky M.W., Hrushesky W.J., Baum M., and Gukas I.D.. 2008. The effects of surgery on tumor growth: a century of investigations. Ann. Oncol. 19:1821–1828. 10.1093/annonc/mdn386 [DOI] [PubMed] [Google Scholar]
- Dempke W.C.M., Fenchel K., Uciechowski P., and Dale S.P.. 2017. Second- and third-generation drugs for immuno-oncology treatment-The more the better? Eur. J. Cancer. 74:55–72. 10.1016/j.ejca.2017.01.001 [DOI] [PubMed] [Google Scholar]
- DeNardo D.G., Brennan D.J., Rexhepaj E., Ruffell B., Shiao S.L., Madden S.F., Gallagher W.M., Wadhwani N., Keil S.D., Junaid S.A., et al. . 2011. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 1:54–67. 10.1158/2159-8274.CD-10-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J., Wang E.S., Jenkins R.W., Li S., Dries R., Yates J., Chhabra S., Huang W., Liu H., Aref A.R., et al. . 2018. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov. 8:216–233. 10.1158/2159-8290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L., Liang H., Xu M., Yang X., Burnette B., Arina A., Li X.D., Mauceri H., Beckett M., Darga T., et al. . 2014. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 41:843–852. 10.1016/j.immuni.2014.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra K.K., Cattaneo C.M., Weeber F., Chalabi M., van de Haar J., Fanchi L.F., Slagter M., van der Velden D.L., Kaing S., Kelderman S., et al. . 2018. Generation of Tumor-Reactive T Cells by Co-culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell. 174:1586–1598.e12. 10.1016/j.cell.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A., Simon A., and van der Meer J.W.. 2012. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 11:633–652. 10.1038/nrd3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre J., Giustiniani J., Garbar C., Antonicelli F., Merrouche Y., Bensussan A., Bagot M., and Al-Dacak R.. 2016. Targeting the Tumor Microenvironment: The Protumor Effects of IL-17 Related to Cancer Type. Int. J. Mol. Sci. 17:1433 10.3390/ijms17091433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faguet G.B. 2015. A brief history of cancer: age-old milestones underlying our current knowledge database. Int. J. Cancer. 136:2022–2036. 10.1002/ijc.29134 [DOI] [PubMed] [Google Scholar]
- Ferrara N., Hillan K.J., Gerber H.P., and Novotny W.. 2004. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 3:391–400. 10.1038/nrd1381 [DOI] [PubMed] [Google Scholar]
- Fraga M.F., Ballestar E., Villar-Garea A., Boix-Chornet M., Espada J., Schotta G., Bonaldi T., Haydon C., Ropero S., Petrie K., et al. . 2005. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat. Genet. 37:391–400. 10.1038/ng1531 [DOI] [PubMed] [Google Scholar]
- Frouws M.A., van Herk-Sukel M.P.P., Maas H.A., Van de Velde C.J.H., Portielje J.E.A., Liefers G.J., and Bastiaannet E.. 2017. The mortality reducing effect of aspirin in colorectal cancer patients: Interpreting the evidence. Cancer Treat. Rev. 55:120–127. 10.1016/j.ctrv.2016.12.008 [DOI] [PubMed] [Google Scholar]
- Gajewski T.F. 2015. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment. Semin. Oncol. 42:663–671. 10.1053/j.seminoncol.2015.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller L.T., Barzily-Rokni M., Danino T., Jonas O.H., Shental N., Nejman D., Gavert N., Zwang Y., Cooper Z.A., Shee K., et al. . 2017. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 357:1156–1160. 10.1126/science.aah5043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germano G., Frapolli R., Belgiovine C., Anselmo A., Pesce S., Liguori M., Erba E., Uboldi S., Zucchetti M., Pasqualini F., et al. . 2013. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 23:249–262. 10.1016/j.ccr.2013.01.008 [DOI] [PubMed] [Google Scholar]
- Gholamin S., Mitra S.S., Feroze A.H., Liu J., Kahn S.A., Zhang M., Esparza R., Richard C., Ramaswamy V., Remke M., et al. . 2017. Disrupting the CD47-SIRPα anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci. Transl. Med. 9:eaaf2968 10.1126/scitranslmed.aaf2968 [DOI] [PubMed] [Google Scholar]
- Goel A., and Boland C.R.. 2012. Epigenetics of colorectal cancer. Gastroenterology. 143:1442–1460.e1. 10.1053/j.gastro.2012.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S., DeCristo M.J., Watt A.C., BrinJones H., Sceneay J., Li B.B., Khan N., Ubellacker J.M., Xie S., Metzger-Filho O., et al. . 2017. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 548:471–475. 10.1038/nature23465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan V., Spencer C.N., Nezi L., Reuben A., Andrews M.C., Karpinets T.V., Prieto P.A., Vicente D., Hoffman K., Wei S.C., et al. . 2018. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 359:97–103. 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S.R., Maute R.L., Dulken B.W., Hutter G., George B.M., McCracken M.N., Gupta R., Tsai J.M., Sinha R., Corey D., et al. . 2017. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 545:495–499. 10.1038/nature22396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto H., Kojima Y., Matsuda K., Kariya R., Taura M., Kuwahara K., Nagai H., Katano H., and Okada S.. 2014. Efficacy of anti-CD47 antibody-mediated phagocytosis with macrophages against primary effusion lymphoma. Eur. J. Cancer. 50:1836–1846. 10.1016/j.ejca.2014.03.004 [DOI] [PubMed] [Google Scholar]
- Greten F.R., Arkan M.C., Bollrath J., Hsu L.C., Goode J., Miething C., Göktuna S.I., Neuenhahn M., Fierer J., Paxian S., et al. . 2007. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 130:918–931. 10.1016/j.cell.2007.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S.I., Greten F.R., and Karin M.. 2010. Immunity, inflammation, and cancer. Cell. 140:883–899. 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero J.L., Sotayo A., Ponichtera H.E., Castrillon J.A., Pourzia A.L., Schad S., Johnson S.F., Carrasco R.D., Lazo S., Bronson R.T., et al. . 2017. Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature. 543:428–432. 10.1038/nature21409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson A.J., Kaneda M.M., Tsujikawa T., Nguyen A.V., Affara N.I., Ruffell B., Gorjestani S., Liudahl S.M., Truitt M., Olson P., et al. . 2016. Bruton Tyrosine Kinase-Dependent Immune Cell Cross-talk Drives Pancreas Cancer. Cancer Discov. 6:270–285. 10.1158/2159-8290.CD-15-0827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B., Fu S., Zhang J., Liu B., and Li Z.. 2016. Targeting inflammasome/IL-1 pathways for cancer immunotherapy. Sci. Rep. 6:36107 10.1038/srep36107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. 2014. Rethinking the war on cancer. Lancet. 383:558–563. 10.1016/S0140-6736(13)62226-6 [DOI] [PubMed] [Google Scholar]
- Haque S., and Morris J.C.. 2017. Transforming growth factor-β: A therapeutic target for cancer. Hum. Vaccin. Immunother. 13:1741–1750. 10.1080/21645515.2017.1327107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding S.M., Benci J.L., Irianto J., Discher D.E., Minn A.J., and Greenberg R.A.. 2017. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 548:466–470. 10.1038/nature23470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley G.P., Chow L., Ammons D.T., Wheat W.H., and Dow S.W.. 2018. Programmed Cell Death Ligand 1 (PD-L1) Signaling Regulates Macrophage Proliferation and Activation. Cancer Immunol. Res. 6:1260–1273. 10.1158/2326-6066.CIR-17-0537 [DOI] [PubMed] [Google Scholar]
- Hengel S.R., Spies M.A., and Spies M.. 2017. Small-Molecule Inhibitors Targeting DNA Repair and DNA Repair Deficiency in Research and Cancer Therapy. Cell Chem. Biol. 24:1101–1119. 10.1016/j.chembiol.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henschke U.K., Luande G.J., and Choppala J.D.. 1977. Aspirin for reducing cancer metastases? J. Natl. Med. Assoc. 69:581–584. [PMC free article] [PubMed] [Google Scholar]
- Hofer S.O., Shrayer D., Reichner J.S., Hoekstra H.J., and Wanebo H.J.. 1998. Wound-induced tumor progression: a probable role in recurrence after tumor resection. Arch. Surg. 133:383–389. 10.1001/archsurg.133.4.383 [DOI] [PubMed] [Google Scholar]
- Hoves S., Ooi C.H., Wolter C., Sade H., Bissinger S., Schmittnaegel M., Ast O., Giusti A.M., Wartha K., Runza V., et al. . 2018. Rapid activation of tumor-associated macrophages boosts preexisting tumor immunity. J. Exp. Med. 215:859–876. 10.1084/jem.20171440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu-Lieskovan S., and Ribas A.. 2017. New Combination Strategies Using Programmed Cell Death 1/Programmed Cell Death Ligand 1 Checkpoint Inhibitors as a Backbone. Cancer J. 23:10–22. 10.1097/PPO.0000000000000246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida N., Dzutsev A., Stewart C.A., Smith L., Bouladoux N., Weingarten R.A., Molina D.A., Salcedo R., Back T., Cramer S., et al. . 2013. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 342:967–970. 10.1126/science.1240527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka J.J., Manguso R.T., Cheruiyot C.K., Bi K., Panda A., Iracheta-Vellve A., Miller B.C., Du P.P., Yates K.B., Dubrot J., et al. . 2019. Loss of ADAR1 in tumours overcomes resistance to immune checkpoint blockade. Nature. 565:43–48. 10.1038/s41586-018-0768-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs C.D., Chun S.G., Yan J., Xie X.J., Pistenmaa D.A., Hannan R., Lotan Y., Roehrborn C.G., Choe K.S., and Kim D.W.. 2014. Aspirin improves outcome in high risk prostate cancer patients treated with radiation therapy. Cancer Biol. Ther. 15:699–706. 10.4161/cbt.28554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R.K. 2001. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat. Med. 7:987–989. 10.1038/nm0901-987 [DOI] [PubMed] [Google Scholar]
- Jiang T., Zhou C., and Ren S.. 2016. Role of IL-2 in cancer immunotherapy. OncoImmunology. 5:e1163462 10.1080/2162402X.2016.1163462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson-Percival A., He B., Li Z.J., Kjellén A., Russell K., Li J., Larma I., and Ganss R.. 2017. De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors. Nat. Immunol. 18:1207–1217. 10.1038/ni.3836 [DOI] [PubMed] [Google Scholar]
- Johnson D.E., O’Keefe R.A., and Grandis J.R.. 2018. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 15:234–248. 10.1038/nrclinonc.2018.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kali A. 2015. TNFerade, an innovative cancer immunotherapeutic. Indian J. Pharmacol. 47:479–483. 10.4103/0253-7613.165190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M.M., Messer K.S., Ralainirina N., Li H., Leem C.J., Gorjestani S., Woo G., Nguyen A.V., Figueiredo C.C., Foubert P., et al. . 2016. PI3Kγ is a molecular switch that controls immune suppression. Nature. 539:437–442. 10.1038/nature19834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura H., Ohno Y., Toyoshima Y., Ohtake J., Homma S., Kawamura H., Takahashi N., and Taketomi A.. 2017. Interleukin-6/STAT3 signaling as a promising target to improve the efficacy of cancer immunotherapy. Cancer Sci. 108:1947–1952. 10.1111/cas.13332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement G., Baruchel S., Rak J., Man S., Clark K., Hicklin D.J., Bohlen P., and Kerbel R.S.. 2000. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J. Clin. Invest. 105:R15–R24. 10.1172/JCI8829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozłowska R., Bożek A., and Jarząb J.. 2016. Association between cancer and allergies. Allergy Asthma Clin. Immunol. 12:39 10.1186/s13223-016-0147-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I., Wei S., Szeliga W., Vatan L., and Zou W.. 2009. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 114:357–359. 10.1182/blood-2008-09-177360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumai T., Kobayashi H., Harabuchi Y., and Celis E.. 2017. Peptide vaccines in cancer-old concept revisited. Curr. Opin. Immunol. 45:1–7. 10.1016/j.coi.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahara H., Shinchi H., Mataki Y., Maemura K., Noma H., Kubo F., Sakoda M., Ueno S., Natsugoe S., and Takao S.. 2011. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J. Surg. Res. 167:e211–e219. 10.1016/j.jss.2009.05.026 [DOI] [PubMed] [Google Scholar]
- Le D.T., Lutz E., Uram J.N., Sugar E.A., Onners B., Solt S., Zheng L., Diaz L.A. Jr., Donehower R.C., Jaffee E.M., and Laheru D.A.. 2013. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J. Immunother. 36:382–389. 10.1097/CJI.0b013e31829fb7a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., and Huang S.H.. 2013. Cancer Epigenetics: Mechanisms and Crosstalk of a HDAC Inhibitor, Vorinostat. Chemotherapy (Los Angel.). 2:14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., and Margolin K.. 2011. Cytokines in cancer immunotherapy. Cancers (Basel). 3:3856–3893. 10.3390/cancers3043856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Qu S., Chen X., Wu Q., and Shi M.. 2017. Promising Targets for Cancer Immunotherapy: TLRs, RLRs, and STING-Mediated Innate Immune Pathways. Int. J. Mol. Sci. 18:E404 10.3390/ijms18020404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C., Broqueres-You D., Brouland J.P., Merkulova-Rainon T., Faussat A.M., Hilal R., Rouquie D., Eveno C., Audollent R., Levy B.I., and Pocard M.. 2013. Hepatic ischemia-reperfusion increases circulating bone marrow-derived progenitor cells and tumor growth in a mouse model of colorectal liver metastases. J. Surg. Res. 184:888–897. 10.1016/j.jss.2013.04.069 [DOI] [PubMed] [Google Scholar]
- Lim S.Y., Yuzhalin A.E., Gordon-Weeks A.N., and Muschel R.J.. 2016. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget. 7:28697–28710. 10.18632/oncotarget.7376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Wei H., Gao P., Yu H., Wang K., Fu Z., Ju B., Zhao M., Dong S., Li Z., et al. . 2017. CD47 promotes ovarian cancer progression by inhibiting macrophage phagocytosis. Oncotarget. 8:39021–39032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Dong Y., Kong L., Shi F., Zhu H., and Yu J.. 2018. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J. Hematol. Oncol. 11:104 10.1186/s13045-018-0647-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majety M., Runza V., Lehmann C., Hoves S., and Ries C.H.. 2018. A drug development perspective on targeting tumor-associated myeloid cells. FEBS J. 285:763–776. 10.1111/febs.14277 [DOI] [PubMed] [Google Scholar]
- Manguso R.T., Pope H.W., Zimmer M.D., Brown F.D., Yates K.B., Miller B.C., Collins N.B., Bi K., LaFleur M.W., Juneja V.R., et al. . 2017. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature. 547:413–418. 10.1038/nature23270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankan A.K., Canli O., Schwitalla S., Ziegler P., Tschopp J., Korn T., and Greten F.R.. 2011. TNF-alpha-dependent loss of IKKbeta-deficient myeloid progenitors triggers a cytokine loop culminating in granulocytosis. Proc. Natl. Acad. Sci. USA. 108:6567–6572. 10.1073/pnas.1018331108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S., Turley S.J., Nickles D., Castiglioni A., Yuen K., Wang Y., Kadel E.E. III, Koeppen H., Astarita J.L., Cubas R., et al. . 2018. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 554:544–548. 10.1038/nature25501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey J.A., Steelman L.S., Chappell W.H., Abrams S.L., Franklin R.A., Montalto G., Cervello M., Libra M., Candido S., Malaponte G., et al. . 2012. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: how mutations can result in therapy resistance and how to overcome resistance. Oncotarget. 3:1068–1111. 10.18632/oncotarget.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano R.F.V., Hunger A., Mendonça S.A., Barbuto J.A.M., and Strauss B.E.. 2017. Immunomodulatory and antitumor effects of type I interferons and their application in cancer therapy. Oncotarget. 8:71249–71284. 10.18632/oncotarget.19531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métayer L.E., Vilalta A., Burke G.A.A., and Brown G.C.. 2017. Anti-CD47 antibodies induce phagocytosis of live, malignant B cells by macrophages via the Fc domain, resulting in cell death by phagoptosis. Oncotarget. 8:60892–60903. 10.18632/oncotarget.18492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molgora M., Supino D., Mantovani A., and Garlanda C.. 2018. Tuning inflammation and immunity by the negative regulators IL-1R2 and IL-1R8. Immunol. Rev. 281:233–247. 10.1111/imr.12609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy S.M., Goldschmidt R.A., Rao L.N., Ammirati M., Buchmann T., and Scanlon E.F.. 1989. The influence of surgical trauma on experimental metastasis. Cancer. 64:2035–2044. [DOI] [PubMed] [Google Scholar]
- Naing A., Papadopoulos K.P., Autio K.A., Ott P.A., Patel M.R., Wong D.J., Falchook G.S., Pant S., Whiteside M., Rasco D.R., et al. . 2016. Safety, Antitumor Activity, and Immune Activation of Pegylated Recombinant Human Interleukin-10 (AM0010) in Patients With Advanced Solid Tumors. J. Clin. Oncol. 34:3562–3569. 10.1200/JCO.2016.68.1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naing A., Infante J.R., Papadopoulos K.P., Chan I.H., Shen C., Ratti N.P., Rojo B., Autio K.A., Wong D.J., Patel M.R., et al. . 2018. PEGylated IL-10 (Pegilodecakin) Induces Systemic Immune Activation, CD8+ T Cell Invigoration and Polyclonal T Cell Expansion in Cancer Patients. Cancer Cell. 34:775–791.e3. 10.1016/j.ccell.2018.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oft M. 2014. IL-10: master switch from tumor-promoting inflammation to antitumor immunity. Cancer Immunol. Res. 2:194–199. 10.1158/2326-6066.CIR-13-0214 [DOI] [PubMed] [Google Scholar]
- Palucka K., and Banchereau J.. 2013. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 39:38–48. 10.1016/j.immuni.2013.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.A., and Minn A.J.. 2018. Combination Cancer Therapy with Immune Checkpoint Blockade: Mechanisms and Strategies. Immunity. 48:417–433. 10.1016/j.immuni.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H., Zhang H., Matei I.R., Costa-Silva B., Hoshino A., Rodrigues G., Psaila B., Kaplan R.N., Bromberg J.F., Kang Y., et al. . 2017. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer. 17:302–317. 10.1038/nrc.2017.6 [DOI] [PubMed] [Google Scholar]
- Perry C.J., Muñoz-Rojas A.R., Meeth K.M., Kellman L.N., Amezquita R.A., Thakral D., Du V.Y., Wang J.X., Damsky W., Kuhlmann A.L., et al. . 2018. Myeloid-targeted immunotherapies act in synergy to induce inflammation and antitumor immunity. J. Exp. Med. 215:877–893. 10.1084/jem.20171435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyonteck S.M., Akkari L., Schuhmacher A.J., Bowman R.L., Sevenich L., Quail D.F., Olson O.C., Quick M.L., Huse J.T., Teijeiro V., et al. . 2013. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 19:1264–1272. 10.1038/nm.3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Chen H., Wu X., Hu L., Huang Q., and Jin Y.. 2017. Interleukin-17 acts as double-edged sword in anti-tumor immunity and tumorigenesis. Cytokine. 89:34–44. 10.1016/j.cyto.2015.09.011 [DOI] [PubMed] [Google Scholar]
- Quail D.F., and Joyce J.A.. 2013. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19:1423–1437. 10.1038/nm.3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quante M., Varga J., Wang T.C., and Greten F.R.. 2013. The gastrointestinal tumor microenvironment. Gastroenterology. 145:63–78. 10.1053/j.gastro.2013.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja J., Ludwig J.M., Gettinger S.N., Schalper K.A., and Kim H.S.. 2018. Oncolytic virus immunotherapy: future prospects for oncology. J. Immunother. Cancer. 6:140 10.1186/s40425-018-0458-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rech A.J., Dada H., Kotzin J.J., Henao-Mejia J., Minn A.J., Twyman-Saint Victor C., and Vonderheide R.H.. 2018. Radiotherapy and CD40 Activation Separately Augment Immunity to Checkpoint Blockade in Cancer. Cancer Res. 78:4282–4291. 10.1158/0008-5472.CAN-17-3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A., Dummer R., Puzanov I., VanderWalde A., Andtbacka R.H.I., Michielin O., Olszanski A.J., Malvehy J., Cebon J., Fernandez E., et al. . 2017. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell. 170:1109–1119.e10. 10.1016/j.cell.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker P.M., MacFadyen J.G., Thuren T., Everett B.M., Libby P., and Glynn R.J.. CANTOS Trial Group . 2017. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 390:1833–1842. 10.1016/S0140-6736(17)32247-X [DOI] [PubMed] [Google Scholar]
- Ries C.H., Cannarile M.A., Hoves S., Benz J., Wartha K., Runza V., Rey-Giraud F., Pradel L.P., Feuerhake F., Klaman I., et al. . 2014. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 25:846–859. 10.1016/j.ccr.2014.05.016 [DOI] [PubMed] [Google Scholar]
- Rivera Vargas T., Benoit-Lizon I., and Apetoh L.. 2017. Rationale for stimulator of interferon genes-targeted cancer immunotherapy. Eur. J. Cancer. 75:86–97. 10.1016/j.ejca.2016.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C., Thomas L., Bondarenko I., O’Day S., Weber J., Garbe C., Lebbe C., Baurain J.F., Testori A., Grob J.J., et al. . 2011. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364:2517–2526. 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- Ruffell B., and Coussens L.M.. 2015. Macrophages and therapeutic resistance in cancer. Cancer Cell. 27:462–472. 10.1016/j.ccell.2015.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel J.A., Otsuka A., and Kabashima K.. 2018. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 8:86 10.3389/fonc.2018.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabo I., Stål O., Olsson H., Doré S., and Svanvik J.. 2008. Breast cancer expression of CD163, a macrophage scavenger receptor, is related to early distant recurrence and reduced patient survival. Int. J. Cancer. 123:780–786. 10.1002/ijc.23527 [DOI] [PubMed] [Google Scholar]
- Simpson T.R., Li F., Montalvo-Ortiz W., Sepulveda M.A., Bergerhoff K., Arce F., Roddie C., Henry J.Y., Yagita H., Wolchok J.D., et al. . 2013. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med. 210:1695–1710. 10.1084/jem.20130579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivan A., Corrales L., Hubert N., Williams J.B., Aquino-Michaels K., Earley Z.M., Benyamin F.W., Lei Y.M., Jabri B., Alegre M.L., et al. . 2015. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 350:1084–1089. 10.1126/science.aac4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer-Phelps A., Hassell B.A., and Ingber D.E.. 2019. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer. 19:65–81. 10.1038/s41568-018-0104-6 [DOI] [PubMed] [Google Scholar]
- Srivatsan S., Patel J.M., Bozeman E.N., Imasuen I.E., He S., Daniels D., and Selvaraj P.. 2014. Allogeneic tumor cell vaccines: the promise and limitations in clinical trials. Hum. Vaccin. Immunother. 10:52–63. 10.4161/hv.26568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue T., Morita S., Plichta D.R., Skelly A.N., Suda W., Sugiura Y., Narushima S., Vlamakis H., Motoo I., Sugita K., et al. . 2019. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 565:600–605. 10.1038/s41586-019-0878-z [DOI] [PubMed] [Google Scholar]
- Tauriello D.V.F., Palomo-Ponce S., Stork D., Berenguer-Llergo A., Badia-Ramentol J., Iglesias M., Sevillano M., Ibiza S., Cañellas A., Hernando-Momblona X., et al. . 2018. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 554:538–543. 10.1038/nature25492 [DOI] [PubMed] [Google Scholar]
- Todoric J., Antonucci L., and Karin M.. 2016. Targeting Inflammation in Cancer Prevention and Therapy. Cancer Prev. Res. (Phila.). 9:895–905. 10.1158/1940-6207.CAPR-16-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohme S., Simmons R.L., and Tsung A.. 2017. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 77:1548–1552. 10.1158/0008-5472.CAN-16-1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M.C., Chen Y., Krewski D., and Ghadirian P.. 2006. An overview of the association between allergy and cancer. Int. J. Cancer. 118:3124–3132. 10.1002/ijc.21752 [DOI] [PubMed] [Google Scholar]
- van den Eertwegh A.J., Versluis J., van den Berg H.P., Santegoets S.J., van Moorselaar R.J., van der Sluis T.M., Gall H.E., Harding T.C., Jooss K., Lowy I., et al. . 2012. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 13:509–517. 10.1016/S1470-2045(12)70007-4 [DOI] [PubMed] [Google Scholar]
- Varga J., and Greten F.R.. 2017. Cell plasticity in epithelial homeostasis and tumorigenesis. Nat. Cell Biol. 19:1133–1141. 10.1038/ncb3611 [DOI] [PubMed] [Google Scholar]
- Vétizou M., Pitt J.M., Daillère R., Lepage P., Waldschmitt N., Flament C., Rusakiewicz S., Routy B., Roberti M.P., Duong C.P., et al. . 2015. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 350:1079–1084. 10.1126/science.aad1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud S., Saccheri F., Mignot G., Yamazaki T., Daillère R., Hannani D., Enot D.P., Pfirschke C., Engblom C., Pittet M.J., et al. . 2013. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 342:971–976. 10.1126/science.1240537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderheide R.H. 2018. The Immune Revolution: A Case for Priming, Not Checkpoint. Cancer Cell. 33:563–569. 10.1016/j.ccell.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Li B., Wei Y., Zhao Y., Wang L., Zhang P., Yang J., He W., Chen H., Jiao Z., and Li Y.. 2018. Tumor-derived exosomes induce PD1+ macrophage population in human gastric cancer that promotes disease progression. Oncogenesis. 7:41 10.1038/s41389-018-0049-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Hu S., Chen X., Shi H., Chen C., Sun L., and Chen Z.J.. 2017. cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc. Natl. Acad. Sci. USA. 114:1637–1642. 10.1073/pnas.1621363114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Kim M.K., Di Caro G., Wong J., Shalapour S., Wan J., Zhang W., Zhong Z., Sanchez-Lopez E., Wu L.W., et al. . 2014. Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity. 41:1052–1063. 10.1016/j.immuni.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R.A. 2014. Coming full circle-from endless complexity to simplicity and back again. Cell. 157:267–271. 10.1016/j.cell.2014.03.004 [DOI] [PubMed] [Google Scholar]
- Whittaker S.R., Mallinger A., Workman P., and Clarke P.A.. 2017. Inhibitors of cyclin-dependent kinases as cancer therapeutics. Pharmacol. Ther. 173:83–105. 10.1016/j.pharmthera.2017.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarin D., Holmgaard R.B., Subudhi S.K., Park J.S., Mansour M., Palese P., Merghoub T., Wolchok J.D., and Allison J.P.. 2014. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl. Med. 6:226ra32 10.1126/scitranslmed.3008095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L., Ma Y., Raoult D., Kroemer G., and Gajewski T.F.. 2018. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science. 359:1366–1370. 10.1126/science.aar6918 [DOI] [PubMed] [Google Scholar]