Abstract
Approaching the end of the second decade of the 21st century, almost the whole demand of vanillin is met by the synthetic product obtained either via a petrochemical process starting from phenol and glyoxylic acid or from energy intensive alkaline oxidative depolymerization of lignin. Only a minor fraction is comprised of natural vanillin obtained from ferulic acid fermentation, and even less of highly valued Vanilla planifolia extracts. Are there alternative green production methods? And, if yes, are they suitable to find practical application?
Keywords: vanillin, SiliaSun, vanillic acid, vanilla, photocatalysis
1. Introduction
Called vanillin by Gobley who first isolated the white crystals from Vanilla planifolia pods ethanol extracts obtained in France in 1858,1 vanillin (4‐hydroxy‐3‐methoxybenzaldehyde, C8H8O3) is a biophenol relatively abundant (in the form of β‐D‐glucoside) in the Vanilla planifolia orchid green pods. It is extracted from the latter, after a curing process lasting months during which the pods turn into dark brown, alongside numerous other compounds, preferably with supercritical CO2, so as to obtain V. planifolia integral extracts, commercialized at a price that in June 2018 reached out that of silver.2
The substance is both a sweet‐smelling fragrance used in perfumery and a flavor widely used in food and flavored beverages.3 “Vanilla beans and their extracts”, has lately written Buccellato, “were used by the ancient Totonaco and Aztec Indians of Mexico. Vanilla beans were used to make a drink called chocolatl, containing powdered cocoa beans, ground corn, and flavored with tlilxochitl (ground black vanilla pods and honey)”.3
Being also a powerful antioxidant biophenol, vanillin is not used only for flavoring and as a fragrance, but also as a food‐preserving agent, able to impart additional nutraceutical properties, due do its antimicrobial, anti‐mould, anti‐yeast, and antioxidant activities.4
Reflecting the ever increasing relevance of this substance, the second edition of a comprehensive treaty on the science and technology of vanilla was published in early 2019.5
Is chemical synthesis starting from coniferyl alcohol (obtained from pine bark) was developed in Germany by Tiemann and Haarmann in 1874.6
The two chemists founded a company in Holzminden (Haarmann's Vanillinfabrik) which by 1876 was joined by Reimer and started to use the Reimer‐Tiemann reaction for the ortho‐formylation of phenols (a 3 molar excess of KOH dissolved in chloroform under reflux) to isoeugenol which in alkaline solution is then oxidised (by nitrobenzene) to vanillin with eugenol profitably sourced from clove oil.7
The company changed its name to Haarmann & Reimer, and under a different name after merging in 2003 with another German company, it continues to supply both natural vanilla and synthetic vanillin, as more than 18,000 products were reported in 2016 to contain vanilla as flavor or fragrance ingredient.8
Nowadays, the global and increasing vanillin demand (around 20,000 tons)7 is almost entirely (>85 %) met by synthetic vanillin produced from petroleum‐derived phenol (converted into guaiacol) and glyoxylic acid. The latter acid and guaiacol react in a two‐step process starting with condensation promoted by base, followed by oxidative decarboxylation of vanillylmandelic acid to vanillin catalyzed by copper(II) in an aqueous alkaline medium at a temperature of 80–130 °C.9 Crude vanillin is then purified via vacuum distillation and recrystallization.
The process is currently carried out at five plants: three in China (one of which is a joint venture with an India‐based company), one in France and one in the U.S. The resulting vanillin produced from guaiacol is sold at $10–20/kg mainly to ice cream and chocolate manufacturers, and to flavor and fragrance companies.
The remainder vanillin is produced from lignin via an alkaline oxidation process consisting in treating an alkaline aqueous solution of lignin with oxidants, at high temperature and pressure, to depolymerize the lignin and obtain crude vanillin containing structurally similar compounds like acetovanillone and syringaldehyde difficult to separate.9
Contrary to what happened with citric acid originally sourced from lemon juice,10 the industrial synthesis did not entirely displace the natural extraction route with Vanilla planifolia harvested in Madagascar, Indonesia, Mexico, Uganda, Belize, Tahiti, and Australia (amid other countries).
Then, driven by the sustainability megatrend translating into an ever‐increasing demand for natural products replacing synthetic ones, by early 2015 the world's largest single user of synthetic vanillin announced plans to eliminate artificial additives including synthetic vanillin from chocolate sold in the U.S.8
That single decision, coupled to bad orchid harvesting season in Madagascar, led prices to more than double to roughly $225/kg by mid 2015.8 Since cured vanilla beans contain only 2 % of extractable vanilla flavor, this translates into pure vanilla price>$11,000/kg.
Since then, not only vanilla prices did not return “to pre‐2012 levels of about $25/kg for beans or $1,250 for vanilla”,8 but further increased to $515/kg for cured vanilla beans as of early June 2018 (almost as expensive as silver which by then was priced at $527/kg).2
The 20‐fold vanilla price increase between 2012 and 2018 opened the route to alternative vanillin production methods that had remained a niche part of the market, such as biobased production by conversion of ferulic acid over native or genetically modified fungi, yeast or bacteria,11 but also to the uptake of V. planifolia greenhouse cultivation in the Netherlands.12
Are there greener vanillin production methods suitable to find practical application? Could the booming global demand for naturals drive innovation in vanillin chemical production? We offer a critical insight whose outcomes can also be used as educational resource in the context of evolving, research‐based chemistry education.13
2. The Case for Green, Bio‐Based Production
In the early 2000s a flavor and fragrance company developed to an industrial scale the biotechnology route for the production of vanillin from ferulic acid over bacterial strains. The process had been introduced by Walton and co‐workers in 1998.14
“The process”, lately emphasized a vanillin industry's practitioner, “is very complex and one needs 1000 kg of rice bran to produce 1 kg of vanillin”.7
Linked to the plant cell wall to whom it confers rigidity by making the crosslink between polysaccharides and lignin,15 ferulic acid is extracted by treating for example rice bran (an agro‐industrial waste with high ferulic acid content) with base. The obtained mixture is then filtered, extracted with organic solvents and purified leaving behind “mountains of biomass… that are expensive to dispose of.”7
Still, three main producers, one based in Europe and two in China, currently use the process on industrial scale to manufacture each 400–500 tons annually, with the biobased vanillin reportedly sold at $400–600/kg.7
Furthermore, today's green chemistry coupled to modern statistical optimization techniques enables to use a versatile and solid‐supported enzyme (feruloyl esterase) for extracting ferulic acid from wheat bran (also an agro‐industrial waste with high ferulic acid content) in high yield under mild conditions in a packed column reactor, followed by adsorption on an Amberlite resin, obtaining 99.5 % recovery rate using methanol as a reusable eluent.16
Upscaling the latter process there would be no “mountains of biomass to be dispensed of”, but only a valued lignocellulosic solid residue ready for saccharification and subsequent utilization of glucose as key platform molecule affording a closed, circular economy process typical of second‐generation biorefineries using abundant agro‐industrial residues and byproducts such as wheat, maize and rice bran as raw materials.17
Already employed as a powerful antioxidant in the production of pharmaceuticals, healthy food and cosmetic products, and in sunscreen formulations (where it stabilizes vitamins C and E and doubles photoprotection of the skin),18 the ferulic acid market is in its nascent stage.19
In the early 2000s a flavor and fragrance company developed the technology for the production of vanillin by fermentation of ferulic acid and licensed it to a fine chemical manufacturer which started production of natural vanillin (then priced at about $700/kg when natural extracts from vanilla beans cost around $1,800/kg and synthetic vanillin was priced at $15/kg).20
Although margins exist for increasing vanillin extraction rates from cured vanilla pods, for example under ultrasound‐assisted processes,21 the price of natural vanillin from vanilla beans now exceeds $25,000/kg.2
With the price of vanillin from V. planifolia extracts having increased by a factor >20 between 2012 and 2018, it may not be surprising to learn that the price of natural vanillin obtained from ferulic acid fermentation was increased by 15 % in 2017,22 and then again by 15 % the subsequent year.23
This powerful economic trend makes vanillin production routes from ferulic acid suitable for industrial scale‐up, particularly those producing no toxic or hazardous effluents, i. e. truly green chemistry processes.
3. The Case for Photocatalysis in Water
In 2012, Palmisano and co‐workers reported the first photocatalytic synthesis of vanillin from ferulic acid mediated by nanostructured TiO2 carried out in water with oxygen as the only oxidant and under UV light irradiation (Figure 1).24
Figure 1.
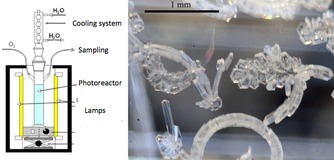
Synthesis of vanillin in water by TiO2 photocatalytic conversion of ferulic acid under UV irradiation in batch photoreactor (left); vanillin crystals formed by deposition of the permeate vapors (right).
The vanillin yield was low (12 % selectivity with 14 % substrate conversion after 90 min irradiation over Merck TiO2), but coupling photocatalysis with a membrane separation unit (pervaporation, Scheme 1) allowed to double the yield and to obtain highly pure vanillin crystals (>99.8 %) downstream to the integrated process.25
Scheme 1.
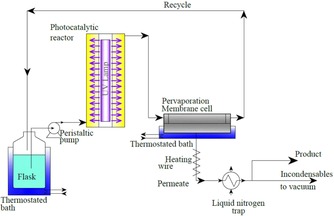
Semi‐continuous reaction system integrating photocatalytic reaction and pervaporation unit used to synthesize highly pure vanillin without purification steps. [Reproduced from Ref. [25], with kind permission].
Vanillin formed via an elegant partial oxidation of the ferulic acid alcohol function to aldehyde involving both semiconductor photocatalysis and catalytic reaction (molecular addition) with O2 at the surface of the TiO2,26 selectively permeates through the non‐porous pervaporation membrane while ferulic acid and most of the other organic photocatalytically produced intermediates are effectively retained in the reactor, mainly due to their low volatility. In this way vanillin can be removed from the reacting medium thus limiting its further oxidation and improving the selectivity of the process.
“In this simple way”, the authors wrote, “an almost complete purification from all the other compounds is obtained without the necessity to use complex extraction and re‐crystallization procedures”.24 Moreover, the membrane completely retains the powdered photocatalyst, avoiding the need of further separation steps.
As expected, the yield of the intensified process was significantly enhanced when compared to the batch process, reaching the level of 4.60 %. Calculations point to a maximum level of 8.48 % yield using a different reactor under further optimized conditions, by optimizing the ratio between the rate of pervaporation and the rate of reaction.27
Coupling photocatalysis and pervaporation is straightforward due to the congruent operating conditions of the two processes. On the other hand, the use of pervaporation to remove vanillin from the fermentation broth, so as to reduce the inhibition effect of vanillin on the enzymatic reaction, is realistic only for a post process separation, due to the low permeate flux at the typical fermentation temperatures.
Numerous other synthetic strategies for the production of vanillin based on the oxidation of trans‐ferulic acid were reported in the subsequent years: since 2015, when Aguilar‐Pliego and co‐workers described the conversion of ferulic acid dissolved in a water/ethanol/acetonitrile mixture in only 1 h with 95 % reaction yield over porous coordination polymer HKUST‐1 as a catalyst and 30 wt% aqueous H2O2 as primary oxidant;28 through 2018 when the same team reported the catalytic transformation of trans‐ferulic acid dissolved in the same mixture to vanillin (yield of 71 % and 97 % selectivity) over the unsaturated Cu metal sites of nanocrystalline Cu−MOF‐74, this time using 50 wt% aqueous H2O2 as primary oxidant.29
In 2016 a joint Italy‐China team reported that ferulic acid dissolved in water is selectively oxidized to vanillic acid in high yield (≈60 %) under remarkably mild and green conditions, namely at room temperature with air as primary oxidant, over a catalytic amount of nanostructured Bi2WO6.30
Vanillic acid to vanillin can be easily reduced to vanillin through metal catalyzed hydrogenation.31
The reaction is both simple (carried out in a closed tube, under stirring a 0.6 mM ferulic acid solution in water containing a 1.5 g L−1 catalyst suspension in the dark), and extremely selective, affording the formation of vanillic acid only.30 The catalyst is a n‐type semiconductor with 2.7 eV bandgap with an orthorhombic structure formed by alternating Bi2O2 2+ and WO4 2− perovskite layers, which has lately emerged as a most promising visible‐light photocatalyst for synthetic organic chemistry.32
The total organic carbon (TOC) analysis before and after reaction highlights a difference between the two TOC values of 26.94 ppm, practically corresponding to the loss of CO2 from the ferulic acid side chain.
The catalytic cycle (Scheme 2) explaining the oxidation mechanism starts with double bond adsorption on a tungsten site (II), followed by O2 insertion into the coordinated intermediate at the tungsten site, and abstraction of a vinyl H (III). The intermediate (III) releases the reduced catalyst (IV) and the partially oxidized 3‐(4‐hydroxy‐3‐methoxyphenyl)‐1,1‐dihydroxy‐1‐propen‐3‐one which, after spontaneous loss of CO2, is oxidized to vanillic acid. Rapid reoxidation of the W sites leads to bismuth tungstate ready for another catalytic cycle.
Scheme 2.
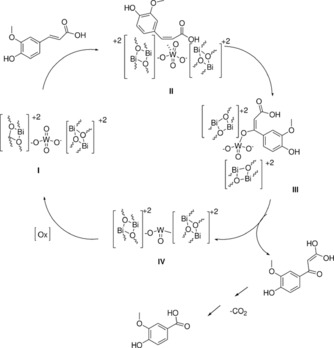
Mechanism for the catalytic oxidation of ferulic acid mediated by Bi2WO6. [Reproduced from Ref. [30], with kind permission].
Oxygen for the reaction originates from static air (no air bubbling conditions), which explains the long (15 h) induction time of the reaction. The reaction under oxygen, rather than under air, is inhibited due to competitive adsorption of O2 on W active sites.
The catalytic nature of the reaction is confirmed by the unmodified crystalline structures of Bi2WO6 before and after reaction, while the catalyst is recyclable, retaining its selective activity in all catalytic tests (>10) in which it was used without any noticeable reduction in activity.
Irradiation of the catalytic suspension in oxygen atmosphere and under simulated solar light afforded almost complete mineralization of ferulic acid. However, entrapping the Bi2WO6 catalyst in silica and organosilica matrices (SiliaSun catalysts) modulates the photocatalytic reaction and endows flower‐like nanostructured Bi2WO6 with new selective activity of synthetic relevance.33
In fact, vanillin and vanillic acid are selectively obtained. The sol‐gel entrapment of flower‐like Bi2WO6 in the non photo‐active silica matrix reduces the overall conversion but doubles the selectivity to vanillic acid with respect to bare Bi2WO6 (Table 1).
Table 1.
Conversion of ferulic acid in air under visible light over Bi2WO6, sol‐gel entrapped SiliaSun catalysts and photocatalytic TiO2. [Reproduced from Ref.33, with kind permission].
| Catalyst | Reaction Time (h) | Ferulic acid Conversion (%) | Vanillic acid Selectivity (%) | Vanillin Selectivity (%) |
|---|---|---|---|---|
| Bi2WO6 | 1 | 42 | 5.0 | 2.7 |
| 2 | 78 | 13.7 | 4.1 | |
| 3 | 80 | 15.3 | 4.5 | |
| 6 | 86 | 15.6 | 4.9 | |
| SiliaSun Me0 % | 1 | 21 | 10.0 | 1.1 |
| 2 | 36 | 30.0 | 1.2 | |
| 3 | 37 | 32.8 | 1.8 | |
| 6 | 59 | 26.0 | 2.0 | |
| SiliaSun Me10 % | 1 | 21 | 12.0 | 0.8 |
| 2 | 31 | 38.5 | 2.1 | |
| 3 | 35 | 33.7 | 3.6 | |
| 6 | 61 | 31.0 | 4.1 | |
| Tio2 | 1 | 93 | – | 1.2 |
| 3 | 99 | 0.2 | 2.1 |
Highlighting the remarkable effect of Bi2WO6 sol‐gel encapsulation, the mass activity of Bi2WO6 catalytic centers is higher in the SiliaSun, where wolframate constitutes only 10 wt% of the sol‐gel material, than in the free standing semiconductor.
As a result, whereas in the presence of free standing Bi2WO6 the conversion of ferulic acid to vanillic acid in the dark required almost 90 h, the photocatalytic reaction over the SiliaSun Me10 % reaches comparable excellent selectivity values to vanillic acid after approximately 2 h (Figure 2).
Figure 2.
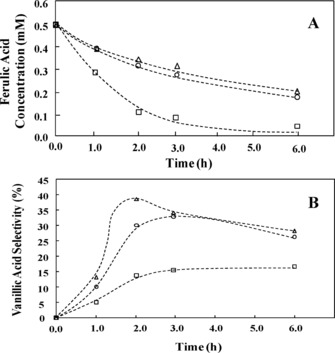
Concentration of ferulic acid during irradiation time (A) and correspondent selectivity values towards vanillic acid (B) for pure Bi2WO6 (□), SiliaSunMe0 % (○), and SiliaSunMe10 % (Δ) photocatalysts in representative runs. [Reproduced from Ref. [33], with kind permission].
Notably, the reduced conversion obtained for the SiliaSun photocatalysts with respect to free Bi2WO6 (Figure 2A, displaying the concentration of trans‐ferulic acid during irradiation over pure Bi2WO6 and over the SiliaSun photocatalysts) translates into twice higher selectivity values.
The selectivity reaches a maximum and then decreases after a critical reaction time (Figure 2B, with the 10 %‐methylated SiliaSun catalyst slightly enhancing the selectivity towards the vanillin).
In each case, switching from O2 to air significantly reduces mineralization while affording formation of vanillic acid and vanillin.
This general trend is due to the competition for oxidation between the formed target compounds and the substrate molecules. For the sake of comparison, the oxidation of trans‐ferulic acid was performed under the same experimental conditions in the presence of TiO2 P25 (Evonik, Germany).
In this case, the substrate is almost completely oxidized after 1 h irradiation and the selectivity towards the desired products is very poor, thus demonstrating the superior synthetic performance of the SiliaSun photocatalyst.
Indeed, differently from TiO2 mediated photooxidations, Bi2WO6 induced photocatalytic oxidation processes do not proceed through .OH radicals but via direct holes and via more selective peroxidic species including the superoxide anion.
4. A Feasible Technology?
Selective photocatalysis of lignin‐derived chemicals carried out under flow in a continuous microreactor is a superior approach for the generation of valuable products emanating from lignin depolymerization and, as put it by Colmenares and co‐workers, can lead to the commercialization of bio‐based chemicals.34
It is instructive to review the feasibility of the sunlight‐driven photocatalytic conversion of ferulic acid over the SiliaSun against the key process quality principle of prevention of errors, emissions and accidents in fine chemical manufacturing, and the related main principles of green chemistry.35
The aforementioned principles are: i) maximize incorporation of the used materials into the final product; ii) use reactants possessing little or no toxicity to human health and environment; iii) avoid the use of auxiliary substances (solvents, separation agents, etc.) or use only innocuous ones; iv) minimize energy requirements and work at ambient temperature and pressure; v) use renewable raw materials, vi) avoid unnecessary derivatization (blocking group, protection/deprotection), and vii) select substances so as to minimize potential for chemical accidents (e. g. releases, explosions, fires).
Conducting the sunlight‐driven photocatalytic conversion of ferulic acid over the SiliaSun in water, at room temperature, employing renewable and safe ferulic acid, air and sunlight photons as reactants meets all the aforementioned principles.
For the purpose of industrial adoption, what remains to be demonstrated is the technical and economic feasibility of contemporary photocatalysis in fine chemical manufacturing. Thanks to the 2005 invention of the continuous tubular photoreactor36 and to the development of new and much more selective photocatalysts working under visible‐light, this has lately become possible.37
The 2017 forecast, according to which the progress in fine chemical manufacturing, after the widespread inception of homogeneous and heterogeneous catalysis, would have been “followed by a second major progress in which a wide range of compounds including active pharmaceutical ingredients will be obtained in high yield and purity under continuous flow in modular photochemical microreactors using energy efficient light emitting diodes (LEDs) as photon sources,37 has materialized: today, several fine chemical companies use photocatalysis under flow for numerous productions.
The photocatalytic production of vanillin from ferulic acid in water extends and complements current bioproduction from the same biobased substrate using fermentation.
The latter is a time demanding process, affording vanillin in low concentration (the aldehyde is toxic to micro organisms) and requires numerous purification steps. However, the resulting vanillin is a natural product conforming for example to European regulation36 on flavoring agents (EC N° 1334/2008) which can be labelled as “natural vanillin” having been obtained from materials of natural origin and thereby complying “with certain criteria which ensure that consumers are not misled”:38
«‘Natural flavouring substance’ shall mean a flavouring substance obtained by appropriate physical, enzymatic or microbiological processes from material of vegetable, animal or microbiological origin either in the raw state or after processing for human consumption by one or more of the traditional food preparation processes listed in Annex II».38
According to the above definition the photocatalytically produced vanillin cannot be labelled as a “natural flavouring substance” as photocatalysis is a chemical, and not a physical process.
This is surprising by considering that, for instance, TiO2 is a safe substance currently used as food additive and component of cosmetics and personal care products, whereas, for comparison, bacterial strains such as potentially contaminating E. Coli are generally used to produce bioderived vanillin which, according to the aforementioned EU definition, can be labeled “natural vanillin”.
The photocatalytic production of vanillin would no longer employ cumbersome photocatalytic batch reactors with immersed large and poorly efficient lamps producing more heat than light. Rather, it would be either carried out under flow,39 or even by immobilization of the photocatalyst on (wireless) internal LED light sources,40 with significant advantages in both cases for up‐scaling the photocatalytic process.
Originating from a liquid undergoing the sol‐gel transition,41 mesoporous sol‐gel photocatalysts provide unique versatility in the production of the thin catalytic films required by the aforementioned applications (for example, TiO2 on hollow glass beads, or SiliaSun thin film coatings).
In a recent account42 on liquid phase aerobic oxidations in continuous flow, Kappe and Hone aptly called molecular oxygen “the ultimate” green oxidant. The team noted therein the limited uptake of O2 or air as primary oxidant in the fine chemical industry, most often due to the high solvent flammability.
“Water”, wrote the scholars, “is the ideal solvent for aerobic oxidations carried out with air's oxygen, However, a significant limitation associated with using water within flow reactors is that the inherent carbon richness of organic substrates mean that most do not dissolve in water, causing slow reaction rates”.42
Ferulic acid solubility in water at room temperature is low (0.78 g L−1, affording a 4 mM solution),43 but large enough to smoothly carry out the biotransformation used to manufacture natural vanillin.11
In conclusion, the photocatalytic conversion of ferulic acid to vanillin and vanillic acid is both feasible and desirable, while the extraction of ferulic acid can similarly be made greener, more efficient, and cheaper.16 Consumers across the world demanding natural vanillin would be further pleased learning that the exquisite flavoring agent was ultimately obtained converting a wheat or rice by‐product dissolved in water using merely air and sunlight photons as reactants.
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Professor of Physical Chemistry and Spectroscopy at Instituto Superior Técnico, Universidade de Lisboa, Laura M. Ilharco also lectures regular postgraduate courses at Mexican and Portuguese universities, including the Master in Biological Engineering at Universidade de Lisboa. Encompassing numerous research fields, from functional silica‐based nanomaterials to green chemistry through the bioeconomy, her research has addressed both fundamental and applied aspects in materials science and technology and spectroscopy as well in green chemistry. In 2003 she was awarded the Solvay Ideas Challenge prize.

Biographical Information
Mario Pagliaro is a chemistry and energy scholar based at Italy's Research Council in Palermo, where for close to 20 years he has led a research group focusing on nanochemistry, sustainability and the bioeconomy. Rapidly approaching 10,000 citations as of early 2019, he ranks amongst Italy's most cited scientists in nanotechnology and materials science. In recognition of his “significant contributions to the chemical sciences” in 2014 he was designated Fellow of the Royal Society of Chemistry. His work has been widely highlighted by national and international press, including by MIT Technology Review, Advanced Science News, Italy's national television, newspapers and magazines.

Acknowledgements
This study is dedicated to Professor Leonardo Palmisano, University of Palermo, for all he has done in the course of the last thirty years (1988–2018) to establish a world's class photocatalysis school in Sicily.
R. Ciriminna, A. Fidalgo, F. Meneguzzo, F. Parrino, L. M. Ilharco, M. Pagliaro, ChemistryOpen 2019, 8, 660.
References
- 1. Gobley N. T., J. Pharm. Chim. 1858, 34, 401–405. [Google Scholar]
- 2.D. Pilling, The real price of Madagascar's vanilla boom, Financial Times, 5 June 2018.
- 3.F. Buccellato, Vanilla in Perfumery and Beverage Flavors. In Handbook of Vanilla Science and Technology, D. Havkin-Frenkel, F. C. Belanger (Eds.), Wiley, New York: 2019; pp.367-373.
- 4. Tomadoni B., Viacava G., Cassani L., Moreira M. R., Ponce A., J. Food Sci. Technol. 2016, 53, 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D. Havkin-Frenkel, F. C. Belanger (Eds., Handbook of Vanilla Science and Technology, Wiley, New York: 2019.
- 6. Tiemann F., Haarmann W., Ber. Dtsch. Chem. Ges. 1874, 7, 608–623. [Google Scholar]
- 7. Havkin-Frenkel D., Vanillin, Kirk-Othmer Encyclopedia of Chemical Technology, Wiley, New York: 2018; pp.1–12. [Google Scholar]
- 8. Bomgardner M. M., Chem. Eng. News 2016, 94, 38–42. [Google Scholar]
- 9. Fache M., Boutevin B., Caillol S., ACS Sustainable Chem. Eng. 2016, 4, 35–46. [Google Scholar]
- 10. Ciriminna R., Meneguzzo F., Delisi R., Pagliaro M., Chem. Cent. J. 2017, 11 : 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Banerjee G., Chattopadhyay P., J. Sci. Food Agric. 2019, 99, 499–506. [DOI] [PubMed] [Google Scholar]
- 12.F. van Noort, Vanilla in Dutch Greenhouses: A Discovery – From Research to Production. In Handbook of Vanilla Science and Technology, D. Havkin-Frenkel, F. C. Belanger (Eds.), Wiley, New York: 2019; pp.157–163.
- 13.M. Pagliaro, Isr. J. Chem. DOI: 10.1002/ijch.201800179.
- 14. Gasson M. J., Kitamura Y., McLauchlan W. Russell, Narbad A., Parr A. J., Parsons E. Lindsay H., Payne J., Rhodes M. J. C., Walton N. J., J. Biol. Chem. 1998, 273, 4163–4170. [DOI] [PubMed] [Google Scholar]
- 15. Kumar N., Pruthi V., Biotechnol. Rep. 2014, 4, 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gopalan N., Nampoothiri K. M., Biocatal. Agric. Biotechnol. 2018, 15, 304–310. [Google Scholar]
- 17. Luque R., Clark J. H, Sustain. Chem. Process 2013, 1 : 10. [Google Scholar]
- 18. Lin F.-H., Lin J.-Y., Gupta R. D., Tournas J. A., Burch J. A., Selim M. A., Monteiro-Riviere N. A., Grichnik J. M., Zielinski J., Pinnell S. R., J. Invest. Dermatol. 2005,125, 826–32. [DOI] [PubMed] [Google Scholar]
- 19.Currently extracted from rice bran oil, the global market forecasts to grow at the level of 6 % compound annual rate during 2019–2025, to reach $84 million by the end of 2025: RNR Market Research, Global Ferulic Acid Market Growth 2019–2024, Pune: 2019.
- 20. Rouhi A. Maureen, Chem. Eng. News 2003, 81, 54. [Google Scholar]
- 21. Jadhav D., Rekha B. N., Gogate P. R., Rathod V. K., J. Food Eng. 2009, 93, 421–426. [Google Scholar]
- 22.Solvay, “Solvay announces price increase for vanillin and ethyl-vanillin across North America, Princeton, NJ, 12 October 2017. See at the URL: www.solvay.us/en/binaries/Solvay_Aroma_price%20increase_01.12.2017.Final-325702.pdf
- 23.Weekly Roundup: Solvay increases price of vanillin and ethyl-vanillin ingredients, Stern-Wywiol Gruppe enlarges production capacity in Mexico, FoodIngredientsFirst, 9 November 2018. See at the URL: www.foodingredientsfirst.com/news/weekly-roundup-solvay-increases-price-of-vanillin-and-ethyl-vanillin-ingredients-stern-wywiol-gruppe-enlarges-production-capacity-in-mexico.html
- 24. Augugliaro V., Camera-Roda G., Loddo V., Palmisano G., Palmisano L., Parrino F., Puma M. A., Appl. Catal. B 2012, 111, 555–561. [Google Scholar]
- 25. Camera-Roda G., Augugliaro V., Cardillo A., Loddo V., Palmisano G., Palmisano L., Chem. Engineer. J. 2013, 224, 136–143. [Google Scholar]
- 26. Parrino F., Palmisano L., Mini-Rev. Org. Chem. 2018. 15, 157–164. [Google Scholar]
- 27. Camera-Roda G., Cardillo, Loddo V., Palmisano L., Parrino F., Membranes 2014. 4, 96–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yepez R., García S., Schachat P., Sánchez-Sánchez M., González-Estefan J. H., González-Zamora E., Ibarra I. A., Aguilar-Pliego J., One-Pot, New J. Chem. 2015, 39, 5112–5115. [Google Scholar]
- 29. Flores J. G., Sánchez-González E., Gutiérrez-Alejandre A., Aguilar-Pliego J., Martínez A., Jurado-Vázquez T., Lima E., González-Zamora E., Díaz-García M., Sánchez-Sánchez M., Ibarra I. A., Dalton Trans. 2018, 47, 4639–4645. [DOI] [PubMed] [Google Scholar]
- 30. Delisi R., Ciriminna R., Parrino F., Palmisano L., Xu Y.-J., Pagliaro M., ChemistrySelect 2016, 1, 626–629. [Google Scholar]
- 31.T. Fenlon, S. Ruedenauer, R. Milicic, EP3109226 A1, 2015.
- 32. Zhang N., Ciriminna R., Pagliaro M., Xu Y.-J., Chem. Soc. Rev. 2014, 43, 5276–5287. [DOI] [PubMed] [Google Scholar]
- 33. Ciriminna R., Delisi R., Parrino F., Palmisano L., Pagliaro M., Chem. Commun. 2017, 53, 7521–7524. [DOI] [PubMed] [Google Scholar]
- 34. Colmenares J. C., Varma R. S., Naira V., Chem. Soc. Rev. 2017, 46, 6675–6686. [DOI] [PubMed] [Google Scholar]
- 35.P. Anastas, J. Warner, Green Chemistry: Theory and Practice, Oxford University Press: New York, 1998.
- 36. Hook B. D. A., Dohle W., Hirst P. R., Pickworth M., Berry M. B., Booker-Milburn K. I., J. Org. Chem. 2005, 70, 7558–7564. [DOI] [PubMed] [Google Scholar]
- 37. Ciriminna R., Delisi R., Xu Y.-J., Pagliaro M., Org. Process Res. Dev. 2016, 20, 403–408. [Google Scholar]
- 38.Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 Regulation (EC) No 1334/2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC, Official Journal of the European Union, 31 December 2008.
- 39.One selected practical example: Emmanuel N., Mendoza C., Winter M., Horn C. R., Vizza A., Dreesen L., Heinrichs B., Monbaliu J.-C. M., Org. Process Res. Dev. 2017, 21, 1435–1438. [Google Scholar]
- 40. Burek B. O., Sutor A., Bahnemann D. W., Bloh J. Z., Catal. Sci. Technol. 2017, 7, 4977–4983. [Google Scholar]
- 41. Ciriminna R., Fidalgo A., Béland F., Pandarus V., Ilharco L. M., Pagliaro M., Chem. Rev. 2013, 113, 6592–6620. [DOI] [PubMed] [Google Scholar]
- 42. Hone C. A., Kappe C. O., Top. Curr. Chem. 2019, 377: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mota F. L., Queimada A. J., Pinho S. P., Macedo E. A., Ind. Eng. Chem. Res. 2008, 47, 5182–5189. [Google Scholar]


