Abstract
Background:
The literature shows that delayed or erroneous diagnosis of respiratory conditions may be common in primary care due to underuse of spirometry or poor spirometric technique. The Community Respiratory Assessment Unit (CRAU) was established to optimise diagnosis and treatment of respiratory disease by providing focused history-taking, quality-assured spirometry, and evidence-based guideline-derived management advice.
Aims:
To review the service provided by the CRAU to primary care health professionals. Methods: Data from 1,156 consecutive GP referrals over 4 years were analysed.
Results:
From the 1,156 referrals, 666 were referred for one of five common reasons: suspected asthma, confirmed asthma, suspected chronic obstructive pulmonary disease (COPD), confirmed COPD, or unexplained breathlessness. COPD was the most prevalent referral indication (445/666, 66.8%), but one-third of suggested diagnoses of COPD by the GP were found to be incorrect (161/445, 36%) with inappropriate prescribing of inhaled therapies resulting from this misdiagnosis. Restrictive pulmonary defects (56/666, 8% of referrals) were overlooked and often mistaken for obstructive conditions. The potential for obesity to cause breathlessness may not be fully appreciated.
Conclusions:
Misdiagnosis has significant financial, ethical, and safety implications. This risk may be minimised by better support for primary care physicians such as diagnostic centres (CRAU) or alternative peripatetic practice-based services operating to quality-controlled standards.
Keywords: community respiratory assessment unit, diagnosis, primary care, asthma, COPD, respiratory disease, spirometry
Introduction
Respiratory disease is the focus of 20% of all general practice consultations, the second most common reason for hospital admission, and the cause of one in five deaths in the UK. It costs the UK National Health Service (NHS) £6.6 billion per year and accounted for 62 million prescriptions in 2004.1 Optimising the recognition and management of lung-related illness is essential to minimise the burden of this ever-growing disease group.
In 2004, a new UK General Medical Services (GMS) contract introduced a pay for performance scheme known as the Quality and Outcomes Framework (QOF). This scheme financially rewards general practices that achieve predetermined targets over a wide range of clinical indicators to improve accuracy of diagnosis, proactive interventions for those with long-term conditions, and improves maintenance of disease registers. There are 1,050 points available, of which 45 are allocated to asthma indicators and 30 to chronic obstructive pulmonary disease (COPD), with additional general points (60 in total) for provision of smoking cessation advice. Points are given for the production of disease registers for asthma and COPD, confirmation of COPD with spirometry, and annual reviews for patients with asthma and COPD. Inclusion of spirometry as an indicator in the QOF has resulted in a significant increase in the number of general practitioners (GPs) offering spirometry.2 However, one national survey showed that very few nurses (12%) who were performing spirometry had undertaken training and few diagnosing and managing COPD had undertaken specialist training.3
A study by Lucas et al. has shown that 30% of patients referred to a primary care diagnostic unit were taking inhaled corticosteroids (ICS) without a clear indication; after 12 months the results showed that 11% had no indication to use ICS and ceased usage.4 Several studies have shown that asthma overdiagnosis/misdiagnosis is not an unusual event in clinical practice.5–7 Underdiagnosis of lung disease is not uncommon,8 but the use of spirometry has been shown to increase recognition.9,10 Current UK guidelines all highlight the importance of accurate diagnosis of asthma,11 COPD,12 and restrictive lung disease13 through a combination of history-taking, examination, and spirometry. A recent standards document has been published which discusses the key areas of quality required for diagnostic spirometry in primary care.2 Despite this, spirometry is often omitted by both primary and secondary care physicians when making respiratory diagnoses. The reasons for this vary,14 but time constraints and staffing15 as well as a lack of confidence in data interpretation10 are important contributors. Furthermore, evidence suggests that good quality spirometry from trained and experienced staff provides more robust and reliable results than office testing,16 although others have shown that well-trained office testing can be accurate.17 This problem is not limited to the UK; in 2009 Schermer et al.18 reported in a study of 15 general practices in the Netherlands that only 39% of the spirometry met acceptability and reproducibility criteria.
Centralisation of spirometry via a dedicated service has been previously discussed as a solution but not trialled in the UK;19 UK models to date have included within-practice services, peripatetic and centralised services, usually within a local hospital. A Community Respiratory Assessment Unit (CRAU) was established in 2004 in West London to provide diagnostic support to primary care physicians working within the Hammersmith and Fulham area.9 This area has a population of around 185,000 with prevalence rates of 6% for asthma and 1% for COPD (compared with modelled prevalence of 3.7%). It aimed to provide high quality spirometry in association with focused history-taking to enhance detection of respiratory disease. Furthermore, it aimed to encourage implementation of national guidelines on management of lung diseases including asthma and COPD by the provision of targeted information to both patients and referring doctors.
The audit reported here reviews the referrals to the service for the first 4 years of the programme and reflects on the role and relevance of the service to primary care.
Methods
The CRAU was based in a London secondary care hospital. The unit was a nurse-led facility, staffed for the first 2 years by two specialist respiratory nurses with extensive experience of caring for those with respiratory diseases in both hospital and the community, and subsequently run by the community respiratory nursing team. Access to a respiratory specialist for advice was always available, initially via a Professor of Respiratory Medicine and subsequently a Consultant in Integrated Respiratory Medicine. All local GPs had access to the service. GPs were informed about the unit by means of a letter from the Executive Director of the PCT, as well as a personal visit from the CRAU nurses to each primary care practice.
Spirometry was carried out following a standard operational procedure based on key national and international guidance.20–25 Briefly, height, weight, ethnic origin, sex, and age were recorded for calculation of normal and predicted values. Date, time of last use, and type of bronchodilator used were recorded. Pre-test conditions followed the usual exclusion criteria (e.g. unstable angina, haemoptysis, active tuberculosis or other respiratory infection, recent thoracic surgery) and normal procedures such as avoidance of smoking for 24 hrs, no use of bronchodilators in the previous 4 hrs, and the test being undertaken while sitting upright with a nose clip. Three technically acceptable manoeuvres were required, two within 100ml (5%) to ensure reproducibility criteria. If prebronchodilator spirometry was normal with a forced expiratory volume in one second/forced vital capacity (FEV1/FVC) ratio above 0.7, and was technically satisfactory, no bronchodilator reversibility testing was required. Spirometer calibration was recorded and completed on a weekly basis and spirometers were cleaned on a daily basis.
Proformas were developed for GP referral, nurse consultation, and diagnostic reports to primary care. A database using the information from the proformas and spirometry results was developed with collated information for each consecutive patient referral to the service between January 2005 and December 2008 using these three sources as well as spirometry results.
Referral forms required GPs to suggest a referral diagnosis by selecting one from a list which included: suspected or confirmed asthma, suspected or confirmed COPD, unexplained breathlessness, and cough. Information was also obtained on the action the GP would have taken if the service was not available. Requests were faxed to the CRAU and patients were encouraged, through an information leaflet dispensed by the GP, to schedule a convenient appointment time by telephone.
Nurse history sheets identified current medications, past medical, family and smoking history as well as current respiratory symptoms and their duration. Assessment of control using the Royal College of Physicians' ‘Three Key Questions’26 were also included for suspected or confirmed asthma referrals.
Standardised semi-structured reports to the GPs contained guideline-focused generic advice on diagnosis and management tailored to the individual based on their history and spirometry results. For example: “There is evidence of fixed airways narrowing on spirometry which does not improve after use of an inhaled bronchodilator. In light of the smoking history and age, COPD is the likeliest diagnosis. You might consider a trial of long-acting anticholinergic therapy. The patient has been given details of the local smoking cessation service and a British Lung Foundation leaflet on COPD”. Data from the referral diagnosis was also confirmed or refuted as part of the report to the GP.
All information from the referral proforma, nurse-completed medical history sheets, spirometry, and letter to the GP was entered into an SPSS database (Version 17.0). Each referral request was entered separately; if patients had attended more than once or been referred more than once, these were matched (where possible) and collated for each patient to assess for repeat visit (if any). Data on each referring GP practice were collected.
GPs were asked to suggest a referral diagnosis from a list of five: (1) suspected asthma, (2) definite asthma, (3) suspected COPD, (4) definite COPD, and (5) unexplained breathlessness. For the sake of clarity this audit focused upon referrals where the GP stipulated just one of the five possible referral diagnoses; those who were referred with multiple possible diagnoses, no diagnoses or ‘unexplained cough’ were excluded.
Results
A total of 1,156 referrals (512 male, age 61.3±15.6 years, body mass index (BMI) 27±6.8 kg/m2) were received by the unit between January 2005 and December 2008 (range 217–348 per year); 754 (65%) of the patients referred to the CRAU were smokers. A total of 162 did not attend and 30 did not complete their consultation due to technical problems (spirometry not completed to suitable standard). This was most frequently because the patient was feeling unwell (including haemoptysis in one case), although several were technically unsatisfactory due to an inability to produce consistently satisfactory results. Several patients reported angina in the previous 48 hrs, therefore spirometry was contraindicated. In some cases patients developed angina/chest symptoms during the test, at which point spirometry testing ceased and appropriate medical care was undertaken.
In total, 964 patients attended the unit and received a final diagnosis, 878 of which were first appointments and 86 were repeat attendances. For 96% of the referrals a suggested diagnosis was given by the GP as well as some additional details about the reason for referral to the Unit. The GPs of 430 (49%) first appointment referrals stated that they would have referred to a specialist respiratory outpatient clinic had the CRAU not been available.
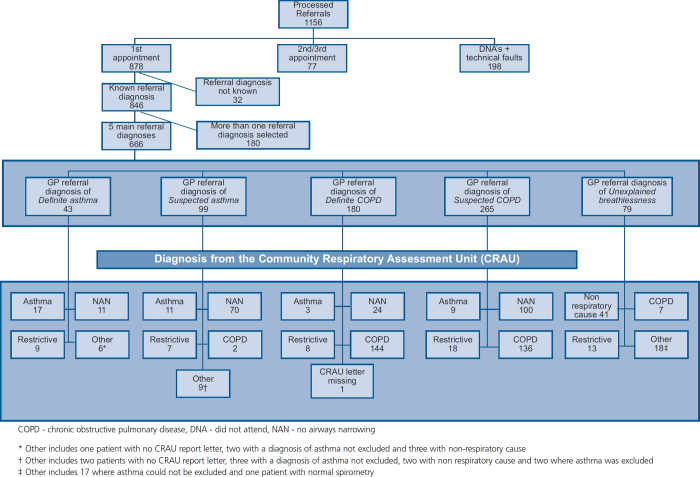
From the 878 referrals for a first attendance, 842 had a completed GP referral diagnosis on the referral form. Of these, 666 had one of the five main referral diagnoses categories ticked (definite asthma, suspected asthma, definite COPD, suspected COPD and unexplained breathlessness). Figure 1 summarises the referral diagnoses and the confirmed diagnoses from the CRAU for each category.
Figure 1. GP referrals to the Community Respiratory Assessment Unit.
Twenty-eight of the 32 general practices in the PCT who were offered the service used it, with an average referral rate of 1–273 per practice (median 27). There were more referrals in the first quartile of the calendar year (1st quartile 373, 2nd quartile 245, 3rd quartile 241, 4th quartile 270). The average distance between the general practice and the CRAU was 2.03 miles (range 0.67–3.76).
Asthma
Of the 43 patients with a GP referral diagnosis of definite asthma (21 males, age 56.6±18.21 years, BMI 29.0±7.4 kg/m2), 17 (40%) were confirmed by virtue of current airway narrowing (FEV1/FVC <0.7) plus either demonstrated reversibility (>200mls) or highly suggestive history. Nine had a restrictive defect and 11 displayed no airways narrowing on testing. Importantly, all of the patients with no airway narrowing and normal spirometry in this group were already on regular inhaled therapy.
Of the 99 with a GP referral diagnosis of suspected asthma (38 males, age 45.5±17.66 years, BMI 26.12±6.3 kg/m2), this diagnosis could be confirmed in only 11 (10%); confirmation was defined as the presence of airway narrowing which was reversed after bronchodilation (>200mls) and/or a highly suggestive history of variable symptoms, personal or family history of atopic disease and/or no history of smoking. Seventy patients (70%) had no evidence of airways narrowing, while seven demonstrated a restrictive defect and two were deemed to have COPD based on a strong smoking history/absence of reported personal or family history of atopic diseases/pressure-dependent airway collapse on flow-volume curves. Once again, the majority (45/70) of the patients with normal spirometry (FEV1/FVC >0.7) in the suspected asthma group were taking regular β-agonists and 21 were taking ICS, making underdiagnosis because of a treatment effect probable.
Of the remaining 15 GP referrals for suspected (n=9) or definite (n=60) asthma, there were five cases for whom it was reported to the GP that asthma could not be excluded. The results from five referrals suggested a non-respiratory cause and there was no documentation available with a final diagnosis in three cases. In the final two cases asthma was excluded.
COPD
The largest number of referrals to the CRAU were for suspected or confirmed COPD (67%, 445/666) (220 male, age 66.6±11.8 years, BMI 26.8±6.9 kg/m2, 396 current/ex-smokers), and in one-third of these (161/445) the diagnosis of COPD was refuted on the basis of there being no evidence of airway narrowing (FEV1/FVC <0.7). Seventy-seven percent reported that symptoms began in their 5th, 6th or 7th decade and 63% reported progressive breathlessness in the last 12–36 months. Of the 180 referred with definite COPD, 144 (80%) were confirmed to have COPD. Three were diagnosed with asthma (positive response to bronchodilators >200mls), eight had a restrictive defect, 24 (13%) were found to have no evidence of airway narrowing and one patient had no CRAU letter available and thus no final CRAU diagnosis could be obtained. These patients were excluded from the analysis.
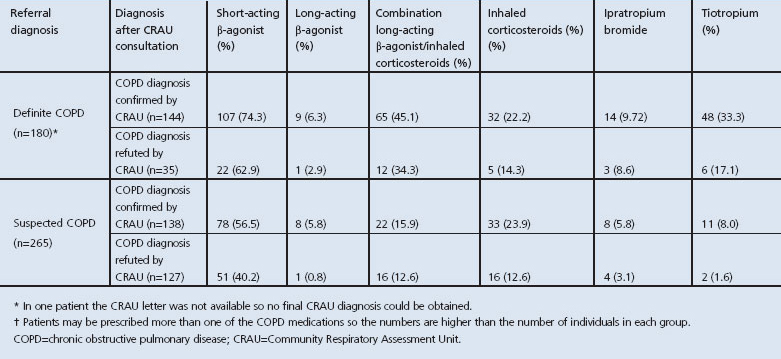
Of the 265 referred with suspected COPD, only half (n=138, 52%) were confirmed to have COPD; 100 (38%) had normal spirometry, precluding a diagnosis of COPD, and 18 were found to have a restrictive rather than an obstructive defect. Nine were diagnosed with asthma on the basis of history and evidence of reversible airway narrowing. Table 1 shows the prescribed medications of those referred by the GP with definite or suspected COPD. Interestingly, 45% (73/162) of patients wrongly diagnosed with COPD were already taking one or more inhaled medications; 30% (49/162) were regularly using ICS and 5% were on tiotropium.
Table 1. Prescribed medications for those with definite or suspected COPD*.
Of the 282 referrals where COPD was confirmed, FEV1/FVC and predicted FEV1 were available in the database for 237 cases (84%); 119 (50%) were classed as moderate COPD (FEV1/FVC <0.7 and FEV1 50-<80% predicted), 84 (35%) were classed as severe (FEV1/FVC <0.7 and FEV1 30-<50% predicted) and 7% were classed as very severe (FEV1/FVC <0.7 and FEV1 <30% predicted).
Unexplained breathlessness
Of the 79 referred with unexplained breathlessness (34 male, age 55.9±17.2 years, BMI 28.8±5.9 kg/m2), the majority (n=41, 52%) had a non-respiratory cause (with the report sent to the GP suggesting consideration of a cardiac cause, obesity, anaemia or other). Fourteen had a restrictive defect and six were found to have COPD. In the remaining 18 cases, asthma could not be excluded in 17 patients and in one patient with normal spirometry no explanation could be suggested for the symptoms.
Restrictive lung disease
Fifty-six of the 666 referrals (8%) were found to have a restrictive defect on spirometry. All of these had breathlessness as a major symptom; 26 were referred with a diagnosis of COPD, 16 with asthma and 14 because of ‘unexplained breathlessness’. Available chest radiographs were reviewed for 42/56 (no radiograph was available for 14) and the cause for the breathless and restriction was postulated for each of the 56 patients using BMI ± radiograph. The cause of the problem was hypothesised to be due to a BMI >30 kg/m2 in 22 patients. In six patients, raised BMI plus another cause was identified (e.g. gastric pull-up surgery, cardiac enlargement). A BMI >30 kg/m2 was the probable sole or a major contributory cause of breathlessness and restrictive spirometry. A further five were classed as overweight (BMI 25–30 kg/m2) which may contribute to breathlessness and restriction with no other cause found. In nine patients no cause could be identified from the information available. In the remaining 14 patients 3 had a mild restrictive defect with queries about sub-optimal effort or ethnicity. Two had unilateral diaphragm elevation, two had infective shadowing and two had TB fibrosis. The remaining five patients had atelectasis (1), ILD (1), asbestos-related pleural disease (1), pneumonia (1) and myotonic dystrophy (1).
Discussion
Main findings
In several countries respiratory assessment units and services have been developed to improve the diagnosis of respiratory diseases such as asthma and COPD and to overcome problems with misdiagnosis. These services are heterogeneous and may include spirometry,27 review of medical history,28 or radiography and oxygen saturation assessments.9 The review may consist of paper-based information or may involve a face-to-face review or consultation29 with components of the diagnostic services mentioned above.9 They are also delivered by a diverse range of healthcare professionals such as respiratory nurses,9 GPs,30 or respiratory specialists.31 Centralisation of spirometry via a dedicated service has previously been discussed as a solution but it has not been trialled in the UK.19 To date, UK models have included within-practice services, peripatetic and centralised services, usually within a local hospital. This report evaluates 4 years of activity at the CRAU which was established in 2004 in West London to provide diagnostic support to primary care physicians. The service aimed to provide high quality spirometry in addition to focused history-taking to enhance detection of respiratory disease.
As shown in the preliminary 1-year report of the CRAU, the majority of referrals were for COPD.9 Hassett et al. hypothesised that the financial benefit for COPD cases confirmed by spirometry under the Government's QOF system contributed to this referral bias. This still holds true. However, the benefit of spirometry clearly supersedes this single gain. In one-third of COPD referrals spirometry did not confirm the initial diagnosis. ‘Mislabelling’ of patients leads to high levels of inappropriate prescribing, as shown in Table 1. If this error rate can be extrapolated and regarded as usual practice, the availability and performance of quality-assured spirometry on every suspected COPD patient prior to prescription of an inhaler could have a substantial impact on the prescribing practice of GPs and their overall medicines budget. Moreover, one-third of wrongly labelled patients were on inhaled steroids and nearly half were on β-agonists, raising self-evident concerns about patient safety and appropriate pharmacotherapy. The mislabelling of patients in this study is in no way a criticism of general practices for they themselves referred the patients for testing, acknowledging the potential fallibility of clinical judgement.
Pulmonary restrictive defects, while not common, were not routinely suggested by GPs as a possible diagnosis. Fifty-six of the 666 referrals (8%) were found to have restrictive defects, the majority having been mistakenly labelled as having obstructive airways diseases and a smaller number being referred with unexplained breathlessness. We were able to trace recent chest radiographs for most of these subjects, the majority of which were normal, and it is likely that obesity was the cause of the symptom of breathlessness and restriction in 39% (22/56) of those found to have such a defect. Significant obesity can reduce the expiratory reserve volume and lead to diminished basal ventilation during tidal breathing,32 although the full effect of a raised BMI on spirometry is debatable.33 Nevertheless, these patients all reported breathlessness to the CRAU nurse, and there may have been a lack of awareness among referrers that raised BMI is an important cause of lung symptoms. Another issue might be reluctance among GPs to address obesity-related issues, as demonstrated in a study by Michie et al. in 2007 which found that 52% of GPs questioned had concerns about discussing weight with patients.34 Education to improve awareness of restrictive lung disease and, in particular, of obesity as a cause of breathlessness is needed to prevent unnecessary referral and investigation in these cases.
It is interesting to note that half of the first appointment referrals (430/878) would have been referred to a respiratory specialist if the services of the CRAU were not available. This would suggest that GPs are looking for support to help them obtain accurate respiratory diagnoses in a significant proportion of cases, and it suggests that the CRAU was recognised as a suitable alternative to secondary care in these cases.
Limitations of this study
We are unable to draw meaningful conclusions about the value of CRAU for asthma referrals for two reasons. First, there is likely to be an ‘on drug’ effect, supported by the fact that approximately half of the patients referred with a diagnosis of asthma were already taking regular inhaled medications at the time of their CRAU appointment. Second, the variable nature of the disease makes spirometry less useful when performed on a single occasion. Monitoring of the peak expiratory flow rate and diary carding or assessment of airway inflammation may produce more dependable results than a one-off measurement. Attempts to obtain spirometry should always be carried out before starting treatment, or spirometry should be repeated on more than one occasion.
Interpretation of findings in relation to previously published work
Mislabelling or misdiagnosis of patients has been reported in other countries. Arne et al. showed that only one-third of Swedish patients with COPD had their diagnosis confirmed by spirometry.35 Tinkelman et al. showed in a study in Scotland and the USA that 51.5% of patients with confirmed COPD reported a previous diagnosis of asthma.36 Walters et al. suggested that a diagnosis of COPD may be intentionally delayed by doctors due to their negative attitudes to prognosis and perceived unwillingness of patients for a diagnosis.10
Implications for future research, policy and practice
The CRAU was devised to improve diagnostic accuracy in primary care; however, other models could be considered to achieve the same goal. Alternatives could include a programme where GPs or nurse practitioners are trained to provide their own quality-assured spirometry and diagnostic assessment service, removing dependency on secondary care. An entirely peripatetic service where the same nurses or clinical scientists rotate throughout the PCT moving from practice to practice on a rolling rota may be a further way of providing quality-assured spirometry and improving its accessibility in each practice. Returning diagnostic decision-making to the GP may instil more confidence in the final diagnosis and would seem preferable for both patient and doctor if it can be done to the necessary exacting standards and if the results can be confidently interpreted.
In summary, this study highlights several benefits to support the development of community respiratory diagnostic services. The CRAU reduced diagnostic inaccuracy in patients referred, and thereby had the potential to facilitate improved prescribing of respiratory medicines in primary care. Community respiratory diagnostic services are likely to reduce the volume of hospital referrals. With regard to the educational benefit of CRAUs, it may be that lessons learnt from early referrals informed GPs and reduced the rate of subsequent referrals.
Conclusions
Underdiagnosis or misdiagnosis of respiratory conditions is common in primary care, in part due to poor technique and underuse of spirometry. This study has shown that a community respiratory assessment unit can improve the diagnosis and treatment of respiratory disease. Approximately one-third of COPD diagnoses made in the community were incorrect, and high levels of inappropriate prescribing were seen as a consequence.
Acknowledgments
Funding This study was unfunded.
We would like to thank the GP practices in Hammersmith and Fulham who participated in this project, as well as the patients who attended the service.
Footnotes
The authors declare that they have no conflicts of interest in relation to this article. ISP is an Associate Editor of the PCRJ, but was not involved in the editorial review of, nor the decision to publish, this article
References
- British Thoracic Society. The Burden of Lung Disease. London: British Thoracic Society, 2006. [Google Scholar]
- Levy ML, Quanjer PH, Booker R, et al. Diagnostic spirometry in primary care: Proposed standards for general practice compliant with American Thoracic Society and European Respiratory Society recommendations. Prim Care Respir J 2009;18(3):130–47. http://dx.doi.org/10.4104/pcrj.2009.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton J, Madoc-Sutton H, Sheikh A, Frank TL, Walker S, Fletcher M. National survey on the roles and training of primary care respiratory nurses in the UK in 2006: are we making progress?. Prim Care Respir J 2007;16(5):284–90. http://dx.doi.org/10.3132/pcrj.2007.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas AAEM. Overtreatment with inhaled corticosteroids and diagnostic problems in primary care patients, an exploratory study. Fam Pract 2008;25(2):86–91. http://dx.doi.org/10.1093/fampra/cmn006 [DOI] [PubMed] [Google Scholar]
- Marklund B, Tunsater A, Bengtsson C. How often is the diagnosis bronchial asthma correct?. Fam Pract 1999;16(2):112–16. http://dx.doi.org/10.1093/fampra/16.2.112 [DOI] [PubMed] [Google Scholar]
- LindenSmith J, Morrison D, Deveau C, Hernandez P. Overdiagnosis of asthma in the community. Can Respir J 2004;11(2):111–16. [DOI] [PubMed] [Google Scholar]
- Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ 2008;179(11):1121–31. http://dx.doi.org/10.1503/cmaj.081332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. Prevalence of chronic obstructive pulmonary disease according to BTS, ERS, GOLD and ATS criteria in relation to doctor's diagnosis, symptoms, age, gender, and smoking habits. Respiration 2005;72(5):471. http://dx.doi.org/10.1159/000087670 [DOI] [PubMed] [Google Scholar]
- Hassett R, Meade K, Partridge MR. Enhancing the accuracy of respiratory diagnoses in primary care: a report on the establishment of a Community Respiratory Assessment Unit. Prim Care Respir J 2006;15(6):354–61. http://dx.doi.org/10.1016/j.pcrj.2006.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters JA. Under-diagnosis of chronic obstructive pulmonary disease: a qualitative study in primary care. Respir Med 2008;102(5):738. http://dx.doi.org/10.1016/j.rmed.2007.12.008 [DOI] [PubMed] [Google Scholar]
- British Thoracic Society/Scottish Intercollegiate Guidelines Network (BTS/SIGN). British guideline on the management of asthma. 2009.
- Department of Health. Consultation on a strategy for services for chronic obstructive pulmonary diseases (COPD) in England. London: Department of Health, 2010. [Google Scholar]
- Wells AAU, Hirani N. Interstitial lung disease guideline. Thorax 2008;63(Suppl 5):v1–58. http://dx.doi.org/10.1136/thx.2008.101691 [DOI] [PubMed] [Google Scholar]
- Roberts N, Smith S, Partridge M. Why is spirometry underused in the diagnosis of the breathless patient: a qualitative study. BMC Pulm Med 2011;11(1):37. http://dx.doi.org/10.1186/1471-2466-11-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PL. Practice management and chronic obstructive pulmonary disease in primary care. Am J Med 2007;120(8):S23–7. http://dx.doi.org/10.1016/j.amjmed.2007.04.009 [DOI] [PubMed] [Google Scholar]
- Lange P, Andersen KK, Munch E, Sorensen TB, Dollerup J, Kasso K. Quality of COPD care in hospital outpatient clinics in Denmark: the KOLIBRI study. Respir Med 2009;103(11):1657–62. http://dx.doi.org/10.1016/j.rmed.2009.05.010 [DOI] [PubMed] [Google Scholar]
- Schermer TR, Jacobs JE, Chavannes NH, et al. Validity of spirometric testing in a general practice population of patients with chronic obstructive pulmonary disease (COPD). Thorax 2003;58(10):861–6. http://dx.doi.org/10.1136/thorax.58.10.861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermer TR, Crockett AJ, Poels PJ, et al. Quality of routine spirometry tests in Dutch general practices. Br J Gen Pract 2009;59(569):e376–82. http://dx.doi.org/10.3399/bjgp09X473088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright P. Does screening for COPD by primary care physicians have the potential to cause more harm than good?. Chest 2006;129:833–5. http://dx.doi.org/10.1378/chest.129.4.833 [DOI] [PubMed] [Google Scholar]
- Nursing and Midwifery Council (NMC). Guidelines for records and record keeping. 2005.
- British Thoracic Society/Association for Respiratory Technology and Physiology (BTS/ARTP). Guideline for the measurement of respiratory function. 1994.
- Hammersmith and Fulham Patient Group Direction. 2005.
- European Community for Coal and Steel. Report Working Party. Standardisation of lung function tests. 1993.
- American Thoracic Society. Standardisation of spirometry update. 1994.
- British Thoracic Society. British guideline on the management of asthma. 2009.
- Steven K, Neville RG, Hoskins G, Sullivan FM, Drummond N, Alder EM. The RCP's Three Key Questions' for asthma: review of practical use. Br J Community Nurs 2002;7(6):300–03. [DOI] [PubMed] [Google Scholar]
- White PW, Wong T, Fleming B. Primary care spirometry: test quality and feasibility and usefulness of specialist reporting. Br J Gen Pract 2007;57:701–05. [PMC free article] [PubMed] [Google Scholar]
- Vaughan RCR, MacIntyre D. An outreach spirometry service for Greater Glasgow Health Board: does it help in diagnosis?. Eur Respir J 2006;28:945–52.16870668 [Google Scholar]
- Wolfenden H, Bailey L, Murphy K, Partridge MR. Use of an open access spirometry service by general practitioners. Prim Care Respir J 2006;15:252–5. http://dx.doi.org/10.1016/j.pcrj.2006.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R, Whittaker M, Hanney K, Shackell B. A pilot study of a mobile spirometry service in primary care. Prim Care Respir J 2005;14:169–71. http://dx.doi.org/10.1016/j.pcrj.2004.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P, Mitchell P, Diamantea F, Warburton CJ, Davies L. Effect of primary care spirometry on the diagnosis and management of COPD. Eur Respir J 2006;28:945–52. http://dx.doi.org/10.1183/?9031936.06.00019306 [DOI] [PubMed] [Google Scholar]
- Partridge MR, Ciofetta G, Hughes JM. Topography of ventilation-perfusion ratios in obesity. Bull Eur Physiopathol Respir 1978;14(6):765–73. [PubMed] [Google Scholar]
- Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol 2010;108(1):206–11. http://dx.doi.org/10.1183/?09031936.06.00019306 [DOI] [PubMed] [Google Scholar]
- Michie S. Talking to primary care patients about weight: a study of GPs and practice nurses in the UK. Psychol Health Med 2007;12(5):521–5. http://dx.doi.org/10.1080/13548500701203441 [DOI] [PubMed] [Google Scholar]
- Arne M, Lisspers K, Stallberg B How often is diagnosis of COPD confirmed with spirometry. Respir Med 2009;104:550–6. http://dx.doi.org/10.1016/j.rmed.2009.10.023 [DOI] [PubMed] [Google Scholar]
- Tinkelman DG, Price DB, Nordyke RJ, Halbert RJ. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma 2006;43:75–80. http://dx.doi.org/10.1080/02770900500448738 [DOI] [PubMed] [Google Scholar]