Abstract
Background:
In primary care, formal functional capacity testing is not always feasible. Guidelines for family practitioners suggest the use of dyspnoea scales to assess exercise tolerance in patients with chronic obstructive pulmonary disease (COPD).
Aims:
To examine whether the use of activity-based dyspnoea scales can substitute for actual functional capacity testing.
Methods:
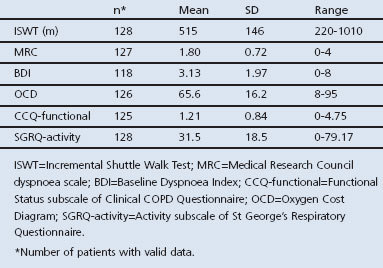
128 subjects (49% at risk of COPD, 24% GOLD stage I, 17% GOLD stage II, 9% GOLD stage III) performed an Incremental Shuttle Walk Test (ISWT) and completed the Medical Research Council dyspnoea scale (MRC), Baseline Dyspnoea Index (BDI), Oxygen Cost Diagram (OCD), Clinical COPD Questionnaire (CCQ), and St George's Respiratory Questionnaire (SGRQ).
Results:
Analysis of variance showed that the relationship between the ISWT and the MRC dyspnoea scale was statistically significant but moderate (p<0.001, R2=0.166). Correlations between the ISWT and the other dyspnoea scales were also moderate (correlation coefficients 0.34–0.42). Combining the dyspnoea scales in one analysis resulted in a proportion of explained variance of the ISWT of 21.4% (R2=0.214).
Conclusions:
Dyspnoea scales cannot substitute for formal functional capacity testing. Authors of COPD guidelines should consider stating more specifically that the MRC and similar scales measure (self-reported) activity-related dyspnoea but cannot replace objectively measured functional capacity.
Keywords: chronic obstructive pulmonary disease, functional capacity, dyspnoea scales, quantitative research
Introduction
In line with the definition of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) committee,1 the severity of COPD is conventionally expressed by the degree of airflow obstruction as measured by spirometry. However, several studies in the past two decades have shown little association between airflow obstruction and other markers of disease severity such as respiratory symptoms, disability, exercise tolerance/physical functional capacity, exacerbations, and health-related quality of life.2,3
In response to these findings, additional measures of disease severity have been proposed.2,4,5 A growing body of research shows that limitation of functional capacity is a better predictor of disability and mortality in COPD patients than airflow limitation.6–9 Apart from its prognostic value, functional capacity testing is useful in evaluating the effect of therapeutic interventions, and improvement of functional capacity is one of the main treatment goals in the management of COPD. These factors have led to a general consensus on the importance of measuring functional capacity in all patients with COPD.10
However, measuring functional capacity requires standardised testing conditions. In a hospital or laboratory setting these requirements are relatively easy to realise, but it is usually difficult to implement standardised functional capacity testing in routine primary care.11 National COPD guidelines recommend annual evaluation of exercise limitations or functional capacity and mention the use of dyspnoea scales (especially the Medical Research Council (MRC) scale12) for performing these measurements.13–15 This suggests that exercise tolerance and daily functioning can be measured by a dyspnoea scale. For instance, the MRC score is used to differentiate between patients referred for physical therapy in primary care.15 There is, however, little evidence to support the use of dyspnoea scales to assess functional capacity in primary care patients.16
In Dutch primary care the majority of patients (∼80%) have mild to moderate COPD (GOLD stages I and II),17 while most research on functional capacity and dyspnoea scales has been performed in patients with severe to very severe COPD. For the current paper our population consisted mainly of patients with mild to moderate disease and of subjects at risk of developing the disease in years to come (i.e. middle-aged men and women who are current smokers and have chronic cough, sputum production, or dyspnoea). These groups make up the majority of the COPD patient population managed in family practice.
The aim of this study was to examine whether assessment of activity-based dyspnoea — using existing and validated scales -can be a useful substitute for the measurement of the actual outcome of interest in COPD (i.e. limitation of functional capacity). Addressing this question is especially important for primary care professionals who are involved in the management of patients with COPD and who do not have access or facilities to perform standardised functional capacity tests.
Methods
Subjects
Our study population consisted of 128 individuals (57 men) recruited through newspaper advertisements and radio announcements for a primary care smoking cessation intervention study between January 2001 and November 2001.18 Thus, all participants were current smokers. Exclusion criteria were smoking history <5 years; history of asthma; acute exacerbation or changes in respiratory treatment regimen in the preceding 4 weeks; and concomitant co-morbidity which may influence functional capacity (i.e. cardiovascular, neurological, endocrine diseases, and/or locomotor limitations). Subjects at risk of COPD were included in this study as a reference group because family practitioners are increasingly encouraged to offer unsolicited advice to patients who smoke, but COPD researchers typically exclude these subjects.
Ethics approval
The Medical Ethics Committee of the Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands approved the study protocol. All subjects were informed about the study protocol and signed a consent form.
Measurements
Pulmonary function tests
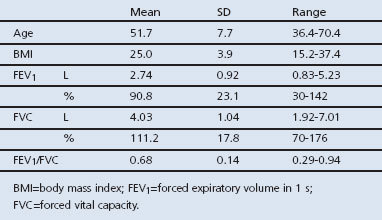
Spirometry was performed before and 20 min after inhalation of two puffs of 200μg salbutamol using a metered-dose inhaler with a spacer device (Volumatic®, GlaxoSmithKline, Zeist, The Netherlands). Inhalation of any bronchodilators was withheld for at least 12 hrs before pulmonary function was tested. Flow-volume curves, forced expiratory volume in 1 s (FEV1), and forced vital capacity (FVC) were measured using a flow screen (Masterlab®, Jaeger, Wurzburg, Germany). The highest FEV1 and FVC values of ≥3 acceptable forced manoeuvres were used for analysis. The FEV1 value obtained was related to a reference value and expressed as percentage of the predicted value (FEV1%).19 Age, height (m), weight (kg), and body mass index (BMI, kg/m2) were assessed in each participant.
Assessment of functional capacity
The Incremental Shuttle Walk Test (ISWT) was used to establish the functional capacity of the study subjects. The ISWT is a standardised test in which subjects have to walk up and down a 10 m course marked with shuttles. Walking speed is dictated by an audiotape and increases every minute. We adhered to the measurement protocol described by Singh and colleagues.20 The outcome measure of the ISWT is the distance walked in metres. The maximum possible score is 1020 m.
Assessment of activity-based dyspnoea
Activity-based dyspnoea was assessed with five validated dyspnoea scales. The MRC dyspnoea scale assesses any limitation or lack of ability to perform an activity in the manner or within the range considered normal for a human being.12,21 The MRC is a single-score scale with a range from 1 (‘I only get breathless with strenuous exercise’) to 5 (‘I am too breathless to leave the house’). In the current study the MRC scale had our specific interest because its use is recommended in several COPD guidelines.13–15,22
The Baseline Dyspnoea Index (BDI) assesses three components that evoke dyspnoea: functional impairment, magnitude of task, and magnitude of effort.23 The scores of the components are added; the total score ranges from 0 to 12. Higher scores indicate more dyspnoea.
The Oxygen Cost Diagram (OCD) consists of a list of several daily activities positioned alongside a 100 mm vertical scale in proportion to their oxygen cost.24 Higher scores indicate fewer limitations due to dyspnoea.
The Clinical COPD Questionnaire (CCQ) was developed to measure functional status and symptoms in daily clinical practice.25 It consists of three subscales: Symptoms, Functional Status, and Mental State. Item and subscale scores range from 0 (best) to 6 (worst). In the current study only the Functional Status subscale was used.
The St George's Respiratory Questionnaire (SGRQ) measures disease-specific health status and has three subscales: Symptoms, Activity, and Impact. The weighted individual item scores are added and divided by their total subscore. Score range is 0–100, higher scores indicating more limitations.26 In this study only the Activity subscale was used.
Statistical analysis
In this cross-sectional study, statistical analyses were performed using SPSS 16.0 statistical software.
The distribution of the MRC scores in the population was unequal; 48 subjects (37.8%) scored 1, 57 (44.9%) scored 2, 16 (12.6%) scored 3, and six (4.7%) scored 4. No patients had the maximal score of 5. Because of the limited number of patients with MRC score 4, we combined scores 3 and 4 for further analysis.
The relationship between the MRC and functional capacity was examined with univariate ANOVA and Tukey's post hoc analyses. The relationship between the other activity-based dyspnoea scales and the distance walked on the ISWT was examined using Pearson's correlation coefficients.
Univariate ANOVA was then performed to examine if the scales combined would explain more of the variance in the ISWT than the MRC alone. The model was subsequently reduced by stepwise exclusion of each (sub)scale with a p value >0.10. Statistical significance was assumed at p<0.05.
Results
Demographics
Of the 128 smokers (57 men, 71 women) recruited to the study, 63 (49%) were considered to be at risk of COPD with an FEV1/FVC ratio >0.70 and the presence of chronic cough, sputum production, and/or dyspnoea. According to the GOLD classification, 31 subjects (24%) had mild COPD, 22 (17%) had moderate COPD, and 11 (9%) had severe COPD.1 Table 1 shows the demographic and lung function characteristics of the study population.
Table 1. Demographic and pulmonary function characteristics of the study population (n=128).
Incremental Shuttle Walk Test
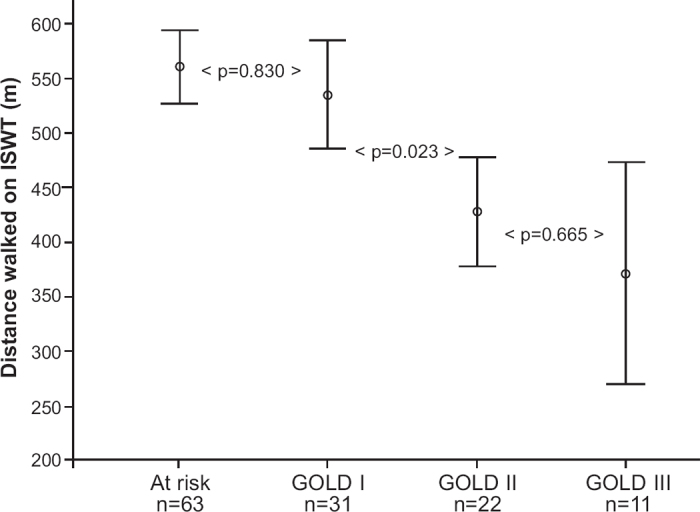
Scores on the ISWT were normally distributed. Mean (SD) distance walked on the ISWT was 515 (146) m, with distances ranging from 220 to 1010 m. Figure 1 shows the relationship between the ISWT and GOLD stage. The mean difference in distance walked was statistically significant between GOLD stages I and II (p=0.023), but not between subjects ‘at risk’ and those with GOLD stage I (p=0.830) or between GOLD stages II and III (p=0.655). Mean distances between all non-subsequent stages were significantly different (i.e. between ‘at risk’ and GOLD II, ‘at risk’ and GOLD III, and between GOLD I and GOLD III).
Figure 1. GOLD stage and mean distance walked during the ISWT. Bars indicate 95% confidence intervals. ISWT=Incremental Shuttle Walk Test in metres; GOLD=Global Initiative for Chronic Obstructive Lung Disease.
Activity-based dyspnoea scales
Table 2 shows the group mean values of the ISWT and the dyspnoea scales.
Table 2. Group mean values of the ISWT and dyspnoea scales in the study population.
Relationship between activity-based dyspnoea scales and ISWT
The mean distance walked was 572 m (range 290–1010) in subjects with MRC score 1, 513 m (range 260–810 m) in subjects with MRC score 2, and 400 m (range 220–730) in subjects with MRC score 3–4 (Figure 2).
Figure 2. Mean values of ISWT per MRC score. Bars indicate 95% confidence intervals. ISWT=Incremental Shuttle Walk Test in metres; MRC=Medical Research Council dyspnoea questionnaire.
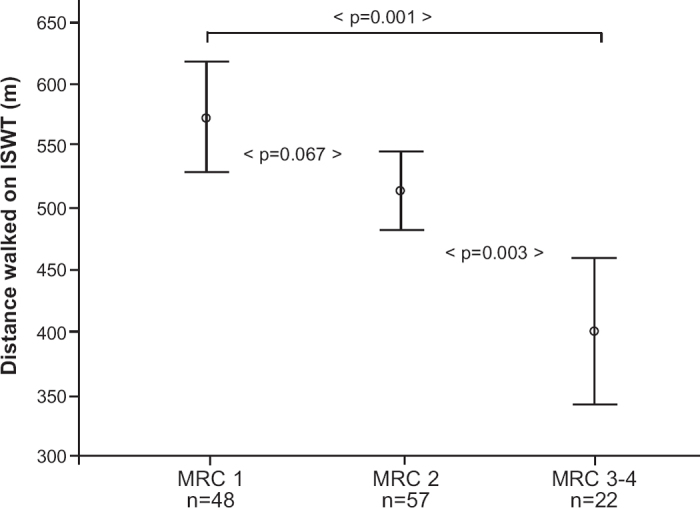
Univariate ANOVA showed that the MRC dyspnoea scale had a statistically significant association with the ISWT (p<0.001). The proportion of explained variance was 16.6% (R2=0.166). Tukey's post hoc analyses revealed a significant difference in walking distance between MRC scores 3–4 and 1 and 2 (p<0.001 and p=0.003, respectively), and borderline significance between MRC scores 2 and 1 (p=0.067).
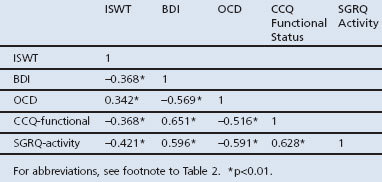
Pearson's correlation coefficients between the ISWT, BDI, OCD, CCQ Functional Status and SGRQ Activity were calculated. Correlations between ISWT and the dyspnoea scales were moderate and varied between −0.421 and 0.342 (Table 3).
Table 3. Pearson's correlation coefficients between ISWT, BDI, OCD, CCQ Functional Status, and SGRQ Activity.
Combined dyspnoea scales
As a final step in our analyses we performed a univariate ANOVA to examine whether a combined set of dyspnoea scales would explain more of the variance in the ISWT distance than the MRC dyspnoea scale alone. The MRC scale was used as an independent variable and the other dyspnoea scales were added as covariates to the model. This full model explained 22.8% of the variance in the observed ISWT distances (R2=0.228). In subsequent steps the OCD, CCQ Functional Status and BDI were excluded from the model as they had p values >0.10. This resulted in a reduced model that contained the MRC and SGRQ Activity, both significantly related to the ISWT (p=0.046 and p=0.007, respectively). This final model explained 21.4% of the variance in the ISWT observations (R2=0.214), which is an additional 4.8% to the 16.6% of variance explained by the MRC alone.
Subgroup analysis of patients with COPD
Exploratory analyses were performed on the 64 patients with COPD to examine whether the results would differ if the subjects at risk were excluded. Pearson's correlation coefficients between the ISWT and BDI, OCD, CCQ Functional Status and SGRQ Activity showed a similar patern as the correlations reported in Table 3 (−0.462, 0.333,−0.355 and −0.521, respectively). The MRC dyspnoea scale could explain 21.9% of the variance of the ISWT. Again, patients with MRC score 3–4 walked significantly shorter distances than patients with MRC scores 1 and 2 (p<0.001 and p=0.02, respectively), whereas there was no difference in the distance walked between those with MRC scores 1 and 2 (p=0.172).
Repeating the univariate ANOVA with the MRC and SGRQ Activity, a total of 29.4% of variance could be explained.
Discussion
Main findings
The aim of this study was to examine whether inquiring about dyspnoea-related functional limitations — using existing and validated scales — can substitute for actual measurement of limitation of functional capacity due to dyspnoea in patients with or at risk of COPD.
The commonly used MRC dyspnoea scale showed a statistically significant but only moderate association with the actual functional capacity test. ISWT scores were highest for subjects with MRC score 1 and lowest in subjects with MRC score 3–4, but this difference failed to reach statistical significance between MRC scores 1 and 2. The relationships between functional capacity and BDI, OCD, CCQ Functional Status subscale, and SGRQ Activity subscale were also moderate. Combining dyspnoea scales in one analysis resulted in a somewhat higher — but still modest — rate of explained variance of the observed ISWT distances. Since the SGRQ Activity subscale has to be weighed and calculated, in our view the extra effort needed if the MRC and SGRQ Activity subscale are combined does not outweigh the limited additional predictive value of the overall instrument.
Interpretation of findings in comparison with previously published work
The moderate relationship between functional capacity and activity-based dyspnoea is not surprising. In the literature, dyspnoea perception and functional capacity are generally viewed as related but separate concepts.27–30 More surprising perhaps is that, apparently without a solid scientific base, guidelines for family practitioners suggest that functional capacity can be assessed with an activity-based dyspnoea questionnaire such as the MRC.13–15
Health care focuses increasingly on prevention and early detection of COPD,1,13 while in most research the pre-clinical stage of COPD is neglected. By including subjects at risk of COPD next to subjects with mild to severe airflow obstruction, we attempted to shed some light on the use of activity-based dyspnoea scales as an indicator of limitations in functional capacity in a primary care setting.
In a study by Taylor et al.,31 mean scores of healthy subjects on the ISWT are reported. After excluding the subjects aged <30 for comparison with our population, the mean distance walked by healthy subjects was 646 m. The subjects in our ‘at risk’ group had a mean ISWT score of 561 m. In a study of the minimal clinically important difference of the ISWT, Singh et al.32 found an improvement of 47.5 m to be ‘slightly better’ and an improvement of 78.7 m to be ‘better’. The difference of 85 m between the healthy subjects in the study by Taylor et al. and the subjects at risk in our study could point to a relevant decline of functional capacity in subjects at risk of COPD, a population that is generally excluded from research.
Strengths and limitations of this study
In this study the distribution of subjects at risk of COPD and patients with mild, moderate and severe disease was unequal. Half of the subjects were not diagnosed with COPD, which could have a profound impact on the results. We therefore repeated all the analyses without the subjects at risk to check this and found similar results. The proportion of explained variance did increase, but not substantially. In addition, the distances walked by smokers at risk of COPD and the patients with GOLD stage I COPD were very similar (561 m vs. 536 m; Figure 1). We therefore have reason to believe that subjects at risk of COPD are not a distinct population from COPD patients in terms of functional capacity. Furthermore, a study on quality of life in a comparable population also found no difference in the results after excluding people at risk of COPD.33
Implications for future research, policy and practice
In this study the association between dyspnoea scales and functional capacity was moderate. The MRC and the SGRQ Activity subscale showed the best results, but the effect sizes were moderate. Furthermore, the difference in the mean ISWT walking distance between MRC scores 1 and 2 was not statistically significant. An important observation for daily clinical practice was the wide spread in the distance walked within the MRC scores. For instance, the minimum distance walked by subjects with MRC score 1 (290 m) was well below the average distance walked by subjects with MRC score 3–4 (400 m), Thus, although for research purposes the MRC or other dyspnoea scales may have sufficient predictive value, in individual patient care this does not seem to be the case. Healthcare professionals should therefore be cautious in deriving conclusions regarding functional capacity based on dyspnoea scales only.
Functional capacity has been shown to influence the course of COPD, and early detection of decline is important.1,13 Approximately 85% of all COPD patients in the UK and the Netherlands are treated by their family practitioner10,34 where standardised functional capacity testing is often not feasible. In that case, referral to a clinic or laboratory with the necessary facilities for testing functional capacity should be considered. Future research on normative and reference data of dyspnoea scales could provide guidelines on when functional capacity testing should be performed (for instance, in patients with MRC score 2), and whether people at risk of COPD could benefit from early detection of limitations in functional capacity.
Conclusions
Activity-based dyspnoea scales cannot be used as a substitute for actual functional capacity tests in a primary care setting. More dyspnoea may indicate lower functional capacity in patients with COPD, but an actual functional capacity test is still needed to confirm such a finding.
We recommend that COPD guidelines state more specifically that the MRC and other dyspnoea scales measure (self-reported) activity-related dyspnoea but cannot replace objectively measured functional capacity.
Acknowledgments
Funding This work was supported by Boehringer Ingelheim b.v., The Netherlands.
Handling editor Niels Chavannes
Statistical review Gopal Netuveli
The authors would like to thank all the subjects who participated in this study for their time and co-operation; Edwin Wagena, Annie Hendriks, Tom de Rooij, Bart van de Ven (Maastricht University), and Marco Akkermans (Asthma Centre Hornerheide) for organising the measurements and collecting and processing the data; and Boehringer Ingelheim b.v. for financial support.
Footnotes
LMB has no conflicts of interest in relation to this article. GMA is an employee of Boehringer Ingelheim b.v. OCPvS is a consultant for Boehringer Ingelheim b.v., AstraZeneca and Pfizer. TRJS is a consultant for Boehringer Ingelheim b.v., GSK and AstraZeneca. OCPvS is an Assistant Editor of, and TRJS an Associate Editor of, the PCRJ; neither were involved in the editorial review of, nor the decision to publish, this article.
References
- Global strategy for the Diagnosis, Management, and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2010. http://www.goldcopd.org/ (accessed 10 Nov 2011).
- Celli BR, Cote CG, Lareau SC, Meek PM. Predictors of survival in COPD: more than just the FEV1. Respir Med 2008;102(Suppl 1):S27–35. http://dx.doi.org/10.1016/S0954-6111(08)70005-2 [DOI] [PubMed] [Google Scholar]
- Curtis JR, Martin DP, Martin TR. Patient-assessed health outcomes in chronic lung disease: what are they, how do they help us, and where do we go from here? Am J Respir Crit Care Med 1997;156:1032–9. http://ajrccm.atsjournals.org/cgi/content/full/156/4/1032 [DOI] [PubMed] [Google Scholar]
- Cazzola M, Hanania NA, Jones PW, et a/. It's about time — directing our attention toward modifying the course of COPD. Respir Med 2008;102(Suppl 1):S37–48. http://dx.doi.org/10.1016/S0954-6111(08)70006-4 [DOI] [PubMed] [Google Scholar]
- Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am J Respir Crit Care Med 2003;167(4):544–9. http://dx.doi.org/10.1164/rccm.200206-583OC [DOI] [PubMed] [Google Scholar]
- Casanova C, Cote C, Marin JM, et al. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest 2008;134(4):746–52. http://dx.doi.org/10.1378/chest.08-0520 [DOI] [PubMed] [Google Scholar]
- Celli BR. Predictors of mortality in COPD. Respir Med 2010;104(6):773–9. http://dx.doi.org/10.1016/j.rmed.2009.12.017 [DOI] [PubMed] [Google Scholar]
- Dolan S, Varkey B. Prognostic factors in chronic obstructive pulmonary disease. Curr Opin Pulm Med 2005;11(2):149–52. http://dx.doi.org/10.1097/01.mcp.0000153548.36054.8f [DOI] [PubMed] [Google Scholar]
- Spruit MA, Polkey MI, Celli B, et al. Predicting outcomes from 6-minute walk distance in chronic obstructive pulmonary disease. J Am Med Dir Assoc 2012;13(3):291–7. http://dx.doi.org/10.1016%2Fj.jamda.2011.06.009 [DOI] [PubMed] [Google Scholar]
- Palange P, Ward SA, Carlsen KH, et al. Recommendations on the use of exercise testing in clinical practice. Eur Respir J 2007;29(1):185–209. http://dx.doi.org/10.1183/09031936.00046906 [DOI] [PubMed] [Google Scholar]
- Haughney J, Gruffydd-Jones K. Patient-centred outcomes in primary care management of COPD — what do recent clinical trial data tell us? Prim Care Respir J 2004;13(4):185–97. http://dx.doi.org/10.1016/j.pcrj.2004.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J 1959;2:257–66. http://dx.doi.org/10.1136/bmj.2.5147.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. London: National Clinical Guideline Centre, 2010. http://guidance.nice.org.uk/ (accessed 10 Nov 2011).
- O'Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease — 2007 update. Can Respir J 2007;14(Suppl B):5B–32B. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2806792/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeele IJM, van Weel C, van Schayck CP, et al. NHG-Standaard COPD. Second Revision. Huisarts Wet 2007;50:362–79. [Google Scholar]
- Wegner RE, Jorres RA, Kirsten DK, Magnussen H. Factor analysis of exercise capacity, dyspnoea ratings and lung function in patients with severe COPD. Eur Respir J 1994;7(4):725–9. http://dx.doi.org/10.1183/09031936.94.07040725 [DOI] [PubMed] [Google Scholar]
- Hoogendoorn M, Rutten-van Molken MP, Hoogenveen RT, et al. A dynamic population model of disease progression in COPD. Eur Respir J 2005;26(2):223–33. http://dx.doi.org/10.1183/09031936.05.00122004 [DOI] [PubMed] [Google Scholar]
- Wagena EJ, Knipschild PG, Huibers MJ, Wouters EF, van Schayck CP. Efficacy of bupropion and nortriptyline for smoking cessation among people at risk for or with chronic obstructive pulmonary disease. Arch Intern Med 2005;165(19):2286–92. http://dx.doi.org/10.1001/archinte.165.19.2286 [DOI] [PubMed] [Google Scholar]
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:5–40. [PubMed] [Google Scholar]
- Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 1992;47(12):1019–24. http://dx.doi.org/10.1136/thx.47.12.1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54(7):581–6. http://dx.doi.org/10.1136/thx.54.7.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie DK, Abramson M, Crockett AJ, et al. on behalf of The Australian Lung Foundation. The COPD-X Plan: Australian and New Zealand. Guidelines for the management of chronic obstructive pulmonary disease 2011. http://www.copdx.org.au/ (accessed 22 Dec 2011).
- Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest 1984;85(6):751–8. http://dx.doi.org/10.1378/chest.85.6.751 [DOI] [PubMed] [Google Scholar]
- McGavin CR, Artvinli M, Naoe H, McHardy GJ. Dyspnoea, disability, and distance walked: comparison of estimates of exercise performance in respiratory disease. Br Med J 1978;2:241–3. http://dx.doi.org/10.1136/bmj.2.6132.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Molen T, Willemse BW, Schokker S, Ten Hacken NH, Postma DS, Juniper EF. Development, validity and responsiveness of the Clinical COPD Questionnaire. Health Qua/ Life Outcomes 2003;1(1):13. http://dx.doi.org/10.1186/1477-7525-1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St George's Respiratory Questionnaire. Am Rev Respir Dis 1992;145(6):1321–7. [DOI] [PubMed] [Google Scholar]
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350(10):1005–12. http://dx.doi.org/10.1056/NEJMoa021322 [DOI] [PubMed] [Google Scholar]
- Foglio K, Carone M, Pagani M, Bianchi L, Jones PW, Ambrosino N. Physiological and symptom determinants of exercise performance in patients with chronic airway obstruction. Respir Med 2000;94(3):256–63. http://dx.doi.org/10.1053/rmed.1999.0734 [DOI] [PubMed] [Google Scholar]
- Kocks JW, Asijee GM, Tsiligianni IG, Kerstjens HA, van der Molen T. Functional status measurement in COPD: a review of available methods and their feasibility in primary care. Prim Care Respir J 2011;20(3):269–75. http://dx.doi.org/10.4104/pcrj.2011.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercoulen JH, Daudey L, Molema J, et al. An Integral assessment framework of health status in chronic obstructive pulmonary disease (COPD). Int J Behav Med 2008;15(4):263–79. http://dx.doi.org/10.1080/10705500802365474 [DOI] [PubMed] [Google Scholar]
- Taylor S, Frost H, Taylor A, Barker K. Reliability and responsiveness of the shuttle walking test in patients with chronic low back pain. Physiother Res Int 2001;6(3):170–8. http://dx.doi.org/10.1002/pri.225 [DOI] [PubMed] [Google Scholar]
- Singh SJ, Jones PW, Evans R, Morgan MD. Minimum clinically important improvement for the incremental shuttle walking test. Thorax 2008;63(9):775–7. http://dx.doi.org/10.1136/thx.2007.081208 [DOI] [PubMed] [Google Scholar]
- Geijer RM, Sachs AP, Verheij TJ, Kerstjens HA, Kuyvenhoven MM, Hoes AW. Quality of life in smokers: focus on functional limitations rather than on lung function? Br J Gen Pract 2007;57(539):477–82. [PMC free article] [PubMed] [Google Scholar]
- Tirimanna PR, van Schayck CP, den Otter JJ, et al. Prevalence of asthma and COPD in general practice in 1992: has it changed since 1977? Br J Gen Pract 1996;46(406):277–81. [PMC free article] [PubMed] [Google Scholar]


